Abstract
Rationale and Objectives:
Few studies have investigated racial disparities in survival among dialysis patients in a manner that considers risk factors and mortality during the phase of kidney disease prior to maintenance dialysis. Our objective was to explore racial variations in survival among dialysis patients and relate them to racial differences in comorbidities and rates of death in the setting of kidney disease not yet requiring dialysis therapy.
Study Design:
Retrospective cohort study.
Settings and Participants:
3288 black and white participants of the Chronic Renal Insufficiency Cohort (CRIC), none of whom were on dialysis at enrollment.
Exposure:
Race.
Outcome:
Mortality.
Analytic approach:
Cox proportional hazards regression was used to examine the association between race and mortality starting at 1) time of dialysis initiation; 2) entry into CRIC cohort.
Results:
During 7.1 years of median follow-up, 678 CRIC participants started dialysis. Starting from time of dialysis initiation, blacks had lower risk of death (unadjusted HR, 0.67; 95% CI, 0.51-0.87) compared to whites. Starting from baseline CRIC enrollment, the strength of the association between some risk factors and dialysis was notably stronger for whites than blacks. For example, the hazard ratio for dialysis onset in the presence (versus absence) of heart failure at CRIC enrollment was 1.30 (95% CI, 1.01-1.68) for blacks versus 2.78 (95% CI, 1.90-4.50) for whites, suggesting differential severity of these risk factors by race. When we included deaths occurring both before and after dialysis, the risk of death was higher among blacks (versus whites) starting from CRIC enrollment (HR, 1.41; 95%CI, 1.22-1.64), but this finding was attenuated in adjusted models (HR, 1.08; 95% CI, 0.91-1.28).
Limitations:
Residual confounding.
Conclusion:
The apparent survival advantage among blacks over whites treated with dialysis may be attributed to selected transition of a subset of whites with more severe comorbid conditions onto dialysis.
Keywords: mortality, race, end-stage renal disease (ESRD), chronic kidney disease (CKD), racial disparities, dialysis, survival paradox, comorbid conditions, non–dialysis-dependent CKD (NDD-CKD), cardiovascular disease, survival analysis, transition to dialysis, Chronic Renal Insufficiency Cohort (CRIC)
Introduction
Non-Hispanic black (henceforth black) adults treated with maintenance dialysis have been consistently observed to have a lower risk of death compared to their non-Hispanic white (henceforth white) counterparts despite the fact that blacks in the general population have worse health outcomes.1-7 This lower risk of death among black hemodialysis and peritoneal dialysis (henceforth dialysis) patients persists despite adjustment for differences in sociodemographic variables and comorbid conditions ascertained at or after dialysis initiation,1-4, 8-15 lower rates of transplantation,1, 7, 16-18 higher prevalence of anemia,15, 19 lower prevalence of arteriovenous fistulas,20, 21 and lower dialysis dosing.14, 15, 19 Some investigators have hypothesized that blacks may be less vulnerable to dialysis-associated inflammation, which could help explain the disparities in survival by race among dialysis patients.22, 23 Others have suggested that black patients may be less sensitive to lower doses of dialysis.14 Some have even speculated that black patients and their families have superior coping mechanisms due to exposure to other adverse socioeconomic stressors.24
At the same time, studies have shown that blacks have a higher risk of death compared to whites during the phase of kidney disease prior to kidney replacement therapy (KRT).25-29 It is possible that the lower absolute mortality rates among blacks treated with dialysis may be related to survival to dialysis initiation of a subset of black CKD patients who are healthier than white CKD patients, rather than unique resilience or biological differences in their response to dialysis.25-29
The objective of this study was to examine racial variation in survival among black and white participants in the Chronic Renal Insufficiency Cohort (CRIC) Study which enrolled CKD patients and continued follow-up of these participants through start of KRT (dialysis or transplant).
Methods
Study population
The CRIC Study is a multi-center prospective cohort study of patients with CKD enrolled at 13 sites from seven clinical centers across the U.S. (Ann Arbor/Detroit, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and San Francisco/Oakland, California).30-32 Participants with estimated glomerular filtration rate (eGFR) at the screening visit between 20-70 mL/min/1.73m2 based on the Modification of Diet in Renal Disease (MDRD) Study equation were recruited for study between June 2003 and September 2008. The inclusion and exclusion criteria have been previously described.30 We included 3288 (out of 3939 total) CRIC participants who self-identified as white or black, and excluded participants of other races (n=154) and Hispanic ethnicity (n=497).
Outcome ascertainment
The main outcome of interest was all-cause mortality. Deaths were identified through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Death Index (SSDI). KRT start among CRIC study participants was ascertained by participant self-report or their named contact with confirmation by study staff, and supplemented with cross-linkage with US Renal Data System (USRDS).33-35 Data from study participants were censored using either the time of last known follow-up visit or March 2013, the administrative censoring date for this study.
