Abstract
Objective:
The aims are to examine the potential association between grand-maternal body mass index (BMI) and grandchild’s birth weight (BW), and whether maternal BW and BMI mediate this association.
Methods:
Data of 209 grandmother-mother pairs and 355 grandchildren from Isle of Wight birth cohort in the UK were analyzed using path analysis.
Results:
An indirect effect of grand-maternal BMI on increasing grandchild’s BW was mediated by maternal BW and BMI at age 18 years (indirect effects: β=2.3 grams per unit increase in grand-maternal BMI via maternal BW, and β=4.4 grams via maternal BMI; p=0.04). These two mediating effects of maternal BW and BMI confounded one another. Grand-maternal smoking during pregnancy (SDP) had an indirect effect on decreasing grandchild’s BW, dependent on maternal SDP and BW (indirect effects: β=−36.1 grams compared to non-smoking grandmothers via maternal SDP, and β=−27.2 grams via maternal BW; p=0.005). Neither direct effect between grand-maternal BMI and grandchild’s BW, nor that between grand-maternal SDP and grandchild’s BW, was statistically significant.
Conclusions:
Larger grand-maternal BMI indirectly increased grandchild’s BW via maternal BW and BMI. Grand-maternal SDP indirectly reduced grandchild’s BW via maternal SDP and BW.
Keywords: Body-Mass Index, BMI, Smoking, Birth Weight
Introduction
Over the past several decades, birth weight (BW) has been recognized to have a long-term impact on the risk of childhood and adulthood diseases, such as obesity, diabetes, lung function, hypertension and cardiovascular diseases (1, 2, 3). Lower BW is well documented to be associated with adverse health outcomes, such as asthma and poorer lung function (1, 4, 5); whereas children born with higher BW are predisposed to higher body mass index (BMI) and overweight or obesity in childhood (6, 7) and adulthood (2, 8). A number of studies demonstrated an intergenerational effect of maternal BMI on BW (9, 10, 11). The observed circle of inheriting obesity from prior generations suggested a possible transgenerational effect of grand-maternal BMI on grandchild’s BW.
To date, researchers have discovered a few grand-maternal risk factors in relation to grandchild’s BW, including grand-maternal BW (12), cardio-metabolic risk factors (13, 14, 15), smoking during pregnancy (16, 17, 18, 19, 20), and socioeconomic status (SES) (21, 22, 23). Nevertheless, only one study focused on the transgenerational effect of grand-maternal BMI (among other cardio-metabolic risk factors, including lipids and glucose levels) on offspring BW (13). Harville et al. were unable to identify a statistically significant association between grand-maternal BMI and grandchild’s BW (13). In their study, maternal BMI was considered a covariate. However, using maternal BMI as a confounder, instead of a potential mediator, might have attenuated the effect of grand-maternal BMI on grandchild’s BW, since maternal BMI is on the path from grand-maternal BMI to grandchild’s BW. Current evidence regarding the transgenerational effect of grand-maternal BMI on grandchild’s BW is extremely limited. We are interested in understanding whether grand-maternal BMI influences grandchild’s BW.
Most studies, assessing the association of transgenerational risk factors with grandchild’s BW, included maternal BW and/or BMI as covariates (13, 15, 16, 17, 18, 19, 20). So far, only two studies examined the potential mediating effect of maternal BW (12) or maternal pre-pregnancy overweight (23) on the association between grand-maternal risk factors and grandchild’s BW. Lahti-Pulkkinen et al. (12) found that maternal BW mediated the positive relationship between grand-maternal BW and BW of the grandchildren. The other study by Huang et al. did not find a significant mediating effect of maternal pre-pregnancy overweight on the association of grand-maternal education level with grandchild’s BW (23). Previous findings are inconclusive; and more studies are needed to determine whether maternal BW and BMI act as mediators or confounders in the transgenerational association of grand-maternal risk factors with grandchild’s BW. Hence, in this study, we hypothesize that maternal BW and BMI may mediate the effect of grand-maternal BMI on grandchild’s BW.
Using the Isle of Wight (IoW) birth cohort in UK (24), we aim to investigate whether the effect of grand-maternal BMI on grandchild’s BW is direct or mediated by maternal BW and/or maternal BMI. The IoW birth cohort prospectively collected three-generation data, thus allowing the investigation of direct and mediating effects by applying path analysis.
