Abstract
Objective:
To explore the associations between prenatal exposure to tobacco and neurocognitive development, in the absence of prematurity or low birth weight.
Study design:
We followed mother-child pairs within Healthy Start through 6 years of age. Children were born at ≥37 weeks of gestation with birth weight ≥2500 g. Parents completed the Third Edition Ages and Stages Questionnaire (ASQ-3; n=246) and children completed a subset of the National Institutes of Health (NIH) Toolbox Cognition Battery (n=200). ASQ-3 domains were dichotomized as fail/monitor and pass. Maternal urinary cotinine was measured at ~27 weeks gestation. Separate logistic regression models estimated associations between prenatal exposure to tobacco (cotinine below vs. above the limit of detection) and the ASQ-3 domains. Separate linear regression models estimated associations between prenatal exposure to tobacco and fully corrected T-scores for inhibitory control, cognitive flexibility and receptive language, as assessed by the NIH Toolbox. A priori covariates included sex, maternal age, maternal education, daily caloric intake during pregnancy, race/ethnicity, household income, maternal psychiatric disorders, and, in secondary models, postnatal exposure to tobacco.
Results:
Compared with unexposed offspring, exposed offspring were more likely to receive a fail/monitor score for fine motor skills (OR: 3.9, 95% CI: 1.5, 10.3) and reduced inhibitory control (B: −3.0, 95% CI: −6.1, −0.7). After adjusting for postnatal exposure, only the association with fine motor skills persisted.
Conclusions:
Pre- and postnatal exposures to tobacco may influence neurocognitive development, in the absence of preterm delivery or low birth weight. Increased developmental screening may be warranted for exposed children.
Keywords: fetal programming, pregnancy, maternal smoking, secondhand smoke, cotinine, neurodevelopment, fine motor development, inhibitory control
Although tobacco use in the United States has declined, approximately 7% of women actively smoke during pregnancy2 and 25% are exposed to secondhand smoke.3 This is concerning because prenatal exposure to tobacco has been linked to impaired neurocognitive development in offspring. Children born to mothers who smoked during pregnancy are less likely to meet age appropriate developmental milestones and may have delays in fine or gross motor function.4-8 Additionally, exposed offspring may exhibit impaired cognitive abilities in focused attention and response inhibition.9-15 These findings also have been shown in animal models.16
Prenatal exposure to tobacco is a well-established risk factor for preterm delivery and low birth weight.17, 18 As children born preterm or at a low birth weight are at greater risk for cognitive and motor impairment,19, 20 these studies may confound risks of prematurity and low birth weight with the risks of tobacco exposure. To date, only one published study restricted their analyses to offspring with normal birth histories.21
Women who smoke during pregnancy, even those who attempt to quit, often smoke in the postpartum period.22 Continued exposure to tobacco during early childhood may influence childhood neurocognitive development.20 However, it is unclear whether the association between prenatal exposure to tobacco and neurocognitive development is independent of postnatal exposure to tobacco.
Finally, there is a need to evaluate the relationship between prenatal exposure to tobacco and offspring neurocognitive development using an objective measure of tobacco exposure. Self-report of smoking during pregnancy may result in exposure misclassification.23 Cotinine, the major metabolite of nicotine,24 is a more accurate indicator of exposure and may reduce exposure misclassification.
In this analysis, we explored the association between prenatal exposure to tobacco (measured by maternal urinary cotinine at 27 weeks’ gestation) and offspring neurocognitive development at age 54 months among mother-child pairs enrolled in the longitudinal Healthy Start study. We hypothesized that prenatal exposure to tobacco would be associated with cognitive and motor impairment in early childhood, in the absence of preterm delivery or low birth weight and independent of exposure to secondhand smoke in early childhood.
Methods
The Healthy Start study enrolled 1,410 women ≥16 years of age and before 24 weeks of gestation with singleton pregnancies from the obstetrics clinics at the University of Colorado Hospital from 2010-14. Participants completed 2 research visits during pregnancy (median 17 and 27 weeks of gestation), and another at delivery (median 1 day post-delivery). Women were not eligible to participate in the Healthy Start study if they had a previous stillbirth or preterm birth at <25 weeks of gestation or had pre-existing diabetes, asthma, cancer, or psychiatric illness.
Mother-child pairs were eligible for the current analysis if they had exposure (urinary cotinine) and developmental outcome data. Mother-child pairs were excluded if born at <37 weeks of gestation or the offspring was low birth weight (<2500 g). The Healthy Start study protocol was approved by the Colorado Multiple Institutional Review Board. All women provided written informed consent before the first study visit. The Healthy Start study was registered as an observational study to explore the fuel-mediated programming of neonatal adiposity (), but has expanded its scope to explore how exposures in early life influence childhood growth and development.
Cotinine was measured in a sub-sample of women with stored urine samples collected at approximately 27 weeks of gestation. Cotinine was measured via solid phase competitive ELISA, with a sensitivity of 1 ng/mL (Calbiotech Cotinine ELISA CO096D). The limit of detection was 0.05 ng/mL. Urinary cotinine was categorized as: no exposure (<limit of detection), exposure to secondhand smoke (≥limit of detection to 550 ng/mL; the established cut point for active smoking25), and active smoking (≥550 ng/mL). As only 15 mothers were classified as active smokers during pregnancy, prenatal exposure to tobacco was defined as maternal urinary cotinine levels >limit of detection (indicating active and secondhand smokers).
