Abstract
Objective
Early life physical activity may help prevent obesity, but objective quantification in infants is challenging.
Methods
A total of 506 infants were examined from 2013 to 2016. Infants wore accelerometers for 4 days at ages 3, 6, 9, and 12 months. Daily log‐transformed physical activity counts were computed, averaged, and standardized across assessments. A linear mixed model was used to examine trends in standardized physical activity counts as well as associations between physical activity and BMI z score, sum of subscapular and triceps skinfold thickness for overall adiposity (SS+TR), and their ratio for central adiposity (SS:TR).
Results
Among infants, 66% were black and 50% were female. For each additional visit, standardized physical activity counts increased by 0.23 (CI: 0.18 to 0.27; P < 0.0001). This translates to 126.3 unadjusted physical activity counts or a 4% increase for each visit beyond 3 months. In addition, a 1‐SD increase in standardized physical activity counts (550 unadjusted physical activity counts) was associated with a 0.01‐mm lower SS:TR (95% CI: −0.02 to −0.001; P = 0.03). However, standardized physical activity counts were not associated with BMI z score or SS+TR.
Conclusions
Physical activity increased over infancy and was associated with central adiposity. Despite limitations, researchers should consider objective measurement in infants.
Study Importance.
What is already known?
-
►
Early life physical activity is difficult to both assess and quantify, and few researchers have used accelerometry because of inherent measurement challenges.
-
►
One prior study assessed energy expenditure in infancy and observed associations with later BMI.
What does this study add?
-
►
It is feasible to monitor and objectively quantify physical activity in infants using ankle‐worn accelerometers.
-
►
Physical activity measured objectively for several days via ankle‐worn accelerometers increases significantly over the course of infancy.
-
►
Higher levels of physical activity in infancy are associated with lower central adiposity.
How might these results change the direction of research?
-
►
This study may encourage other researchers to measure and quantify physical activity in infancy in order to assess its potential associations with later obesity.
Introduction
Childhood obesity is a significant public health problem, even for very young children. Nearly 10% of children younger than 2 years have overweight or obesity, which represents an increase in this age group in recent years 1. Early life obesity and excessive weight gain have been associated with adverse health conditions later in childhood, including higher blood pressure, wheezing, and hospitalizations 2, 3. Furthermore, heavier infants and infants who gain weight rapidly are more likely than other infants to have obesity later in childhood 4, 5. The number of adipocytes, which is a determinant of fat mass in adults, appears to be set very early in life, emphasizing the importance of obesity prevention in infancy 6. Obesity also disproportionally affects children in racial and ethnic minorities. Rates of obesity have increased more rapidly among Native American, African American, and Latino/a children than among whites, and these disparities are evident early in life 7, 8.
Infancy appears to be a sensitive and even critical period within the life course 9, and researchers are beginning to explore biological and behavioral factors that may impact obesity during this time. Early life physical activity may help prevent obesity later in childhood, but it is difficult to both measure and quantify, especially in infants. However, based on early evidence, promoting opportunities for movement and decreasing sedentary time may be the most effective way to increase physical activity in infants 10, 11, 12, 13. Infants may not be engaging in adequate physical activity. A study of 455 infants in Australia found that most were not sufficiently active 14. Furthermore, a study of 90 young children in Canada, including some infants, found that the majority were sedentary during waking hours 15. However, these prior studies relied exclusively on parent report of infant physical activity, which may not be accurate.
Accelerometers have been used to objectively measure physical activity in toddlers 16, 17, 18, 19 but very rarely in infants 13, 20. An added complexity is that infant movement may be generated and controlled by outside forces (e.g., the mother may be carrying the infant, causing movement but not energy expenditure). As a result, accelerometer measurement may target overall movement, which is likely a combination of movement of the infant and movement induced by outside forces. Consequently, most researchers have either avoided more objective physical activity measurement in infants or approached it with caution 21. Despite limitations, researchers have started to collect accelerometer data in infants in a handful of studies. A 2016 review identified six studies that measured physical activity via accelerometers, with sample sizes ranging from 1 to 31 infants 20. A more recent study using accelerometers in approximately 150 South African infants and toddlers found that boys were more active than girls 22 and that maternal and child physical activity levels were highly correlated 13. However, these findings were limited by relatively small sample sizes and cross‐sectional designs. The purpose of this study was to assess accelerometer‐measured physical activity over the course of infancy and quantify the associations between physical activity and adiposity in a longitudinal context (repeated measures of both) over four equally spaced visits in the first year of life in a sample of racially diverse infants.
