Abstract
Data for liver transplant recipients (LTRs) regarding the benefit of care concordant with clinical practice guidelines for management of blood pressure (BP) are sparse. This paper reports on clinician adherence with BP clinical practice guideline recommendations and whether BP control is associated with mortality and cardiovascular events (CVEs) among LTRs. We conducted a longitudinal cohort study of adult LTRs who survived to hospital discharge at a large tertiary care network between 2010 and 2016. The primary exposure was a BP of <140/<90 mmHg within year 1 of LT. Among 602 LTRs (mean age 56.7 years, 64% men), 92% had hypertension and 38% had new onset hypertension. Less than 30% of LTRs achieved a BP of <140/<90 mmHg over a mean of 43.2 months. In multivariable models, adjusted for key confounders, BP control post-LT compared with lack of control was associated with a significantly lower hazard of mortality (Hazard Ratio (HR) 0.48, 95% confidence interval (CI) 0.39, 0.87) and of CVEs (HR 0.65, 95%CI 0.43, 0.97). The association between BP control of <140/<90 mmHg with improved survival and decreased CVEs in LTRs suggests that efforts to improve clinician adherence to BP clinical practice recommendations should be intensified.
1. Introduction
Liver transplantation is a high-risk, high-cost intervention that, at present, extends life in over 8,000 patients in the U.S. each year, with 1-year survival rates > 90%.1 However, cardiovascular (CV) disease is the leading cause of early (< 1 year) mortality, and the third leading cause of late (≥ 1 year) mortality after transplant.2, 3 Up to 30% of LTRs will experience a CV event (CVE), including myocardial infarction (MI), heart failure, cardiac arrest, atrial fibrillation, thromboembolism, or stroke after transplant.4, 5
In the general population, CVEs are potentially preventable with intensive management of CV disease risk factors, such as blood pressure (BP).6 Clinical practice guidelines for BP management in both the general population7 and LTRs8 recommend BP lowering in order to prevent CVEs. However, prevalence data and outcomes of controlled BP among LTRs are limited. In a cross-sectional study of 490 LTRs with hypertension in Spain, 68% of participants had controlled BP (<140/<90 or <130/<80 mmHg if diabetic).9 However, this study only assessed BP at a single outpatient visit and did not assess the relationship between BP control and clinical outcomes. BP control (<140/<90 mmHg) has also been reported to be poor among kidney transplant recipients, ranging from 40%−56%, and poor control has been linked to graft failure, increased CVEs, and mortality.10–13 To our knowledge, BP control has not previously been linked to clinical outcomes among LTRs. We therefore sought to assess clinician adherence to clinical practice guidelines for BP management of LTRs at a large urban tertiary care network and to assess whether BP control predicts mortality and clinical CVEs in this high-risk population.
2. Materials and Methods
2.1. Study Design:
A longitudinal cohort study was conducted at a large urban tertiary care network in the U.S. The Institutional Review Board of Northwestern University approved the study.
2.2. Study Population:
Patients, who underwent a LT between January 1, 2010 and December 31, 2016 were included. In order to study patients with stable immunosuppression and graft function, we excluded patients who died within the first 6 months after transplant.
2.3. Data Source and Collection:
Eligible LTRs were identified using International Classification of Diseases ninth or tenth revision (ICD-9 or ICD-10) codes and clinical information was ascertained from the Northwestern Medicine Enterprise Data Warehouse (NMEDW), which contains clinical data for 7.5 million unique patients at all Northwestern Medicine sites. Supplemental manual chart review was used for data elements not easily captured in an electronic health record (EHR), such as clinical reasoning for not adhering to a clinical guideline (e.g., documentation of why it would be inappropriate). Vital status was obtained from the Organ Procurement and Transplantation Network (OPTN) database, which is linked to the U.S. Social Security Death Index (SSDI). Data were linked to each LTR’s clinical data based on a previously published methodology.3, 14
2.4. Covariate Definitions, Immunosuppression and Clinical Visit Protocol:
Hypertension was identified by ICD-9/10 code or order of a BP-lowering medication or systolic BP ≥ 140 or diastolic BP ≥ 90 mmHg on at least two separate outpatient visits, consistent with clinical practice guidelines during the study period. Chronic kidney disease (CKD) was identified by ICD-9/10 code or estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 on at least two separate outpatient visits separated by ≥ 90 days. Diabetes was identified by ICD-9/10 code or hemoglobin A1C ≥ 6.5%, random blood glucose > 200 mg/dL or use of glucose-lowering medication and prednisone daily dose ≤ 10mg. Atherosclerotic cardiovascular disease (ASCVD) was identified by ICD-9/10 code for acute coronary syndrome, MI, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2 or ICD-9/10 code for obesity. Hyperlipidemia was identified by ICD-9/10 code or treatment with lipid-lowering therapy or total cholesterol ≥ 200 mg/dL. Obstructive sleep apnea (OSA) was defined by ICD-9/10 code. Smoking status was assessed using natural language processing, which is a technique that converts free text information relevant to smoking status that is commonly found in clinical documentation into a structured form that can be readily processed and analyzed, as described previously.15 A hypertension specialist was defined as a cardiologist or nephrologist. Standard immunosuppression during the study period (2010–2016) included induction therapy with steroids alone followed by early calcineurin inhibitor (CNI) initiation. Patients with immune-mediated liver disease were treated additionally with mycophenolate mofetil. For patients with renal injury, mycophenolate mofetil or everolimus was added at the discretion of the treating physician with the goal of reducing target CNI levels as a renal protective strategy. All patients were seen weekly during month 1 and then at 1, 2, 3, 4, 6, 9 and 12 months post-transplant per protocol. In general, transplant clinicians prescribe BP-lowering medications during year 1 post-transplant and, subsequently, a primary care physician provides care beyond year 1. If a patient is co-managed by a hypertension specialist, then BP management is often deferred to specialist care.
