Abstract
Rationale & Objective.
Risk factors for kidney failure are the basis of live kidney donor candidate evaluation. We quantified risk of end-stage kidney dieases (ESKD) by the biological relationship of the donor to the recipient, a risk factor that is not addressed by current clinical practice guidelines.
Study Design:
Retrospective cohort study.
Setting & Participants.
A cohort of 143,750 United States kidney donors between 1987 and 2017.
Exposure.
Biological relationship of donor and recipient.
Outcome.
ESKD. Donors records were linked to national dialysis and transplant registries to ascertain development of the outcome.
Analytic Approach.
Donors were observed over a median of 12 years (interquartile range, 6–18 years; maximum, 30 years). Survival analysis methods that account for the competing risk of death were used.
Results.
Risk of ESKD varied by orders of magnitude across donor-recipient relationship categories. For Asian donors, risks compared with unrelated donors were 259.4-fold greater for identical twins (95%CI, 19.5–3445.6), 4.7-fold greater for full-siblings (95% CI, 0.5–41.0), 3.5-fold greater for offspring (95%CI, 0.6–39.5), 1.0 for parents, and 1.0 for half-sibling or other biological relative. For black donors, the risk was 22.5-fold greater for identical twin donors (95%CI, 4.7–107.0), 4.1-fold for full-sibling (95%CI, 2.1–7.8), 2.7-fold for offspring (95%CI, 1.4–5.4), 3.1-fold for parents (95%CI, 1.4-.6.8), and 1.3-fold for half-sibling or other biological relative (95%CI, 0.5–3.3). For white donors, the risk was 3.5-fold greater for identical twin donors (95%CI, 0.5–25.3), 2.0-fold for full-sibling (95%CI, 1.4–2.8), 1.4-fold for offspring (95%CI, 0.9–2.3), 2.9-fold for parents (95%CI, 2.0–4.1), and 0.8-fold for half-sibling or other biological relatives (95%CI, 0.3–1.6).
Limitations.
Insufficient sample size in some race and relationship groups. Absence of data on family history of kidney disease for donors biologically unrelated to their recipients.
Conclusions.
Marked differences in risk for ESKD across types of donor-recipient relationship were observed for Asian, black, and white donors. These findings warrant further validation with more robust data to better inform clinical practice guidelines.
Keywords: kidney donation, living-related donor, renal transplantation, family history of disease, health risks, kidney failure, end-stage renal disease (ESRD), ancestry, donor-recipient relationship, parent, sibling, offspring, biological relationship, risk of ESRD, race/ethnicity
INTRODUCTION
Live kidney donors who are biologically related to the recipient have been central to the success of kidney transplantation. Before the advent of potent immunosuppressive therapy, identical twin live donor kidney transplantation provided a proof-of-concept for the fledgling practice.1 Subsequent innovations in immunosuppressive therapy permitted kidney transplantation from full-sibling, offspring, parent, second- and third-degree relatives, and even biologically unrelated donors.2–4
Yet despite the growth of live donor kidney transplantation, the implications of a family history of end-stage kidney disease (ESKD) have not been reflected in clinical practice guidelines, except for autosomal dominant polycystic kidney disease in persons with European ancestry.5–7 While the most important goals of donor evaluation are to identify donors with kidney disease that would preclude donation, eliminate the potential transfer of infection/cancer, and ensure there is no coercion, standard donor evaluation is hinged on excluding candidates who are at high-risk of ESKD based on clinical risk factors such as hyperglycemia, high blood pressure, and proteinuria.8–14 Our previous attempts to characterize risk of ESKD in related donors at the national level were constrained by limited follow-up,15 and by limited statistical power that allowed only a single estimate for risk of ESRD for related vs. unrelated donors (1.7-fold greater risk for related donors).16 But a single estimate of risk of ESKD for related donors does not address the substantial variation in risk by ancestry,17–24 nor the potential gradient of risk within more closely related donors. Relatives of African Americans with non-diabetic ESKD are enriched for apolipoprotein L1 (APOL1) high-risk variants;18 likewise, related donors with ancestries other than African might be enriched for some other high-risk variants.25
To better quantify risk of ESKD among donors biologically related to the recipient to whom they donated a kidney, we used national registry data from the United States to investigate the risk along gradients of biological relationships between donor and recipient.25, 26 For racial groups with sufficient data, we compared the 20-year risk of ESKD for different types of donor-recipient relationships to biologically unrelated donors.