Methods
Study design and population
Participants were from the Nurture study, a birth cohort of 666 women and their infants residing in the southeastern United States 23. Between 2013 and 2016, we enrolled women in pregnancy, reconsented them shortly after the birth of their infants, and conducted home visits when infants were 3, 6, 9, and 12 months of age. One goal of the Nurture study was to identify factors related to physical activity that contributed to excessive weight gain in infancy. Additional information about the Nurture study is available elsewhere 23.
Briefly, we recruited women from a private prenatal clinic and a local county health department prenatal clinic. To participate in the Nurture study, women were required to be at 20 to 36 weeks gestation, have a singleton pregnancy with no known congenital abnormalities, be at least 18 years old, speak and read English, intend to keep their infants, and plan to live in the area for at least 12 months. At birth, we further excluded infants who were born prior to 37 weeks gestation, had congenital abnormalities that could affect development as determined by the study pediatrician, were in the hospital for 3 or more weeks after birth, or were not able to take food by mouth at hospital discharge. Women provided written informed consent for themselves and their infants. We provided women with gift cards and other items appropriate for infants (e.g., bath towel, blanket) for participating in the study. The Duke University Medical Center Institutional Review Board approved this study and its protocol.
Exposure: infant physical activity
Infants wore an wActiSleep+ monitor (ActiGraph, Pensacola, Florida) continuously on the right ankle for 4 days (2 weekdays and 2 weekend days) at 3, 6, 9, and 12 months. We instructed mothers to keep accelerometers on infants at all times, including when infants were sleeping and bathing. The device records triaxial acceleration in 30 Hz, and it is water resistant. We downloaded the raw data and then converted it to counts using ActiLife v6.13.4 software. Thus, we utilized the vector magnitude of activity counts across three axes. We defined a valid day as having at least 10 hours of monitor wear time and included infants who wore the device for all 4 days. We aggregated 30 Hz acceleration time series data into minute‐by‐minute activity counts from 7 am and 7 pm to assess daytime physical activity only. We classified accelerometer data with more than 60 minutes of consecutive zeros as nonwear time 24. However, to remove the device, mothers had to cut the plastic band used to attach it to the ankle. We gave mothers a replacement band in the rare event that the device needed to removed and reattached. Thus, we had few instances of nonwear time during the 4‐day assessment period. To reduce skewness in the data and be consistent with prior studies, we log‐transformed minute‐level activity counts by applying the transformation log(1 + activity counts) 25. We summed the log‐transformed activity counts for each minute to obtain daily activity counts, a measure of total volume of physical activity per day. Next, we averaged daily physical activity counts across all 4 days for each infant at 3, 6, 9, and 12 months. We also took the SD of daily activity counts across these days to reflect the day‐to‐day variability of daytime activity. We used the SD as an adjustment variable in analyses examining physical activity over infancy. Finally, we standardized the average physical activity counts to improve the interpretability of results. More specifically, we centered average log activity count sums at the mean and scaled them with the SD at the 3‐month assessment. For brevity, we refer to the results as standardized physical activity counts.
Outcome: infant adiposity
Trained research assistants measured infant weight and length in triplicate during each home visit when infants were 3, 6, 9, and 12 months old and used an average of the three measurements. We measured infant length without shoes using a ShorrBoard portable length board (Weight and Measure, LLC, Olney, Maryland) to the nearest 1/8 inch. We measured weight with infants in light clothing without shoes using a Seca infant scale (Seca, Chino, California) to the nearest 0.1 lb. Infant scales were professionally calibrated every 6 months throughout the duration of the study. We then calculated BMI z scores using World Health Organization age‐ and sex‐specific reference data 26. We also measured subscapular (SS) and tricep (TR) skinfold thickness in triplicate to the nearest 0.2 mm by using standard techniques 27. We calculated the sum of SS and TR (SS+TR) as a marker for overall adiposity and their ratio (SS:TR) for central adiposity, consistent with our work and other prior studies of adiposity in young children 28, 29, 30.
