Abstract
Introduction:
Patients who undergo endovascular abdominal aortic aneurysm repair (EVR) remain at risk for reintervention and rupture. We sought to define the five-year rate of reintervention and rupture following EVR in the Vascular Quality Initiative (VQI).
Methods:
We identified all patients in the VQI who underwent EVR from 2003 – 2015. We linked patients in the VQI to Medicare claims for long-term outcomes. We stratified patients on baseline clinical and procedural characteristics to identify those at risk for reintervention. Our primary outcomes were five-year rates of reintervention and late aneurysm rupture after EVR. We assessed these with Kaplan-Meier survival estimation.
Results:
We studied 12,911 patients who underwent EVR. Mean age 75.5 years, 79.9% male, 3.9% black, and 89.1% of operations were performed in the elective setting. The five-year rate of reintervention for the entire cohort was 21%, and the five-year rate of late aneurysm rupture was 3%. Reintervention rates varied across categories of EVR urgency. Patients who underwent EVR in the elective setting had the lowest five-year rate of reintervention at 20%. Those who underwent surgery for symptomatic aneurysms had higher rates of reintervention at 25%. Patients undergoing EVR emergently for rupture had the highest rate of reintervention, 27% at four years (log-rank across the 3 groups, p<.001). Black race and aneurysm size ≥6.0 centimeters were associated with significantly elevated reintervention rates (black: 31% versus white: 20%, log-rank p<.001; aneurysm size ≥6.0: 27% versus all others: <20%, log-rank p<.001). There were no significant associations between age or gender and the five-year rate of reintervention.
Conclusions:
More than one in five Medicare patients undergo reintervention within five years after EVR in the VQI, while late rupture remains low at 3%. Black patients, those with large aneurysms, and those who undergo EVR in urgent and emergent settings, have a higher likelihood of adverse outcomes and should be the focus of diligent long-term surveillance.
Table of Contents Summary:
This retrospective study of 12,911 endovascular aortic aneurysm repair (EVAR) patients enrolled in the VQI-Medicare matched database found that one in five patients underwent reintervention, and that black patients, those with large aneurysms, and those after urgent or emergent EVAR had higher rates of reintervention. These high risk subgroups need diligent long-term surveillance.
Introduction:
Four out of five Americans who undergo surgical repair of an abdominal aortic aneurysm do so by means of an endovascular repair (EVR).1 The transition to EVR as the de facto method of aneurysm repair in the United States has been associated with several benefits. EVR has lower in-hospital mortality, fewer adverse cardiac events, and patients are discharged from the hospital earlier, when compared to traditional open surgery.2–4
However, this transition has also created new challenges for patients undergoing aneurysm repair and the providers caring for them. One of the most impactful challenges is reintervention – procedures performed after the index EVR that are related to the aneurysm or the aneurysm repair.4 While patients who undergo open surgical repair require minimal imaging and infrequently require repeat procedures related to their aneurysm, patients who undergo EVR are recommended to undergo lifelong annual imaging surveillance as they remain at risk for aneurysm and endograft-related reintervention.2, 4–6 The rate at which these procedures occur in real world practice is not well defined, and subgroups of patients who may be at elevated risk for reintervention remain unknown.7
Therefore, the purpose of this study, was to define the five-year rate of reintervention among patients undergoing EVR at Vascular Quality Initiative (VQI) participating centers. To do this, we leveraged granular clinical information from the VQI, and linked it with reintervention events found in Medicare using a validated claims based algorithm.8 We sought to describe overall reintervention rates, and to identify high risk subgroups of individuals who remain at elevated risk for reintervention after EVR.
Methods:
Data sources and analytic cohort
We used data from the VQI registry linked to Medicare claims at the patient level. Patients were linked directly using unique identifiers with 95.0% matching success.9 Data from Medicare claims were available from January 1st 2003 until September 30th 2015, which was used as the end of the study period. Corresponding VQI data was used from these dates, and billing codes were identified using validated algorithms.8, 9
All patients who underwent EVR within the VQI for whom matched Medicare claims were available were eligible for inclusion in the study (n=14,549; Supplementary Figure 1). We excluded patients for whom baseline characteristics were missing if that variable was missing in <1.0% of patients (n=1,638). Therefore, baseline characteristics were known for 100% of included patients. We found no meaningful difference in the rate of reintervention between the two cohorts. We therefore report the rate of reintervention for the cohort where baseline characteristics were known to allow for a consistent cohort across subgroup analyses.