METHODS
Live Kidney Donors
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere.27, 28 The Health Resources and Services Administration, U.S. Department of Health and Human Services, oversees the activities of the OPTN and SRTR contractors. Through this reporting, all adult live donors between October 1, 1987, and December 31, 2017, were included in this study (including those who donated to children). This study was exempted from formal approval by the institutional review board of Johns Hopkins University because it uses de-identified data. Similarly, informed consent requirements ere waived due to de-identified nature of the information herein. ESKD outcomes were ascertained by linkage to the Centers for Medicare & Medicaid Services’ (CMS’s) medical evidence Form 2728 (certification of ESKD; including records through May 31, 2016) using a combination of Social Security number, last name, first, middle name, or all 3; date of birth; and sex. ESKD was defined as the initiation of maintenance dialysis or receipt of a living or deceased donor kidney transplant, as previously reported.15 Donor-recipient relationship was ascertained by self-report at donor evaluation and was grouped as identical twin, full-sibling, offspring, parent, half-sibling or other biological relative, and biologically unrelated (which included spouse and life partner).
Cause of ESKD
We classified donor and recipient causes of ESKD into eight broad categories: diabetes, hypertension or large vessel disease, glomerulonephritis (GN), cystic kidney disease, other urological disease, other cause, unknown cause, and missing cause. Diabetes includes type II (adult-onset type or unspecified type) and type I (juvenile type, ketosis prone diabetes). Hypertension includes kidney disease caused by hypertension (no primary kidney disease), renal artery stenosis, renal artery occlusion, and cholesterol and renal emboli. GN includes GN (histology not examined), focal segmental GN, membranous nephropathy, membranoproliferative GN, dense deposit disease, IgA nephropathy, IgM nephropathy (proven by immunofluorescence), rapidly progressive GN, Goodpasture’s syndrome, postinfectious GN and other proliferative GN. Cystic kidney disease included polycystic- and medullary cystic kidney disease. All other diagnoses were grouped under other cause. Unknown cause was documented as such. Missing cause included all records with no information on cause of ESKD.
Cumulative Incidence of ESKD
The outcome of interest was time to ESKD where time zero for all donors was the date of donation. Death prior to ESKD was a competing event. Cumulative incidence functions methods were used to estimate 20-year risk of ESKD, with a time scale of years since time zero.
Adjusted Odds Ratios of ESKD
Because of anticipated small sample size in some donor groups jointly defined by race and specific donor-recipient relationship,15 we also used multivariable logistic regression to calculate age- and sex-adjusted odds ratios (OR), a measure of association between the exposure (related vs. unrelated) and the outcome (ESKD vs. no ESKD). The following assumptions were needed for interpreting the odds ratios. First, adjusted odds ratios of ESKD were estimated over 20 years. Second, donors with follow-up time shorter than 20 years without ESKD were assumed to have no ESKD after 20 years of follow-up.
Adjusted Hazard Ratios of ESKD
Cause-specific hazards models were used to estimate multivariable-adjusted hazard ratios that accounted for age and sex by treating the competing events as censoring. A priori, we decided to model age as a continuous variable with a linear spline-term with a knot at 50-years-old since we previously observed a direct association between age at donation and 15-year risk of ESKD, but only for those ≥50 years. For donors <50 years, we previously observed an inverse association between age at donation and 15-year risk of ESKD: those 18–39 years old had a higher risk of ESKD compared with those aged 40–49 years.15 Because we were interested in investigating the familial basis of ESKD risk in donors, a priori we decided to perform the above analyses in each racial group. Finally, we explored models in which we additionally adjusted for systolic blood pressure, eGFR, history of smoking, and BMI. All analyses were performed using Stata 14.0/MP for Linux (Stata Corp, College Station, TX). All hypothesis tests were 2 sided (α = .05).
RESULTS
Study Population
Among 143,750 live kidney donors, the median age at donation was 40 years, 59% were female, 71% were white, 12% were Hispanic, 12% were black, and 3% were Asian. No donor had diabetes at baseline, but 2% had hypertension. The median eGFR for this population was 97 mL/min/1.73m3, median systolic/diastolic blood pressure was 121/74 mmHg, median BMI was 27 kg/m2, 25% had a history of smoking cigarettes, 26% graduated from college, and 11% had postgraduate education. With regard to relationship to recipient, 35% were unrelated (including 11% who were spousal relations, but biologically unrelated), 8% were half-sibling or other biological relative, 13% were parents, 16% were offspring, 29% were full-sibling, and 0.2% were identical twins. These characteristics varied by race (Table 1).
Table 1.