Other measures
We collected additional information from mothers via interviews and questionnaires at birth and during each home visit. Maternal variables included education (high school graduate or less or some college or more) and prepregnancy BMI as a continuous variable. Infant variables included race (black, white, other race, or more than one race), gender, and birth weight for gestational age as a continuous z score calculated using Intergrowth‐21st newborn birth weight standards and z scores 31. We also included breastfeeding (months of any breastfeeding) and annual household income at 3 months (< $20,000 or ≥ $20,000).
Analysis
We computed means and SD for continuous variable frequencies and percentages for categorical variables. We compared participants who were excluded with those we included in analyses. Next, we averaged (unadjusted) daily physical activity counts across all accelerometer assessment days for infants and calculated the SD of daily activity counts at 3, 6, 9, and 12 months. We calculated hourly unadjusted mean (nonstandardized) physical activity counts, averaged across all four assessment days at each visit. We also computed total unadjusted nonstandardized and standardized physical activity counts, averaged over hours and days at each visit.
We then conducted two adjusted analyses. First, we used a linear mixed‐effects model to examine trends over time (3, 6, 9, and 12 months) in standardized physical activity counts. Specifically, let be the standardized physical activity counts of the th infant during the th assessment () and be a matrix of adjusting variables. Model 1 took the following form:
which included both fixed and random intercepts and time slopes. Our parameter of interest was , which represents the “average” rate of change for at each visit. The adjusting variables were identified a priori and included the SD of daily physical activity counts within each visit, maternal education and prepregnancy BMI, infant race, gender, and birth weight for gestational age z score, breastfeeding duration, and household income.
Second, we fit three distinct linear mixed‐effects models with standardized physical activity counts as the exposure of interest and BMI z score, SS+TR, and SS:TR as separate adiposity outcomes. Let denote one of the three adiposity outcomes for infant at visit , let denote the corresponding standardized physical activity counts at visit , and let be the matrix of adjusting variables. Model 2 is expressed as follows:
which included both fixed and random intercepts and time slopes. Our parameter of interest was , which represents the average change in adiposity at visit when standardized physical activity counts increased by one unit. This model estimates the association between exposure and outcome, both measured at each 3‐month visit, taking into account the correlation among repeated measures for each infant. We adjusted for the same covariates noted earlier, and because we were interested in fat distribution after controlling for overall body size, we further adjusted for BMI z score in our analyses with SS:TR. This approach is consistent with our prior studies 28, 29. We present results in terms of means, SD, parameter estimates, 95% CI, and two‐sided P values. We conducted all analyses using R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and a significance level of α = 0.05.
Results
Of the 666 infants enrolled at birth, 506 had complete exposure, outcome, and covariate data. When we compared the 160 infants that we excluded with the 506 we included, we observed a few differences. Infants in the excluded sample were breastfed for fewer weeks than those in the analytic sample (9.6 [14.6] weeks vs. 16.3 [18.9] weeks; P < 0.001). In addition, the excluded sample had more black infants than the analytic sample (76% vs. 66%; P = 0.06). Among mothers, the excluded sample was slightly younger than the analytic sample (26.3 [5.8] years vs. 27.6 [5.7] years; P = 0.02) and had lower prepregnancy BMI (28.4 [9.6] vs. 30.3 [9.2]; P = 0.02). We did not observe any other significant differences between the two groups.
Among the 506 infants included, 66% were black, 16% were white, and 18% were other race or multiple races (Table 1). A total of 50% of the infants were female, and 10% were Hispanic or Latino/a. Infants were breastfed for an average of 16.3 (SD 18.9) weeks. Among mothers, 47% had a high school diploma or less. Just more than half (55%) of the infants lived in households with incomes of < $20,000 per year.
Table 1.