Development was assessed at 48, 54 or 60 months using the Ages and Stages Questionnaire, Third Edition (ASQ-3)26,27 The ASQ-3 assesses fine motor, gross motor, communication, problem-solving, and personal/social developmental domains. Scores for each domain were categorized as either “Fail”, “Monitor”, or “Pass”, based on the cut-offs provided in the ASQ-3 User’s Guide.26 Very few children received a failing score (n= 9 for fine motor, n=7 for gross motor, n=1 for communication, n=3 for problem-solving, and n=5 for personal/social skills). Therefore, the ASQ-3 domains were dichotomized as “Fail/Monitor” and “Pass.”
The National Institutes of Health (NIH) Toolbox Cognition Battery is a series of tests designed to measure executive function across the lifespan (ages 3 to 85 years).28 Three tests in the Cognition Battery were relevant for our study population: the Flanker test (inhibitory control),28 the Dimensional Change Card Sort test (DCCS, cognitive flexibility),29 and the Picture Vocabulary test (receptive language). During the in-person research visit, children completed the tests on a tablet computer while a trained professional research assistant supervised. Raw scores were based on accuracy and response time (Flanker and DCCS) or accuracy (Picture Vocabulary). Fully-corrected T-scores were utilized,30 with a normative mean fully-corrected T-score is 50 for all tests and a standard deviation (SD) of 10.30
Mothers were asked to report the number of adults in the household (including themselves) who were regular smokers when their child was 5, 18, and 54 months of age. Responses to this question ranged from 0-6. We dichotomized these data into no household smokers versus any household smokers (≥ 1 household smoker at 5, 18, or 54 months of age). This variable was used as the indicator for postnatal exposure to tobacco.
Covariates were offspring sex, birth weight, and gestational age (obtained from medical records). Maternal race/ethnicity, maternal education, and annual household income were self-reported. Although maternal psychiatric illness was part of the initial exclusion criteria, some women did not self-report a condition or were diagnosed after recruitment into our study. Therefore, we obtained information about maternal psychiatric disorders (non-specified) via medical records. Maternal daily caloric intake was measured using the Automated Self-Administered 24-hour Dietary Recall (ASA24), an online platform developed and hosted by the National Cancer Institute (ASA24-Beta and ASA24-2011, Bethesda, Maryland). The duration of exclusive breastfeeding was ascertained via questionnaire at age 5 months and was dichotomized as <5 months and >5 months.
Statistical analyses:
Logistic regression models were used to estimate associations between prenatal cotinine categories (no exposure vs any exposure) and the 5 dichotomized domains of the ASQ-3 as separate outcomes. Our base models adjusted for covariates that are related to both childhood development and maternal smoking during pregnancy (listed in Table I [available at www.jpeds.com]),31,32 as well as maternal psychiatric disorder (yes, no), and maternal daily caloric intake during pregnancy (kCal). In addition to these covariates, our secondary models also adjusted for postnatal exposure to tobacco (none, any).
Table 1; online only.
Characteristics of the Healthy Start cohort, the ASQ-3 sample, and the NIH Toolbox subsample
| Healthy Start cohort (n=1,410) |
ASQ-3 sample (n=246) |
NIH Toolbox sample (n=200) |
|
|---|---|---|---|
| Maternal age, yrs | 28±6 | 29±6 | 30±5 |
| Race/Ethnicity | |||
| Non-Hispanic white | 53% | 55% | 69% |
| Non-Hispanic black | 15% | 12% | 11% |
| Hispanic | 25% | 28% | 16% |
| Other | 6% | 5% | 5% |
| Household income | |||
| <$40,000 | 30% | 26% | 19% |
| $40,001 to $70,000 | 20% | 13% | 18% |
| >$70,000 | 32% | 39% | 52% |
| Don’t know | 18% | 21% | 12% |
| Highest level of education | |||
| <High school | 14% | 15% | 7% |
| High school degree | 18% | 15% | 13% |
| Some college | 67% | 70% | 80% |
| Male offspring | 53% | 52% | 55% |
Abbreviations: BMI, body mass index
Continuous variables are expressed as means ± standard deviation. Categorical variables are expressed as proportions of column totals.
Linear regression models estimated associations between the prenatal cotinine categories and fully corrected T-scores for inhibitory control (Flanker task), cognitive flexibility (DCCS), and receptive language (Picture Vocabulary test) as separate outcomes. The fully corrected T-scores adjust for age, sex, race/ethnicity, and maternal education. Base models adjusted for maternal age, annual household income, non-specified maternal psychiatric disorder (yes, no), and maternal daily caloric intake during pregnancy (kCal). In addition to these covariates, our secondary models also adjusted for postnatal exposure to tobacco (none, any).
We calculated adjusted odds ratios (ORs) or means and beta coefficients with corresponding 95% confidence intervals (CIs) for each separate model. An alpha level of 0.05 was used to determine statistical significance. All analyses were performed using Stata, version 14 (StataCorp LP).
Secondary analyses explored the association between prenatal exposure to tobacco and ASQ-3 categories at 18 months of age. We also repeated our base model analyses among those with ASQ-3 results at 18 and 54 months of age (n=133).