Demographic and health characteristics of live kidney donors in the United States by race/ethnicity, 1987–20171
| Asian (n=4,440) | Black (n=17,884) | Hispanic (n=18,535) | White (n=102,891) | |
|---|---|---|---|---|
| Age, y | 39 [31–49] | 35 [28–43] | 36 [28–44] | 42 [33–50] |
| Women, % | 59 | 57 | 58 | 60 |
| Diabetes, % | 0 | 0 | 0 | 0 |
| Hypertension, % | 2.3 | 1.8 | 1.3 | 3.3 |
| eGFR, ml/min/1.73m2 | 103 [91–114] | 107 [92–123] | 107 [93–117] | 94 [82–106] |
| Blood pressure, mmHg | ||||
| Systolic | 119 [110–128] | 121 [113–130] | 119 [110–127] | 120 [112–130] |
| Diastolic | 73 [67–80] | 74 [69–80] | 72 [66–80] | 74 [68–80] |
| BMI, kg/m2 | 24 [22–27] | 28 [24–31] | 27 [24–30] | 26 [24–29] |
| Smoke, % | 17 | 19 | 18 | 27 |
| Education, % | ||||
| High School or Less | 27 | 34 | 51 | 28 |
| Attended College | 24 | 33 | 27 | 27 |
| Graduate | 33 | 23 | 17 | 30 |
| Postgraduate | 16 | 9 | 5 | 13 |
| Year of Donation | 2008 [2002–2013] | 2004 [1999–2010] | 2006 [2000–2012] | 2005 [1999–2011] |
| Recipient Age, years | 44 [33–56] | 43 [31–54] | 40 [28–53] | 46 [32–56] |
| Spouse or life partner of recipient, % | 16 | 9 | 11 | 11 |
| Donor-recipient relationship, % | ||||
| Biologically unrelated to recipient | 32 | 23 | 26 | 38 |
| Half-sibling or other biological relationship | 8 | 11 | 8 | 7 |
| Parent of recipient | 13 | 11 | 15 | 13 |
| Offspring of recipient | 15 | 25 | 18 | 14 |
| Full-sibling of recipient | 31 | 30 | 32 | 28 |
| Identical twin of recipient | 0.2 | 0.2 | 0.2 | 0.2 |
| Recipient cause of ESKD, % | ||||
| Diabetes | 17 | 21 | 23 | 21 |
| HTN or large vessel disease | 18 | 32 | 19 | 12 |
| Glomerulonephritis | 42 | 30 | 32 | 30 |
| Cystic kidney disease | 5 | 3 | 5 | 12 |
| Urological disease | 1 | 1 | 1 | 3 |
| Other Cause | 6 | 5 | 8 | 12 |
| Unknown Cause | 9 | 8 | 11 | 9 |
| Missing Cause | 1.0 | 0.4 | 0.7 | 1.2 |
Values for continuous variables given as median [interquartile range]. Urinary albumin-creatinine ratio was not available.
Abbreviations: NA, not available; eGFR, estimated glomerular filtration rate; BMI, body-mass index; HTN, hypertension
All values are rounded to the nearest whole number except the % of donors with HTN and identical twins. Data before April 1, 1994 were left censored. Data on age, sex, race or ethnicity, and donor/recipient relationship were available throughout the study. Donor HTN was defined as “using anti-hypertensive medication.”
Not available before 1999, 49% with missing values in 1999, 11% with missing values in 2000 and 6% with missing values in 2001. Estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Not available before 1999, 43% with missing values in 1999, 20–30% with missing values from 2000 to 2003, 9–16% with missing values from 2004 to 2007.
Not available before 1998, 57% with missing values in 1999, 20% with missing values in 2000 and 10% with missing values in 2005.
Not available before 1999, 50% with missing values in 2000, 30% with missing values in 2005 and 10% with missing values in 2009.
Cause of Kidney Failure
Recipient causes of kidney failure varied in relative frequency by race. For white donors, the recipient cause of kidney failure was most frequently GN (30%) and diabetes (21%), with hypertension (12%) and cystic kidney disease (12%) tying for third place. For black donors, the ordering of recipient cause of kidney failure was hypertension (32%), GN (30%), and diabetes (21%). For Hispanic donors, recipient cause of kidney failure was GN (32%), diabetes (23%), and hypertension (19%). And for Asian donors, it was GN (42%), hypertension (18%), and diabetes (17%). Compared with the expected agreement between donor and recipient cause of kidney failure that would be only by chance (15.8%), the observed agreement for donor-recipient pairs was 19.4%, reflecting only slight but statistically significant concordance (kappa 0.04, p = 0.02) (Table S1). After stratifying the donor-recipient pairs as biologically related (thus potentially sharing genetic risks), spouse or lifetime partner (thus sharing lifestyle risks), and unrelated nonspousal pairs (sharing genetic or lifestyle risks only by chance), we observed a slight but statistically significant agreement in cause of kidney failure for biologically related pairs (kappa 0.05, p=0.01). A similarly slight but not statistically significant agreement in cause of kidney failure was observed for spousal or life partner pairs (kappa 0.04, p=0.3). No agreement was observed among biologically unrelated nonspousal pairs (kappa −0.01, p=0.6) (Table S2).
Cumulative Incidence of ESKD
Donors were followed for a median of 12 years (interquartile range, 6–18 years; maximum 30 years) and a total of 1,824,825 person-years, during which 407 donors developed ESKD. We estimated the incidence of ESKD according to race and donor-recipient biological relationship.
