Abstract
The role of extracellular purine nucleotides, including adenosine triphosphate (ATP) and adenosine, as modulators of post-transplantation outcome and ischemia-reperfusion injury is becoming increasingly evident. Upon pathological release of ATP, binding and activation of P2 purinergic surface receptors promote tissue injury and inflammation, while the expression and activation of P1 receptors for adenosine have been shown to attenuate inflammation and limit ischemia-induced damage, which are central to the viability and long-term success of allografts. Here we review the current state of the transplant field with respect to the role of extracellular nucleotide signaling, with a focus on the sources and functions of extracellular ATP. The connection between ischemia-reperfusion, purinergic signaling, and graft preservation, as well as the role of ATP and adenosine as driving factors in the promotion and suppression of post-transplant inflammation and allograft rejection, are discussed. We also examine novel therapeutic approaches that take advantage of the ischemia-reperfusion-responsive and immunomodulatory roles for purinergic signaling with the goal of enhancing graft viability, attenuating post-transplant inflammation, and minimizing complications including rejection, graft failure and associated comorbidities.
1. Introduction
Numerous intra- and extracellular factors contribute to the success or failure of solid-organ transplants, and the contribution of purinergic signaling mediated by purine nucleotides and nucleosides such as adenosine and adenosine triphosphate (ATP) is now recognized to be important in all stages of the transplant process.1 For example, acute graft dysfunction as a result of ischemia-reperfusion injury (IRI) after transplant causes damage to graft tissues, involving the release of ATP that acts as a danger signal and promotes immune cell activation and infiltration.2 Because the purinergic system is important to T cell biology, it has also been a therapeutic target for the prevention of acute rejection and to promote long-term graft survival.
A comprehensive review by Zeiser et al. in 2016 provides in-depth information on the role of purinergic signaling in the setting of transplantation.1 In addition, Boros et al. recently provided a concise review on adenosine regulation of the immune response to ischemia-reperfusion injury.3 In the current review, we highlight recent advances in our understanding of the role of extracellular nucleotides as modulators of solid organ transplantation, with a particular focus on sources of adenosine nucleotides, especially ATP, their role in IRI, rejection and resolution of inflammation post-transplant. We also discuss various possibilities for intervention via pharmacologic or genetic strategies to minimize nucleotide-induced damage and enhancing the pro-resolving and anti-inflammatory potential of nucleotide-mediated signaling to enhance short-term success of engraftment as well as control post-transplant inflammation and rejection.
2. Extracellular ATP: Sources and Functions
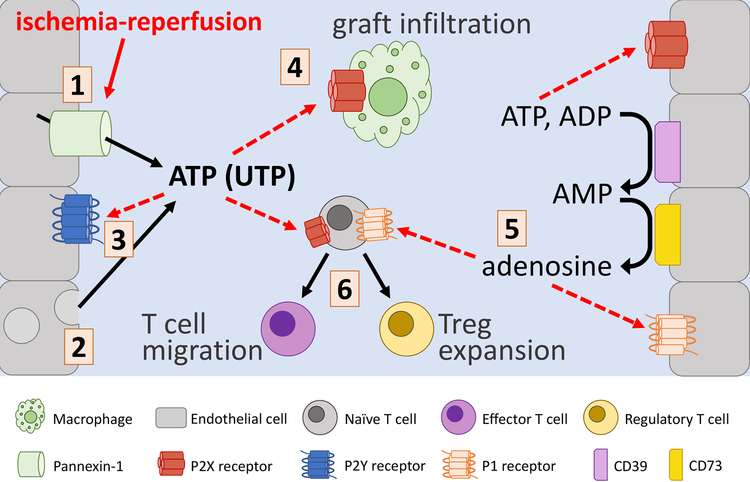
ATP is not only the universal energy currency of all cells, but it is also a potent signaling molecule defined by unique mechanisms of release that contribute to differential signaling in multiple cell types. ATP is typically sequestered inside the cell, but in response to cell damage or specific stimuli (such as IRI) it can be massively released into the extracellular environment, either through cell rupture, fusion of vesicles with the plasma membrane or via the membrane channel pannexin-1 (Panx1), which upon activation by caspase-dependent cleavage or downstream GPCR-mediated signaling events, facilitates the controlled local release of ATP (see Figure 1).4 ATP released from necrotic or apoptotic cells is considered to be a pro-inflammatory damage-associated molecular pattern (DAMP) molecule that activates the innate immune response,2 but the localization and concentration of ATP is critical for determining the downstream consequences of ATP signaling (purinergic regulation of immune cell function is reviewed by Cekic and Linden5). Two distinct classes of cell-surface receptors bind ATP: the P2X family of receptors are ligand-gated ion channels that are thought to require higher concentrations of ATP to facilitate channel opening, while the P2Y receptors are ubiquitously expressed G-protein-coupled receptors that mediate diverse functions in response to lower concentrations of ATP, adenosine diphosphate (ADP), and other nucleotides (see Table 1 for a summary of the major receptor subtypes involved in the response to IRI and allotransplantation). In the context of organ transplantation, differential activation of P2X and/or P2Y receptors may be dependent on the localization of extracellular nucleotides: local accumulation of ATP released from necrotic cells may activate P2X-dependent signaling locally within tissues, whereas controlled release of ATP, such as that mediated by Panx1, may be sufficient to agonize P2Y receptors at sites distant from injury.