2.5. Assessment of Blood Pressure Guideline Adherence:
We selected 18 metrics, supported by strong scientific evidence and that could be reliably assessed from the EHR, to assess BP guideline concordant care (Table 1) from 36 total recommendations in the 2012 American Association for the Study of Liver Diseases (AASLD) and American Society of Transplantation (AST) guidelines for the long-term management of LTRs,8 the 2014 Eighth Joint National Committee (JNC8) guideline for the management of high BP in adults16 and the 2017 American Heart Association (AHA) and American College of Cardiology (ACC) guidelines for the prevention, detection, evaluation and management of high BP in the general population.7 Multiple guideline documents were consulted to inform BP guideline concordance for the study, since the study time period (2010–2016) spans a period that had many changes in BP management guideline recommendations in adults. Adherence scores were calculated using the method established by the Center for Medicare and Medicaid Services, defined as the number of LTRs in whom the BP metric was offered divided by the total number of LTRs who were eligible for the BP metric.17, 18 Controlled BP was defined as mean systolic BP < 140 and mean diastolic BP < 90 mmHg, measured during an outpatient clinic visit between 30- and 365-days post-LT. This timeframe was chosen to minimize the effect of fluctuations in blood volume status commonly seen in the early perioperative period post-LT. If no BP was recorded, the patient was classified as “not controlled.” A guideline-concordant care intervention was considered “offered” if it was documented in the EHR as “received” or if the intervention was “considered but not received” for a medical reason. If no documentation was found in the EHR, then the intervention was considered as “not offered.”
Table 1.
Adherence Scores* for Selected Blood Pressure Performance and Process Metrics among Liver Transplant Recipients, 2010–2016
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
|---|---|---|---|---|---|---|
| Total Eligible LTRs | 602 | 542 | 451 | 353 | 255 | 160 |
| LTR with BP measured at least yearly after transplant | 100.0% | 94.8% | 87.4% | 83.3% | 76.5% | 80.0% |
| 602/602* | 514/542 | 394/451 | 294/353 | 195/255 | 128/160 | |
| LTR with SBP ≥ 140 or DBP ≥ 90 mmHg, and who had BP reassessed within 1 month after elevated reading | 35.6% | 41.8% | 34.6% | 35.6% | 37.0% | 42.0% |
| 180/505 | 167/400 | 110/318 | 89/250 | 67/181 | 50/119 | |
| LTR with SBP ≥140 or DBP ≥90 mmHg, and who had a BP-lowering agent offered | 41.8% | 22.4% | 19.4% | 20.9% | 13.3% | 16.2% |
| 211/505 | 88/393 | 57/294 | 46/220 | 20/150 | 19/117 | |
| LTR with diagnosis of HTN, and who had a BP-lowering agent offered | 37.0% | 40.1% | 34.0% | 34.6% | 34.5% | 41.7% |
| 198/535 | 196/489 | 139/409 | 112/324 | 82/238 | 63/151 | |
| LTR with diagnosis of CKD and SBP ≥ 130 or DBP ≥ 80 mmHg, and who had a BP-lowering agent offered | 37.2% | 40.3% | 36.1% | 35.7% | 35.9% | 43.4% |
| 160/430 | 155/385 | 114/316 | 90/252 | 69/192 | 53/122 | |
| LTR with diagnosis of DM and SBP ≥ 130 or DBP ≥ 80 mmHg, and who had a BP-lowering agent offered | 35.2% | 44.1% | 40.7% | 38.7% | 39.7% | 48.7% |
| 102/290 | 115/261 | 85/209 | 63/163 | 50/126 | 38/78 | |
| LTR with diagnosis of HTN and CKD, and who had an ACEI or ARB offered | 9.0% | 8.4% | 7.5% | 8.8% | 8.8% | 14.1% |
| 37/412 | 33/391 | 25/332 | 24/272 | 18/205 | 19/135 | |
| LTR with BP controlled to SBP <140 and DBP <90 mmHg | 16.1% | 26.2% | 29.5% | 29.2% | 29.0% | 25.6% |
| 97/602 | 142/542 | 133/451 | 103/353 | 74/255 | 41/160 | |
| LTR with BP controlled to SBP <130 and DBP <80 mmHg | 5.0% | 10.0% | 12.9% | 13.0% | 11.0% | 13.8% |
| 30/602 | 54/542 | 58/451 | 46/353 | 28/255 | 22/160 | |
| LTR with diagnosis of HTN and in whom BP was controlled to SBP <140 and DBP <90 mmHg | 10.1% | 21.5% | 26.7% | 28.1% | 26.5% | 25.2% |
| 54/535 | 105/489 | 109/409 | 91/324 | 63/238 | 38/151 | |
| LTR with diagnosis of HTN and in whom BP was controlled to SBP <130 and DBP <80 mmHg | 3.0% | 6.7% | 10.0% | 11.7% | 8.4% | 13.2% |
| 16/535 | 33/489 | 41/409 | 38/324 | 20/238 | 20/151 | |
| LTR with diagnosis of CKD and in whom BP was controlled to SBP < 130 and DBP <80 mmHg | 3.4% | 7.0% | 9.7% | 11.9% | 9.9% | 12.2% |
| 15/445 | 29/414 | 34/350 | 34/286 | 21/213 | 17/139 | |
| LTR with diagnosis of HTN and CKD and in whom BP was controlled to SBP < 130 and DBP <80 mmHg | 2.9% | 6.1% | 8.7% | 11.0% | 8.8% | 11.9% |
| 12/412 | 24/391 | 29/332 | 30/272 | 18/205 | 16/135 | |
| LTR with diagnosis of DM and in whom blood pressure was controlled to SBP < 130 and DBP <80 mmHg | 3.7% | 7.8% | 8.3% | 6.3% | 7.4% | 13.3% |
| 11/301 | 22/283 | 19/228 | 11/174 | 10/136 | 12/90 | |
| LTR with diagnosis of HTN and DM and in whom BP was controlled to SBP < 130 and DBP <80mmHg | 2.5% | 6.1% | 6.6% | 6.1% | 4.6% | 12.6% |
| 7/282 | 16/264 | 14/213 | 10/164 | 6/130 | 11/87 | |
| LTR with a diagnosis of coronary artery disease and BP <140/90 mmHg, or LTR with BP ≥ 140/90 mmHg who were prescribed ≥ 2 BP-lowering medications | 23.6% | 23.3% | 16.3% | 15.9% | 17.7% | 14.0% |
| 124/526 | 104/447 | 59/361 | 45/283 | 37/209 | 19/136 | |
| LTR with diagnosis of HTN and in whom goal BP (SBP < 140 and DBP < 90 mmHg) was not achieved, who then were offered referral to a HTN specialist** | 32.6% | 20.6% | 19.7% | 19.3% | 17.1% | 15.0% |
| 140/481 | 76/384 | 51/300 | 42/233 | 27/175 | 16/113 | |
| LTRs with diagnosis of HTN who are on at least 2 or more BP-lowering agents and in whom goal BP (SBP < 140 and DBP < 90 mmHg) was not achieved, who were then offered referral to a HTN specialist** | 35.5% | 38.8% | 36.4% | 35.0% | 26.5% | 46.7% |
| 43/121 | 38/98 | 20/55 | 14/40 | 9/34 | 7/15 | |
Abbreviations: LTR, liver transplant recipient; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension; CKD, chronic kidney disease; DM, diabetes mellitus; ACEI, angiotension converting enzyme inhibitor; ARB, angiotension receptor blocker
Adherence score = number of liver transplant recipients who received the intervention divided by the total number of eligible liver transplant recipients for the intervention X 100.