Outcomes and definitions
Our primary exposure was EVR. All patients in the study underwent the procedure. Patients who received multiple procedures on the same day, such as EVR converted to open repair, were assigned according to the first procedure performed on them.
We defined our outcomes of interest as in prior studies.4, 8 Our primary outcome was reintervention. We defined this as any procedure performed after hospital discharge from the index EVR that was related to the aneurysm or the index aneurysm repair. Procedures occurring during the index hospitalization were excluded. Patients were censored at their date of death. Patients dying during the index hospitalization were given a survival time of 0.5 days. We identified reintervention using a validated ICD-9 claims algorithm documented to have 92.0% sensitivity and 96.0% specificity.8
Our secondary outcome of interest was late aneurysm rupture. We defined this outcome as any patient encounter with a primary diagnosis code of aortic aneurysm rupture that was associated with a reintervention or death within 14 days of the admission date.
Statistical analysis
We report continuous variables as means with standard deviations, and categorical variables as percentages. The increase in centers participating in the VQI also means that more patients are entered into the registry in later years of the study. Therefore, we choose Kaplan-Meier estimation to allow us to handle censoring associated with this phenomenon. Reintervention and late aneurysm rupture are described using this method, and subgroups were compared with the log-rank test. A p-value of <.05 was considered statistically significant. Statistical analyses were performed with Stata version 15 (College Station, Texas).
Human subjects protection
All data are collected under the auspices of an Agency for Healthcare Research and Quality designated Patient Safety Organization and were de-identified. Our study was approved by the Center for the Protection of Human Subjects at Dartmouth. All patient personal health information was protected, records and outcomes were de-identified, and no testing or procedures were required for this study. Thus, the need for specific consent was waived.
Results:
Cohort characteristics
We studied 12,911 patients who underwent EVR in the VQI, who could also be identified in Medicare claims, and met our inclusion and exclusion criteria. The median follow-up was 1.72 years (interquartile range of 0.80 – 3.00). The mea n age among EVR patients was 75.5 years (standard deviation 7.3 years), 79.9% of patients were male, and 3.9% of patients were black (Table I). We found that 84.8% of patients had a history of smoking, 30.0% had a history of coronary artery disease, 32.9% had a history of pulmonary disease, and 8.2% of patients had renal insufficiency. Most patients were taking aspirin (65.2%) or a statin (68.7%) at the time of their index repair.
Table 1:
Characteristics of the cohort n=12,911.
| Variable | % (n) |
|---|---|
| Demographics | |
| Age, mean (SD), years | 75.5 (7.3) |
| Male | 79.9 (10,310) |
| Race | |
| White | 93.0 (12,012) |
| Black | 3.9 (503) |
| Other | 3.1 (396) |
| Clinical conditions | |
| Hypertension | 84.0 (10,841) |
| Smoking history | 84.8 (10,954) |
| Coronary disease | 30.0 (3,867) |
| Heart failure | 11.9 (1,539) |
| Diabetes | 20.4 (2,628) |
| COPD | 32.9 (4,251) |
| Renal insufficiency | 8.2 (1,053) |
| Obesity | 28.8 (3,718) |
| Operative characteristics | |
| Urgency | |
| Elective | 89.1 (11,498) |
| Symptomatic | 7.3 (946) |
| Ruptured | 3.6 (467) |
| Aneurysm size | |
| <5.0 cm | 15.8 (2,040) |
| 5.0 – 5 .4 cm | 29.5 (3,809) |
| 5.5 – 5 .9 cm | 24.4 (3,151) |
| ≥6.0 cm | 30.3 (3,911) |
| Endograft manufacturer | |
| Medtronic™ | 58.9 (7,607) |
| Gore™ | 18.8 (2,421) |
| Cook™ | 17.0 (2,195) |
| Endologix™ | 2.9 (377) |
| Trivascular™ | 1.2 (158) |
| Lombard™ | 0.2 (26) |
| Bolton™ | 0.1 (15) |
| Other | 0.9 (112) |
| Medications | |
| Antiplatelet therapy | |
| Aspirin | 65.2 (8,412) |
| P2y12 inhibitor | 11.3 (1,458) |
| Beta-blocker | 60.9 (7,868) |
| Statin | 68.7 (8,868) |
All listed characteristics were known for 100% of the cohort.