Figure 1. Schematic overview of extracellular nucleotide signaling in IRI and organ transplant.
ATP is released (by various cells such as endothelial cells) in response to ischemia-reperfusion by Pannexin-1 channels (1) or via vesicular release or cell rupture (2). Extracellular ATP activation of P2Y receptors (3) is protective in cardiac IRI, while activation of P2X receptors (4) promotes vascular dysfunction and macrophage graft infiltration. CD39 and CD73 convert ATP into adenosine (5), which activates adenosine (P1) receptors, with largely protective effects in IRI. T cells (6) adopt activated or regulatory phenotypes in response to P2X- and P1-dependent signaling.
Table 1.
Differential roles of selected P2X, P2Y and P1 receptors in ischemia-reperfusion injury (IRI) and solid organ transplantation.
| Receptor Category | Receptor | Role in IRI and Organ Transplantation | Reference |
|---|---|---|---|
| P2X | P2X4R | Agonism leads to activation and enhanced migration of T cells | 51 |
| Pharmacological inhibition causes Treg expansion and limits graft lymphocyte infiltration | 7 | ||
| P2X7R | Inhibition leads to expansion of Treg population and is protective in renal IRI | 32 | |
| Forms complex with Panx1 to cause autocrine activation of receptor by Panx1-mediated ATP release | 42 | ||
| Activation in cardiac IRI induces downstream signaling that results in release of cardioprotective molecules | 42 | ||
| Antagonism limits macrophage infiltration and development of vascular damage after cardiac ischemia | 49 | ||
| P2Y | P2Y2R | Agonism reduces functional deficits after myocardial ischemia | 40 |
| Pharmacologic blockade of P2Y receptors blocks UTP-mediated benefits | 41 | ||
| P1 | A1R | Receptor activation is protective in pulmonary IRI | 18 |
| A2AR | A2AR signaling is protective in lung IRI | 18 | |
| Pharmacologic activation reduces IRI in porcine lung transplant model | 20 | ||
| A2BR | Activation is protective in cardiac IRI | 21 | |
| Inhibition of A2BR prolongs pulmonary graft viability during ex vivo perfusion | 23 | ||
| Treatment with A2BR antagonist allowed for successful lung transplantation | 24 | ||
| A3R | Activation is protective in pulmonary IRI | 18 |
Activation of proinflammatory signals resulting from increases in extracellular ATP are combated by cell-surface ectonucleotidase enzymes which catalyze the breakdown of ATP and its metabolites (see Figure 1). A review by Roberts et al. in 2014 provides in-depth information on the role of ectonucleotidases in solid organ transplantation.6 The ectonucleoside triphosphate diphosphohydrolase CD39 is the rate-limiting enzyme in extracellular nucleotide breakdown and catalyzes the conversion of ATP and ADP into adenosine monophosphate (AMP). The ecto-5’-nucleotidase CD73 then converts AMP to adenosine, which has a potent anti-inflammatory signaling capacity mediated by a variety of adenosine (P1) receptors. CD39 and CD73 serve as controllers of the balance between levels of extracellular ATP and adenosine, and the shift from ATP to adenosine is essential for the resolution of inflammation and suppression of the adaptive immune response, which are critical to prevent transplant rejection and promote graft survival.7
3. Targeting ATP in IRI and Graft Preservation
Ischemia (the loss of perfusion to an organ or tissue) is unavoidable during organ transplantation. Although reperfusion (the reintroduction of oxygenated blood to the organ or tissue) induces inflammation and injury, it is necessary for organ viability. Cellular injury that occurs during ischemia results in the release of ATP into the local extracellular environment, which is further exacerbated during reperfusion, where sudden reoxygenation and resulting production of reactive oxygen species cause further release of DAMPs including ATP.8–11 Although the length of ischemic organ preservation time is minimized to reduce damage at the time of engraftment, IRI has been shown to impact both acute and chronic graft survival.12,13