Ascertainment of comorbidities
Demographic data and socioeconomic status, tobacco and alcohol use, and comorbidities were ascertained via participant self-report during annual visits in CRIC. Cardiovascular disease (including heart failure [HF], stroke, myocardial infarction or revascularization, and peripheral vascular disease) status was based on self-report at time of baseline enrollment of patients with CKD into CRIC study (henceforth termed baseline) and updated additionally based on self-reported or adjudicated cardiovascular events that occurred during CRIC follow-up as previously described.36 Weight and height were measured annually in CRIC study and used for body mass index (BMI) determination, and categorized as presence or absence of obesity (BMI ≥30 kg/m2) as there were few underweight CRIC participants.30-32 If data were missing for a covariate of interest, values from the last CRIC visit were carried forward.
For comorbidities at time of dialysis initiation, data obtained at the last CRIC visit before kidney failure onset were used and updated with data collected from the Centers for Medicare and Medicaid Services (CMS)-2728 form submitted to the USRDS at time of dialysis initiation.37, 38
Risk of death after dialysis initiation, by race
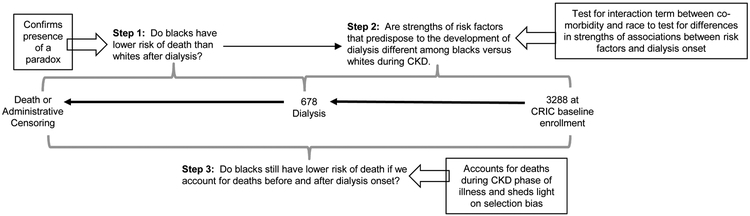
We first examined the association between race and risk of death among participants who underwent dialysis initiation using unadjusted proportional hazards models. We focused specifically on the subset of patients treated with dialysis, since the “racial survival paradox” has not been observed consistently in kidney transplant39, 40 (Figure 1, Step 1). We confirmed that these models did not violate the proportional hazards assumption by race using tests based on Schoenfeld residuals.41
Figure 1.
Study design and rationale for analytic approach
Next, we adjusted for age at dialysis initiation, sex, income, insurance, and education category (Model 1a). We also performed additional adjusted analyses, accounting for demographic characteristics and time-updated co-morbid conditions from baseline through start of KRT, including tobacco use, obesity, hypertension, diabetes mellitus, MI or revascularization, PVD, stroke, HF, and cancer as performed in prior analyses (Model 1b).1-4, 8-15,6, 42
Potential contributory factors for the association between race and all-cause mortality after dialysis initiation
Next, we compared characteristics of black versus white participants at baseline CRIC enrollment and at the time of dialysis initiation. Subsequently, we determined baseline risk factors for future dialysis initiation using multi-variable proportional hazards models that include demographic characteristics. For comorbidities that were statistically significantly associated with risk of dialysis, we then tested for potential interactions between each comorbid condition and race using proportional hazards regression models starting at baseline CRIC enrollment for the outcome of dialysis initiation (Figure 1, Step 2). Comorbidities of interest were those ascertained at baseline that were previously associated with risk of dialysis or risk of death after dialysis and included tobacco use, obesity, hypertension, diabetes, MI, PVD, stroke, HF, and cancer.43-48 In these analyses, patients who died prior to dialysis initiation were censored at time of death, although in sensitivity analysis we also used Fine-Gray models and treated death as a competing risk (given our observation that blacks had both higher dialysis and higher death rates compared with whites). The goal of these analyses was to capture racial differences in the nature or severity of comorbid conditions (i.e. accounting for more than presence or absence) as quantified by the differential strengths of their associations with dialysis initiation among black and white participants. We identified six risk factors that were statistically significantly associated with risk of dialysis (obesity, hypertension, diabetes, MI, PVD, and HF), so we added interaction terms between race and each of these risk factors and repeated our proportional hazards models, then used stepwise elimination of interaction terms that did not achieve statistical significance. With this approach, only obesity and HF met statistical significance for tests for interaction with race (p=0.003 and p=0.001, respectively). Our final model included race, other demographic characteristics, co-morbidities, and interaction terms between obesity and race and HF and race. From these models, we determined the risk of dialysis in the presence or absence of obesity and HF separately for blacks versus whites.
Risk of death starting from CRIC enrollment, by race
To further elucidate the role of potential selection bias in the “racial survival paradox” among dialysis patients, we used unadjusted proportional hazards models to examine the association between race and risk of death starting from baseline CRIC enrollment (Figure 1, Step 3) after confirming that these results did not violate proportional hazards assumption using tests based on Schoenfeld residuals.41 We also adjusted for baseline demographic and co-morbidities ascertained at baseline for analyses of risk of death starting from baseline CRIC enrollment. We then tested for the interaction between race and KRT (as a time-varying covariate) for the risk of death. Because we found a statistically significant interaction between race and KRT, we also repeated our analyses isolating time of follow-up from baseline CRIC enrollment to time of KRT start using unadjusted and adjusted proportional hazards models.
Stata 14 (StataCorp, TX) was used for all analyses and then verified separately by a different analyst using SAS version 9.4 (Cary, NC). We adhered to the Principles of the Declaration of Helsinki and obtained written informed consent from participants and institutional review board approval at all sites.
Results
Among 3288 analyzed CRIC participants, approximately half were black (Table 1). Black participants were likely to be younger, had lower household income and educational attainment, and higher prevalence of tobacco use, obesity, hypertension, diabetes, stroke, and heart failure (HF) at baseline (Table 1). The mean baseline eGFR was 43 mL/min/1.73 m2 in blacks and 45 mL/min/1.73 m2 in whites.