HTN specialist is defined as a cardiologist or nephrologist
2.6. Exposure and Outcome Measures:
The primary exposure variable was achievement of systolic BP <140 and diastolic BP <90 mmHg (“controlled BP”) within year 1 of liver transplant. The secondary exposure variable was achievement of additional BP control of <130/<80 mmHg. Mortality was determined from the OPTN database, by matching the Social Security death master file located within the SSDI. Recipient cause of death was determined by physician review (L.B.V.) of primary underlying and contributory causes of death (including all free text inputs) listed in the OPTN database, in addition to EHR review of any death that was documented in the EHR. Any LTR with death due to CV disease was reviewed by an independent panel of three physicians [1 cardiologist (D.M. L-J.), 1 surgeon (A.S.), 1 internist (S.C.)].2 The primary outcome variables were all-cause mortality and CVEs, defined as death from a CV cause or hospitalization for MI or revascularization, cardiac arrest, heart failure, atrial fibrillation, thromboembolism, or stroke.
2.7. Statistical Analysis:
Clinical characteristics of LTRs from the integrated dataset were described using frequency counts and percentages for categorical variables and means ± standard deviations for continuous variables. A t-test, chi-square or Fisher’s exact test were used to examine predictors of BP control among LTRs for continuous or categorical variables, as appropriate. Cox proportional hazard models were used to estimate survival and CVEs between LTRs who had controlled BP within 1 year post-LT and those who did not. Variables significant at a P value <0.05 in univariate analysis for the BP control versus uncontrolled groups were then tested in age- and sex-adjusted multivariable models to estimate survival and CVEs. Only variables significant at a P value < 0.05 were retained. The final models were adjusted for sex, and time-varying age, diabetes, CKD and hyperlipidemia status. Patients were censored at date of death or at the end of the study period (December 31, 2017). A set of a priori subgroup analyses among high CV risk populations were conducted and included analyses stratified by older age (≥ 65 years), sex, diabetes, CKD, hypertension, obesity, hyperlipidemia, OSA, ASCVD, and smoking status. Multiplicative interaction terms were generated to assess interactions between BP control <140/<90 mmHg and these CV risk populations in terms of mortality or CVEs. SAS software version 9.4 (SAS institute, Cary, NC) was used to complete all analyses. All P values are two-sided and a P value < 0.05 was considered to indicate statistical significance.
3. Results
Of 705 patients transplanted during the study period, 56 patients died within 6 months of transplant (CV death n=22) and 47 patients had less than 1 year follow up time (CVE n=12) and were excluded from analysis. The remaining 602 LTRs formed the sample population. The average age at LT was 56.7 ± 11.1 years, 63.8% of LTRs were men, and 60.8% of LTRs were non-Hispanic White; 16.4% of the LTRs were of Hispanic ethnicity. The majority of LTRs were transplanted for hepatitis C (33.5%), followed by alcohol (23.5%), and NASH (16.1%). The median lab Model for End-Stage Liver Disease (MELD) score at transplant was 21 (range 6–44). Preexisting CV conditions were common: 53.7% had hypertension, 38.9% met criteria for obesity, 32.9% had diabetes, 41.5% had CKD, and 41.2% reported current or former smoking at the time of transplant (Supplemental Table 1).
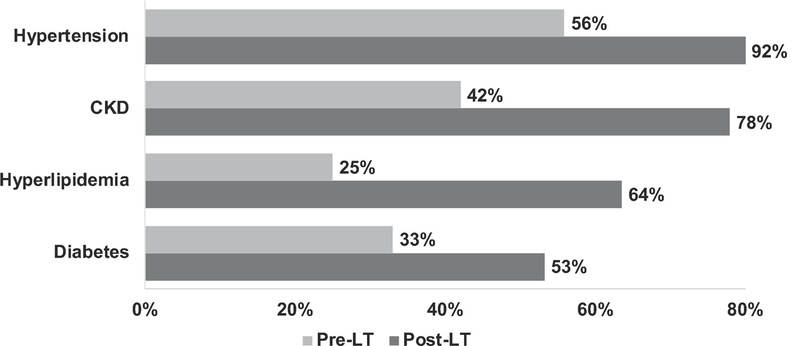
Exacerbation of underlying CV risk factors was observed post-LT (Figure 1). Hypertension, CKD, hyperlipidemia, and diabetes prevalence increased substantially. The greatest CV risk factor prevalence was observed for hypertension, with 92% of LTRs having hypertension within 6 years of transplant.
Figure 1.
Cardiovascular risk factor burden in relation to liver transplant among 602 liver transplant recipients, 2010–2016
Adherence (to guideline recommendations) scores for selected BP performance and process metrics are shown in Table 1. Overall, adherence to BP measurement was high throughout the 6-year study period and, not surprisingly, BP measurement decreased as time from transplant increased and visit frequency decreased. However, despite frequent measurement, BP was poorly controlled in most LTRs. Only 16.1% of LTRs had BP controlled to <140/<90 mmHg in year 1 after transplant and only 29% of LTRs had BP controlled to <140/<90 mmHg at any point post-LT. As expected, adherence was even lower for BP controlled to <130/<80 mmHg, ranging from 5.0% to 13.8%. Among the 97 LTRs with BP controlled to <140/90 mmHg in year 1, only 16 (16.5%) were treated with BP-lowering medications and among the 30 LTRs with BP controlled to <130/80 mmHg, 5 (16.7%) were treated with BP-lowering medications. Less than one-third of LTRs who did not have BP controlled were offered referral to a hypertension specialist for co-management. Guideline-adherent BP control was even lower among high CV risk populations, including those with diabetes and CKD. Only 13.3% of LTRs with diabetes and 12.2% of LTRs with CKD had BP controlled to a guideline-recommended threshold of <130/<80 mmHg at any point after transplant. In addition, a minority of LTRs with hypertension and CKD (8.4%−14.1%) were offered evidence-based treatment with an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB).