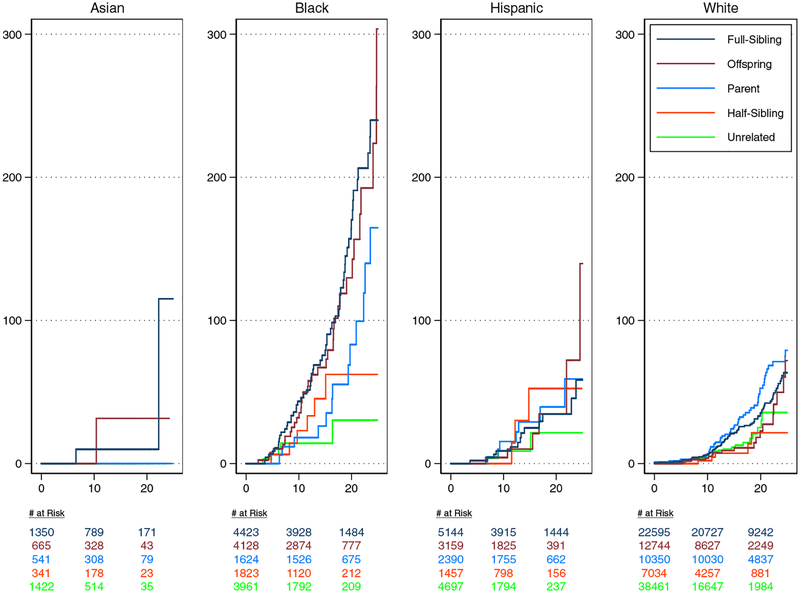
For Asian donors, the 20-year risk of ESKD, expressed per 10,000 donors, was 10 (95%CI, 1–70) for full-siblings, 32 (95%CI, 4–222) for offspring, 0 for parents, 0 for half-sibling/other biological relatives, and 0 for biologically unrelated donors. For black donors, the 20-year risk of ESKD, expressed per 10,000 donors, was 170 (95%CI, 126–230) for full-siblings, 130 (95%CI, 86–195) for offspring, 83 (95%CI, 42–164) for parents, and 62 (95%CI, 27–144) for half-sibling/other biological relatives, and 30 (95%CI, 10–95) for biologically unrelated donors. For Hispanic donors, the 20-year risk of ESKD, expressed per 10,000 donors, was 35 (95%CI, 19–64) for full-siblings, 35 (95%CI, 12–100) for offspring, 39 (95%CI, 17–91) for parents, 52 (95%CI, 17–165) for half-sibling/other biological relatives, and 22 (95%CI, 6–79) for biologically unrelated donors. For white donors, the 20-year risk of ESKD, expressed per 10,000 donors, was 35 (95%CI, 27–46) for full-siblings, 23 (95%CI, 12–44) for offspring, 53 (95%CI, 38–72) for parents, 22 (95%CI, 8–58) for half-sibling/other biological relatives, and 25 (95%CI, 14–44) for biologically unrelated donors (Figure 1).
Figure 1.
Cumulative Incidence of ESKD per 10,000 full-sibling-, offspring-, parent-, half-sibling or other biologically related-, and unrelated donors of varied racial groups. The cumulative incidence function was used to estimate the incidence of ESKD.
Adjusted Odds of ESKD
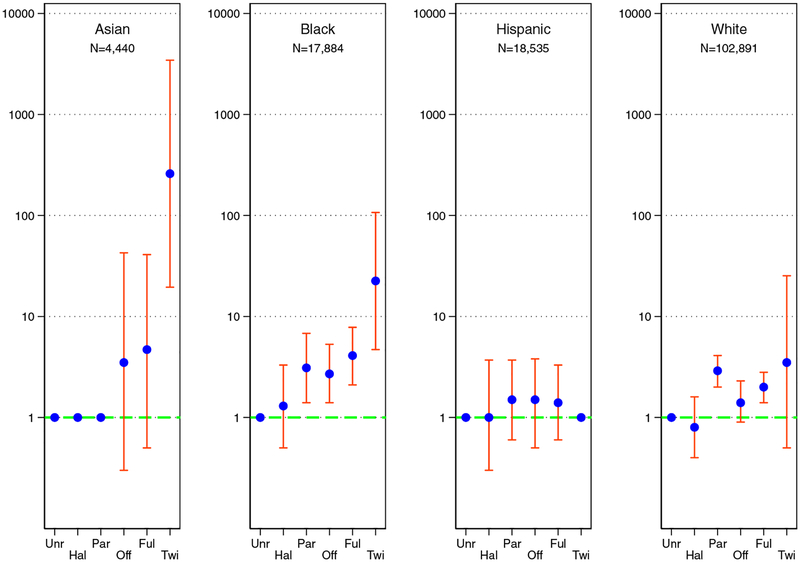
Risk of ESKD as estimated by the age- and sex-adjusted odds ratio varied by orders of magnitude across race. For Asian donors, risks compared with unrelated donors were elevated 259.4-fold (95%CI, 19.5–3445.6) for identical twins, 4.7-fold (95%CI, 0.5–41.0) for full-siblings, 3.5-fold (95%CI, 0.2–42.7) for offspring, 1.0 for parents, and 1.0 for half-sibling or other biological relative. For black donors, risks compared with unrelated donors were elevated 22.5-fold (95%CI, 4.7–107.0) for identical twins, 4.1-fold (95%CI, 2.1–7.8) for full-sibling, 2.7-fold (95%CI, 1.4–5.4) for offspring, 3.1-fold (95%CI 1.4–6.8) for parents, and 1.3-fold (95%CI, 0.5–3.3) for half-sibling or other biological relative. For Hispanic donors, risks compared with unrelated