A number of recent studies have established that extracellular ATP accumulation and subsequent purinergic signaling is an important mediator of solid organ transplantation. In the lung, ATP is established as a driver of IRI, and recent work has demonstrated that Panx1-dependent release from endothelial cells mediates IRI and that endothelial-specific genetic ablation or pharmacologic inhibition of Panx1 activity attenuates immune cell infiltration and pulmonary damage after IRI.14 Ibrahim et al. showed that direct elimination of extracellular ATP by pharmacologic administration of apyrase attenuates injury in a canine model of pulmonary IRI, while lung allograft patients with graft dysfunction have higher levels of circulating ATP.15 Similarly, inhibition of the purinergic pathway, via the nonselective inhibitor suramin or a P2X7 receptor inhibitor, was shown to prolong mouse lung allograft survival.16
Activation of adenosine receptors has been shown to be protective in lung IRI in most cases.17 For example, activation of adenosine A1 receptor (A1R), A2AR, or adenosine A3 receptor (A3R) have been shown to attenuate lung IRI,18,19 and pharmacologic activation A2AR attenuated IRI in a porcine lung transplant model.20 Although activation of adenosine 2B receptor (A2BR) has been shown to be protective in cardiac IRI21, Anvari et al. demonstrated that lung IRI is improved in A2BR−/− mice22, and Huerter et al. showed that pharmacologic inhibition of A2BR attenuates murine lung IRI, which may involve targeting of A2BRs on alveolar epithelial cells to prevent IL-8 production.23 Furthermore, pharmacologic inhibition of A2BR during ex vivo lung perfusion allowed for the successful transplantation of donor lungs after circulatory death.24 On the other hand, Hoegl et al. demonstrated that A2BR activation on alveolar epithelial cells was protective in a two-hit model of acute lung injury involving intratracheal LPS treatment followed by injurious mechanical ventilation.25 Eckle et al. provided evidence that termination of pulmonary adenosine signaling is predominantly mediated by equilibrative nucleotide transporter 2 (ENT2) and reveal a novel crosstalk pathway between ENT2 and alveolar epithelial A2BRs in promoting protection during acute lung injury.26 Further insight into the conflicting role of A2BR activation has been provided by Seo et al., who used a tissue-specific approach for A2BR signaling during ischemic preconditioning or IRI and found different functions for A2BR in different tissues.27
In the kidney, the activity of ectonucleotidases CD39 and CD73 and resultant breakdown of extracellular ATP and enrichment of adenosine have been directly tied to graft survival.28 CD73 has been demonstrated to be protective in renal IRI29 via multiple mechanisms30 including enhancement of local adenosine concentration and subsequent activation of adenosine receptors. On the other hand, deficiency of CD73 activity was shown to be beneficial in mild kidney IRI, suggesting a novel protective role for AMP-mediated signaling.31 Koo et al. demonstrated that a P2X7 receptor antagonist (or P2X7 receptor deficiency in hematopoietic cells) ameliorates murine renal IRI by expansion of regulatory T (Treg) cells.32 Similarly, apyrase treatment to degrade extracellular ATP protected mice from both acute and chronic renal IRI.33
Outcomes from liver transplant have also been tied to adenine nucleotide levels and manipulation of ATP-mediated signaling. Interestingly, it has been documented that liver transplant patients with higher circulating ATP had an increased likelihood of successful outcome, and that lower ATP levels were associated with complications such as infection, liver damage and graft failure.34 However, multiple recent studies examining the role of nucleotide-mediated signaling in liver IRI and transplant have demonstrated the importance of ectonucleotidases in liver transplant success. For example, deficiency of CD39 in liver allografts aggravated inflammatory injury and immune cell mediated rejection in a mouse model of cold-ischemia transplantation, and exogenous administration of soluble CD39 prolonged survival of CD39 deficient allografts.35