Table 1.
Characteristics of CRIC participants at different time points throughout the course of kidney disease.
| Characteristic | At time of baseline CRIC enrollment | At time of dialysis initiation2 | ||||
|---|---|---|---|---|---|---|
| White (n=1638) |
Black (n=1650) |
P- value |
White (n=203) |
Black (n=475) |
P-value | |
| age | 58 ± 11 | 58 ± 11 | 0.004 | 62 ± 12 | 60 ± 12 | 0.009 |
| Men | 982 (60.0) | 806 (48.9) | <0.001 | 141 (69.5) | 253 (53.3) | <0.001 |
| Baseline income category | <0.001 | <0.001 | ||||
| ≤$20,000 | 254 (15.5) | 646 (39.2) | 58 (28.6) | 209 (44) | ||
| 20,001-50,000 | 416 (25.4) | 417 (25.3) | 58 (28.6) | 108 (22.7) | ||
| 50,001-100,000 | 455 (27.8) | 215 (13.0) | 42 (20.7) | 54 (11.4) | ||
| ≥100,000 | 295 (18.0) | 62 (3.8) | 18 (8.9) | 16 (3.4) | ||
| Don’t wish to answer | 218 (13.3) | 310 (18.8) | 27 (13.3) | 88 (18.5) | ||
| Education Completed | <0.001 | <0.001 | ||||
| < high school | 90 (5.5) | 437 (26.5) | 13 (6.4) | 146 (30.7) | ||
| High school | 291 (17.8) | 366 (22.2) | 52 (25.6) | 97 (20.4) | ||
| Some college | 467 (28.5) | 567 (34.4) | 69 (34.0) | 162 (34.1) | ||
| ≥College graduate | 790 (48.2) | 280 (17.0) | 69 (34.0) | 70 (14.7) | ||
| Insurance | <0.001 | <0.001 | ||||
| None | 48 (3.2) | 96 (5.8) | 4 (2.0) | 31 (6.5) | ||
| Medicaid | 96 (5.9) | 327 (19.8) | 16 (7.9) | 80 (16.8) | ||
| Medicare | 564 (34,4) | 494 (29.9) | 67 (33.0) | 153 (32.2) | ||
| VA/military | 73 (4.5) | 110 (6.7) | 5 (2.5) | 20 (4.2) | ||
| Private | 284 (17.3) | 190 (11.5) | 101 (49.8) | 157 (33.1) | ||
| Unknown | 573 (35.0) | 433 (26.2) | 10 (4.9) | 34 (7.2) | ||
| serum albumin (g/dL) | 4.0 [3.8-4.3] | 4.0 [3.7-4.2] | <0.001 | 3.6 [3.2-3.9] | 3.5 [3.1-3.8] | 0.04 |
| proteinuria (g/d) | 0.10 [0.05-0.38] | 0.19 [0.06-0.87] | <0.001 | --- | --- | --- |
| Tobacco use | 155 (9.5) | 320 (19.4) | <0.001 | 14 (6.9) | 42 (8.8) | 0.4 |
| BMIa (kg/m2) | 29.9 [26.2-35.0] | 32.3 [28.0-37.8] | <0.001 | 29.5 [24.9-35.6] | 29.5 [25.3-36.2] | 0.6 |
| Obesity | 811 (49.5) | 1055 (63.9) | <0.001 | 98 (48.3) | 226 (47.6) | 0.9 |
| Hypertension | 1293 (78.9) | 1533 (92.9) | <0.001 | 200 (98.5) | 473 (99.6) | 0.1 |
| eGFR1 (mL/min/1.73m2) | 46.2 ± 14.7 | 43.7 ± 14.9 | <0.001 | 9.8 ± 4.1 | 9.8 ± 5.0 | 0.61 |
| baseline eGFR (mL/min/1.73m2) | 45.1 [35.6-56.0] | 42.7 [32.2-53.2] | <0.001 | 9.2 [6.5-12.5] | 9.0 [6.6-11.8] | 0.61 |
| Diabetes mellitus | 649 (39.6) | 848 (51.4) | <0.001 | 143 (70.4) | 334 (70.3) | 0.9 |
| MI or revascularization* | 376 (23.0) | 361 (21.9) | 0.5 | 105 (51.7) | 160 (33.7) | <0.001 |
| PVD * | 105 (6.4) | 117 (7.1) | 0.4 | 50 (24.6) | 82 (17.3) | 0.03 |
| Stroke * | 118 (7.2) | 227 (13.8) | <0.001 | 29 (14.3) | 101 (21.3) | 0.04 |
| Heart failure * | 117 (7.1) | 217 (13.2) | <0.001 | 81 (39.9) | 206 (43.4) | 0.4 |
| Cancer | 172 (10.5) | 82 (5.0) | <0.001 | 46 (22.7) | 46 (9.7) | <0.001 |
Values for continuous variables given as Mean ± SD or median [interquartile range]; values for categorical variables count (percentage).