Methods
The Isle of Wight Birth Cohort
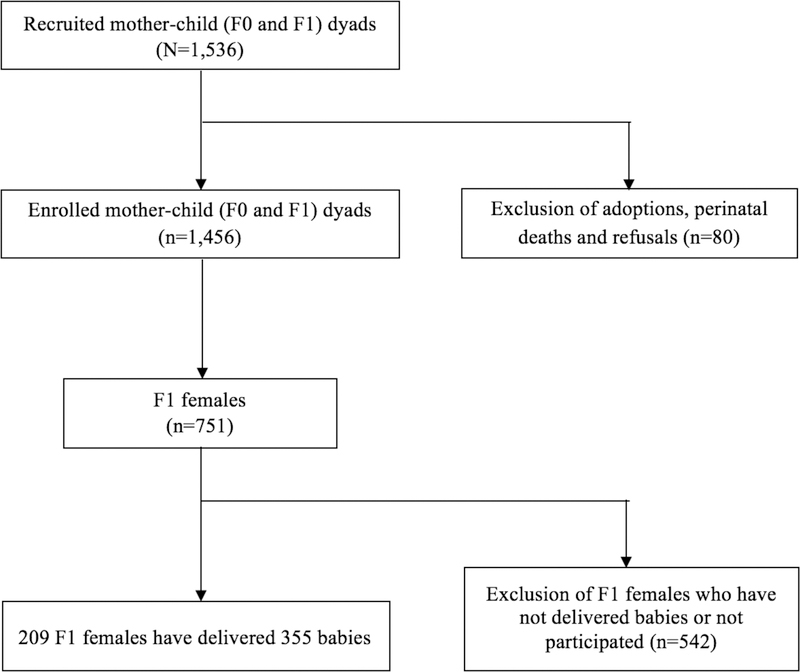
The IoW birth cohort was established to study the natural history and etiology of asthma and allergic diseases on Isle of Wight, UK in 1989. The IoW study recruited 1,536 mother-child dyads (F0 and F1 generations) between January 1, 1989 and February 28, 1990. Among these, 1,456 (94.8%) were enrolled after exclusion of adoptions, perinatal deaths and refusals. The maternal F1 generation was followed up at the ages of 1 (n=1,167), 2 (n=1,174), 4 (n=1,218), 10 (n=1,373), 18 (n=1,313), and 26 years (n=1,033), with detailed questionnaires and clinical examinations. F1 cohort females and spouses of F1 cohort males, who became pregnant after age 18 years, were followed up for their delivery. Their offspring, the F2 generation, are currently under follow-up (n=472). This birth cohort has been described in detail elsewhere (25, 26). The IoW study was approved repeatedly by the local research ethics committee (NRES Committee South Central – Hampshire B, U.K.), and University of Memphis Institutional Review Board in Memphis, U.S. (FWA00006815). Written consents were obtained from all participants at recruitment and all follow-ups. The total sample had 1,456 F0 (grandmother) and F1 (mother/father) pairs, and 472 F2 grandchildren. Since we were interested in the direct and mediating effect via the maternal line, the analytical sample included 355 offspring of the 209 grandmother-mother dyads (Figure 1).
Figure 1.
Flowchart of creating the analytical sample of the three-generational IoW cohort study
Measurements
Outcome.
Grandchild’s BW in kilograms was measured at birth and transcribed from hospital records.
Exposure of Interest.
Grandmother’s height and weight were measured at antenatal visit, and used to calculate grandmother’s early pregnancy BMI as weight in kilograms divided by the square of height in meters.
Potential Mediators (Intervening Variables).
Maternal BW was measured in kilograms at birth and collected from birth records. Maternal BMI was calculated from their height and weight, which were measured at age 18 years during the follow-up visit, or self-reported when a visit was not possible. Due to the adverse effect of maternal smoking during pregnancy on BW (27), maternal smoking status during pregnancy was considered as a mediator. Its information was obtained via questionnaires, asking about the number of cigarettes smoked during each trimester. The total number of cigarettes was summed up and grouped into three levels: no smoking, light smoking (total number of cigarettes smoked during pregnancy between 1 and 9), and moderate smoking (total number of cigarettes smoked during pregnancy at or above 10).
Potential Confounders.
Three potential confounders were included in the analysis: grand-maternal smoking status during pregnancy (Yes/No), grand-maternal SES, and gender of the grandchild. Grandmother’s smoking status during pregnancy was included as a confounder, because maternal smoking during pregnancy is a risk factor for BW (27). Grand-maternal SES was measured by a composite variable using cluster analysis as described before (28). Three indicators were used in the cluster analysis: the British socioeconomic classes (1–6) derived from parental occupation reported at birth, number of children in the index child’s bedroom (collected at age 4 years), and family income when the child was 10 years of age. The derived grand-maternal SES variable was grouped into three levels: low, medium and high.
Statistical Analysis
Descriptive analyses were conducted to describe the characteristics of participants. Path analysis, a special case of structural equation modeling (29), was conducted to assess whether the association between grand-maternal BMI and grandchild’s BW was mediated by maternal BW, maternal BMI at age 18 years, and maternal smoking during pregnancy. Path analysis was used to estimate the direct, indirect, and total effects between exogenous and endogenous variables. The sum of direct and indirect effects constitutes the total effect. Indirect effects are due to the mediating effect of a third variable. Note, path analysis does not estimate causal relations; however, compared to other regression models path analytical models have the advantage of considering data from consecutive generations not as independent effects, but as part of a path over generations. The full information maximum likelihood (FIML) method was applied to incorporate incomplete observations into the analysis (30). To reduce the number of parameters, the effect of maternal BMI at age 18 years on grandchild’s BW was constrained to be equal to that of grand-maternal BMI on maternal BW. This constraint was justified since the association between grand-maternal BMI and maternal BW (15.59 grams per kg/m2) was not significantly different from that between maternal BMI and grandchild’s BW (17.09 grams per kg/m2) (p=0.90). We started with a full model including all reasonable paths, then dropped paths one at a time according to stepwise multivariate Wald test, until a parsimonious model was reached. We evaluated the goodness of fit using the following criteria: 1) p-value for the overall model fit > 0.05, 2) standardized root mean square residual (SRMR) < 0.08, 3) adjusted goodness of fit (AGFI) > 0.95, and 4) root mean square error of approximation (RMSEA) < 0.05. The following paths were excluded from the final model due to insignificance: 1) from grand-maternal SES to maternal smoking status during pregnancy, 2) from grand-maternal smoking status during pregnancy to maternal BMI at age 18 years, and 3) from maternal BW to maternal BMI at age 18 years. All analyses were performed using the Statistical Analysis System (SAS 9.4, Cary, NC, USA).