IRI is a hallmark not only of organ transplant but also cardiovascular disease, and activation of purinergic signaling is now recognized as a driver of IR-induced cardiovascular damage as evidenced in various recent studies.14,36,37 Signaling via P2X receptors has been shown to be detrimental in cardiovascular IRI, and blockade of P2X7 receptors reverses vasomotor dysfunction in saphenous vein grafts during coronary artery bypass.38 Although mainstay antithrombotic therapies act by blocking the P2Y12-mediated aggregation of platelets, growing evidence has revealed a potential protective role for P2Y receptor signaling in cardiac IRI.39 Here, direct agonism of P2Y2 receptors by exogenous uridine-5’-triphosphate (UTP) reduces infarct size and functional deficits in a rat model of myocardial infarction40, and inhibition of P2Y receptors blocks the protective effects of UTP-mediated purinergic signaling.41 Recent work has also elucidated a role for P2X receptors in cardioprotection, and pretreatment with P2X7 receptor agonists during ischemic preconditioning has been shown to be protective in cardiac IRI, possibly via a mechanism whereby Panx1 channels and P2X7 receptor form a complex that, in response to exogenous ATP or P2X7 receptor agonists, activate downstream signals that cause the release of cardioprotective molecules including sphingosine-1-phosphate.42 These findings have implications not only for treatment of ischemic cardiovascular disease, but may also elucidate novel avenues for prevention of IR-induced damage in cardiac transplant, as well as other organ systems. Although differences in mechanisms of IRI among solid organ transplant exist, taken together, these studies provide evidence that purinergic signaling pathways provide important protective and detrimental effects common among solid organ transplants.
IRI itself can alter the cellular and systemic responses to extracellular nucleotides, which can lead to enhancement or attenuation of inflammatory responses in the acute period after reperfusion. IRI by definition results in profound tissue hypoxia, which causes the stabilization and activation of hypoxia-inducible factors (HIFs), a group of transcription factors that regulate not only general inflammatory transcriptional programs, but are also a key mediator of nucleotide receptor and transporter transcription. In the context of organ transplantation, the action of HIFs may serve as a central modulator of the outcomes of nucleotide signaling (see reviews by Bowser et al.43 and Le et al.44 for a more thorough examination of the role of HIFs in adenosine nucleotide signaling). For example, A2BR-dependent stabilization of the rhythm protein Per2 has been shown to be required for the full protective effect of ischemic preconditioning in myocardial infarction models in a HIF-dependent manner.45 Studies suggest that A2BR signaling plays a central role in tissue adaptation to hypoxia whereby A2BR has emerged as a therapeutic target for dampening hypoxia-induced inflammation.46,47 Additionally, in a clinically relevant model of mechanical ventilation-induced acute lung injury, the upregulation of A2BR expression was controlled by HIF-1α.48 Since HIFs can also be stabilized by hypoxia-independent pathways, including via Toll-like receptor activation, it is also likely that the inflammatory response post-IRI also plays a role in modulating nucleotide receptor expression and the response to extracellular ATP and adenosine.