Missing in n=183; eGFR was not carried forward from last visit
Supplemented from CMS-2728 form; data was carried forward from last CRIC visit if missing on form. IQR = interquartile range
BMI missing in N=9; serum albumin missing in N=55
self-report or adjudicated
We noted that between baseline enrollment and time of dialysis initiation, substantial evolution had occurred in the balance of comorbidities between black and white CRIC participants who started dialysis (Table 1). For example, while obesity, diabetes, and HF were more common in blacks at baseline, by the time of dialysis initiation, there were no longer statistically significant differences in these conditions between groups (Table 1). In fact, the prevalence of MI was higher among whites compared to blacks at the time of dialysis initiation.
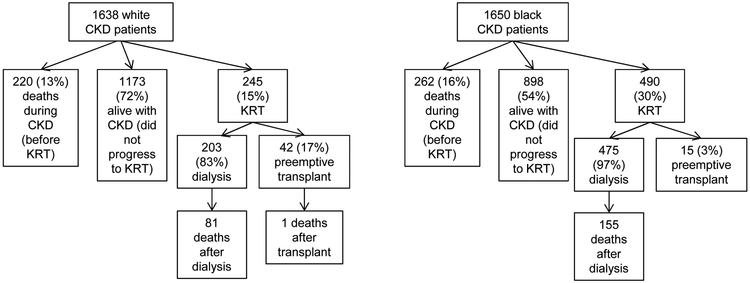
Among 1638 white participants, 245 initiated KRT (rate 2.2 per 100 person-years including dialysis or transplant), and among 1650 black participants, 490 (rate of 5.0 per 100 person-years) initiated KRT (Figure 2). Within white participants who initiated KRT, 42 out of 245 (17%) received preemptive transplant and an additional 42 (17%) received kidney transplant after dialysis. In contrast, only 15 out of 490 (3%) black participants received preemptive transplant and 65 additional (13%) black participants received kidney transplant during follow-up. Thus, 34% of whites versus 16% of blacks who initiated KRT were treated with kidney transplantation during our study period (p<0.001).
Figure 2.
Distribution of deaths by race starting from CRIC baseline enrollment.
RR death (blacks vs whites) after dialysis =(155/475blacks)/(81/203whites) =0.81 → (survival paradox) RR death (blacks vs whites) from CKD through ESRD= ((155+262)/1650blacks)/((81+1+220)/1638whites) =1.38 (no survival paradox)
Risk of death after dialysis initiation by race
A total of 678 black and white participants received dialysis as their first treatment modality during follow-up (584 [86.1%] hemodialysis and 94 [13.9%] peritoneal dialysis).
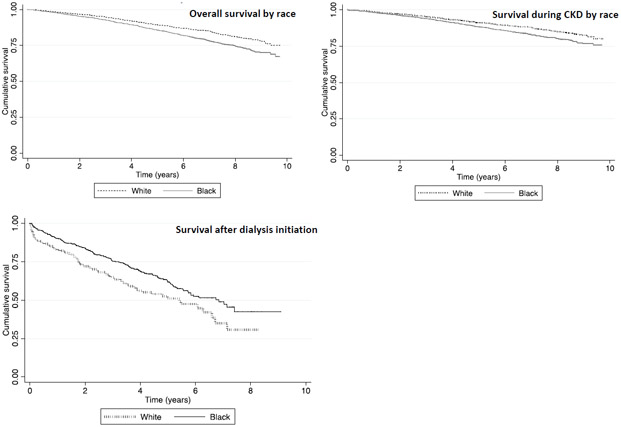
We first confirmed the presence of a racial survival paradox using proportional hazards regression models starting from time of dialysis initiation and found that black participants had lower risk of death than white participants after dialysis initiation (HR, 0.67 [95% CI, 0.51-0.87]; Table 2 and Figure 1, Step 1 and Figure 3). We performed tests for Schoenfeld residuals to ensure that these models did not violate proportional hazards assumptions (p= 0.9). This disparity in risk of death among blacks versus whites persisted even in our demographic and fully adjusted Models 1a and 1b (Table 2).
Table 2.
Overall risk of death and before and after dialysis initiation.
| HR (95% CI) for Black vs White |
|
|---|---|
| Starting from time of dialysis initiation | |
| Unadjusted | 0.67 (0.51-0.87) |
| Adjusted for demographic characteristics1 (Model 1a) | 0.65 (0.48-0.88) |
| Adjusted for demographic characteristics,1 and other co-morbidities2 (Model 1b) | 0.72 (0.53-0.98) |
| Starting from baseline enrollment until KRT start | |
| Unadjusted | 1.36 (1.13-1.62) |
| Adjusted for demographic characteristics3 (Model 1a) | 1.03 (0.84-1.26) |
| Adjusted for demographic characteristics,3 and other co-morbidities4 (Model 1b) | 1.02 (0.83-1.25) |
| Overall risk of death | |
| Unadjusted | 1.41 (1.22-1.64) |
| Adjusted for demographic characteristics3 (Model 1a) | 1.11 (0.94-1.31) |
| Adjusted for demographic characteristics,3 and other co-morbidities4 (Model 1b) | 1.08 (0.91-1.28) |
Demographic characteristics = age at KRT start; sex, income, and education based on CRIC baseline data. Insurance data are from CRIC baseline data and supplemented with updated data reported at time of KRT start to the USRDS (CMS-2728 form) if available.