Results
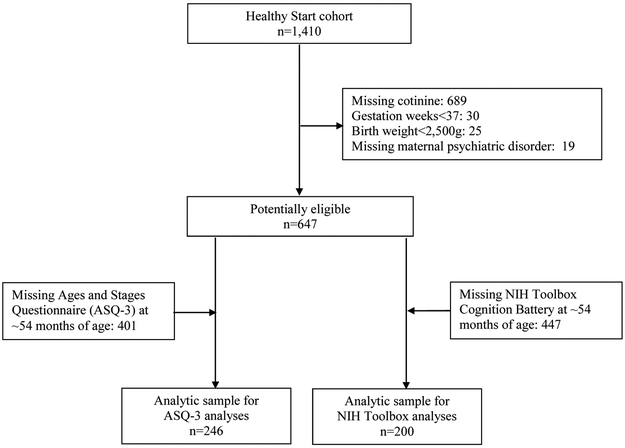
Healthy Start initially enrolled 1,410 participants (Figure; available at www.jpeds.com). Of these, 689 did not have cotinine measured in stored urinary samples collected during pregnancy and 19 were missing information on maternal psychiatric disorders. An additional 55 participants were excluded due to birth before 37 weeks (n=30) or a birth weight < 2500 g (n=25). Of the eligible sample (n=647), 401 did not complete the ASQ-3 at ~54 months of age; the ASQ-3 is valid through 66 months of age and some children were no longer eligible when they attended the visit. Therefore, the final analytic sample for the ASQ-3 analyses was 246. Of the eligible sample (n=647), 447 did not complete the NIH Toolbox Cognition Battery because this assessment was introduced later during the study. Therefore, the final analytic sample for the NIH Toolbox analyses was 200.
Figure 1.
Flow of participants through the study.
There were no differences in maternal age, race/ethnicity, education, or offspring sex between the entire cohort (n=1,410) and the ASQ-3 sample (n=246) (Table 1). Women whose children completed the NIH Toolbox Cognition Battery were more likely to be non-Hispanic White, to have an annual household income >$70,000, and to have at least some college education. Maternal age or offspring sex did not differ between participants from the entire cohort (n=1,410) and the NIH toolbox sample (n=200).
Characteristics of the analytic sample are summarized in Table 2. Based on maternal urinary cotinine, a majority of the women were classified as having no exposure (n=181, 74%). Women with any exposure to tobacco during pregnancy were younger than those with no exposure (p<0.01). Non-exposed women were more likely to be non-Hispanic white (p<0.01), to have an annual household income above $70,000 (p<0.01), and to have attended college (p<0.01). Offspring born to exposed women had a lower birth weight than offspring born to non-exposed women (p=0.02). There was a statistically significant difference in the duration of exclusive breastfeeding among exposed and non-exposed women. Half of the non-exposed women exclusively breastfed their infants ≥5 months, whereas only 25% of the exposed women did so. Exposed and non-exposed participants did not differ in maternal daily caloric intake during pregnancy (P = .07), offspring sex (p=0.46), gestational age (among term births) (p=0.32), child age at NIH Toolbox Cognition Battery assessment (p=0.46), child age at ASQ-3 assessment (p=0.38), or the ASQ-3 version completed (p=0.14).
Table 2.
Characteristics of eligible mothers and children in the Healthy Start study
| All (n=246) |
Prenatal cotinine categories | p-value | ||
|---|---|---|---|---|
| No exposure (cotinine<LOD) (n=181) |
Any exposure (cotinine≥LOD) (n=65) |
|||
| Mother characteristics | ||||
| Age (years) | 29±6 | 30±6 | 27±6 | p<0.01 |
| Race/Ethnicity | ||||
| Non-Hispanic white | 55% | 61% | 37% | |
| Non-Hispanic black | 12% | 4% | 34% | |
| Hispanic | 28% | 31% | 20% | |
| Other | 5% | 4% | 9% | p<0.01 |
| Household income | ||||
| <$40,000 | 26% | 20% | 42% | |
| $40,001 to $70,000 | 13% | 14% | 12% | |
| >$70,000 | 39% | 48% | 17% | |
| Don’t know | 21% | 18% | 30% | p<0.01 |
| Highest level of education | ||||
| <High school | 15% | 11% | 25% | |
| High school degree | 15% | 12% | 25% | |
| Some college or more | 70% | 77% | 51% | p<0.01 |
| Maternal psychiatric disorder (non-specified) | ||||
| No | 85% | 89% | 74% | |
| Yes | 15% | 11% | 26% | p<0.01 |
| Maternal daily caloric intake during pregnancy (kCal) | 2,009±648 | 1,966±574 | 2,133±818 | p=0.07 |
| Child characteristics | ||||
| Male | 52% | 53% | 48% | p=0.46 |
| Birth weight (g) | 3333±420 | 3364±421 | 3245±408 | p=0.02 |
| Gestational age at birth (weeks) | 40±1 | 40±1 | 40±1 | p=0.32 |
| Age at NIH Toolbox Cognition Battery assessment (months) | 55±4 | 54±4 | 54±3 | p=0.46 |
| Age at ASQ-3 assessment (months) | 54±3 | 54±3 | 54±4 | p=0.38 |
| ASQ-3 version | ||||
| 48 months | 22% | 22% | 27% | |
| 54 months | 66% | 67% | 54% | |
| 60 months | 12% | 11% | 18% | p=0.14 |
| Duration of exclusive breastfeeding | ||||
| <5 months | 55% | 49% | 75% | |
| ≥5 months | 45% | 51% | 25% | p<0.01 |
Continuous variables are expressed as means ± standard deviation. Independent samples t-tests were used to examine the differences in means by cotinine categories. Categorical variables are expressed as proportions of column totals. Chi-square tests were used to examine differences in proportions by cotinine categories
NIH Toolbox Cognition Battery and ASQ-3:
Response inhibition (Flanker) was lower among children with an ASQ-3 fail/monitor score for fine motor (Table 3 [available at www.jpeds.com]; p=0.02), gross motor (p=0.03), personal-social (p=0.02), and problem-solving skills (p=0.02). There were no differences in response inhibition across the categories of the ASQ-3 communication domain (p=0.28). There were no differences in cognitive flexibility (DCCS) or receptive language (Picture Vocabulary) across the five ASQ-3 domains.