donors were undefined for identical twins, elevated 1.4-fold (95%CI, 0.6–3.3) for full-sibling, 1.5-fold (95%CI, 0.5–3.8) for offspring, 1.5-fold (95%CI, 0.6–3.7) for parents, and 1.0-fold (95%CI, 0.3–3.7) for half-sibling or other biological relatives. For white donors, risks compared with unrelated donors were elevated 3.5-fold (95%CI, 0.5–25.3) for identical twins, 2.0-fold (95%CI, 1.5–2.8) for full-sibling, 1.4-fold (95%CI, 0.9–2.3) for offspring, 2.9-fold (95%CI, 2.0–4.1) for parents, and 0.8-fold (95%CI, 0.4–1.6) for half-sibling or other biological relatives (Figure 2).
Figure 2. Age- and sex-adjusted odds ratios of ESKD.
Donor-recipient relationship and twenty-year odds of ESKD in live kidney donors of varied racial groups. The blue dot is the age- and sex-adjusted odds ratio comparing a biologically related donor group with unrelated donors; the orange bar is the 95% confidence interval of the odds ratio. Key: biologically unrelated (Unr), half-sibling or other biological relative (Hal), parent (Par), offspring (Off), full-sibling (Ful), and Identical Twin (Twi)
Adjusted Risk of ESKD
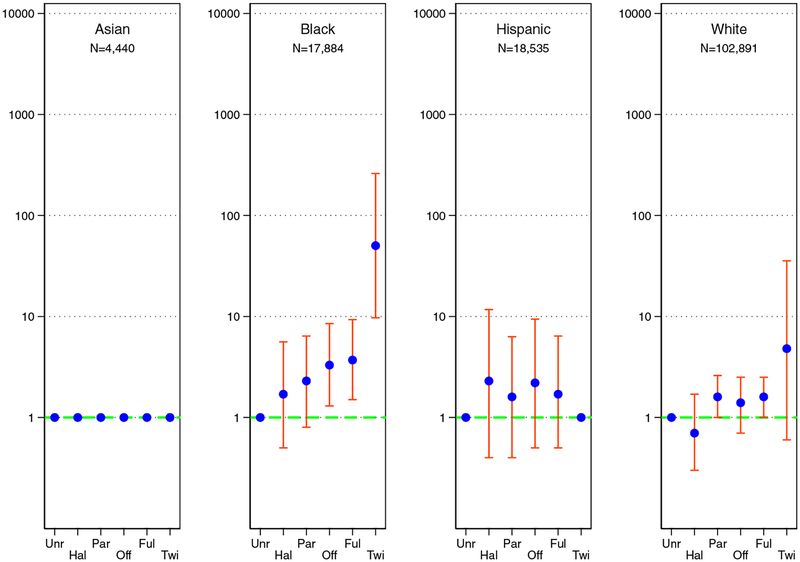
Age- and sex-adjusted cause-specific hazards regression were similar. For black donors, risks compared with unrelated donors were elevated 50.3-fold (95%CI, 9.7–260.2) for identical twins, 3.7-fold (95%CI, 1.5–9.3) for full-siblings, 3.3-fold (95%CI, 1.3–8.6) for offspring, 2.3-fold (95%CI, 0.8–6.5) for parents, and 1.7-fold (95%CI, 0.5–5.6) for half-siblings or other relative. For Hispanic donors, risks compared with unrelated donors were undefined for identical twins, elevated 1.7-fold (95%CI, 0.5–6.4) for full-siblings, 2.2-fold (95%CI, 0.5–9.5) for offspring, 1.6-fold (95%CI, 0.4–6.3) greater risk for parents, and 2.3-fold (95%CI, 0.4–11.7) for half-siblings or other relative. For white donors, risks compared with unrelated donors were elevated 4.8-fold (95%CI, 0.6–35.6) greater risk for identical twins, 1.6-fold (95%CI, 1.0–2.5) for full-siblings, 1.4-fold (95% CI, 0.7–2.5) for offspring, 1.6-fold (95%CI 1.0–2.6) for parents, and 0.7-fold (95%CI, 0.3–1.7) risk for half-siblings or other relative. There were insufficient data to estimate age- and sex-adjusted cause-specific hazards for Asian donors (Figure 3).