Table 2 demonstrates characteristics at transplant stratified by presence or absence of BP control to <140/<90 mmHg during the first year after transplant. Patients with uncontrolled BP were more likely to have higher BMI, a prevalent diagnosis of hypertension, hyperlipidemia, ASCVD or OSA and more likely to be on steroids or a CNI between 6 and 12 months post-LT. Similar findings were seen in the small group who had BP controlled to <130/<80 mmHg (data not shown).
Table 2.
Cohort characteristics stratified by controlled BP <140/<90 mmHg versus uncontrolled BP groups with the first year after liver transplantation
| Characteristic | Controlled BP | Uncontrolled BP | P value* |
|---|---|---|---|
| Population N, % | 97 (16.1) | 505 (83.9) | |
| Age at transplant, Mean, SD | 52.6 (14.9) | 57.5 (10.0) | .51 |
| Female Sex, % | 38 (39.2) | 180 (35.6) | .51 |
| Race/Ethnicity, % | .61 | ||
| Non-Hispanic White | 57 (58.8) | 309 (61.2) | |
| Non-Hispanic Black | 7 (7.2) | 47 (9.3) | |
| Hispanic | 21 (21.6) | 78 (15.4) | |
| Asian | 4 (4.1) | 20 (4.0) | |
| Other | 8 (8.2) | 51 (10.1) | |
| Less than high school education, % | 1 (1.0) | 22 (4.4) | .04 |
| Primary Payer, % | .83 | ||
| Private insurer | 62 (63.9) | 300 (59.4) | |
| Medicare | 29 (29.9) | 165 (32.7) | |
| Medicaid | 5 (5.2) | 31 (6.1) | |
| Other | 1 (1.0) | 9 (1.8) | |
| Lab MELD score at transplant, median (range) | 14 (11–37) | 22 (6–44) | .27 |
| BMI at transplant, mean, SD | 27.8 (5.5) | 29.6 (6.8) | .02 |
| BMI ≥ 30 kg/m2 at transplant | 30 (30.9) | 204 (40.4) | .14 |
| BMI at 1 year post transplant, mean, SD | 25.0 (4.7) | 27.0 (5.5) | .003 |
| Cause of end-stage liver disease | |||
| Hepatitis C | 27 (27.8) | 189 (37.4) | .07 |
| Alcohol | 28 (28.9) | 143 (28.3) | .91 |
| NASH | 9 (9.3) | 67 (13.3) | .28 |
| Other | 41 (42.3) | 150 (29.7) | .02 |
| HCC status at transplant | 37 (38.1) | 218 (43.2) | .36 |
| Smoking status at transplant | |||
| Never | 54 (55.7) | 270 (53.5) | .64 |
| Current/Former | 40 (41.2) | 208 (41.2) | |
| Missing | 3 (3.1) | 27 (5.3) | |
| CV comorbidity at transplant | |||
| ASCVD | 21 (21.6) | 187 (37.0) | .004 |
| Heart Failure | 11 (11.3) | 91 (18.0) | .11 |
| Atrial Fibrillation | 10 (10.3) | 52 (10.3) | 1.0 |
| Stroke | 12 (12.4) | 67 (13.3) | .81 |
| PE | 1 (1.0) | 8 (1.6) | .68 |
| Hypertension | 42 (43.3) | 281 (55.6) | .03 |
| Hyperlipidemia | 17 (17.5) | 136 (26.9) | .05 |
| Peripheral Vascular Disease | 8 (8.2) | 62 (12.3) | .26 |
| Other comorbidities at transplant | |||
| Chronic kidney disease | 40 (41.2) | 210 (41.6) | .33 |
| Stage 1 | 25 (36.2) | 94 (24.9) | |
| Stage 2 | 15 (21.7) | 94 (24.9) | |
| Stage 3 | 15 (21.7) | 114 (30.2) | |
| Stage 4 | 10 (14.5) | 50 (13.3) | |
| Stage 5 | 4 (5.8) | 25 (6.6) | |
| Renal replacement therapy | 13 (13.4) | 77 (15.2) | .64 |
| Diabetes | 27 (27.8) | 171 (33.9) | .25 |
| Obstructive Sleep Apnea | 4 (4.1) | 57 (11.3) | .03 |
| BP-Lowering Therapy | 16 (16.5) | 171 (33.9) | <.001 |
| Thiazide | 2 (2.1) | 7 (1.3) | .64 |
| Calcium channel blocker | 6 (6.2) | 81 (16.0) | .01 |
| ACEI or ARB | 5 (5.2) | 39 (7.7) | .37 |
| Vasodilator | 0 (0.0) | 0 (0.0) | 1.0 |
| Alpha2 agonist | 0 (0.0) | 23 (4.6) | .04 |
| Beta blocker | |||
| Nonselective beta blocker | 0 (0.0) | 22 (4.4) | .03 |
| Selective | 4 (4.1) | 48 (9.5) | .08 |
| Immunosuppression Regimen | |||