Table 3; online only.
Means and standard deviations of fully-adjusted T-scores of NIH Toolbox cognition tasks by ASQ-3 domains
| Fine motor | Gross motor | Communication | Personal-social | Problem solving | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fail/monitor | Pass | p | Fail/monitor | Pass | p | Fail/monitor | Pass | p | Fail/monitor | Pass | p | Fail/monitor | Pass | p | |
| Flanker | 47±9 | 50±8 | 0.02 | 45±13 | 50±8 | 0.03 | 48±5 | 50±8 | 0.28 | 45±10 | 50±8 | 0.02 | 45±5 | 50±8 | 0.02 |
| DCCS | 49±6 | 50±10 | 0.34 | 50±10 | 52±10 | 0.17 | 45±8 | 50±10 | 0.11 | 46±10 | 50±10 | 0.13 | 45±6 | 50±10 | 0.07 |
| Picture Vocabulary | 51±13 | 52±11 | 0.65 | 49±12 | 51±13 | 0.23 | 47±10 | 51±13 | 0.15 | 45±11 | 51±13 | 0.06 | 48±15 | 51±13 | 0.24 |
Abbreviation: ASQ-3, Third Edition Ages and Stages Questionnaire; DCCS, Dimensional Change Card Sort.
Table 4 presents the association between prenatal exposure to tobacco and the ASQ-3 domains. Compared with non-exposed offspring, exposed offspring had 3.9 times the odds of a fail/monitor score for fine motor skills (95% CI: 1.5, 10.3). The association between prenatal exposure to tobacco and fine motor skills remained statistically significant after adjusting for postnatal exposure to tobacco (adjusted OR: 3.3, 95% CI: 1.1, 10.2). No significant associations were observed between prenatal exposure to tobacco and the other ASQ-3 domains (gross motor, personal-social, communication, or problem solving skills).
Table 4.
Adjusted odds ratios for a fail/monitor score on separate ASQ-3 domains, by maternal urinary cotinine categories
| Adjusted odds ratios for fail/monitor score for separate ASQ-3 domains (95% CIs) | ||||||
|---|---|---|---|---|---|---|
| Cotinine categories | n | Fine Motor | Gross Motor | Personal-social | Communication | Problem Solving |
| Model 1a | ||||||
| <0.5 ng/mL (LOD) | 181 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥0.5 ng/mL | 65 | 3.9 (1.5, 10.3); p<0.01 | 1.6 (0.4, 5.9); p=0.46 | 0.9 (0.2, 4.0); p=0.96 | 1.2 (0.2, 6.1); p=0.80 | 1.2 (0.4, 4.0); p=0.72 |
| Model 2b | ||||||
| <0.5 ng/mL (LOD) | 172 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥0.5 ng/mL | 62 | 3.3 (1.1, 10.2); p=0.03 | 1.8 (0.4, 7.5); p=0.45 | 0.8 (0.2, 3.9); p=0.80 | 0.7 (0.1, 3.9); p=0.65 | 0.9 (0.2, 3.1); p=0.82 |
Abbreviations: ASQ-3, Third Edition Ages and Stages Questionnaire; CI, confidence interval; LOD, limit of detection.
Model 1 adjusted for maternal age (years), maternal education (<high school, high school diploma, some college), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), offspring sex, a non-specified maternal psychiatric diagnosis (yes, no), and maternal daily caloric intake during pregnancy (kCal).
Model 2 adjusted for Model 1 covariates, as well as self-report of a household smoker in early childhood (none, any).
Table 5 presents the association between prenatal exposure to tobacco and fully corrected T-scores for the NIH Toolbox assessments. Compared with non-exposed offspring, exposed offspring exhibited decreased inhibitory control on the Flanker test (adjusted beta coefficient: x2212; 3.0, 95% CI: −6.1, −0.7). This association was no longer statistically significant following adjustment for postnatal exposure to tobacco (adjusted beta coefficient: −2.5; 95% CI: −5.9, 1.0). No significant associations were observed between prenatal exposure to tobacco and cognitive flexibility (Dimensional Change Card Sort) or receptive language (Picture Vocabulary Test).
Table 5.