Other co-morbidities = tobacco use, obesity, hypertension, diabetes mellitus, MI or revascularization, PVD, stroke, HF, and cancer using most recent CRIC data available prior to KRT start and supplemented with updated data reported at time of KRT start to the USRDS (CMS-2728 form) if available.
Demographic characteristics = age, sex, income, and education at baseline CRIC enrollment. Insurance data are from CRIC baseline data.
Other co-morbidities = tobacco use, obesity, hypertension, diabetes mellitus, MI or revascularization, PVD, stroke, HF, and cancer at baseline CRIC enrollment.
Figure 3.
Kaplan-Meier Survival Estimates
Examination of contributory factors for the association between race, dialysis initiation, and all-cause mortality
Motivated by our finding in Table 1, we formally tested for effect modification of the association between risk factors and dialysis by race (Figure 1, Step 2). We found that the association between baseline factors such as obesity and HF and the risk of dialysis indeed were stronger for whites than blacks (p<0.05 for interaction, Table 3 and Table S1). As shown in Table 2, in adjusted models including interaction terms (for obesity and race, as well as heart failure and race), the risk of dialysis was higher for those with HF versus no HF in whites (HR, 2.78; 95% CI, 1.90-4.05) compared to blacks (HR, 1.30; 95% CI, 1.01-1.68). Similarly, the risk of dialysis was lower in the presence of obesity (versus no obesity) in blacks but not in whites (Table 2), indicating the presence of effect modification by race. In sensitivity analysis treating death as a competing risk using Fine-Gray models, results were very similar (Table 3 and Table S2).
Table 3.
Association between various baseline risk factors for the outcome of dialysis initiation in adjusted models, including interactions between race and risk factors of interest.*
| Adjusted HR** (95% CI) for presence vs. absence of each co-morbidity | |||
|---|---|---|---|
| Black | White | P-value for interaction | |
| Proportional hazards model | |||
| Obesity | 0.69 (0.57-0.84) | 1.17 (0.88-1.56) | 0.003 |
| Heart failure | 1.30 (1.01-1.68) | 2.78 (1.90-4.05) | 0.001 |
| Fine-Gray model | |||
| Obesity | 0.69 (0.57-0.85) | 1.21 (0.91-1.60) | 0.001 |
| Heart failure | 1.14 (0.87-1.49) | 2.42 (1.67-3.52) | 0.001 |
Models adjusted for age at baseline enrollment in CRIC, sex, income, insurance, education, tobacco use, obesity, hypertension, diabetes mellitus, MI or revascularization, PVD, stroke, HF, and cancer and includes interaction terms between race and obesity and race and HF.
For Fine-Gray model HR is subdistribution HR.
Risk of death starting from CRIC enrollment, by race
The distribution of deaths that occurred before and after KRT among CRIC participants starting from baseline enrollment are shown in Figure 2 and Table S3. We tested for and found a statistically significant interaction between race and KRT (as a time-dependent covariate, p=0.002). We therefore examined estimates of the risk of death in overall analyses, and analyses limited to non-KRT-requiring CKD versus the time of dialysis therapy (Figure 3).
When we began our survival analysis starting at the time of baseline CRIC enrollment before any participant developed kidney failure (Figure 1, Step 3) and counting deaths observed both before and after KRT start, the risk of death in black participants was higher than that of whites (unadjusted HR, 1.41; 95% CI, 1.22-1.64], Table 2 and Figure 3) and did not violate proportional hazards assumptions (p=0.9). In adjusted analysis accounting for demographic and baseline comorbid conditions, the risk of death in black participants compared to whites starting from baseline enrollment was not different by race (HR, 1.02; 95% CI, 0.83-1.25).
When we began our survival analysis starting at the time of baseline CRIC enrollment and censoring follow-up at time of KRT start, our results were similar to results of our overall survival analyses (incorporating follow-up before and after KRT start) using unadjusted and adjusted models (Table 2 and Figure 3).
Discussion
Black adults treated with dialysis have been shown to have a survival advantage over their white counterparts in multiple studies.7-9, 24, 25, 42, 49-52 Prior studies have not been able to explain this association despite adjustment for racial differences in the presence or absence of comorbidities at dialysis initiation. Consistent with these prior studies, we observed a 33% lower crude risk of death among black compared to white participants treated with dialysis in the CRIC Study. However, our results suggest that racial differences in the strengths of the associations between certain key risk factors and adverse outcomes (i.e. presence of effect modification between these key factors and race) may be an important explanation for the lower risk of death among black versus white CRIC participants after dialysis onset.