4. ATP and Adenosine in Graft Rejection and Resolution of Inflammation
Rejection is the process by which an allograft is recognized as foreign and subsequently attacked by the host immune system, and suppression of the immune response to combat rejection is a major focus of therapeutic treatment as well as research to prolong graft survival. Immune-mediated rejection is a complex process that results from interplay of multiple different cell types, including macrophages, dendritic cells and T cells. Immune cells including macrophages infiltrate grafts and contribute to chronic rejection, and pharmacologic inhibition of P2X7 receptor signaling has been shown to inhibit macrophage infiltration and prevent vascular damage in a model of heart allograft transplantation.49 In addition, Vergani et al. demonstrated that P2X7 receptor expression is upregulated in graft-infiltrating lymphocytes in cardiac-transplanted humans and mice and that P2X7 receptor antagonism resulted in improved long-term cardiac transplant survival,50 while direct production of ATP and subsequent activation of the P2X4 receptor has been shown to regulate T cell migration51 (recent advances in specific roles of ATP in lymphocyte activation and rejection are reviewed by Castillo-Leon and colleagues7). In the setting of cardiac transplantation, D’Addio et al. showed that the P2X7 receptor/NLR Family Pyrin Domain Containing 3 (NLRP3) complex maintains a physiological NLRP3-mediated Th2 program, while intracellular mutation of P2X7 receptor induces NLRP3 displacement in T cells, causing Th17 skewing and subsequent poor allograft outcome.52 Furthermore, ATP production in activated circulating lymphocytes was significantly increased in lung transplant patients undergoing acute rejection.53
Additional work into the role of ATP and adenosine signaling on memory T cell development and maintenance may also provide insight into development and control of rejection. A2AR deletion has been shown to cause a shrinking of the pool of naïve T cells, but not of memory T cells54, while extracellular ATP, acting through the P2X7 receptor, maintains long-term CD8+ memory T cells.55 On the other hand, the effects of adenosine signaling on Th17 cell development is more nuanced whereby Liang et al. showed that a nonselective adenosine receptor agonist can have either a pro- or anti-inflammatory effect on Th17 development56, while CD39 has been implicated in the development and maintenance of Th17 cells with a proinflammatory phenotype in Crohn’s Disease.57 On the other hand, CD39 activity on intestinal Th17 cells was shown to promote cell survival and development of a pro-resolution phenotype through depletion of ATP in a model of experimental colitis.58 These varied functions of purinergic signaling in controlling specific T cell populations underscore the importance of localization and timing of nucleotide signaling in modulating immune cell activation. Consequently, a more thorough understanding is needed for how timing and cell type-specific modulation of adenine nucleotide signaling affects immune cell activation in the context of transplantation.
Adenosine is a potent immunosuppressant, and adenosine can accumulate rapidly after ATP release due to the activity of CD39 and CD73 on various cells, which acts to inhibit function of lymphocytes, macrophages and dendritic cells via the Gs-coupled A2AR.5 Local concentrations of adenosine are also modulated by the expression of ectonucleotidases and adenosine deaminase (ADA), which are differentially expressed in different classes of immune cells. Treg cells directly generate adenosine via expression of high levels of CD39 and CD37 on their cell membrane that degrade extracellular ATP59, and inhibition of adenosine degradation by blockade of ADA was shown to enhance the immunosuppressive function of Treg cells60. In the lung, the CD73-dependent production of adenosine ameliorates airway rejection through stimulation of A2ARs.61 In contrast to mouse models, recent work has shown that the activation of both human regulatory and effector T cells is inhibited by extracellular adenosine, and in a cohort of liver transplant patients in an immunosuppression withdrawal trial, both circulating levels of adenosine and expression of ADA was increased in patients tolerant to immunosuppression withdrawal (when compared with patients intolerant of withdrawal).62 This suggests that dynamic regulation of adenosine levels is essential for development of immune tolerance post-transplant.
As targeted genetic and pharmacologic manipulation of specific adenosine receptors becomes more available, this will no doubt further help to unravel the complex network of adenosine-mediated signaling events locally in graft tissue and in immune cells that serve to drive organ damage or promote graft preservation. The roles of extracellular ATP and adenosine in activation and inhibition of the immune system are varied and complex, and integrating diverse and sometimes conflicting findings in the context of allograft rejection and immunosuppression underpins the importance of considering nucleotide localization, production, as well as time- and context-dependent effector functions.
5. Purinergic Signaling as a Target for Improving Transplant Outcomes
Advances in our understanding of the molecular mechanisms that drive nucleotide-mediated pathogenesis in the context of IRI and inflammation have highlighted potential new avenues for targeted intervention, to both block ATP-driven promotion of inflammation and organ damage as well as to augment the beneficial effects of adenosine, with the end result of enhancing graft survival and limiting development of acute and chronic allograft rejection (highlighted in Figure 1). One logical approach is to target the initial release of ATP during the graft procurement process, thereby limiting ATP-induced activation of immune cells and preventing acute graft injury. Indeed, pharmacologic inhibition of ATP release using the Panx1 inhibitors carbenoxolone or probenecid attenuated vascular inflammation and pulmonary dysfunction in mice after lung IRI14, while inhibition of P2X7 receptor, in addition to immunosuppressive functions, may also limit extracellular ATP accumulation by blocking the activity of P2X7-Panx1 complexes.42 In addition to preventing ATP release, another strategy has been to treat with selective adenosine receptor agonists or enhance the degradation of extracellular ATP, which has the added benefit of enhancing circulating adenosine levels. Treatment with soluble CD39 was sufficient to promote survival of ectonucleotidase-deficient liver allografts35, while transgenic overexpression of CD39 protected mice from renal IRI, with expression on either circulating cells or the vasculature was sufficient to provide protective effects.63 Additional therapeutic targets, including blockade of ENT activity and enhancement of HIF-induced receptor expression as discussed above, are also actively being investigated in models of IRI and warrant further exploration in pre-clinical transplant models.