Adjusted means and beta coefficients for fully-adjusted T-scores for NIH Toolbox Cognition Battery tests, according to maternal urinary cotinine categories
| Adjusted means and beta coefficients (95% CIs) | ||||
|---|---|---|---|---|
| Cotinine categories | n | Flanker Test | Dimensional Change Card Sort | Picture Vocabulary Test |
| Model 1a | ||||
| <0.5 ng/mL (LOD) | 152 | 51.5 (50.2, 52.9) | 49.6 (47.9, 51.2) | 50.8 (48.6, 53.0) |
| ≥0.5 ng/mL | 48 | −3.0 (−6.1, −0.7); p=0.04 | 1.8 (−1.8, 5.5); p=0.32 | 1.8 (−3.0, 6.6); p=0.83 |
| Model 2b | ||||
| <0.5 ng/mL (LOD) | 149 | 51.3 (49.9, 52.7) | 49.4 (47.7, 51.0) | 51.6 (49.3, 53.8) |
| ≥0.5 ng/mL | 48 | −2.5 (−5.9, 1.0); p=0.16 | 2.8 (−1.3, 6.8); p=0.18 | −0.1 (−5.5, 5.2); p=0.96 |
Abbreviations: CI, confidence interval; LOD, limit of detection.
Model 1 adjusted for maternal age (years), annual household income (<$40,000, $40,001 to $70,000, >$70,000, missing or do not know), a non-specified maternal psychiatric diagnosis (yes, no), and maternal daily caloric intake during pregnancy (kCal).
Model 2 adjusted for Model 1 covariates, as well as self-report of a household smoker in early childhood (none, any).
Secondary analyses: When we restricted our analyses to the subsample of children with ASQ-3 assessed at 18 and 54 months of age (n=133), we did not detect an association between prenatal exposure to tobacco and a fail/monitor score for fine motor skills at 18 months of age (Table 6; available at www.jpeds.com). Consistent with our main analyses, no significant associations were observed between prenatal exposure to tobacco and the other ASQ-3 domains (gross motor, personal-social, communication, or problem solving skills) at age 18 months.
Table 6; online only.
Adjusted odds ratios for a fail/monitor score on separate ASQ-3 domains, among a subset of children with ASQ-3 assessed at 18 months of age, n=133
| Adjusteda odds ratio for fail/monitor ASQ score (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Cotinine categories | n | Fine Motor | Gross Motor | Personal-social | Communication | Problem Solving |
| Age 18 months | ||||||
| <0.5 ng/mL (LOD) | 97 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥0.5 ng/mL | 36 | 0.9 (0.2, 3.8) | 1.4 (0.1, 20.9) | empty | 0.7 (0.2, 2.9) | 1.0 (0.1, 4.6) |
Abbreviations: ASQ-3, Third Edition Ages and Stages Questionnaire; CI, confidence interval.
All models adjusted for maternal age (years), maternal education (<high school, high school diploma, some college), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), offspring sex, and a non-specified maternal psychiatric diagnosis (yes, no).
Discussion
Our results confirm earlier findings of less optimal neurocognitive development among children exposed to tobacco in utero. In addition, we provide evidence that early-life exposure to tobacco smoke is associated with less optimal fine motor development and reduced inhibitory control in children born at ≥37 weeks of gestation and birth weights ≥2500 g.
Tobacco smoke is a complex mixture of over 5,000 chemicals and compounds.33 Although many of these individual constituents may contribute to neurocognitive delays, neuroimaging studies suggest that nicotine is especially toxic to the developing brain. Nicotine is a vasoconstrictor and reduces uterine blood flow to the placenta.34 This results in fetal hypoxia with sustained deprivation of nutrients and oxygen. Nicotine-induced fetal hypoxia may lead to significant changes in brain structures involved in learning and memory, such as decreased volume in the cortical areas35 and the amygdala.36 Additionally, nicotine may act as a neuro teratogen by over-stimulating nicotinic acetylcholine receptors.37 These receptors are abundant in the cerebellum, which plays an important role in motor control and coordination,38 and the hippocampus, which is responsible for memory and learning.39 Nicotine exposure may contribute to damage of the nicotinic cholinergic system in the offspring cerebellum, resulting in subsequent motor dysfunction.38 Dysfunction of nicotinic acetylcholine receptors in the offspring hippocampus has been linked to cognitive deficits.39
Consistent, positive associations between prenatal exposure to tobacco and delayed motor development have been described in the literature.4-8, 21 Most of these studies were conducted among preschool-aged children. Only three studies have examined these associations among toddlers.4, 6, 21 Contrary to our findings, Gusella and Fried4 reported weaker fine motor skills at age 13 months whereas Evlampidou et al reported weaker gross motor skills at age 18 months.6 These studies included infants born at <37 weeks of gestation or with a low birth weight, which may have contributed to the positive results. Among a population of children in Korea with normal birth histories, Lee et al failed to detect an association between prenatal exposure to tobacco and motor development at age 24 months.21 Thus, the impact of prenatal exposure to tobacco on delayed motor development may not emerge until later in life among children who are not typically considered to be at risk for developmental delays.