Results
Characteristics of the total and analytical samples are presented in Table 1. Note that results related to maternal BW and grandchild’s BW are presented in grams. Compared to the total sample, grandmothers in the analytical sample had a higher prevalence of low SES (21.3% vs. 15.4%, p=0.03; Table 1) and a higher prevalence of smoking during pregnancy (34.1% vs. 25.3%, p=0.006). In addition, the analytical sample had lower maternal BW (3324.3 vs. 3393.1 grams, p=0.04) and higher maternal BMI at age 18 years (24.8 vs. 23.2 kg/m2, p<0.0001). When comparing the smoking status during pregnancy of grandmothers and mothers, a Chi-square test showed that grand-maternal smoking was significantly associated with maternal smoking during pregnancy (34.1% smoking grandmothers vs. 17.9% and 21.8% light and heavy smoking mothers, p=0.003, data not shown). Among the 209 grandmother-mother dyads, 118 pairs had information on both grand-maternal and maternal BMI, and 74 pairs were discordant on BMI by more than 2 units. Of the grandchildren, 54.7% are male.
Table 1.
Comparison of population characteristics between the total sample and analytical sample
| Continuous variables | Total sample a (n=1536; 472) |
Analytical sample b (n=209; 355) |
p-value c | ||
|---|---|---|---|---|---|
| N | Mean (SD) | n | Mean (SD) | ||
| Grand-maternal BMI (kg/m2) | 1175 | 24.43 (4.20) | 158 | 25.11 (5.05) | 0.21 |
| Maternal birth weight (g) | 1511 | 3393.1 (537.71) | 208 | 3324.3 (527.33) | 0.04 |
| Maternal BMI at age 18 (kg/m2) | 964 | 23.19 (4.33) | 156 | 24.82 (4.91) | <0.0001 |
| Grandchild’s birth weight (g) | 342 | 3371.4 (554.71) | 263 | 3358.8 (573.64) | 0.79 |
| Categorical variables | N | N (%) | n | n (%) | p-value d |
| Grand-maternal SES | 1357 | 192 | 0.03 | ||
| Low | 209 (15.4) | 41 (21.3) | |||
| Medium | 1037 (76.4) | 143 (74.5) | |||
| High | 111 (8.2) | 8 (4.2) | |||
| Grand-maternal SDP | 1521 | 208 | 0.006 | ||
| No | 1137 (74.7) | 137 (65.9) | |||
| Yes | 384 (25.3) | 71 (34.1) | |||
| Maternal SDP | 427 | 325 | 0.85 | ||
| No smoking | 265 (62.1) | 196 (60.3) | |||
| Light smoking | 70 (16.4) | 58 (17.9) | |||
| Moderate smoking | 92 (21.5) | 71 (21.8) | |||
| Grandchild’s gender | 444 | 333 | 0.88 | ||
| Male | 245 (55.2) | 182 (54.7) | |||
| Female | 199 (44.8) | 151 (45.3) | |||
Abbreviation: SD, standard deviation; BMI, body mass index; SES, socioeconomic status; and SDP, smoking during pregnancy.
The sample size of the total sample for F0 and F1 generations is 1536, and that for F2 generation is 472.
The sample size of the analysis sample for F0 and F1 generations is 209, and that for F2 generation is 355.
Two-sample t-test or Wilcoxon rank-sum test.
Chi-square test.