Legend: SD, standard deviation; MI, myocardial infarction; UA, unstable angina; BMI, body mass index; kg/m2, kilograms per meter squared; g/dL, grams per deciliter; cm, centimeters.
The majority (89.1%) of patients underwent their index EVR in the elective setting, while the remaining patients underwent surgery for a symptomatic (7.3%) or ruptured (3.6%) abdominal aortic aneurysm. Device type was distributed across the major EVR manufacturers, including 58.9 % Medtronic™, 18.8% GORE™, and 17.0% Cook™ end ovascular prostheses.
Reintervention
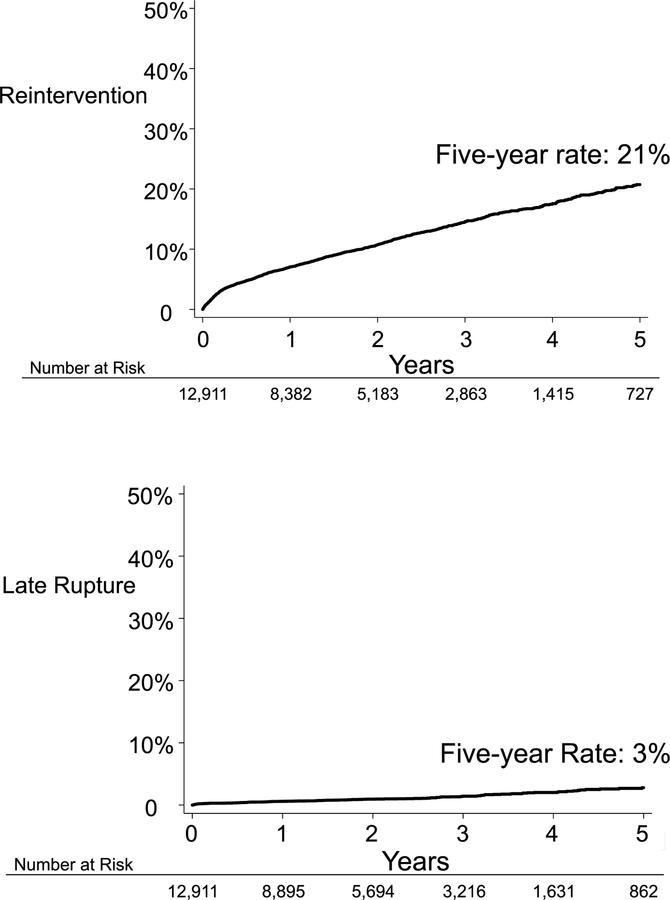
The Kaplan-Meier five-year rate of reintervention among all EVR patients at VQI participating centers was 21% (Figure 1). The rate of reintervention did not plateau over time. Rather, the Kaplan-Meier rate of reintervention rose rapidly to 5% at 3 months post index procedure, then increased linearly at approximately 3 – 4% percent per year each year. Reinterventions included procedures such as: coil embolization of type 2 endoleak, endograft relining, and femoral-femoral bypass for EVR limb occlusion.8 Procedures for type 1 endoleak, endograft relining, or femoral femoral bypass for limb thrombosis were distributed throughout follow-up. With some patients undergoing these procedures early on, and others later during their course. Coiling procedures tended to be more common after 6–12 months of follow-up.8
Figure 1:
Five-year rate of reintervention and late aneurysm rupture.
