Figure 5.
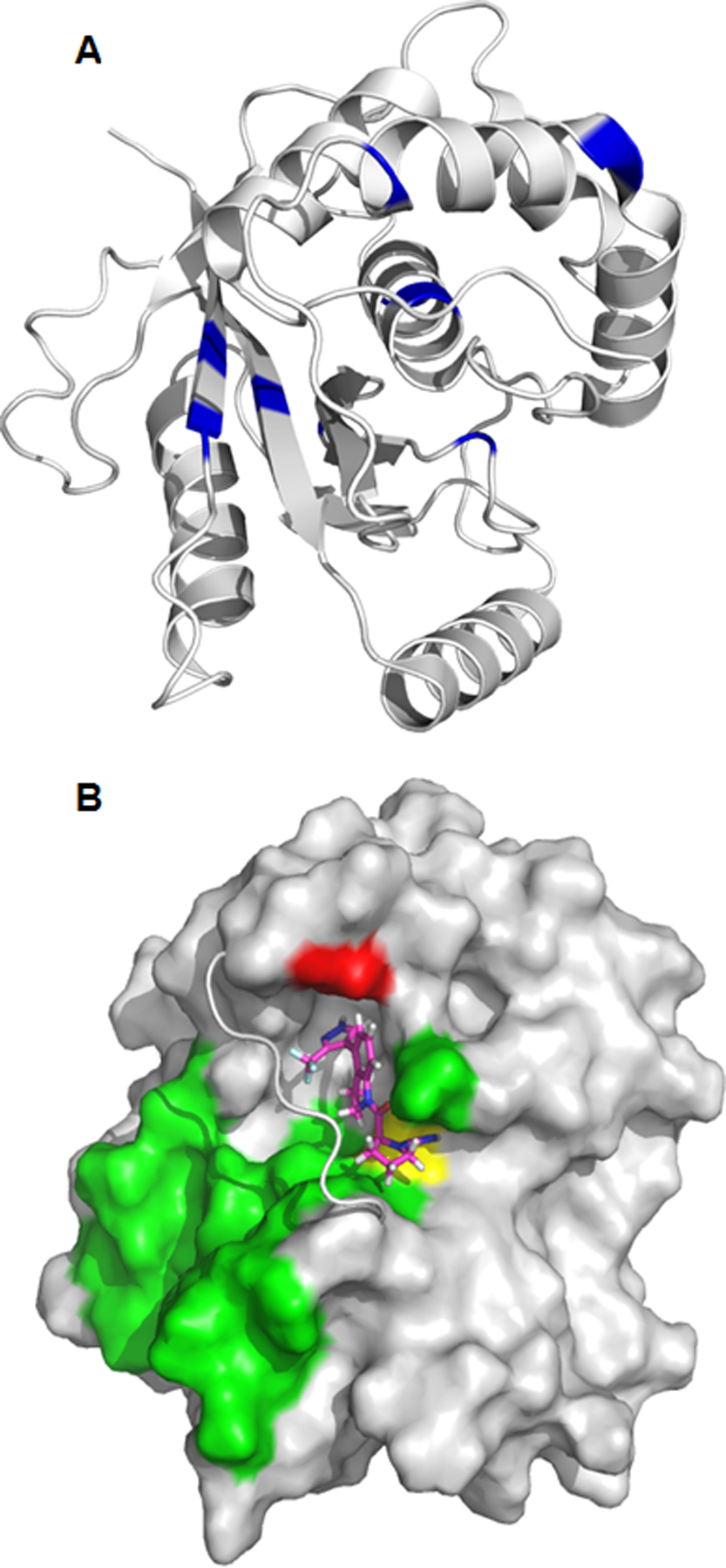
A) Chemical shift changes () greater than 0.15 ppm are mapped to the structure of UCHL1 (PDB ID: 2ETL). The difference in 15N-HSQC chemical shifts between unligated UCHL1 and after addition of 1 were measured at a molar ratio [1:1]. The orientation of UCHL1 is approximately the same as Figure 5B. B) Predicted binding pose of compound 1 (magenta sticks) with UCHL1 (grey), supported by 3D NOE NMR experiments. The crossover loop of UCHL1 is shown as a cartoon for clarity. Compound 1 binds to UCHL1 on the same side of the crossover loop as the ubiquitin binding interface of UCHL1 (green surface). Compound 1 forms a covalent bond with cysteine 90 (yellow surface) and has an experimentally determined NOE with alanine 147 (red surface).