Research has established an association between self-report of maternal active smoking during pregnancy and poorer offspring performance with inhibitory tasks at age 4-18 years.9-15 Additionally, neuroimaging studies suggest that prenatal exposure to tobacco is associated with increased activation in brain regions related to response inhibition during a Flanker/NoGo task among adolescents40 and young adults.41, 42 Our data demonstrated that prenatal exposure to tobacco alone was associated with impaired inhibitory control, in the absence of low birth weight or preterm delivery. Consistent with our results, no clear relationships have been established for the association between self-report of maternal active smoking during pregnancy with receptive language43-45 or cognitive flexibility.14, 46
Our results may have implications for the impact of prenatal exposure to tobacco smoke on overall neurocognitive development. Fine motor skills are essential for early learning. A majority of classroom activities in kindergarten involve fine motor skills, such as coloring, copying, cutting, and drawing.47 Early-life fine motor function is often associated with later academic achievement, especially in mathematics.48 Furthermore, some studies, including data from the present study, have shown that fine motor coordination is associated with inhibitory control.49 Inhibitory control is an essential first step in solving complex problems. As children become capable of inhibiting responses to distractions, other executive functions (such as working memory and cognitive flexibility) can develop to allow them to negotiate increasingly complex problems.50 Furthermore, both fine motor skills and inhibitory control have been linked to fluid intelligence, or the ability to think logically and solve problems in novel situations.51
It is difficult to disentangle the interplay between pre- and postnatal exposure to tobacco in their relationship with offspring neurocognitive development. Pre- and postnatal exposures to tobacco may act synergistically to influence neurodevelopment. However, due to the low sample sizes within exposure subgroups, we were unable to specifically test for an interaction. In secondary analyses, we included postnatal exposure to tobacco as a covariate. After adjusting for postnatal exposure to tobacco, the association between prenatal exposure to tobacco and fine motor skills remained significant, but the association with inhibitory control was attenuated. Fine motor skills are well-established by preschool age whereas cognition continues to develop rapidly throughout adolescent.52 This suggest that pregnancy may be the most susceptible developmental period for offspring motor development whereas postnatal exposures continue to influence offspring cognitive development. Prospective cohorts with sufficiently large subgroups of children with objective assessment of both prenatal and postnatal exposure to tobacco are needed to explore these questions more conclusively.
A strength of our approach was the use of a biomarker to objectively characterize prenatal exposure to tobacco. Compared with self-report of exposure during pregnancy, maternal urinary cotinine is more likely to capture prenatal exposure to tobacco. This was especially true for secondhand exposures, which are more likely to be under-reported than active smoking among pregnant women.23 However, cotinine cannot differentiate the source and type of exposure. In addition to tobacco products, nicotine exposure can arise from nicotine replacement therapy, as well as consumption of certain foods, such as tomatoes, potatoes, and black tea.53 Although cotinine is not tobacco-specific, cotinine tends to agree with tobacco-specific nitrosamines, such as 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL).54 Therefore, it is likely that the potential for exposure misclassification is low. The reduction in exposure measurement error may have provided a more accurate representation of the true association between prenatal exposure to tobacco and childhood neurocognitive development.
A limitation of our study is the diminished ability to establish causality, given the observational nature of this study. Another limitation is the relatively small number of participants with the ASQ-3 and NIH Toolbox assessments. Maternal smoking during pregnancy is associated with lower socioeconomic status, lower educational attainment, maternal psychopathology, malnutrition of the mother during pregnancy, and a shorter duration of breastfeeding. Although we adjusted for many of these covariates, it remains difficult to causally attribute the impaired developmental outcomes to smoking itself. Co-use of tobacco and other substances, cannabis, cocaine or opioids, during pregnancy is common55 and may contribute to neurocognitive delays through similar mechanisms.56 However, these data were not available in the present study. Therefore, we cannot rule out the possibility of confounding by co-use of other substances during pregnancy.
Early-life exposure to tobacco continues to be an important public health concern. Although many women attempt to quit smoking during pregnancy, at least 1 in 3 children in the United States are exposed to some level of tobacco in utero.2, 57 After birth, exposure to tobacco becomes more prevalent. Parents who smoke during pregnancy continue to smoke after delivery.22 Among women who quit smoking during pregnancy, relapse in the early postpartum period is common.22 Furthermore, there is concern that the prevalence of this exposure may increase, as more youth, the future generation of parents, adopt the use of e-cigarettes.58 The results of our study, coupled with recent trends in smoking prevalence and market shifts to different nicotine products, suggest that it is important to encourage parents to quit smoking and limit their children’s exposure to nicotine and tobacco during and after pregnancy.
Acknowledgments
Supported by the National Institutes of Health (R01DK076648, 5UG3OD023248, R01ES022934, R01GM121081, K99ES028711). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented as a poster at the 31st Annual Conference for the International Society of Environmental Epidemiology (ISEE 2019), August 25-29, 2019, Utrecht, The Netherlands.