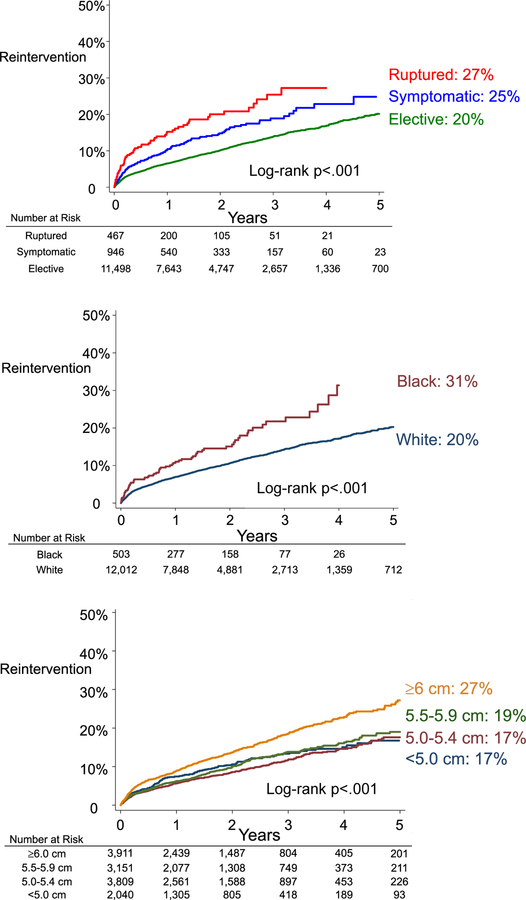
We identified several subgroups that had an elevated likelihood of undergoing reintervention (Figure 2). Urgency demonstrated a stepwise increase in the likelihood of reintervention. Patients who underwent EVR in the elective setting had a 20% likelihood of undergoing reintervention at five years, while those who underwent EVR for symptomatic aneurysms had a 25% reintervention rate, and those who underwent EVR for rupture had a 27% reintervention rate (log rank p<.001). Patients with large aneurysms also demonstrated a higher rate of reintervention. Those who underwent EVR for an abdominal aortic aneurysm that was 6.0 centimeters or larger had a five-year rate of reintervention of 27%, while patients with aneurysms less than 6.0 centimeters had reintervention rates less than 20% (log rank p<.001). Furthermore, black patients had a substantially higher rate of reintervention when compared to their white counterparts, with a reintervention rate 31% at four years, compared to a rate of just 20% for white patients (log rank p<.001). Lastly, Figure 2 demonstrates that the rate of reintervention had a persistent linear rise over the study period, regardless of urgency of the index operation, aneurysm size, or race.
Figure 2:
Five-year rate of reintervention stratified by urgency, race, and aneurysm size.
We noted no difference in the rate of reintervention by age group, or by gender. Patients who were aged less than 65 years had a similar reintervention rate to patients who were older (log rank p=.10, when age groups were compared in five-year strata). In addition, we found that men and women had the same rate of reintervention, 21%, at five years (log rank p=.17). There was no difference in the rate of reintervention across device manufacturers (Medtronic™, GORE™, and Cook™, log-rank p=.776).
Late aneurysm rupture
Late rupture was much less common than reintervention. The five-year rate of late aneurysm rupture after EVR among all patients at VQI participating centers was 3% (Figure 1).
Discussion:
In our large, observational study of nearly 13,000 patients who underwent EVR at VQI participating centers across the United States, we found that more than one in five Medicare patients underwent reintervention after EVR within the first five years after surgery. Furthermore, we identified high risk subgroups which had an even higher likelihood of reintervention after discharge from their index hospitalization. These included patients who underwent EVR for rupture, those with aneurysms larger than six centimeters, and patients who were of black race. Interestingly, we also found that men and women, and patients of all age strata, had similar rates of reintervention at five years. While reintervention rates were high, late rupture was not, with a five-year rate of late aneurysm rupture after EVR of three percent. These findings indicate that patients who undergo EVR should continue long term follow-up, and specific high-risk subgroups should be the focus of diligent targeted imaging surveillance. Furthermore, tailored surveillance strategies may be possible as our knowledge of factors predicting reintervention improves.