Characteristics of infants and mothers participating in the Nurture study (n = 506), 2013 to 2016
| Infant characteristics | Mean (SD) |
| Birth weight for gestational age z score | 0.1 (1.0) |
| BMI z score at 3 months | 0.0 (1.1) |
| BMI z score at 6 months | 0.3 (1.1) |
| BMI z score at 9 months | 0.5 (1.0) |
| BMI z score at 12 months | 0.7 (1.0) |
| Any breastfeeding, wk | 16.3 (18.9) |
| Percent (n) | |
| Gender, female | 50 (251) |
| Race | |
| Black | 66 (335) |
| White | 16 (81) |
| Other race/more than one race | 18 (90) |
| Ethnicity, Hispanic or Latino/a | 10 (49) |
| Overweight/obesity (z score at 1 SD or above) at 12 months | 30 (152) |
| Obesity (z score at 2 SD or above) at 12 months | 8 (40) |
| Maternal characteristics | Mean (SD) |
| Age, y | 27.6 (5.7) |
| Prepregnancy BMI, kg/m2 | 30.3 (9.2) |
| Percent (n) | |
| Race | |
| Black | 70 (352) |
| White | 20 (101) |
| Other race/more than one race | 10 (53) |
| Ethnicity, Latina | 7 (33) |
| Education | |
| ≤ High school | 47 (236) |
| Some college/college graduate/graduate degree | 53 (270) |
| Annual household income | |
| < $20,000 | 55 (278) |
| ≥ $20,000 | 45 (228) |
Infants wore the device for a mean (SD) of 23.4 (2.2) hours per day at 3 months, 23.2 (2.5) at 6 months, 23.2 (2.6) at 9 months, and 23.1 (2.7) at 12 months. In Figure 1, we present hourly unadjusted mean physical activity counts, averaged across assessment days at each visit. Starting at about 9 am, infants at each visit (i.e., different ages) began to show separate mean physical activity count patterns. Furthermore, the mean (SD) for unadjusted original physical activity counts was 3,066.4 (549.3) at 3 months, 3,237.4 (586.2) at 6 months, 3,381.1 (652.7) at 9 months, and 3,435.9 (651.4) at 12 months. Similarly, in Figure 2, we present both unadjusted nonstandardized and standardized physical activity counts, averaged over hours and days at each visit.
Figure 1.
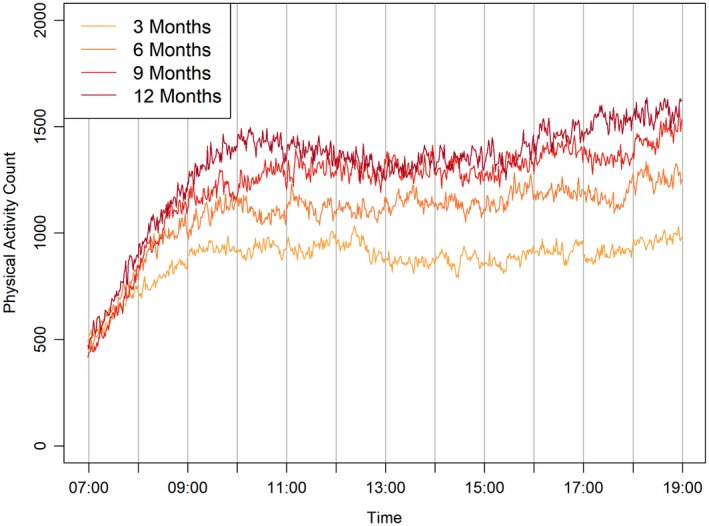
Mean nonstandardized physical activity counts, averaged over a given day, for infants at ages 3, 6, 9, and 12 months.
Figure 2.
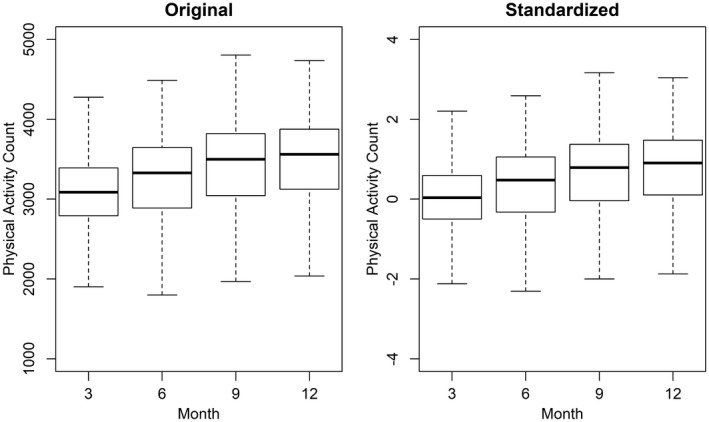
Original and standardized physical activity counts for infants, averaged over each visit at ages 3, 6, 9, and 12 months.