In addition to promising preclinical research to decipher the complex role of nucleotide signaling in solid organ transplantation, several clinical trials are ongoing or have concluded that address purinergic signaling in the context of transplantation (see Table 2). To date, only one study () has been published whereby Flyer et al. showed that adenosine induces atrioventricular block in healthy pediatric and young adult heart transplant recipients with minimal risk when low initial doses are used.64 Another study () is currently evaluating the potential protective role of adenosine treatment in vasculopathy in heart transplantation, while another ongoing study () is evaluating the maximum safe dose and duration of Regadenoson (an A2AR agonist) in lung transplant patients as a means to prevent primary graft dysfunction. Another recent trial () investigated the role of the P1 receptor antagonist theophylline in modulating kidney function post-transplant.
Table 2.
Examples of clinical trials that address purinergic signaling in the contaxt of organ transplantation (source: ClinicalTrials.gov).
| Study Title | Conditions | ClinicalTrials.gov Identifier | Drug | Status | References |
|---|---|---|---|---|---|
| Analysis of Adenosine on Sinus and Atrioventricular Nodal Conduction in the Pediatric Transplanted Heart | Sinus bradycardia | Adenosine | Completed | 64 | |
| Myocardial Protection With Adenosine Preconditioning | Myocardial reperfusion injury | Adenosine | Completed | none | |
| Coronary Artery Vasculopathy in Pediatric Heart Transplant Patients (CFR-OHT) | Orthotopic heart transplant | Adenosine | Completed | none | |
| Study to Evaluate Adenosine 2A Receptor Agonist in Patients Undergoing Lung Transplantation | Lung transplant | Regadenoson | Recruiting | none | |
| Perioperative Aminophylline to Improve Early Kidney Function After a Kidney Transplant | Liver Transplantation | Aminophylline (theophylline) | Completed | none | |
| Stress Cardiac MRI in Heart Transplant | Heart transplant failure and rejection | Regadenoson | Recruiting | none |
6. Conclusions
Extracellular nucleotides such as ATP are important signaling molecules that mediate allograft health and survival such as IRI and rejection. Extracellular ATP activates a variety of proinflammatory pathways, whereas adenosine (a product of ectonucleotidase-mediated breakdown of ATP) has prominent immunomodulatory functions. Understanding this balance of ATP and adenosine signaling pathways, and how to pharmacologically manipulate such balance, will be key to the development therapeutic strategies to improve both short- and long-term outcomes after solid organ transplantation.
Acknowledgments
This work was supported by National Institutes of Health grants R01 HL133293 (VEL), R01 HL130053 (VEL), T32 HL007849 (VEL), P01 HL120840 (NL), T32 GM007267 (SY) and T32 GM007055 (SY). The authors also acknowledge the many excellent papers in the field that, due to space constraints, could not be directly referenced in the current review.