Intergenerational associations within path analyses
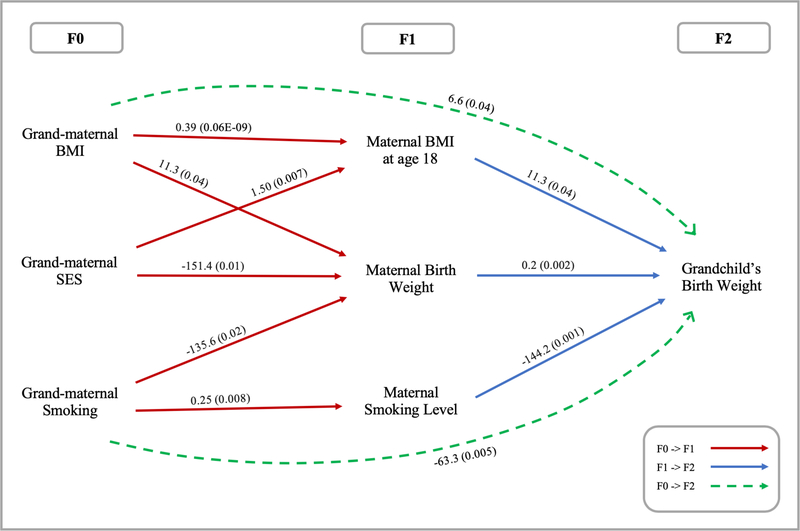
The path analytical model fitness statistics indicated an excellent fit of the data to the overall model (χ2 (15)=15.73, p-value=0.40; SRMR=0.04; AGFI=0.96; RMSEA=0.01; Figure 2). The final model with unstandardized coefficients is presented in Table 2. Note that results of the intergenerational associations in this section and those of the transgenerational associations in the following section were from three paths in one single model. As expected, every unit (kg/m2) increase in grand-maternal BMI significantly contributed to an increase in maternal BW by 11.3 grams (p=0.04; Table 2). The same association was found between maternal BMI at age 18 years and grandchild’s BW, as an equal effect constraint was requested to reduce the number of parameters. Grand-maternal BMI was also positively associated with maternal BMI at age 18 years (β=0.4 kg/m2, p<0.001). Additionally, one-gram increase in maternal BW led to 0.2 grams increase in grandchild’s BW (p=0.002).
Figure 2.
Path diagram showing statistically significant effects of grand-maternal BMI and smoking during pregnancy on grandchild’s birth weight (n=355). Note: χ2 (15)=15.73, p-value=0.40; SRMR=0.04; AGFI=0.96; RMSEA=0.01. This figure reports significant direct and indirect effects whereas insignificant effects were not reported, and p-values are in parentheses. Solid lines refer to direct effects; dashed lines refer to indirect effects. Square box was not used because only manifest variables were included.
Abbreviation: BMI, body mass index; SES, socioeconomic status; standardized root mean square residual, SRMR; adjusted goodness of fit, AGFI; and root mean square error of approximation RMSEA.
Table 2.
Analytical path model showing the unstandardized coefficients for the associations of grand-maternal BMI and smoking during pregnancy with grandchild’s birth weight and p-values in parentheses (n=355) a
| Grand-maternal BMI |
Grand-maternal SDP |
Grand-maternal SES |
Maternal BW |
Maternal BMI |
Maternal SDP | Grandchild’s gender |
|
|---|---|---|---|---|---|---|---|
| Maternal BW | |||||||
| Direct | 11.3 (0.04) | −135.6 (0.02) | −151.4 (0.01) | – | – | – | – |
| Indirect | – | – | – | – | – | – | – |
| Total | 11.3 (0.04) | −135.6 (0.02) | −151.4 (0.01) | – | – | – | – |
| Maternal BMI | |||||||
| Direct | 0.39 (0.06E-09) | – | 1.50 (0.007) | – | – | – | – |
| Indirect | – | – | – | – | – | – | – |
| Total | 0.39 (0.06E-09) | – | 1.50 (0.007) | – | – | – | – |
| Maternal SDP | |||||||
| Direct | – | 0.25 (0.008) | – | – | – | – | – |
| Indirect | – | – | – | – | – | – | – |
| Total | – | 0.25 (0.008) | – | – | – | – | – |
| Grandchild’s BW | |||||||
| Direct | 1.3 (0.87) | 93.6 (0.20) | 16.8 (0.83) | 0.2 (0.002) | 11.3 (0.04) | −144.2 (0.001) | −62.3 (0.36) |
| Indirect | 6.6 (0.04) | −63.3 (0.005) | −13.4 (0.48) | – | – | – | – |
| Total | 8.0 (0.32) | 30.3 (0.68) | 3.4 (0.96) | 0.2 (0.002) | 11.3 (0.04) | −144.2 (0.001) | −62.3 (0.36) |
Abbreviation: BMI, body mass index; SDP, smoking during pregnancy; SES, socioeconomic status; and BW, birth weight.
Values presented in the above table are unstandardized regression coefficients (β) and p-values (in parentheses).
Grand-maternal smoking during pregnancy was significantly associated with reduced maternal BW (β=−135.6 grams, p=0.02; Table 2). Similarly, a higher level of maternal smoking during pregnancy reduced grandchild’s BW by 144.2 grams (p=0.001). Grand-maternal smoking during pregnancy was also associated with maternal smoking during pregnancy (β=0.25, p=0.008). Additionally, one level increase in grand-maternal SES was associated with a reduction of 151.4 grams in maternal BW (p=0.01) and an increase of 1.5 units in maternal BMI at age 18 years (p=0.007).