Figure 3. Age- and sex-adjusted hazard ratios of ESKD.
Donor-recipient relationship and risk of ESKD in live kidney donors of varied racial groups. The blue dot is the age- and sex-adjusted hazard ratio comparing a biologically related donor group with unrelated donors; the orange bar is the 95% confidence interval of the adjusted hazard ratio. The hazard ratios could not be estimated for Asian donors due to small sample size. Key: biologically unrelated (Unr), half-sibling or other biological relative (Hal), parent (Par), offspring (Off), full-sibling (Ful), and Identical Twin (Twi)
DISCUSSION
In this national study of donor-recipient relationship and risk of ESKD in live kidney donors of varied racial groups, we observed that risks varied by several orders of magnitude across donor-recipient relationship and racial group. When compared with biologically unrelated donors, the risk of ESKD in Asian related donors was graded: 259-fold greater for identical twins, 4.7-fold for full-siblings, 3.5-fold for offspring, 1.0-fold for parents, and 1.0-fold for half-sibling or other biological relative. Similarly, there was graded risk for related donors of black race: 22.5-fold greater for identical twins, 4.1-fold for full-siblings, 2.7-fold for offspring, 3.1-fold for parents, and 1.3-fold for half-siblings or other relatives. Risk was undefined for Hispanic twins, and greater by 1.4-fold for full-siblings, 1.5-fold for offspring, 1.5-fold for parents, and 1-fold for half-siblings or other biological relatives. The magnitude of risk for white related donors was 3.5-fold greater risk for identical twins, 2.0-fold for full-siblings, 1.4-fold for offspring, 2.9-fold for parents, and 0.8-fold for half-siblings or other biological relatives. Such variation in magnitude of risk greatly exceeds what is documented for well-established risk factors such as age, sex, hypertension, diabetes, urine albumin, eGFR, obesity, and smoking, especially among otherwise healthy, screened persons.12
Previous large studies (N≥100,000) that quantified the risk of ESKD for individuals with a known family history of ESKD in donor and nondonor populations reported a single measure of risk of modest magnitude (1.7-fold among donors and 1.4-fold among nondonors).16, 29 Consistent with these two studies, another report did not account for gradients of biological relationships or for variations in risk by race or ancestry.30 Our findings in this study suggest that the implications of a family history of ESKD are both race- and relationship-specific: the magnitude of risk observed in identical twin donors of Asian race (259-fold) might be approximately 12 times that observed in black identical twin donors (22-fold); this in turn might be 6 times what is observed in white identical twins (3.5-fold); in fact, the measure of risk for white identical twins did not reach statistical significance despite the white population being 23 times the size of the Asian donor population and 6 times the size of the black donor population. Because of our race- and relationship-specific approach to risk of ESKD in donors, we have uncovered a new observation regarding risks among Asian living kidney donors.
A similar observation to ours was recently reported in a population-based study of all 23,422,955 persons registered in the Taiwan National Health Insurance Research Database in 2013.25 In this national study including participants of predominantly Han Chinese ancestry, there were 47%, 57%, 47%, and 1.5% who had a known parent, offspring, sibling, or twin, respectively; 87,849 had a diagnosis of ESKD. The relative risk of ESKD was 387 (95%CI, 148–1,016) for female identical twins and 85 (95%CI, 38–189) for male identical twins; this yields an unweighted average relative risk of 235 for identical twins, close to our estimate of 259. The relative risk for full-siblings in the Taiwanese study was 4.9 and, as such, very comparable to our estimate of 4.7. Taken together, the graded strength of the association in our US national study and in the Taiwanese national study points to genetic determinants of ESKD risk that are specific to persons of Han Chinese ancestry (95% of Taiwan, 92% of China, and 17% of the global population).25 This proposition is analogous to inferences from emerging literature on genetic determinants of risk in persons with West African ancestry. The prevalence of high-risk APOL1 variants is 24–45% among the Yoruba in Nigeria, 13–40% among the Asante in Ghana, and 13–22% among African Americans.31 Persons with two high-risk variants are at very high risk of focal segmental glomerulosclerosis and rapid progression to ESKD.19, 22