Abbreviations:
- A1R
adenosine A1 receptor
- A2AR
adenosine 2A receptor
- A2BR
adenosine 2B receptor
- A3R
adenosine A3 receptor
- ADA
adenosine deaminase
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- DAMP
damage-associated molecular pattern
- ENT2
equilibrative nucleotide transporter 2
- HIF
hypoxia-inducible factor
- IRI
ischemia-reperfusion injury
- NLRP3
NLR Family Pyrin Domain Containing 3
- Panx1
pannexin-1
- Treg
regulatory T cell
- UTP
uridine-5’-triphosphate
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Sharing
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Zeiser R, Robson SC, Vaikunthanathan T, Dworak M, Burnstock G. Unlocking the potential of purinergic signaling in transplantation. Am J Transplant. 2016;16(10):2781–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chekeni FB, Elliott MR, Sandilos JK, et al. Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boros D, Thompson J, Larson DF. Adenosine regulation of the immune response initiated by ischemia reperfusion injury. Perfusion. 2016;31(2):103–110. [DOI] [PubMed] [Google Scholar]
- 4.Chiu Y-H, Jin X, Medina CB, et al. A quantized mechanism for activation of pannexin channels. Nat. Commun 2017;8:14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. [DOI] [PubMed] [Google Scholar]
- 6.Roberts V, Stagg J, Dwyer KM. The role of ectonucleotidases CD39 and CD73 and adenosine signaling in solid organ transplantation. Front Immunol. 2014;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Leon E, Dellepiane S, Fiorina P. ATP and T-cell-mediated rejection. Curr Opin Organ Transplant. 2018;23(1):34–43. [DOI] [PubMed] [Google Scholar]
- 8.Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci. 2018;19(10):3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Kilgas S, Alam A, Eguchi S, Ma D. The role of extracellular adenosine triphosphate in ischemic organ injury. Crit Care Med. 2016;44(5):1000–1012. [DOI] [PubMed] [Google Scholar]
- 10.Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21(3):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28(3):229–248. [DOI] [PubMed] [Google Scholar]
- 12.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant. 2010;29(10):1104–1118. [DOI] [PubMed] [Google Scholar]
- 13.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73(4):1041–1048. [DOI] [PubMed] [Google Scholar]
- 14.Sharma AK, Charles EJ, Zhao Y, et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol-Lung Cell Mol Physiol. 2018;315(2):L301–L312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim M, Wang X, Puyo CA, et al. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015;34(2):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K, Vergani A, Zhao P, et al. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol. Biol 2014;51(2):300–310. [DOI] [PubMed] [Google Scholar]
- 17.Laubach VE, French BA, Okusa MD. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin Ther Targets. 2011;15(1):103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazoni LM, Walters DM, Unger EB, et al. Activation of A1, A2A, or A3 adenosine receptors attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2010;140(2):440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazoni LM, Laubach VE, Mulloy DP, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg 2008;135(1):156–165. [DOI] [PubMed] [Google Scholar]
- 20.LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg 2011;142(4):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Y, Piras BA, Kron IL, French BA, Yang Z. Adenosine 2B receptor activation reduces myocardial reperfusion injury by promoting anti-inflammatory macrophages differentiation via PI3K/Akt pathway. Oxid Med Cell Longev 2015; 2015:585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anvari F, Sharma AK, Fernandez LG, et al. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2010;140(4):871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huerter ME, Sharma AK, Zhao Y, et al. Attenuation of pulmonary ischemia-reperfusion injury by adenosine A2B receptor antagonism. Ann Thorac Surg 2016;102(2):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles EJ, Mehaffey JH, Sharma AK, et al. Lungs donated after circulatory death and prolonged warm ischemia are transplanted successfully after enhanced ex vivo lung perfusion using adenosine A2B receptor antagonism. J Thorac Cardiovasc Surg 2017;154(5):1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoegl S, Brodsky KS, Blackburn MR, et al. Alveolar-epithelial A2B adenosine receptors in pulmonary protection during acute lung injury. J Immunol 2015;195(4):1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckle T, Hughes K, Ehrentraut H, et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J 2013;27(8):3078–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo S, Koeppen M, Bonney S, et al. Differential tissue-specific function of Adora2b in cardioprotection. J Immunol 2015;195(4):1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung S-SJ, Li L, Huang L, et al. Proximal tubule CD73 Is critical in renal ischemia-reperfusion injury protection. J Am Soc Nephrol 2017;28(3):888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu B, Rajakumar SV, Robson SC, et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86(12):1707–1712. [DOI] [PubMed] [Google Scholar]
- 31.Rajakumar SV, Lu B, Crikis S, et al. Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation. 2010;90(12):1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo TY, Lee J-G, Yan J-J, et al. The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int 2017;92(2):415–431. [DOI] [PubMed] [Google Scholar]