Transgenerational associations within path analyses
The total and direct effects between grand-maternal BMI and grandchild’s BW were not statistically significant (total effect: β=8.0 grams of increase in grandchild’s BW per unit increase of grand-maternal BMI, p=0.32; direct effect: β=1.3 grams, p=0.87; Table 2). However, we discovered a positive indirect association between grand-maternal BMI and grandchild’s BW (β=6.6 grams of increase in grandchild’s BW per unit increase of grand-maternal BMI, p=0.04), and such transgenerational association was mediated by maternal BW and BMI at age 18 years. Among 6.6 grams of increase in grandchild’s BW due to indirect effects of grand-maternal BMI, 2.3 grams was via maternal BW and 4.4 grams via maternal BMI at age 18 years. As we mentioned earlier, there was no causal path between maternal BW and BMI at age 18 years. Besides, we examined whether the indirect effect can be explained by a single mediator, either maternal BW or maternal BMI at age 18 years. However, the indirect effect of grand-maternal BMI on grandchild’s BW became insignificant if we removed either maternal BW (β=4.7 grams, p=0.20) or maternal BMI at age 18 years (β=1.7 grams, p=0.23), indicating that the transgenerational indirect effect depended on the presence of both mediators. Hence, the two mediating effects may confound one another, suggesting two possible pathways: First, a higher grand-maternal BMI could lead to higher maternal BW, which may result in an increased grandchild’s BW. Second, a higher grand-maternal BMI may increase maternal BMI at age 18 years, resulting in higher grandchild’s BW.
We found an indirect, negative relationship between grand-maternal smoking during pregnancy and grandchild’s BW, mediated by maternal BW and maternal smoking during pregnancy (β=−63.3 grams, p=0.01; Table 2). Thus, when a grandmother smoked during pregnancy, her grandchild was predicted to have a decreased BW by 63.3 grams, because the mother had a lower BW and/or smoked more during pregnancy (light or moderate smoking). Among 63.3 grams of decrease in grandchild’s BW due to indirect effects of grand-maternal smoking during pregnancy, −27.2 grams were via maternal BW and −36.1 grams via maternal smoking during pregnancy. There was neither a significant total nor direct effect of grand-maternal smoking during pregnancy on grandchild’s BW (total effect: β=30.3 grams, p=0.68; direct effect: β=93.6 grams, p=0.20). Moreover, we examined whether the indirect effect between grand-maternal smoking during pregnancy and grandchild’s BW can be explained by a single mediator, either maternal BW or smoking during pregnancy. When maternal BW was removed from the final model, the indirect effect of grand-maternal smoking during pregnancy on grandchild’s BW via maternal smoking during pregnancy remained statistically significant (β=−33.9 grams, p=0.04). However, the indirect effect via maternal BW became marginally significant (β=−25.3 grams, p=0.07) if maternal smoking during pregnancy was removed. This suggests that maternal smoking during pregnancy was the main mediator whereas the mediating effect of maternal BW depended on that of maternal smoking during pregnancy. In addition, there was no significant association between grand-maternal SES and grandchild’s BW (total effect: β=3.4 grams, p=0.96; direct effect: β=16.8 grams, p=0.83; indirect effect: β=−13.4 grams, p=0.48).
Additional sensitivity analyses were conducted. We assessed whether grand-maternal height may act as a confounder, though it was not correlated with grand-maternal BMI (r=−0.02, p=0.80). Adding grand-maternal height as a covariate did not change the results significantly (results not shown), so it was not included in the final model. Maternal gestational diabetes and insulin-dependent diabetes showed no association with BW and thus were not included. Although maternal hypertension during pregnancy was associated with offspring BW, adding this variable to the path analytical model did not change its estimation and thus it was dropped.
Discussion
Using prospectively collected three-generation data, we found an increasing, indirect effect of grand-maternal BMI on grandchild’s BW. This transgenerational effect was mediated by maternal BW and BMI at age 18 years. It is worth noting that these two mediators may confound one another, because the indirect effect between grand-maternal BMI and grandchild’s BW depended on the existence of both mediators. Contrary to the previous studies that reported an increasing effect of grand-maternal smoking during pregnancy on grandchild’s BW (16, 17, 18, 20), we observed an inverse association of grand-maternal smoking during pregnancy with grandchild’s BW. This association was primarily dependent on maternal smoking during pregnancy, and then on maternal BW. In addition, there were no significant direct or total effects between grand-maternal BMI and grandchild’s BW, and between grand-maternal smoking and grandchild’s BW.
Mediation of transgenerational effects through the mother is not a new assumption; however, missing heritability of complex conditions (31), i.e. genetic variance explains very little of transgenerational effects, is a major challenge. Hence, the results of our three-generation analyses demonstrating mediating roles of maternal birthweight, BMI, and maternal smoking linking grand-maternal BMI to grandchild’s birthweight, provide new insights. For the first time, we demonstrate that a higher grand-maternal BMI could predict a higher grandchild’s BW, mediated by maternal BW and BMI at age 18 years. As mentioned in the introduction, Harville et al. identified that grandchild’s BW showed a positive association with pre-pregnancy glucose levels in grandmothers, and a negative association with triglycerides and low-density lipoprotein in grandmothers (13). However, they found no transgenerational relationship between grand-maternal BMI and grandchild’s BW, though also using three-generational data. This may be due to the methodological limitation of not using path-analytical models. Another possible reason may be that grand-maternal BMI in the study of Harville et al. was measured during adolescence (at mean age of 16.2) (13), which may result in an underestimation of the grand-maternal BMI effect.