In adjusted analyses based on model 1, we found that for each additional visit, standardized physical activity counts increased by 0.23 (95% CI: 0.18 to 0.27; P < 0.0001). This translates to 126.3 unadjusted (nonstandardized) physical activity counts or a more than 4% increase for each additional visit beyond 3 months. Some covariates identified a priori were significant predictors in the model, including infant gender, infant race, and family income. Next, in adjusted analyses based on model 2, we found that a 1‐SD increase in standardized physical activity counts, which translates to about 550 unadjusted physical activity counts, was associated with a 0.01‐mm lower SS:TR (95% CI: −0.02 to −0.001; P = 0.03) (Table 2). However, standardized physical activity counts were not associated with BMI z score (0.04 units; 95% CI: −0.01 to 0.08; P = 0.14) or SS+TR (0.08 mm; 95% CI: −0.09 to 0.24; P = 0.35).
Table 2.
Adjusteda estimates and 95% CI in regression analyses examining standardized physical activity counts and adiposity in infants
| BMI z score | Subscapular + tricep skinfolds | Subscapular:tricep skinfoldsb | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Standardized physical activity counts | 0.04 unit (−0.01 to 0.08) | 0.14 | 0.08 mm (−0.09 to 0.24) | 0.35 | −0.01 mm (−0.02 to −0.001) | 0.03 |
Adjusted for SD of daily physical activity counts within each visit; infant gender, race, birth weight for gestational age z score, and breastfeeding duration; maternal education and prepregnancy BMI; and household income.
Additionally adjusted for BMI z score.
Discussion
In this study of approximately 500 racially diverse infants measured at 3, 6, 9, and 12 months, we found that physical activity, quantified as mean standardized physical activity counts, increased significantly over infancy. To our knowledge, this is the first study to longitudinally assess physical activity in this age group. We also found that higher levels of physical activity in infancy were associated with lower central adiposity but not overall adiposity or BMI z score.
Although the methods were different, one prior study observed similar findings in associations of physical activity and adiposity. Sijtsma et al. 32 examined maternal report of early infant movement in 1,722 infants and observed associations with lower waist circumference at 9 months. In a second study of 23 infants by Wells et al. 33, total energy expenditure measured using doubly labeled water when infants were 9 to 12 months of age was associated with SS+TR skinfolds at 2 years. This study used overall adiposity but did not examine the ratio of skinfold thicknesses for central adiposity. In our study, we did not observe associations between physical activity and BMI or overall adiposity. One possible explanation may be that the effect of physical activity may need to accumulate over early childhood before an association with the other adiposity measures emerges. Moreover, there is debate about the most appropriate measure of adiposity during infancy. Skinfold thickness may be more closely associated with body fat than BMI in infants 34. Conversely, another study found that in infancy, skinfold thickness and BMI were relatively equivalent in their ability to predict later body fat 35.
In our study, we observed associations between physical activity and central adiposity. This is not entirely surprising given that higher levels of physical activity have been associated with lower central adiposity in older children 36. No prior studies, however, have examined physical activity and adiposity using repeated measures of both the exposure and outcome in infants. Thus, our study is the first to demonstrate an association between higher levels of objectively measured physical activity and lower central adiposity in infancy.