A key insight from our study is that comorbidities such as obesity or HF have different prognostic implications in black versus white CKD patients as it relates to kidney disease progression. We demonstrate this through our observation of a higher hazard ratio of dialysis in the presence of HF and obesity at baseline CRIC enrollment among whites compared to blacks. These results suggest that differential severity of baseline co-morbidities as risk factors for kidney failure may be present in black or white patients during non-KRT-requiring CKD (i.e. effect modification by race). We hypothesize that the same effect modification of the association between baseline risk factors and dialysis by race apply when the outcome is survival after dialysis initiation. The fact that race is an effect modifier of associations between risk factors and adverse outcomes may explain why only adjusting for presence or absence of risk factors at time of incident dialysis fails to explain the “racial survival paradox” among dialysis patients (Table 2 Model 1b), since these risk factors are not equivalent in terms of their health effects in blacks and whites.
Furthermore, we found that when we compared deaths that occurred prior to the start of dialysis (by starting our survival analysis at baseline CRIC enrollment), there was no “racial paradox.” In fact, blacks had a 41% higher unadjusted risk of death than whites during non-KRT-requiring CKD, in contradistinction to the 33% lower unadjusted risk of death than whites after dialysis onset. This observation belies prior suggestions that blacks are somehow intrinsically more resilient to dialysis. Our observations in CRIC also differ from that in other CKD populations, where the survival advantage of blacks over whites have also been observed (at least in the later stages of CKD).53, 54 The sharp contrast between the two unadjusted hazard ratios for the risk of death among blacks versus whites (0.67 and 1.41) excluding and including deaths during non-KRT-requiring CKD, respectively (Table 2), are instructive since they are derived from the same CRIC study population. The heterogeneity of CKD could be one of the factors that contributes to the observation of a higher rate of death among blacks during non-KRT-requiring CKD but lower rate after start of KRT. For example, blacks who develop CKD due to diabetes may be at higher risk of death prior to dialysis, whereas blacks who start dialysis may be more likely to have APOLl-associated kidney disease55 and be younger or healthier at time of dialysis initiation, which could explain their better survival.
We believe that a plausible explanation for the “racial survival paradox” may be that black race (which encompasses risk factors such as APOL1 genetic variants55) is a very strong risk factor for kidney failure (Figure 2).55, 56 Thus, whites who develop kidney failure presumably have other predisposing comorbid conditions, such as more severe HF. In turn, more severe HF among whites initiating dialysis could explain the higher risk of death among whites after dialysis. This may be an example of the general issue of selection (collider) bias in studies when certain diagnoses (such as end-stage renal disease) are used as the condition for entry into study.27-29, 57, 58 Taking into account race and risk factor interactions is traditionally less straightforward in epidemiology studies, given that it is easier to control only for differences in the presence or absence of a risk factor rather than its differential effects or severity.
The issue of the survival of patients by race is complex, as differences in access to healthcare before kidney failure onset that may become more equalized after the start of KRT (due to universal healthcare coverage of dialysis patients for citizens in the US) may be a contributor to differential survivorship, although our study accounts for insurance status in all analyses.59-61 In CRIC study, these issues may be less prominent compared to the general population given CRIC participants were research volunteers recruited mostly from nephrology clinics or academic centers. Blacks are also known to have lower rates of referral and longer dialysis vintage at time of referral for transplant evaluation compared to their white counterparts. 39, 52, 62 Some authors have expressed concern that the perception of enhanced survival among black dialysis populations may contribute to complacency with regards to the known lower rates of transplantation among racial minorities in the United States,20, 40 even though transplantation is the preferred form of KRT. Given that our data suggest that black patients who survive to dialysis are a younger and potentially healthier subset of the greater CKD population compared to their white counterparts, the lower rate of transplantation among black CRIC study participants is especially concerning.
The strengths of our study included the well-characterized CRIC cohort, which is national in scope with longitudinal follow-up during CKD, and detailed annual assessments of behaviors, comorbidities, laboratory findings, and outcomes of interest by research protocol. We used a systematic approach to the identification of potential effect modification by race and the impact of comorbidities on risk of dialysis. Our study is strengthened by the lower likelihood of ascertainment bias compared to studies using administrative or clinical data. Limitations include the lack of availability of granular measurements regarding co-morbid conditions, including data surrounding the severity of these co-morbid conditions, chronicity, adequacy of treatment, or differences in disease awareness which may contribute to misclassification of risk factors or residual confounding.38 In addition, while several studies have noted the importance of age of dialysis onset (whereby the racial survival paradox is only observed in older patients) as an effect modifier of the relationship between race and survival, we were unable to examine this issue due to limited power (only 118 of the 678 CRIC participants who started KRT were younger than 50 years of age).6, 54 Our study results may not be generalizable to all patients with CKD given that CRIC was not designed to be a random sample of the U.S. CKD population. For instance, the age difference between black and white CRIC participants at time of dialysis initiation was less pronounced that than that seen in the general US population.6 Finally, we acknowledge that the study of race is complex, and the various contributions of genetics, culture, and physical phenotype is often difficult to parse out.63
In conclusion, our study highlights that the lower risk of death for black patients after dialysis need to be considered in the context of our finding in of a higher unadjusted risk of death for black patients during non-KRT-requiring CKD. We believe this approach provides a more comprehensive view of the “racial survival paradox”, which informs practice and potentially policy. In contrast, studies that limit survival analyses to start at time of dialysis initiation are prone to selection bias. Our results suggest substantial racial disparities in the severity of risk factors that appear and accumulate during non-KRT-requiring CKD. Further research is needed to mitigate disparities in risk factors that contribute to a higher risk of death in the black population prior to the onset of kidney failure. And greater effort needs to be devoted towards providing equity in access to kidney transplantation among the black population who may survive longer on dialysis due to their better health status at the start of dialysis.