| 0–6 months | |||
| Steroids | 96 (99.0) | 502 (99.4) | .63 |
| Calcineurin inihibitor | 97 (100.0) | 500 (99.0) | .33 |
| Mycophenolate | 68 (70.1) | 378 (74.9) | .33 |
| mTOR inhibitor | 7 (7.2) | 60 (11.9) | .18 |
| 6–12 months | |||
| Steroids | 19 (19.6) | 162 (32.1) | .01 |
| Calcineurin inihibitor | 60 (61.9) | 377 (74.7) | .01 |
| Mycophenolate | 23 (23.7) | 184 (36.4) | .02 |
| mTOR inhibitor | 6 (6.2) | 64 (12.7) | .07 |
| > 1 year | |||
| Steroids | 30 (30.9) | 148 (29.3) | .75 |
| Calcineurin inihibitor | 78 (80.4) | 417 (82.6) | .61 |
| Mycophenolate | 41 (42.3) | 227 (45.0) | .62 |
| mTOR inhibitor | 15 (15.5) | 84 (16.6) | .78 |
Abbreviations: SD, standard deviation; MELD, model for end-stage liver disease; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; ASCVD, atherosclerotic cardiovascular disease; PE, pulmonary embolism; mTOR, mammalian target of rapamycin
T-test for continuous variables and chi-square for categorical variables
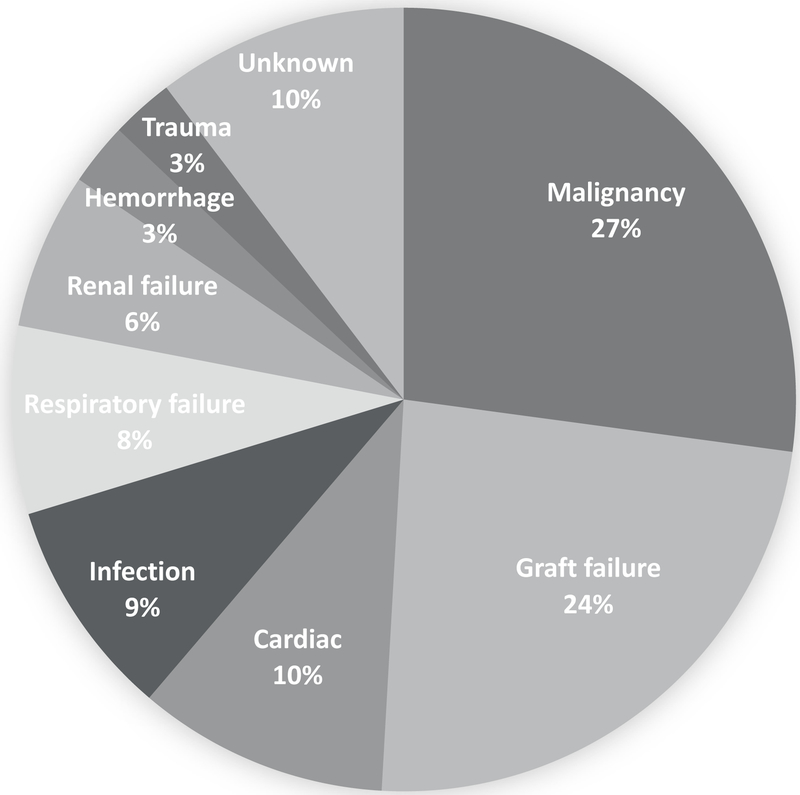
During the follow up period (mean 43.2 months), 86 LTRs who survived to 6 months post-LT experienced a CVE and 77 died, 8 of whom died from a CV cause (Figure 2). The most common cause of death was malignancy (27.3%), followed by graft failure (23.9%) and cardiac disease (10.4%) (Figure 2). The majority of CVEs (54.7%) occurred within the first 365 days post-LT. The most common underlying etiology of a CVE was stroke (27.9%), followed by MI/revascularization (20.9%), atrial fibrillation (19.8%), heart failure (18.6%), and thromboembolism (12.8%, Supplemental Figure 1). Preexisting and incident hypertension were associated with higher CVEs compared to LTRs without hypertension (18.0% vs. 10.0%, p=0.006 and 12.2% vs. 0%, p=0.01, respectively).
Figure 2.
Primary cause of death among liver transplant recipients who survived to at least 6 months post-transplant (n=77)
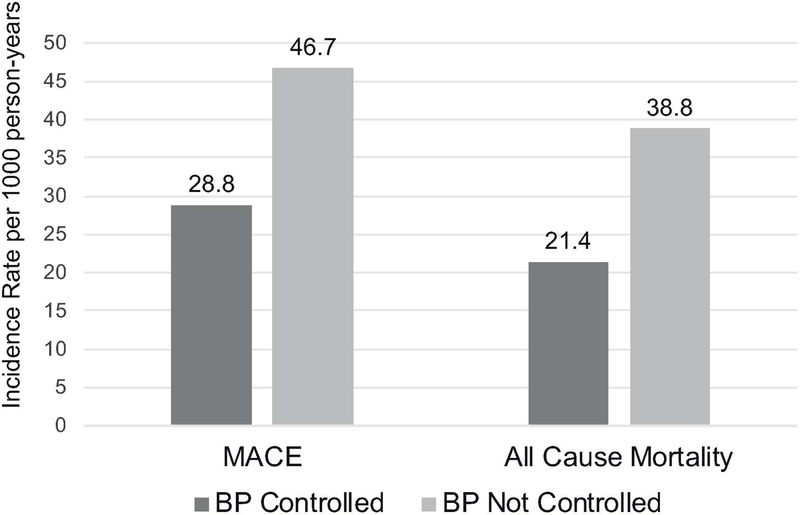
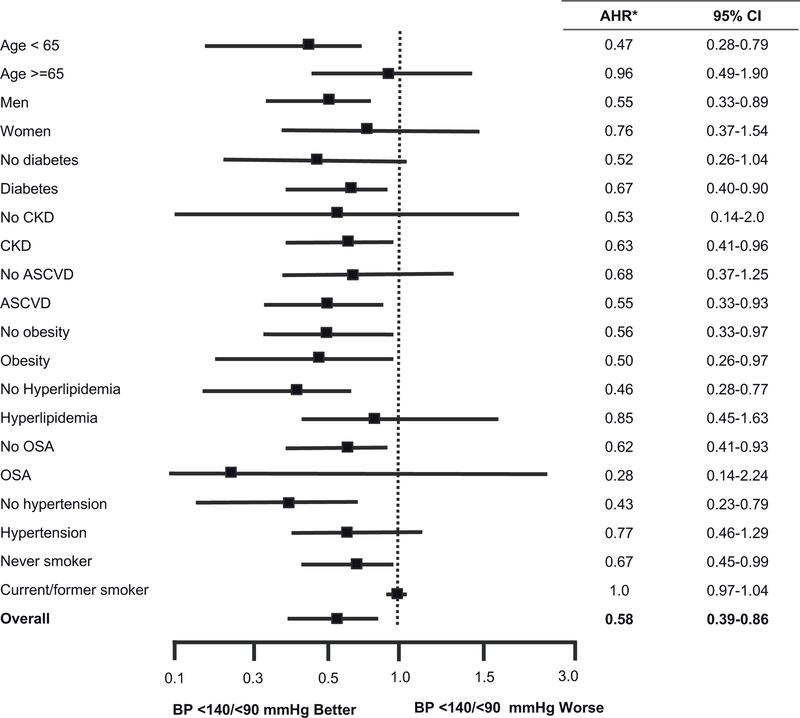
Figure 3 shows the incidence rate per 1000 person-years of CVEs and all-cause mortality of LTRs with controlled versus uncontrolled BP. Having BP controlled to <140/<90 mmHg was associated with a 38% lower rate of CVEs (28.8 vs. 46.7 per 1000 person-years) and a 45% lower rate of all-cause mortality (21.4 vs. 38.8 per 1000 person-years). After multivariable adjustment for sex, and time-varying age, diabetes, CKD, and hyperlipidemia status, BP controlled to <140/<90 mmHg was associated with a 35% lower hazard of CVEs (Hazard Ratio (HR) 0.65, 95% Confidence Interval (CI) 0.43, 0.97; p=0.03) and a 42% lower hazard of mortality (HR 0.58, 95%CI 0.39, 0.87; p=0.008) among LTRs (Table 3). In age and sex-adjusted analyses, the association of lower mortality and CVEs among LTRs with controlled BP <140/<90 mmHg within year 1 post-LT was similar across clinically relevant subgroups stratified by age, sex, diabetes, CKD, ASCVD, obesity, hyperlipidemia, OSA, hypertension, or smoking status (Figure 4). There were no interactions between BP control to <140/90 and these subgroups in terms of CVEs or mortality (data not shown). In age and sex-adjusted analyses, there was no statistically significant difference in CVE among LTRs who received BP-lowering treatment and those who did not (HR 0.87, 95%CI 0.24, 3.13; p=0.84). However, among the 505 LTRs who had uncontrolled BP (≥140/90 mmHg) in the first year post-LT, 83 (16.4%) were reclassified as controlled in Y2. In an age- and sex-adjusted model, moving from uncontrolled to controlled BP was associated with a 46% lower hazard of CVE (HR 0.54, 95%CI 0.30, 0.97; p=0.039).