A key strength of our study is that it yields several inferences with direct relevance to clinical science and practice. Our findings may motivate efforts to identify high-risk alleles in Asian live kidney donors with Han Chinese ancestry.25, 32 The findings indirectly reaffirm the observation that relatives of African Americans with non-diabetic ESKD are enriched for high-risk variants of APOL1.18 Our study implies that ESKD risk calculators, including those that have been endorsed by the 2017 KDIGO clinical practice guideline on the evaluation and care of living kidney donors,13, 14 should be used with caution when evaluating non-white donor candidates. The Grams et al. calculator does not account for family history of ESKD;12 the Massie et al. calculator does not account for the substantial variation in risk by race and specific relationship to the recipient;16 and the Ibrahim et al. calculator – which unlike the others provides risk estimates for a composite of earlier stages of CKD and ESKD – was trained on data from a predominantly white donor population.33
Some limitations of our study are worth noting. First, although we describe risk of ESKD across donor-recipient relationship and in varied racial groups, our study has no information on family members other than the recipient. This leads us to misclassify some unrelated donors who might have family members – other than the recipient – with ESKD. For this reason, we may actually underestimate the magnitude of risk of ESKD for biologically related donors since our inferences are potentially biased towards the null (some high-risk donors with a family history of ESKD are included in the reference group). Our inferences ideally target the potential donor with a family history of ESKD even when they are unrelated to the intended recipient. Second, ESKD is a very rare outcome among healthy, screened donors and for this reason we lacked sufficient statistical power to investigate the risk in various racial and ethnic groups. For the specific case of Hispanic donors (n=18,535), we had a comparable size to the black donor population (n=17,884), which 4 times the size of the Asian donor population (n=4,440), but we still did not find any evidence of a risk gradient. This may partly reflect the fact that a majority of donors of Hispanic ethnicity are white, by far the largest subgroup but with a muted risk of ESKD (n=102,890). Third, as a registry-based observational study, we were unable to define the genes underlying the donor-recipient risks we have observed. While APOL1 high-risk variants are a plausible candidate for what we observed in black donors, the sickle-cell trait is just as common in African Americans and the effect-size with regard to ESKD risk is of a comparable magnitude.34
Fourth, we were not able to adjust for well-established confounders other than age and sex. In other words, we were not able to adjust for diabetes, hypertension, urine albumin, eGFR, obesity, and smoking. Since some of these risk factors are associated with lifestyle and environmental exposures, they might contribute to risk more in biologically related donors (who share both genes and other exposures) than in unrelated donors. Granted, the observed variation in risk across donor race and relationship is much greater in magnitude than what is documented for these well-established risk factors and so our findings are unlikely to be explained solely by unmeasured confounding. Fifth, non-biological familial factors including psychosocial factors, income, level of education, environment, and other epigenetic factors may contribute to the risk of ESKD in ways we did not explore – beyond the issue of confounding outlined above. That said, our examination of donor-recipient concordance of cause of ESKD yielded slight, but graded, agreement across groups that potentially shared genetic (biologically related), lifestyle (spouse or lifetime partner), or did not share genetic or lifestyle risk factors (nonspousal biologically unrelated). This provides cursory evidence of the influence of environment on the risk of cause-specific ESKD. Again, this might partly explain our inferences regarding risk of ESKD in biologically related donors. It might also explain the 25-year eGFR trajectories reported for donors with (vs. without) a first-degree relative with ESKD.35
In conclusion, substantial variations in risks of ESKD were observed across race and donor-recipient relationship. Whether counseling and screening guidelines for donor candidates should reflect these race- and relationship-specific risks is debatable. But our findings point to signals that warrant further validation with more robust data.
Supplementary Material
Table S1. Frequency of observed donor-recipient pair cause of ESKD agreement.
Table S2. Kappa statistics for biologically related, spousal, nonspousal unrelated.
Acknowledgements:
Scientific Registry of Transplant Registry staff performed all Social Security number linkages to Social Security Death Master File and Centers for Medicare & Medicaid Services data to ensure confidentiality of Social Security number data provided to the OPTN.