Supplementary Material
Table S1. Presence or absence of baseline comorbidities during CRIC and risk of dialysis onset using fully adjusted proportional hazards models.
Table S2. Presence or absence of baseline comorbidities during CRIC and risk of dialysis onset, accounting for competing risk of death using fully adjusted Fine-Gray models.
Table S3. Association between race/ethnicity and mortality rates among CRIC enrollees who started dialysis between 1995-2015.
Acknowledgments
Support: This work was supported by the National Kidney Foundation Satellite Dialysis Clinical Investigator Grant and R01 DK115629 to EK and CEM and K24 DK92291 to CYH. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Disclaimer: The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bleyer AJ, Tell GS, Evans GW, Ettinger WH Jr., Burkart JM. Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis. 1996;28(1): 72–81. [DOI] [PubMed] [Google Scholar]
- 2.Held PJ, Pauly MV, Diamond L. Survival analysis of patients undergoing dialysis. JAMA. 1987;257(5): 645–650. [PubMed] [Google Scholar]
- 3.Medina RA, Pugh JA, Monterrosa A, Cornell J. Minority advantage in diabetic end-stage renal disease survival on hemodialysis: due to different proportions of diabetic type? Am J Kidney Dis. 1996;28(2): 226–234. [DOI] [PubMed] [Google Scholar]
- 4.Pei YP, Greenwood CM, Chery AL, Wu GG. Racial differences in survival of patients on dialysis. Kidney Int. 2000;58(3): 1293–1299. [DOI] [PubMed] [Google Scholar]
- 5.Szczech L Association of race and age with survival among patients undergoing dialysis: racial differences on many levels. Kidney Int. 2011;80(8): 792. [PubMed] [Google Scholar]
- 6.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. Jama. 2011;306(6): 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee CM, Lertdumrongluk P, Streja E, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. American journal of nephrology. 2014;39(3): 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowie CC, Port FK, Rust KF, Harris MI. Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care. 1994;17(7): 681–687. [DOI] [PubMed] [Google Scholar]
- 9.Frankenfield DL, Rocco MV, Roman SH, McClellan WM. Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol. 2003;14(1): 180–186. [DOI] [PubMed] [Google Scholar]
- 10.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol. 1994;5(5): 1231–1242. [DOI] [PubMed] [Google Scholar]
- 11.Morris D, Samore MH, Pappas LM, Ramkumar N, Beddhu S. Nutrition and racial differences in cardiovascular events and survival in elderly dialysis patients. Am J Med. 2005;118(6): 671–675. [DOI] [PubMed] [Google Scholar]
- 12.Pugh JA, Tuley MR, Basu S. Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: the emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis. 1994;23(6): 803–807. [DOI] [PubMed] [Google Scholar]
- 13.Mesler DE, McCarthy EP, Byrne-Logan S, Ash AS, Moskowitz MA. Does the survival advantage of nonwhite dialysis patients persist after case mix adjustment? Am J Med. 1999;106(3): 300–306. [DOI] [PubMed] [Google Scholar]
- 14.Owen WF Jr., Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998;280(20): 1764–1768. [DOI] [PubMed] [Google Scholar]
- 15.Sehgal AR. Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA. 2003;289(8): 996–1000. [DOI] [PubMed] [Google Scholar]
- 16.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients' preferences on racial differences in access to renal transplantation. The New England journal of medicine. 1999;341(22): 1661–1669. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RM, Sankar A, Pittman J. Disparities in access to kidney transplantation between donor service areas in Texas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(10): 2303–2309. [DOI] [PubMed] [Google Scholar]
- 18.Purnell TS, Powe NR, Troll MU, et al. Measuring and explaining racial and ethnic differences in willingness to donate live kidneys in the United States. Clinical transplantation. 2013;27(5): 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF Jr., Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis. 1999;34(4): 721–730. [DOI] [PubMed] [Google Scholar]
- 20.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13(8): 2117–2124. [DOI] [PubMed] [Google Scholar]
- 21.Allon M, Ornt DB, Schwab SJ, et al. Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study. Hemodialysis (HEMO) Study Group. Kidney Int. 2000;58(5): 2178–2185. [DOI] [PubMed] [Google Scholar]
- 22.Crews DC, Sozio SM, Liu Y, Coresh J, Powe NR. Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol. 2011;22(12): 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Norris KC. Is the malnutrition-inflammation complex the secret behind greater survival of African-American dialysis patients? J Am Soc Nephrol. 2011;22(12): 2150–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kovesdy CP, Norris KC. Racial survival paradox of dialysis patients: robust and resilient. Am J Kidney Dis. 2012;60(2): 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7): 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedewa SA, McClellan WM, Judd S, Gutierrez OM, Crews DC. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC nephrology. 2014;15: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banack HR, Kaufman JS. The "obesity paradox" explained. Epidemiology (Cambridge, Mass.). 2013;24(3): 461–462. [DOI] [PubMed] [Google Scholar]
- 28.Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Annals of epidemiology. 2015;25(5): 342–349. [DOI] [PubMed] [Google Scholar]
- 29.Lajous M, Bijon A, Fagherazzi G, et al. Body mass index, diabetes, and mortality in French women: explaining away a "paradox". Epidemiology (Cambridge, Mass.). 2014;25(1): 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2): S148–153. [DOI] [PubMed] [Google Scholar]