- 33.Roberts V, Campbell DJ, Lu B, et al. The differential effect of apyrase treatment and hCD39 overexpression on chronic renal fibrosis after ischemia-reperfusion injury. Transplantation. 2017;101(7):e194–e204. [DOI] [PubMed] [Google Scholar]
- 34.Lanir A, Jenkins RL, Caldwell C, et al. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatol. 1988;8(3):471–475. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida O, Dou L, Kimura S, et al. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl Immunol 2015;32(2):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristiansen SB, Skovsted GF, Berchtold LA, et al. Role of pannexin and adenosine triphosphate (ATP) following myocardial ischemia/reperfusion. Scand Cardiovasc J 2018;52(6):340–343. [DOI] [PubMed] [Google Scholar]
- 37.Good ME, Eucker SA, Li J, et al. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight. 2018;3(6):e96272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W, Feldman D, McCallister R, Brophy C, Cheung-Flynn J. P2X7R antagonism after subfailure overstretch injury of blood vessels reverses vasomotor dysfunction and prevents apoptosis. Purinergic Signal. 2017;13(4):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djerada Z, Feliu C, Richard V, Millart H. Current knowledge on the role of P2Y receptors in cardioprotection against ischemia-reperfusion. Pharmacol Res 2017;118:5–18. [DOI] [PubMed] [Google Scholar]
- 40.Hochhauser E, Cohen R, Waldman M, et al. P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal. 2013;9(4):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wee S, Peart JN, Headrick JP. P2 purinoceptor-mediated cardioprotection in ischemic-reperfused mouse heart. J Pharmacol Exp Ther 2007;323(3):861–867. [DOI] [PubMed] [Google Scholar]
- 42.Vessey DA, Li L, Kelley M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X₇ channels. Am J Physiol Heart Circ. Physiol 2011;301(3):H881–887. [DOI] [PubMed] [Google Scholar]
- 43.Bowser JL, Phan LH, Eltzschig HK. The hypoxia-adenosine link during intestinal inflammation. J Immunol 2018;200(3):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le T-TT, Berg NK, Harting MT, et al. Purinergic signaling in pulmonary inflammation. Front Immunol 2019;10:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch critical for myocardial adaptation to ischemia. Nat Med 2012;18(5):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med 2013;19(6):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol 2011;61:145–186. [DOI] [PubMed] [Google Scholar]
- 48.Eckle T, Kewley EM, Brodsky KS, et al. Identification of hypoxia-inducible factor HIF1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol 2014;192(3):1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Zhao Y, Xiao X, et al. Graft-infiltrating macrophages adopt an M2 phenotype and are inhibited by purinergic receptor P2X7 antagonist in chronic rejection. Am J Transplant 2016;16(9):2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vergani A, Tezza S, D’Addio F, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013;127(4):463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ledderose C, Liu K, Kondo Y, et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 2018;128(8):3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Addio F, Vergani A, Potena L, et al. P2X7R mutation disrupts the NLRP3-mediated Th program and predicts poor cardiac allograft outcomes. J Clin Invest 2018;128(8):3490–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shino MY, Weigt SS, Saggar R, et al. Usefulness of immune monitoring in lung transplantation using adenosine triphosphate production in activated lymphocytes. J Heart Lung Transplant 2012;31(9):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cekic C, Sag D, Day Y-J, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med 2013;210(12):2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Silva HB, Beura LK, Wang H, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature. 2018;559(7713):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang D, Zuo A, Shao H, et al. Anti-inflammatory or proinflammatory effect of an adenosine receptor agonist on the Th17 autoimmune response is inflammatory environment-dependent. J Immunol 2014;193(11):5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai A, Moss A, Kokkotou E, et al. CD39 and CD161 modulate T helper type 17 responses in Crohn’s disease. J Immunol 2014;193(7):3366–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernández D, Flores-Santibáñez F, Neira J, et al. Purinergic signaling as a regulator of Th17 cell plasticity. PloS One. 2016;11(6):e0157889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204(6):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 2010;285(10):7176–7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohtsuka T, Changelian PS, Bouïs D, et al. Ecto-5’-nucleotidase (CD73) attenuates allograft airway rejection through adenosine 2A receptor stimulation. J Immunol 2010;185(2):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baroja-Mazo A, Revilla-Nuin B, de Bejar Á, et al. Extracellular adenosine reversibly inhibits the activation of human regulatory T cells and negatively influences the achievement of the operational tolerance in liver transplantation. Am J Transplant 2019;19(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 2010;10(12):2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flyer JN, Zuckerman WA, Richmond ME, et al. Prospective study of adenosine on atrioventricular nodal conduction in pediatric and young adult patients after heart transplantation. Circulation. 2017;135(25):2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]