Figure 3.
Incidence rates for all-cause mortality and CVE according to BP controlled to < 140/<90 mmHg within the first year of liver transplantation. BP controlled to < 140/<90 mmHg was associated with a 38% absolute reduction in CVE and a 45% absolute reduction in all-cause mortality. Abbreviations: CVE, cardiovascular event; BP, blood pressure.
Table 3.
Adjusted Hazard Ratios* for overall mortality or cardiovascular events among liver transplant recipients with BP controlled vs. those with uncontrolled BP during year 1 post-transplant
| Overall Mortality | CVEs^ | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazards Ratio | 95% CI | P value | Hazards Ratio | 95% CI | P value | |||
| BP controlled < 140/90 mmHg | 0.58 | 0.39 | 0.87 | 0.008 | 0.65 | 0.43 | 0.97 | 0.03 |
| Age (per year) | 1.06 | 1.04 | 1.08 | <.0001 | 1.001 | 0.98 | 1.012 | 0.95 |
| Female sex | 0.95 | 0.72 | 1.27 | 0.74 | 0.62 | 0.44 | 0.87 | 0.005 |
| Diabetes | 0.72 | 0.55 | 0.95 | 0.02 | 1.29 | 0.95 | 1.76 | 0.10 |
| CKD | 1.19 | 0.83 | 1.71 | 0.35 | 2.03 | 1.24 | 3.33 | 0.005 |
| Hyperlipidemia | 0.64 | 0.48 | 0.85 | 0.002 | 1.38 | 1.01 | 1.89 | 0.05 |
| Hazards Ratio | 95% CI | P value | Hazards Ratio | 95% CI | P value | |||
| BP controlled < 130/80 mmHg | 0.91 | 0.53 | 1.55 | 0.73 | 0.93 | 0.52 | 1.65 | 0.80 |
| Age (per year) | 1.06 | 1.05 | 1.08 | <.0001 | 1.002 | 0.99 | 1.02 | 0.80 |
| Female sex | 0.95 | 0.71 | 1.26 | 0.71 | 0.62 | 0.44 | 0.86 | 0.005 |
| Diabetes | 0.74 | 0.56 | 0.97 | 0.03 | 1.32 | 0.97 | 1.79 | 0.08 |
| CKD | 1.21 | 0.84 | 1.74 | 0.30 | 2.09 | 1.27 | 3.43 | 0.004 |
| Hyperlipidemia | 0.65 | 0.49 | 0.87 | 0.003 | 1.40 | 1.02 | 1.93 | 0.04 |
Time-dependent Cox Proportional Hazard Analyses; all comorbidities included as time-varying covariates except sex which was recorded at time of transplant
CVE defined as death from a CV cause or hospitalization for post-transplant myocardial infarction or revascularization, cardiac arrest, heart failure, atrial fibrillation, thromboembolism, or stroke
Abbreviations: CKD, chronic kidney disease; CVE, cardiovascular events; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease.
Figure 4.
Adjusted Hazard Ratios for BP-Controlled <140/90 mmHg vs Uncontrolled, stratified by key patient characteristics for A) mortality and B) cardiovascular events. *Adjusted hazard ratios for age- and sex-adjusted models. Abbreviations: AHR, adjusted hazard ratio: ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; LT, liver transplant; OSA, obstructive sleep apnea
In multivariable analyses, there were no statistically significant associations between LTRs with BP controlled to a lower threshold of <130/<80 mmHg and all-cause mortality (HR 0.91, 95%CI 0.53,1.55; p=0.73) or CVEs (HR 0.93, 95%CI 0.52,1.65; p=0.80), although few patients achieved this level of BP control, thus limiting the power to detect statistically significant associations. In sensitivity analyses excluding BP values within the first 90 days of transplant, we observed a similar effect size as presented in the main results though power to detect statistically significant differences was lower.