- 31.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58(2): 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. JAMA. 1992;268(21): 3085–3091. [PubMed] [Google Scholar]
- 34.Agodoa LY, Jones CA, Held PJ. End-stage renal disease in the USA: data from the United States Renal Data System. American journal of nephrology. 1996; 16(1): 7–16. [DOI] [PubMed] [Google Scholar]
- 35.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18(10): 2644–2648. [DOI] [PubMed] [Google Scholar]
- 36.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65(2): 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(5): 845–851. [DOI] [PubMed] [Google Scholar]
- 38.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11(3): 520–529. [DOI] [PubMed] [Google Scholar]
- 39.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2): 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young CJ, Gaston RS. Renal transplantation in black Americans. The New England journal of medicine. 2000;343(21): 1545–1552. [DOI] [PubMed] [Google Scholar]
- 41.Grambsch PM, and Therneau TM. 1994. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526. [Google Scholar]
- 42.Yan G, Norris KC, Yu AJ, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(6): 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charytan DM, Solomon SD, Ivanovich P, et al. ESRD After Heart Failure, Myocardial Infarction, or Stroke in Type 2 Diabetic Patients With CKD. Am J Kidney Dis. 2017;70(4): 522–531. [DOI] [PubMed] [Google Scholar]
- 44.Rhee CM, Kovesdy CP, Ravel VA, et al. Association of Glycemic Status During Progression of Chronic Kidney Disease With Early Dialysis Mortality in Patients With Diabetes. Diabetes Care. 2017;40(8): 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stack AG, Mohammed A, Hanley A, Mutwali A, Nguyen H. Survival trends of US dialysis patients with heart failure: 1995 to 2005. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(8): 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stack AG, Yermak D, Roche DG, et al. Differential impact of smoking on mortality and kidney transplantation among adult Men and Women undergoing dialysis. BMC nephrology. 2016;17: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka A, Inaguma D, Shinjo H, Murata M, Takeda A. Relationship Between Mortality and Cancer-Bearing Status at Time of Dialysis Initiation. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2017;21(4): 345–353. [DOI] [PubMed] [Google Scholar]
- 48.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3)(suppl 1):S1–S676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amaral S, Patzer RE, Kutner N, McClellan W. Racial disparities in access to pediatric kidney transplantation since share 35. J Am Soc Nephrol. 2012;23(6): 1069–1077. [DOI] [PubMed] [Google Scholar]
- 50.Patzer RE, Sayed BA, Kutner N, McClellan WM, Amaral S. Racial and ethnic differences in pediatric access to preemptive kidney transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(7): 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tjaden LA, Noordzij M, van Stralen KJ, et al. Racial Disparities in Access to and Outcomes of Kidney Transplantation in Children, Adolescents, and Young Adults: Results From the ESPN/ERA-EDTA (European Society of Pediatric Nephrology/European Renal Association-European Dialysis and Transplant Association) Registry. Am J Kidney Dis. 2016;67(2): 293–301. [DOI] [PubMed] [Google Scholar]
- 52.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95(2): 309–318. [DOI] [PubMed] [Google Scholar]
- 53.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(5): 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovesdy CP, Quarles LD, Lott EH, et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62(2): 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369(23): 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 Risk, and eGFR Decline in the General Population. J Am Soc Nephrol. 2016;27(9): 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flanders WD, Eldridge RC, McClellan W. A nearly unavoidable mechanism for collider bias with index-event studies. Epidemiology (Cambridge, Mass.). 2014;25(5): 762–764. [DOI] [PubMed] [Google Scholar]
- 58.Stensrud MJ, Valberg M, Aalen OO. Can Collider Bias Explain Paradoxical Associations? Epidemiology (Cambridge, Mass.). 2017;28(4): e39–e40. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and Ethnic Disparities in Health Care Access and Utilization Under the Affordable Care Act. Medical care. 2016;54(2): 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(9): 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkelmayer WC, Glynn RJ, Levin R, Owen WF Jr., Avorn J Determinants of delayed nephrologist referral in patients with chronic kidney disease. Am J Kidney Dis. 2001;38(6): 1178–1184. [DOI] [PubMed] [Google Scholar]
- 62.Arce CM, Goldstein BA, Mitani AA, Lenihan CR, Winkelmayer WC. Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(12): 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vander Weele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology (Cambridge, Mass.). 2014;25(4): 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Presence or absence of baseline comorbidities during CRIC and risk of dialysis onset using fully adjusted proportional hazards models.
Table S2. Presence or absence of baseline comorbidities during CRIC and risk of dialysis onset, accounting for competing risk of death using fully adjusted Fine-Gray models.
Table S3. Association between race/ethnicity and mortality rates among CRIC enrollees who started dialysis between 1995-2015.