Defining rates of reintervention after EVR and those who are at highest risk for these procedures has proven difficult. While rates within clinical trials have been well delineated, real world rates have been more difficult to determine.7, 10 Studies are often single center, limited in follow-up, or lack granular clinical information on the index EVR procedure.5, 7, 11, 12 Our study informs this clinical problem by describing the five-year rates of reintervention that can be expected in real world clinical practice, and by identifying high risk subgroups of patients that should expect an elevated likelihood of reintervention.
Our cohort demonstrated that a significant proportion of patients undergo reintervention in the early post-operative phase, less than 3 months. Interestingly, the need for reintervention thereafter did not plateau, but rather continued to rise over five years at a steady, linear rate. This phenomenon has been noted both in randomized trials including EVAR-1 and DREAM, and in institutionally-based reports, where a similar persistent need for reintervention was found.4, 13,14 Our large cohort indicates that in real-world practice, the persistent need for reintervention is indeed observed. These findings can inform surgeons and their referring physicians caring for patients who have undergone EVR.
Surveillance after EVR has proven challenging in clinical practice. While the Society for Vascular Surgery and the United States Food and Drug Administration have recommended annual imaging surveillance after EVR, approximately 50% of Medicare EVR patients are lost to imaging follow-up.6, 15–18 This surveillance failure has been associated with inferior clinical outcomes, highlighting the need for improvement in patient surveillance mechanisms, which could include patient education as to the implications of loss to follow-up, and collaborative efforts with primary care physicians and patient-care networks, among others.19
Conversely, the rate of late aneurysm rupture was much lower, at three percent over five years. Whether the high rate of reintervention procedures is driving a lower rate of late rupture, remains unknown. Recent investigators have attempted to describe the effect of reintervention on late rupture using clinical trial data.10 However, determining if there is an association between reintervention and second reintervention, or late rupture is a complex task. Reintervention is itself a time-dependent outcome, as examined herein. Transitioning this to an exposure which occurs at variable times after the index EVR makes interpreting the association between reintervention and any other outcome challenging. Appropriately addressing these challenges, predicting reintervention after EVR, and eliciting the association between reintervention and secondary reinterventions, and late rupture is an area of active investigation for our group.
Our study has limitations. Data from our study was not derived from a randomized controlled trial and is therefore subject to inherent bias. Nearly one in eight EVRs were performed for aneurysms less than 5 centimeters. Closer investigation into the reasons for these procedures is beyond the scope of this project. We stratified our analysis based on factors that could be known preoperatively in order to inform decision making for patients and providers. We did not examine endoleak at the time of repair, and we did not have data available on the change in aneurysm morphology over time. In addition, reinterventions were ultimately performed at the discretion of the operating surgeon, and thresholds for procedures likely vary across institutions. However, our study represents real world results, and no matter what thresholds are used at institutions, this study describes the actual experience that patients can expect to have at VQI participating centers. We examined the types of reinterventions occurring at our center by chart review and patient telephone interview, and applied the ICD-9 codes used to identify reinterventions at other centers. We are not able to comment on the types of reinterventions that were performed at these centers as procedures were identified using ICD-9 codes and not manual chart review.
Conclusions:
More than one in five Medicare patients who undergo EVR in contemporary practice can expect to undergo reintervention. Few patients will experience a late rupture event, but this outcome still remains higher for EVR than historical series of open surgical repair. Black patients, those with large aneurysms, and those who undergo EVR in urgent and emergent settings, have a higher likelihood of adverse outcomes and should be the focus of diligent long-term surveillance.
Supplementary Material
Article Highlights.
Type of Research:
Retrospective analysis of the VQI-Medicare matched national database
Key Findings:
At 5 years, reintervention rate after 12, 911 endovascular abdominal aortic aneurysm repairs (EVARs) was 21%, and late aneurysm rupture rate was 3%. Black patients, those with aneurysms large than six centimeters, and those who underwent repair uergently or emergently had higher rates of reintervention.
Take home Message:
More than one in five Medicare patients who undergo EVAR can expect to undergo reintervention. High risk subgroups have more adverse outcomes and should be the focus of diligent long-term surveillance.