4. Discussion
These findings demonstrate an association between receipt of guideline concordant care with BP controlled to <140/<90 mmHg and lower all-cause mortality and CVEs among LTRs receiving care at a large, urban, tertiary care network, independent of comorbidity burden. However, overall adherence guideline concordant BP control care post-LT was low, despite a high prevalence of elevated BP and hypertension in the population. These findings suggest that system redesign to support improved guideline concordant BP control care by the multidisciplinary team providing care for LTRs is needed.
The association between guideline concordant BP care and reduction in CVEs and all-cause mortality was preserved among high-risk patients, although these patients were actually less likely to receive guideline concordant care. Therefore, future guideline updates for LTRs should focus on the importance of broad application of the guidelines and inclusion of patients with comorbidities, particularly those with CKD and diabetes, who are arguably at highest risk for adverse CV outcomes.3, 19, 20 The major questions arising from our results are (a) whether the difference in mortality or CVE between LTRs with controlled and uncontrolled BP reflects a true association between the delivery of guideline concordant care and a durable morbidity and mortality benefit, (b) whether BP control is merely a marker of healthier patients, or (c) whether it is a marker of those receiving better overall post-LT care. To address this potential confounding, we used multivariable Cox analysis to adjust for the relationship between time-varying confounders and long-term outcomes. It should be noted however, that all patients in the cohort were being cared for by a single team of transplant clinicians, thus, mitigating substantial variation in overall care. In addition, the stratified subgroup analyses consistently showed a significant improvement in overall mortality and CVE for controlled versus uncontrolled BP groups.
Up to 92% of LTRs met the criteria for a diagnosis of hypertension within 6 years post-LT. Prior studies have reported hypertension prevalence in LTRs ranging from 60% to 85%.20, 21 Of note, these prevalence estimates are based on a BP threshold ≥ 140/90 mmHg. Recently released U.S. guidelines recommend a new BP classification system that characterizes hypertension as an average systolic BP ≥ 130 mmHg or diastolic BP ≥ 80 mmHg, based on clinical trials and meta-analyses of optimal BP levels to reduce CVEs in the general population.7 Application of this new hypertension classification would result in 95% of the cohort meeting criteria for BP-lowering therapy. Regardless of the BP threshold used, this study demonstrates that elevated BP is a major health challenge for LTRs. The rate of CVEs in this study (14.2%) is consonant with previously estimated CVE rates post-LT, which range from 7% to 30% depending on time from transplant, the definition of CVEs, and the reporting period.2, 3, 5, 22 Notably, the leading underlying etiology of CVEs in the current study was stroke (28%), which is highly related to elevated BP.
The mechanisms underlying the high prevalence of hypertension in LTRs are predominantly related to renal (and systemic) vasoconstriction, as well as, impaired eGFR and sodium excretion.23 In addition, older age and a high pre-transplant prevalence of hypertension contribute to the high post-transplant prevalence of hypertension, as placement of the new allograft results in increased venous return to the heart and an increase in systemic vascular resistance. Hypertension in LTRs is also highly associated with CNI therapy that results in renal arterial vasoconstriction.23 This is most prominently seen with cyclosporine and can be exacerbated with combination therapy with mammalian target of rapamycin (mTOR) inhibitors.24, 25 Finally, steroids have adverse effects on BP control through their mineralocorticoid effects and by increasing systemic vascular resistance and cardiac contractility.23, 26 Notably, persons with uncontrolled BP in the cohort were more likely to be on prolonged steroid treatment and have higher immunosuppression utilization, as indicated by higher use of mTOR inhibitors and mycophenolate.
Despite the high prevalence of elevated BP, medications for lowering BP were offered in only 37% of LTRs during year 1 post-LT and adherence dropped precipitously over time. Interestingly, adherence was lowest (range 3.4%−13.3%) for LTRs who, arguably, should be prioritized for BP control, including patients with underlying CKD and diabetes.7, 8, 21 Guideline-adherent BP treatment in LTRs was much lower than reports about the general U.S. population, wherein approximately 61.7% (95% CI: 58.6%−64.8%) of persons with hypertension are estimated to receive BP-lowering treatment and 70.8% (95% CI, 64.8–76.2%) of persons with CKD and diabetes are treated.27 We also found that BP control was achieved in a minority of LTRs with hypertension (range 10.1–28.1%); also lower than the rates of BP control in the general population [mean 71.7% (95% CI, 68.5–74.7)].27
Based on current U.S. treatment guidelines for elevated BP, antihypertensive drug therapy, in addition to nonpharmacologic therapy, is recommend for U.S. adults with stage 1 hypertension (systolic BP 130 to 139 mmHg or diastolic BP 80 to 89 mm Hg) with a 10-year risk of ASCVD ≥ 10% and all those with systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg.7 The AASLD and AST recommend treating all LTRs with systolic BP ≥ 130 or diastolic BP ≥ 80 mmHg.8 When these guidelines are applied to LTRs, who likely all have 10-year ASCVD risk above the 10% threshold, over 95% of LTRs meet criteria for some form of BP-lowering therapy. The BP threshold of <140/<90 mmHg, used in this study, is aligned with prior, contemporaneous JNC recommendations for BP control and with numerous other clinical practice guidelines for the management of hypertension.16, 28–30 A small minority of patients in the current study achieved the newly recommended lower BP threshold of <130/<80 mmHg, and we were unable to demonstrate any statistical association between BP controlled to <130/<80 mmHg in terms of mortality or CVEs. A larger prospective study is needed to identify the optimal target for BP-lowering in LTRs.