References Cited
- [1].Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, et al. Tobacco Product Use Among Adults - United States, 2017. MMWR Morbidity and mortality weekly report. 2018;67:1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drake P, Driscoll AK, Mathews TJ. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief. 2018:1–8. [PubMed] [Google Scholar]
- [3].Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers' exposure to secondhand smoke--United States, 1999-2012. MMWR Morb Mortal Wkly Rep. 2015;64:103–8. [PMC free article] [PubMed] [Google Scholar]
- [4].Gusella JL, Fried PA. Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehavioral toxicology and teratology. 1984;6:13–7. [PubMed] [Google Scholar]
- [5].Polanska K, Hanke W, Sobala W, Trzcinka-Ochocka M, Ligocka D, Brzeznicki S, et al. Developmental effects of exposures to environmental factors: the Polish Mother and Child Cohort Study. BioMed research international. 2013;2013:629716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Evlampidou I, Bagkeris M, Vardavas C, Koutra K, Patelarou E, Koutis A, et al. Prenatal Second-Hand Smoke Exposure Measured with Urine Cotinine May Reduce Gross Motor Development at 18 Months of Age. J Pediatr. 2015;167:246–52.e2. [DOI] [PubMed] [Google Scholar]
- [7].Hsieh CJ, Liao HF, Wu KY, Hsieh WS, Su YN, Jeng SF, et al. CYP1A1 Ile462Val and GSTT1 modify the effect of cord blood cotinine on neurodevelopment at 2 years of age. Neurotoxicology. 2008;29:839–45. [DOI] [PubMed] [Google Scholar]
- [8].Trasti N, Vik T, Jacobsen G, Bakketeig LS. Smoking in pregnancy and children's mental and motor development at age 1 and 5 years. Early human development. 1999;55:137–47. [DOI] [PubMed] [Google Scholar]
- [9].Boucher O, Jacobson JL, Burden MJ, Dewailly É, Jacobson SW, Muckle G. Prenatal Tobacco Exposure and Response Inhibition in School-Aged Children: An Event-Related Potential Study. Neurotoxicology and teratology. 2014;44:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cornelius MD, De Genna NM, Leech SL, Willford JA, Goldschmidt L, Day NL. Effects of Prenatal Cigarette Smoke Exposure on Neurobehavioral Outcomes in Ten-Year-Old Children of Teenage Mothers. Neurotoxicology and teratology. 2011;33:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huijbregts SCJ, Warren AJ, de Sonneville LMJ, Swaab-Barneveld H. Hot and Cool Forms of Inhibitory Control and Externalizing Behavior in Children of Mothers who Smoked during Pregnancy: An Exploratory Study. Journal of Abnormal Child Psychology. 2008;36:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Julvez J, Ribas-Fito N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. International journal of epidemiology. 2007;36:825–32. [DOI] [PubMed] [Google Scholar]
- [13].Micalizzi L, Marceau K, Brick LA, Palmer RH, Todorov AA, Heath AC, et al. Inhibitory control in siblings discordant for exposure to maternal smoking during pregnancy. Developmental psychology. 2018;54:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Daseking M, Petermann F, Tischler T, Waldmann H. Smoking during Pregnancy Is a Risk Factor for Executive Function Deficits in Preschool-aged Children. Geburtshilfe und Frauenheilkunde. 2015;75:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Piper BJ, Corbett SM. Executive Function Profile in the Offspring of Women That Smoked During Pregnancy. Nicotine & Tobacco Research. 2012;14:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Critical reviews in toxicology. 2012;42:279–303. [DOI] [PubMed] [Google Scholar]
- [17].Kallen K Maternal smoking during pregnancy and infant head circumference at birth. Early human development. 2000;58:197–204. [DOI] [PubMed] [Google Scholar]
- [18].Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatric and perinatal epidemiology. 2008;22:162–71. [DOI] [PubMed] [Google Scholar]
- [19].Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatric research. 2013;74 Suppl 1:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Current opinion in pediatrics. 2008;20:184–90. [DOI] [PubMed] [Google Scholar]
- [21].Lee M, Ha M, Hong Y-C, Park H, Kim Y, Kim E-J, et al. Exposure to prenatal secondhand smoke and early neurodevelopment: Mothers and Children's Environmental Health (MOCEH) study. Environmental health : a global access science source. 2019;18:22-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000-2005. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2009;58:1–29. [PubMed] [Google Scholar]
- [23].Moore BF, Starling AP, Magzamen S, Harrod CS, Allshouse WB, Adgate JL, et al. Fetal exposure to maternal active and secondhand smoking with offspring early-life growth in the Healthy Start study. International journal of obesity (2005). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Benowitz N, Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, et al. Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiol Biomarkers Prev. 2010;19:2795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zielinska-Danch W, Wardas W, Sobczak A, Szoltysek-Boldys I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12:484–96. [DOI] [PubMed] [Google Scholar]
- [26].Squires J, Twombly E, Bricker D, Potter L. ASQ-3 User’s Guide. Baltimore, MD: Brookes Publishing; 2009. [Google Scholar]
- [27].Schonhaut L, Armijo I, Schonstedt M, Alvarez J, Cordero M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics. 2013;131:e1468–74. [DOI] [PubMed] [Google Scholar]
- [28].Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beck DM, Schaefer C, Pang K, Carlson SM. Executive Function in Preschool Children: Test-Retest Reliability. Journal of cognition and development : official journal of the Cognitive Development Society. 2011;12:169–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, et al. Demographically Corrected Normative Standards for the English Version of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society : JINS. 2015;21:378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alvik A Variables predicting low infant developmental scores: maternal age above 30 years is a main predictor. Scandinavian journal of public health. 2014;42:113–9. [DOI] [PubMed] [Google Scholar]