Acknowledgments
Funding:
This work was supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (ME-1503–28261), the United States Food and Drug Administration (U01-FD005478), the National Institute on Aging (PO1-AG19783), and the American Heart Association (18SFRN33900147). All statements in this paper, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of our funding agencies. Specifically, the funders played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 45th Annual Meeting of the New England Society for Vascular Surgery October 2018, Cape Neddick, Maine, USA
References
- 1.Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, et al. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J vasc surg. 2018;67(6):1690–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. The Cochrane database of systematic reviews. 2014(1):CD004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontopodis N, Antoniou SA, Georgakarakos E, Ioannou CV. Endovascular vs Open Aneurysm Repair in the Young: Systematic Review and Meta-analysis. J Endovasc Ther. 2015;22(6):897–904. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. The Lancet. 2016;388(10058):2366–74. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zierler RE, Jordan WD, Lal BK, Mussa F, Leers S, Fulton J, et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J vasc surg. 2018;68(1):256–84. [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Allen C, Grima MJ, Brownrigg JRW, Patterson BO, Holt PJE, et al. A Systematic Review of Predictors of Reintervention After EVAR: Guidance for Risk-Stratified Surveillance. Vasc endovasc surg. 2017;51(6):417–28. [DOI] [PubMed] [Google Scholar]
- 8.Columbo JA, Kang R, Hoel AW, Kang J, Leinweber KA, Tauber KS, et al. A comparison of reintervention rates after endovascular aneurysm repair between the Vascular Quality Initiative registry, Medicare claims, and chart review. J vasc surg. 2019;69;(1):74–79.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoel AW, Faerber AE, Moore KO, Ramkumar N, Brooke BS, Scali ST, et al. A pilot study for long-term outcome assessment after aortic aneurysm repair using Vascular Quality Initiative data matched to Medicare claims. J vasc surg 2017;66(3):751–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grootes I, Barrett JK, Ulug P, Rohlffs F, Laukontaus SJ, Tulamo R, et al. Predicting risk of rupture and rupture-preventing reinterventions following endovascular abdominal aortic aneurysm repair. BJS. 2018;105(10):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123(24):2848–55. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Gloviczki P, Duncan AA, Kalra M, Oderich GS, Fleming MD, et al. Maximal aortic diameter affects outcome after endovascular repair of abdominal aortic aneurysms. J vasc surg. 2017;65(5):1313–22 e4. [DOI] [PubMed] [Google Scholar]
- 13.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1881–9. [DOI] [PubMed] [Google Scholar]
- 14.Conrad MF, Crawford RS, Pedraza JD, Brewster DC, Lamuraglia GM, Corey M, et al. Long-term durability of open abdominal aortic aneurysm repair. J vasc surg 2007;46(4):669–75. [DOI] [PubMed] [Google Scholar]
- 15.Garg T, Baker LC, Mell MW. Postoperative Surveillance and Long-term Outcomes After Endovascular Aneurysm Repair Among Medicare Beneficiaries. JAMA Surg. 2015;150(10):957–63. [DOI] [PubMed] [Google Scholar]
- 16.Judelson DR, Simons JP, Flahive JM, Patel VI, Healey CT, Nolan BW, et al. Determinants of Follow-Up Failure in Patients Undergoing Vascular Surgery Procedures. Ann vasc surg. 2017;40:74–84. [DOI] [PubMed] [Google Scholar]
- 17.The United States Food and Drug Administration. Safety Alerts for Human Medical Products. Accessed August 1st, 2017 Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm611193.htm
- 18.Schanzer A, Messina LM, Ghosh K, Simons JP, Robinson WP 3rd, Aiello FA, et al. Follow-up compliance after endovascular abdominal aortic aneurysm repair in Medicare beneficiaries. J vasc surg. 2015;61(1):16–22 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks CW, Zarkowsky DS, Bostock IC, Stone DH, Black JH 3rd, Eldrup-Jorgensen J, et al. Endovascular aneurysm repair patients who are lost to follow-up have worse outcomes. J vasc surg. 2017;65(6):1625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.