Another novel finding is the inverse, indirect effect of grand-maternal smoking during pregnancy on grandchild’s BW, primarily dependent on maternal smoking during pregnancy, and then on maternal BW. Instead of treating maternal characteristics as confounders as in previous studies (13, 15, 16, 17, 18, 19, 20), we considered maternal BW and smoking during pregnancy as mediators for the association between grand-maternal smoking and grandchild’s BW. Mediation should not be confused with confounding, because a confounder should not be on the causal path from an exposure to an outcome (32). A mediating effect is consistent with the model, when the direct and indirect effects of an exposure on an outcome have the same sign (33). It also can be inconsistent, but still meaningful, when the direct and indirect effects have opposite signs, which is called confounding mediation or suppression (33). It is reasonable to have an insignificant total effect (i.e., the sum of direct and indirect effects), but significant direct and/or indirect effects, because the direct and indirect effects may cancel each other out. In this study, the two mediators of maternal BW and smoking during pregnancy had suppressing effects, because the direct and indirect effects of grand-maternal smoking on grandchild’s BW had similar magnitude but opposite signs (93.6 vs. −63.3 grams).
Contradictory to the results of this study, four previous studies found increasing effects of grand-maternal smoking during pregnancy (1) on grandchild’s BW (16, 17, 20) and (2) on grandsons’ BW (18). Detailed findings of these four studies are summarized in Table S1. However, given the widely demonstrated association between maternal smoking during pregnancy and lower BW (27), one would not expect an increasing effect of grand-maternal smoking on grandchild’s BW. The discrepancies between the results of this study and those of the previous studies can again be explained by the statistical methods used. Instead of using (grand-)maternal smoking as a confounder, we used path analysis, which enabled us to reveal the hidden indirect effects between grand-maternal smoking during pregnancy and offspring BW. We further investigated whether this difference was due to statistical methods. Linear mixed models were conducted using IoW data and three conventional methods: 1) adjustment for maternal smoking, 2) stratified analysis based on maternal smoking status during pregnancy, and 3) using an interaction between grand-maternal and maternal smoking during pregnancy. Results of the three conventional methods showed an increasing (but insignificant) association between grand-maternal smoking during pregnancy and grandchild’s BW (Table S2), as did in prior studies. The lack of statistical significance may result from the small sample size in our study. The effect of grand-maternal smoking during pregnancy on increasing grandchild’s BW is likely due to falsely applying linear regression, when the longitudinal set-up would require path analysis or structural equation models. Other studies’ findings showed that maternal smoking during pregnancy reduces BW, but grand-maternal smoking during pregnancy has the opposite effect, is unexpected and hard to explain. Future studies should take into account the longitudinal nature of three-generation prospective cohort data, and consider using more appropriate statistical methods, such as structural equation models.
The underlying relation between grand-maternal risk factors and grandchild’s BW has yet to be unveiled. One candidate of the mechanisms is DNA methylation, which may memorize and transfer information on grand-maternal metabolism (BMI) or smoking to the next generation. Maternal BMI (34, 35) and smoking during pregnancy (36) have been identified to be associated with differential DNA methylations in the offspring. It is possible that the influences of grand-maternal BMI and smoking on epigenetic changes can persist into later life of the mothers, and then be transferred to the third generation. A few recent studies investigated the mediating role of DNA methylation, and found that differential DNA methylations mediated the association between maternal smoking during pregnancy and birth outcomes, including lower birth weight (37), small for gestational age (38) and preterm birth (39). Further studies are needed to corroborate the epigenetic mechanism across two generations, and explore how it may play a role in transgenerational effects.
The strengths of this study include: First, this prospective birth cohort study enabled us to assess the transgenerational effect of grand-maternal BMI on grandchild’s BW across three consecutive generations. Second, using path analysis facilitated the evaluation of both direct and indirect effects of grand-maternal BMI on grandchild’s BW, as well as the separation of mediating and confounding effects. Third, birth weight was based on obstetric and delivery records, and height and weight of grandmothers and mothers were directly measured at prenatal and follow-up visits, respectively, providing a reliable ascertainment.
This study has some limitations. First, the sample size was small with 209 grandmother-mother pairs and 355 grandchildren. Second, we were only able to analyze the data of the maternal line (i.e., grandmother-mother pairs and their offspring), because only a small number of grandchildren have been born to cohort males in the intermediate generation. Male cohort members were maximal 27 years old when the third-generation data were extracted. Hence, some male cohort males have not reached the childbearing age yet. In addition, non-cohort males who had a child with an original female F1-cohort member joined the cohort later. These males had no grand-parental information collected at the inception of the study. Hence, no data was available. Although some studies showed that the transmission of BW and BMI was more apparent through the maternal line (15, 41), the transmission via the paternal line still deserves further examination. Third, some mothers in this study delivered more than one child. BW of grandchildren within the same family could be correlated, which may cause a violation of independence requirement when analyzing grandchildren. To assess the robustness of our results, we conducted a sensitivity analysis including first-born children only (n=209). Results of the sensitivity analysis showed that the indirect effect of grand-maternal BMI on increasing grandchild’s BW became marginally significant (β=6.9 grams, p=0.09; Table S3). The direction of the association remained positive and the effect size was very close to that in the main result (6.9 vs 6.6 grams). Additionally, the inverse, decreasing association between grand-maternal smoking and grandchild’s BW remained statistically significant (β=−85.0 grams, p=0.01). Thus, our results are unlikely to be affected by the violation of independence requirement. Fourth, the mean maternal age of 23.1 years at delivery was younger in this study than that of 28.8 years in 2016 from the national statistics in UK (40). This was due to the study setting, which was based on the first pregnancies of cohort mothers, in contrast to including all pregnancies of a representative group of mothers in the F0 generation. Fifth, we did not test nonlinear association in the structural equations models since the data did not show any suggestion of such. Sixth, we could not control for dietary factors or physical activity during pregnancy because we did not collect data on these factors.