Support: This study was supported by grant K01DK101677 (awarded to Dr Massie), K01DK114388 (awarded to Dr Henderson), K01HS024600 (awarded to Dr Purnell), F32DK109662 & an American College of Surgeons Resident Research Scholarship (awarded to Dr Holscher), K23DK103918 and R01DK113980 (awarded to Dr. Locke), R01DK096008 (awarded to Drs Lentine and Segev), and K24DK101828 (awarded to Dr Segev). Dr. Snyder is supported under the SRTR contract HHSH250201500009C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: Disclaimer: The analyses described herein are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
REFERENCES
- 1.Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc. 1956;160(4): 277–282. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE. Landmark perspective: The landmark identical twin case. Jama. 1984;251(19): 2572–2573. [PMC free article] [PubMed] [Google Scholar]
- 3.Murray JE. The Nobel Lectures in Immunology. The Nobel Prize for Physiology or Medicine, 1990. The first successful organ transplants in man. Scandinavian journal of immunology. 1994;39(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Hou S, Bush HL, Jr. Kidney transplantation from unrelated living donors. Time to reclaim a discarded opportunity. The New England journal of medicine. 1986;314(14): 914–916. [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB, Devuyst O, Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88(1): 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong AC, Devuyst O, Knebelmann B, Walz G, Diseases E-EWGfIK. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981): 1993–2002. [DOI] [PubMed] [Google Scholar]
- 7.Simms RJ, Travis DL, Durkie M, Wilson G, Dalton A, Ong AC. Genetic testing in the assessment of living related kidney donors at risk of autosomal dominant polycystic kidney disease. Transplantation. 2015;99(5): 1023–1029. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Ravenscraft M, Ramos EL, Gaston RS, Bia MJ, Danovitch GM. The evaluation of living renal transplant donors: clinical practice guidelines. Ad Hoc Clinical Practice Guidelines Subcommittee of the Patient Care and Education Committee of the American Society of Transplant Physicians. Journal of the American Society of Nephrology: JASN. 1996;7(11): 2288–2313. [DOI] [PubMed] [Google Scholar]
- 9.Delmonico F, Council of the Transplantation S. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl): S53–66. [PubMed] [Google Scholar]
- 10.Mandelbrot DA, Pavlakis M, Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(10): 2333–2343. [DOI] [PubMed] [Google Scholar]
- 11.Poggio ED, Braun WE, Davis C. The science of Stewardship: due diligence for kidney donors and kidney function in living kidney donation--evaluation, determinants, and implications for outcomes. Clinical journal of the American Society of Nephrology: CJASN. 2009;4(10): 1677–1684. [DOI] [PubMed] [Google Scholar]
- 12.Grams ME, Sang Y, Levey AS, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med. 2016;374(5): 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lentine KL, Kasiske BL, Levey AS, et al. Summary of Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8): 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8S Suppl 1): S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6): 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massie AB, Muzaale AD, Luo X, et al. Quantifying Postdonation Risk of ESRD in Living Kidney Donors. J Am Soc Nephrol. 2017;28(9): 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011;92(7): 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman BI, Langefeld CD, Turner J, et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int. 2012;82(7): 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofman T, Audard V, Narjoz C, et al. APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis. 2014;63(5): 816–819. [DOI] [PubMed] [Google Scholar]
- 20.Riella LV, Sheridan AM. Testing for High-Risk APOL1 Alleles in Potential Living Kidney Donors. Am J Kidney Dis. 2015;66(3): 396–401. [DOI] [PubMed] [Google Scholar]
- 21.Ross LF, Thistlethwaite JR Jr. Introducing Genetic Tests With Uncertain Implications in Living Donor Kidney Transplantation: ApoL1 as a Case Study. Prog Transplant. 2016;26(3): 203–206. [DOI] [PubMed] [Google Scholar]
- 22.Zwang NA, Shetty A, Sustento-Reodica N, et al. APOL1-Associated End-Stage Renal Disease in a Living Kidney Transplant Donor. Am J Transplant. 2016;16(12): 3568–3572. [DOI] [PubMed] [Google Scholar]
- 23.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390(10105): 1888–1917. [DOI] [PubMed] [Google Scholar]
- 24.Thomas CP, Mansilla MA, Sompallae R, et al. Screening of Living Kidney Donors for Genetic Diseases Using a Comprehensive Genetic Testing Strategy. Am J Transplant. 2017;17(2): 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HH, Kuo CF, Li IJ, et al. Family Aggregation and Heritability of ESRD in Taiwan: A Population-Based Study. Am J Kidney Dis. 2017;70(5): 619–626. [DOI] [PubMed] [Google Scholar]
- 26.Persu A, Duyme M, Pirson Y, et al. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004;66(6): 2132–2136. [DOI] [PubMed] [Google Scholar]
- 27.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 2): 1228–1242. [DOI] [PubMed] [Google Scholar]
- 28.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8): 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4): 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wainright JL, Robinson AM, Wilk AR, Klassen DK, Cherikh WS, Stewart DE. Risk of ESRD in Prior Living Kidney Donors. Am J Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Advances in chronic kidney disease. 2014;21(5): 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limou S, Vince N, Parsa A. Lessons from CKD-Related Genetic Association Studies-Moving Forward. Clin J Am Soc Nephrol. 2018;13(1): 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim HN, Foley RN, Reule SA, et al. Renal Function Profile in White Kidney Donors: The First 4 Decades. J Am Soc Nephrol. 2016;27(9): 2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik RP, Irvin MR, Judd S, et al. Sickle Cell Trait and the Risk of ESRD in Blacks. J Am Soc Nephrol. 2017;28(7): 2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matas AJ, Vock DM, Ibrahim HN. GFR </=25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant. 2018;18(3): 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Frequency of observed donor-recipient pair cause of ESKD agreement.
Table S2. Kappa statistics for biologically related, spousal, nonspousal unrelated.