This study has several limitations which warrant mention. First, the findings represent practice patterns at a single institution and may not generalize to all transplant centers. However, this is the first analysis to assess the association, in LTRs, of guideline concordant BP control with measurable clinical outcomes. Future studies, using large, national, transplant datasets that link detailed clinical BP data with pharmacy fill records of BP lowering medications are needed in order to validate these findings. Currently, we are unaware of any datasets that contain all of these data. This study may be subject to recording bias as EHRs are more likely to capture performed actions (e.g., medication prescribed) versus non-performed actions (e.g., clinical reasoning as to why a medication was not prescribed). To minimize this bias, we performed extensive EHR review involving review of all clinical notes from any care provider within the reporting period to capture appropriate clinical reasoning to ascertain actions that may not have been appropriately performed. In addition, there is the potential of residual confounding by factors that are difficult to measure in the EHR, such as smoking status, alcohol use, and weight management and physical activity and competing risks from rejection and graft failure. We used natural language processing methods to improve ascertainment of smoking status, and addressed weight management by considering the effect of BMI %change on all outcomes, which did not change the magnitude or direction of the observed associations (data not shown). Finally, assuming clinically valid BP values, two major factors contribute to BP control in treated patients: (1) prescription of an adequate number and appropriate dose(s) of BP medications and (2) patient adherence to treatment. The current study addresses the former, demonstrating that few LTRs were prescribed guideline concordant 2-drug therapy for BP control and even fewer were referred to a hypertension specialist for co-management. These findings identify important high-priority targets for quality improvement efforts to improve BP management in this high-risk population.
In conclusion, given the consistent association between BP controlled to <140/<90 mmHg and improved clinical outcomes, specifically CVEs, in LTRs, efforts to improve clinician adherence to guideline concordant care needs to be intensified. Prospective studies are needed to examine whether these observations generalize to the larger transplant community, and whether comprehensive risk assessment of current clinical care practices and user-centered redesign of the delivery of guideline concordant care for BP in LTRs can lead to improved clinical outcomes in this high CV risk population.
Supplementary Material
Acknowledgments
Dr. VanWagner is supported by the National Heart, Lung and Blood Institute grant number, K23 HL136891. The Northwestern Medicine Enterprise Data Warehouse (NMEDW) is funded, in part, by the National Center for Advancing Translational Sciences (NCATS) of the NIH research grant UL1TR001422 to the Northwestern University Clinical and Translational Sciences (NUCATS) Institute.
Abbreviations:
- AASLD
American association for the study of liver diseases
- ACC
American college of Cardiology
- AHA
American heart association
- AST
American society for transplantation
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- CKD
chronic kidney disease
- CNI
calcineurin inhibitor
- CMS
center for medicare and Medicaid services
- CVE
cardiovascular event
- CV
cardiovascular
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- ICD
international classification of diseases
- LT
liver transplant
- LTR
liver transplant recipient
- MI
myocardial infarction
- mTOR
mammalian target of rapamycin
- NASH
nonalcoholic steatohepatitis
- NMEDW
northwestern medicine enterprise data warehouse
- OPTN
organ procurement and transplantation network
- OSA
obstructive sleep apnea
- SSDI
social security death index
- US
United States
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information Section at the end of the article.
References
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant 2019;19 Suppl 2:184–283. [DOI] [PubMed] [Google Scholar]
- 2.VanWagner LB, Lapin B, Levitsky J, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl 2014;20:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanWagner LB, Serper M, Kang R, et al. Factors Associated With Major Adverse Cardiovascular Events After Liver Transplantation Among a National Sample. Am J Transplant 2016;16:2684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fussner LA, Heimbach JK, Fan C, et al. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl 2015;21:889–96. [DOI] [PubMed] [Google Scholar]
- 5.Albeldawi M, Aggarwal A, Madhwal S, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl 2012;18:370–5. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 8.Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19:3–26. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Saldivar B, Prieto J, Berenguer M, et al. Control of blood pressure in liver transplant recipients. Transplantation 2012;93:1031–7. [DOI] [PubMed] [Google Scholar]
- 10.Malyszko J, Malyszko J, Bachorzewska-Gajewska H, et al. Inadequate blood pressure control in most kidney transplant recipients and patients with coronary artery disease with and without complications. Transplant Proc 2009;41:3069–72. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis 2004;43:1071–81. [DOI] [PubMed] [Google Scholar]
- 12.Tutone VK, Mark PB, Stewart GA, et al. Hypertension, antihypertensive agents and outcomes following renal transplantation. Clin Transplant 2005;19:181–92. [DOI] [PubMed] [Google Scholar]
- 13.Adrych D, Kuzmiuk-Glembin I, Tylicki L, et al. Improvement of Blood Pressure Control in Renal Transplant Recipients-Retrospective Longitudinal Study. Transplant Proc 2018;50:155–159. [DOI] [PubMed] [Google Scholar]
- 14.Salvalaggio PR, Dzebisashvili N, MacLeod KE, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transplantation 2011;17:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalifa A, Meystre S. Adapting existing natural language processing resources for cardiovascular risk factors identification in clinical notes. J Biomed Inform 2015;58 Suppl:S128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)2014 Guideline for Management of High Blood Pressure2014 Guideline for Management of High Blood Pressure. JAMA 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 17.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. Jama 2006;295:1168–70. [DOI] [PubMed] [Google Scholar]
- 18.Ryan AM. Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv Res 2009;44:821–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanWagner LB, Montag S, Zhao L, et al. Cardiovascular Disease Outcomes Related to Early Stage Renal Impairment After Liver Transplantation. Transplantation 2018;102:1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watt KD, Pedersen RA, Kremers WK, et al. Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-Up Study. Am J Transplant 2010. [DOI] [PMC free article] [PubMed]
- 21.Watt KD. Keys to long-term care of the liver transplant recipient. Nat Rev Gastroenterol Hepatol 2015;12:639–48. [DOI] [PubMed] [Google Scholar]
- 22.Safadi A, Homsi M, Maskoun W, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation 2009;120:1189–94. [DOI] [PubMed] [Google Scholar]
- 23.Watt KD, Charlton MR. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol 2010;53:199–206. [DOI] [PubMed] [Google Scholar]
- 24.Canzanello, Vincent J, Textor SC, Taler SJ, et al. Late hypertension after liver transplantation: A comparison of cyclosporine and tacrolimus (FK 506). Liver Transplantation and Surgery 1998;4:328–334. [DOI] [PubMed] [Google Scholar]
- 25.Gonwa T, Mendez R, Yang HC, et al. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75:1213–20. [DOI] [PubMed] [Google Scholar]
- 26.Schäcke H, Döcke W-D, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology & Therapeutics 2002;96:23–43. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 28.Zanchetti A, Dominiczak A, Coca A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 29.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureThe JNC 7 Report. JAMA 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.