In conclusion, findings of this study are the first to suggest a transgenerational role of grand-maternal BMI on increased grandchild’s BW, via the mediating effects of maternal BW and BMI during early adulthood (age 18 years). Contrary to previous studies, grand-maternal smoking during pregnancy was found to indirectly decrease grandchild’s BW, primarily dependent on maternal smoking during pregnancy, and then on maternal BW. Future studies should examine whether these transgenerational effects exist in the paternal line, and whether epigenetic mechanisms play a role in the transgenerational associations.
Supplementary Material
Study Importance Questions.
Provide no more than 3 short bullet-point answers to these two study importance questions:
Q1. What is already known about this subject?
-
▪
Prior research demonstrated intergenerational effects of maternal body mass index (BMI) on offspring birth weight (BW).
-
▪
Only one study assessed the transgenerational link between grand-maternal BMI and grandchild’s BW. The investigators did not consider mediating effects, and were unable to find a significant association.
Q2. What does your study add?
-
▪
This is the first study that demonstrates an indirect and positive effect between grand-maternal BMI and grandchild’s BW via the mediating effect of maternal BW and BMI in early adulthood.
-
▪
The study also reports an indirect and inverse link between grand-maternal smoking during pregnancy and grandchild’s BW, primarily dependent on maternal smoking during pregnancy, and then on maternal BW. The direction of the association was opposite to that reported in previous studies, which did not use intervening variables.
-
▪
This study emphasizes the importance of using path-analytical models to analyze transgenerational effects. Using conventional statistical methods, including multiple linear regression and linear mixed model, may produce misleading results.
Acknowledgments
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases (grant number: 1R01AI091905) and by the National Heart, Lung, and Blood Institute (grant number: R01HL132321).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Balte P, Karmaus W, Roberts G, Kurukulaaratchy R, Mitchell F, Arshad H. Relationship between birth weight, maternal smoking during pregnancy and childhood and adolescent lung function: A path analysis. Resp Med 2016;121: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes 2014;10: 77–83. [DOI] [PubMed] [Google Scholar]
- 3.Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 2014;237: 391–399. [DOI] [PubMed] [Google Scholar]
- 4.Ortqvist AK, Ullemar V, Lundholm C, Kuja-Halkola R, Magnusson PKE, Lichtenstein P, et al. Fetal Growth and Childhood Lung Function in the Swedish Twin Study on Prediction and Prevention of Asthma. Ann Am Thorac Soc 2017;14: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 5.Saad NJ, Patel J, Burney P, Minelli C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2017;14: 994–1004. [DOI] [PubMed] [Google Scholar]
- 6.Hui LL, Schooling CM, Leung SSL, Mak KH, Ho LM, Lam TH, et al. Birth Weight, Infant Growth, and Childhood Body Mass Index Hong Kong’s Children of 1997 Birth Cohort. Archives of Pediatrics and Adolescent Medicine 2008;162: 212–218. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell EA, Stewart AW, Braithwaite I, Hancox RJ, Murphy R, Wall C, et al. Birth weight and subsequent body mass index in children: an international cross-sectional study. Pediatr Obes 2017;12: 280–285. [DOI] [PubMed] [Google Scholar]
- 8.Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes (Lond) 2012;36: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 9.Brewster AJ, Hardock V, Bhattacharya S. Exploring the relationship between maternal body mass index and offspring birth weight: Analysis of routinely collected data from 1967 to 2010 in Aberdeen, Scotland. J Obstet Gynaecol 2015;35: 810–816. [DOI] [PubMed] [Google Scholar]
- 10.Strom-Roum EM, Tanbo TG, Eskild A. The associations of maternal body mass index with birthweight and placental weight. Does maternal diabetes matter? A population study of 106 191 pregnancies. Acta Obstet Gyn Scan 2016;95: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PloS one 2013;8: e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahti-Pulkkinen M, Bhattacharya S, Raikkonen K, Osmond C, Norman JE, Reynolds RM. Intergenerational Transmission of Birth Weight Across Three Generations. Am J Epidemiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harville EW, Jacobs MB, Qi L, Chen W, Bazzano LA. Multigenerational Cardiometabolic Risk as a Predictor of Birth Outcomes: The Bogalusa Heart Study. J Pediatr-Us 2017;181: 154-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarron P, Smith GD, Hattersley AT, Team AS. Type 2 diabetes in grandparents and birth weight in offspring and grandchildren in the ALSPAC study. Journal of epidemiology and community health 2004;58: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrivastava A, Murrin C, O’Brien J, Viljoen K, Heavey P, Grant T, et al. Grandparental morbidity and mortality patterns are associated with infant birth weight in the Lifeways cross-generation cohort study 2001–2010. Journal of developmental origins of health and disease 2012;3: 458–468. [DOI] [PubMed] [Google Scholar]