- [32].Kerstjens JM, Bos AF, ten Vergert EM, de Meer G, Butcher PR, Reijneveld SA. Support for the global feasibility of the Ages and Stages Questionnaire as developmental screener. Early human development. 2009;85:443–7. [DOI] [PubMed] [Google Scholar]
- [33].Borgerding M, Klus H. Analysis of complex mixtures--cigarette smoke. Exp Toxicol Pathol. 2005;57 Suppl 1:43–73. [DOI] [PubMed] [Google Scholar]
- [34].Walsh RA. Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol. 1994;66:1059–92. [PubMed] [Google Scholar]
- [35].El Marroun H, Schmidt MN, Franken IH, Jaddoe VW, Hofman A, van der Lugt A, et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haghighi A, Schwartz DH, Abrahamowicz M, Leonard GT, Perron M, Richer L, et al. Prenatal exposure to maternal cigarette smoking, amygdala volume, and fat intake in adolescence. JAMA psychiatry. 2013;70:98–105. [DOI] [PubMed] [Google Scholar]
- [37].Slotkin TA, Southard MC, Adam SJ, Cousins MM, Seidler FJ. Alpha7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: nicotine and chlorpyrifos. Brain research bulletin. 2004;64:227–35. [DOI] [PubMed] [Google Scholar]
- [38].Blood-Siegfried J, Rende EK. The long-term effects of prenatal nicotine exposure on neurologic development. Journal of midwifery & women's health. 2010;55:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yakel JL. Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Archiv : European journal of physiology. 2013;465:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bennett DS, Mohamed FB, Carmody DP, Bendersky M, Patel S, Khorrami M, et al. Response inhibition among early adolescents prenatally exposed to tobacco: an fMRI study. Neurotoxicology and teratology. 2009;31:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holz NE, Boecker R, Baumeister S, et al. Effect of prenatal exposure to tobacco smoke on inhibitory control: Neuroimaging results from a 25-year prospective study. JAMA Psychiatry. 2014;71:786–96. [DOI] [PubMed] [Google Scholar]
- [42].Longo CA, Fried PA, Cameron I, Smith AM. The long-term effects of prenatal nicotine exposure on response inhibition: an fMRI study of young adults. Neurotoxicology and teratology. 2013;39:9–18. [DOI] [PubMed] [Google Scholar]
- [43].Huijbregts SCJ, Séguin JR, Zelazo PD, Parent S, Japel C, Tremblay RE. Interrelations Between Maternal Smoking During Pregnancy, Birth Weight and Sociodemographic Factors in the Prediction of Early Cognitive Abilities. Infant and child development. 2006;15:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5 years. American journal of epidemiology. 1995;142:S19–29. [DOI] [PubMed] [Google Scholar]
- [45].Makin J, Fried PA, Watkinson B. A comparison of active and passive smoking during pregnancy: long-term effects. Neurotoxicology and teratology. 1991;13:5–12. [DOI] [PubMed] [Google Scholar]
- [46].Ramsay H, Barnett JH, Murray GK, Mäki P, Hurtig T, Nordstrom T, et al. Smoking in pregnancy, adolescent mental health and cognitive performance in young adult offspring: results from a matched sample within a Finnish cohort. BMC Psychiatry. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marr D, Cermak S, Cohn ES, Henderson A. Fine motor activities in Head Start and kindergarten classrooms. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2003;57:550–7. [DOI] [PubMed] [Google Scholar]
- [48].Pitchford NJ, Papini C, Outhwaite LA, Gulliford A. Fine Motor Skills Predict Maths Ability Better than They Predict Reading Ability in the Early Primary School Years. Frontiers in psychology. 2016;7:783-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rigoli D, Piek JP, Kane R, Oosterlaan J. An examination of the relationship between motor coordination and executive functions in adolescents. Developmental medicine and child neurology. 2012;54:1025–31. [DOI] [PubMed] [Google Scholar]
- [50].Pennequin V, Sorel O, Fontaine R. Motor planning between 4 and 7 years of age: changes linked to executive functions. Brain and cognition. 2010;74:107–11. [DOI] [PubMed] [Google Scholar]
- [51].van der Fels IM, Te Wierike SC, Hartman E, Elferink-Gemser MT, Smith J, Visscher C. The relationship between motor skills and cognitive skills in 4-16 year old typically developing children: A systematic review. Journal of science and medicine in sport. 2015;18:697–703. [DOI] [PubMed] [Google Scholar]
- [52].Zysset AE, Kakebeeke TH, Messerli-Bürgy N, Meyer AH, Stülb K, Leeger-Aschmann CS, et al. Predictors of Executive Functions in Preschoolers: Findings From the SPLASHY Study. Front Psychol. 2018;9:2060-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Davis RA, Stiles MF, deBethizy JD, Reynolds JH. Dietary nicotine: a source of urinary cotinine. Food Chem Toxicol. 1991;29:821–7. [DOI] [PubMed] [Google Scholar]
- [54].Moore BF, Clark ML, Bachand A, Reynolds SJ, Nelson TL, Peel JL. Interactions Between Diet and Exposure to Secondhand Smoke on Metabolic Syndrome Among Children: NHANES 2007-2010. J Clin Endocrinol Metab. 2016;101:52–8. [DOI] [PubMed] [Google Scholar]
- [55].Oga EA, Mark K, Coleman-Cowger VH. Cigarette Smoking Status and Substance Use in Pregnancy. Matern Child Health J. 2018;22:1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. The European journal of neuroscience. 2011;34:1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Vital signs: disparities in nonsmokers' exposure to secondhand smoke--United States, 1999-2012. MMWR Morbidity and mortality weekly report. 2015;64:103–8. [PMC free article] [PubMed] [Google Scholar]
- [58].Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morbidity and mortality weekly report. 2015;64:381–5. [PMC free article] [PubMed] [Google Scholar]