- 16.Ding M, Yuan C, Gaskins AJ, Field AE, Missmer SA, Michels KB, et al. Smoking during pregnancy in relation to grandchild birth weight and BMI trajectories. PloS one 2017;12: e0179368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hypponen E, Smith GD, Power C. Effects of grandmothers’ smoking in pregnancy on birth weight: intergenerational cohort study. BMJ 2003;327: 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller LL, Pembrey M, Smith GD, Northstone K, Golding J. Is the Growth of the Fetus of a Non-Smoking Mother Influenced by the Smoking of Either Grandmother while Pregnant? PloS one 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra DP, Astone N, Lynch CD. Maternal smoking and birth weight - Interaction with parity and mother’s own in utero exposure to smoking. Epidemiology 2005;16: 288–293. [DOI] [PubMed] [Google Scholar]
- 20.Rillamas-Sun E, Harlow SD, Randolph JF Jr,. Grandmothers’ smoking in pregnancy and grandchildren’s birth weight: comparisons by grandmother birth cohort. Maternal and child health journal 2014;18: 1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins JW Jr., David RJ, Rankin KM, Desireddi JR. Transgenerational effect of neighborhood poverty on low birth weight among African Americans in Cook County, Illinois. Am J Epidemiol 2009;169: 712–717. [DOI] [PubMed] [Google Scholar]
- 22.Collins JW, Rankin KM, David RJ. Low birth weight across generations: the effect of economic environment. Maternal and child health journal 2011;15: 438–445. [DOI] [PubMed] [Google Scholar]
- 23.Huang JY, Gavin AR, Richardson TS, Rowhani-Rahbar A, Siscovick DS, Enquobahrie DA. Are Early-Life Socioeconomic Conditions Directly Related to Birth Outcomes? Grandmaternal Education, Grandchild Birth Weight, and Associated Bias Analyses. Am J Epidemiol 2015;182: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arshad SH, Holloway JW, Karmaus W, Zhang H, Ewart S, Mansfield L, et al. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arshad SH, Hide DW. Effect of environmental factors on the development of allergic disorders in infancy. J Allergy Clin Immunol 1992;90: 235–241. [DOI] [PubMed] [Google Scholar]
- 26.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 2010;65: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira PPD, da Mata FAF, Figueiredo ACG, de Andrade KRC, Pereira MG. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine Tob Res 2017;19: 497–505. [DOI] [PubMed] [Google Scholar]
- 28.Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax 2009;64: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Rourke N, Hatcher L. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling, 2nd edn. SAS Institute: Cary, NC, 2013. [Google Scholar]
- 30.Yung YF, Zhang W. Making use of incomplete observations in the analysis of structural equation models: The CALIS procedure’s full information maximum likelihood method in SAS/STAT® 9.3 SAS Global Forum SAS Institute Inc: Las Vegas, NV, 2011. [Google Scholar]
- 31.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature 2009;461: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman K, Greenland S, & Lash TL Modern Epidemiology, 3rd edn. Lippincott Williams & Wilkins: Philadelphia, PA, 2008. [Google Scholar]
- 33.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci 2000;1: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Chen Q, Tsai HJ, Wang GY, Hong XM, Zhou Y, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: Exploration of early life origins of disease. Environ Mol Mutagen 2014;55: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharp GC, Salas LA, Monnereau C, Allard C, Yousefi P, Everson TM, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Human molecular genetics 2017;26: 4067–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richmond RC, Joubert BR. Contrasting the effects of intra-uterine smoking and one-carbon micronutrient exposures on offspring DNA methylation. Epigenomics-Uk 2017;9: 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witt SH, Frank J, Gilles M, Lang M, Treutlein J, Streit F, et al. Impact on birth weight of maternal smoking throughout pregnancy mediated by DNA methylation. Bmc Genomics 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouwland-Both MI, van Mil NH, Tolhoek CP, Stolk L, Eilers PHC, Verbiest MMPJ, et al. Prenatal parental tobacco smoking, gene specific DNA methylation, and newborns size: the Generation R study. Clinical epigenetics 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maccani JZJ, Koestler DC, Houseman EA, Marsit CJ, Kelsey KT. Placental DNA methylation alterations associated with maternal tobacco smoking at the RUNX3 gene are also associated with gestational age. Epigenomics-Uk 2013;5: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Births by parents’ characteristics in England and Wales: 2016 In: Haines N (ed). Live births by age of mother and father, type of registration, median interval between births, number of previous live-born children and National Statistics Socio-economic Classification (NS-SEC). Office for National Statistics: Titchfield, UK, 2017. [Google Scholar]
- 41.Kelly GE, Murrin C, Viljoen K, O’Brien J, Kelleher C. Body mass index is associated with the maternal lines but height is heritable across family lines in the Lifeways Cross-Generation Cohort Study. Bmj Open 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.