Despite this, our study has several limitations. First, Nurture participants were not fully representative of the population in the southeastern United States, in that the demographic composition of our sample included a higher representation of black infants. However, black participants are underrepresented in most US birth cohorts 37, so this study makes a unique contribution. Second, has been some prior evidence that body fat distribution in children varies by race 38. But, given the composition of our sample, we were also not able to examine differences by race. Third, we did not include other factors that may contribute to adiposity in this analysis, such as dietary intake and sleep. Fourth, as with most birth cohorts in which families are experiencing rapid change because of the birth of an infant, we experienced attrition. Despite this, from birth to the 12‐month visit, we retained over 70% of the Nurture sample. This retention rate is higher than that of a similar birth cohort from the same geographic region, in which only 56% of women completed the 12‐month visit 39. Fifth, we did not include 7 days of monitor wear time. However, recent studies have suggested that at least 2, but preferably 3, days of ankle accelerometry are sufficient for measuring physical activity in infants 40, 41. Sixth, we limited our assessment to 7 am to 7 pm (daytime hours). An alternative would be to use physical activity between the waking and bedtime hours for each individual infant. However, methods for estimating waking hours for infants have not been validated. Moreover, a similar approach of restricting accelerometer data to daytime hours has been used in prior studies of physical activity in toddlers 18 and school‐aged children 42.
Finally, as with any study of infant physical activity measured via accelerometers, we were not able to separate movement generated by the infant and adult caregiver. Mothers, for example, may be carrying infants, which will generate acceleration that is currently indistinguishable from the acceleration generated by infants’ movement. This lack of precision is inherent in any study of physical activity in populations of infants who are not yet fully mobile 21. However, we would expect to see increases in actual physical activity as infants age and become more mobile (begin to roll over, crawl, and eventually walk). Our finding that physical activity increased significantly over the course of infancy provides partial validation for our measure. Future studies could, however, assess the extent to which maternal and infant physical activity are correlated. There may also be differences based on responsive parenting that we did not measure. Mothers who were more physically engaged with their infants may have been engaged in behaviors that prevent obesity (e.g., healthy sleep and appropriate dietary intake), independent of physical activity.
However, activity count may not be the most appropriate quantitative summary metric of raw accelerometer data. Indeed, activity counts are proprietary measures that are device, platform, and location specific and are difficult to translate into specific recommendations without activity‐specific validation. Moreover, cut points are not yet available for infants, so we were not able to classify physical activity into sedentary, light, moderate, or vigorous activity, consistent with more conventional approaches. Cut points have been widely used for children ages 3 to 5 years 43, 44 and have recently been used in children as young as 2 years 16, 45, but they have not have been developed for younger children. Despite this, in a study of 2‐ to 3‐year‐old children, researchers compared three existing cut points for preschool‐aged children and found they all substantially overestimated moderate to vigorous physical activity 45. In a second study of 2‐year‐olds, researchers found that cut points for preschool‐aged children overestimated sedentary time and underestimated light physical activity 16. A third study of children aged 2 years found that when compared with direct observation, cut points exhibited poor to fair classification of sedentary time, light physical activity, and moderate to vigorous physical activity 17. Instead, the researchers found that activity count was most associated with direct observation 17.
There is some evidence that activity count, the metric we used in this study, is less sensitive to sedentary and light‐intensity physical activity compared with moderate and vigorous physical activity 46. There are, however, alternative summary metrics that can be derived from raw accelerometer data. Future studies should compare Euclidean norm minus one, mean amplitude deviation, vector magnitude, and activity index to activity count. Mean amplitude deviation and activity index are all systematic ways to extract potentially meaningful information about physical activity from the raw, dense sample multi‐axis accelerometer data. One prior study of infants and toddlers in South Africa used vector magnitude, which is expressed in Earth gravitational units 22. We recommend future research explore algorithms using machine learning. However, activity count is the default metric provided by Actigraph, which is a natural first step for data analysis.
Conclusion
This analysis addressed the compelling and important issue of early life physical activity. We found that infants increased their physical activity from 3 to 12 months of age, and higher physical activity during this time was associated with lower central adiposity. However, larger studies with longer follow‐up periods are needed to assess the sustained impact of infant physical activity. There is, however, a critical need to measure and quantify physical activity as accurately as possible in infancy in order to assess its potential associations with later obesity. In the absence of such knowledge, the development of effective intervention strategies to promote early life physical activity and prevent the development of obesity will likely remain problematic. Given our findings, interventions in young children may consider targeting physical activity as a potential early life contributor to obesity.
Funding agencies
This study was supported by a grant from the National Institutes of Health (R01DK094841). The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declared no conflicts of interest.
Clinical trial registration
https://www.ClinicalTrials.gov identifier NCT01788644.
Acknowledgments
The authors would like to thank the women and infants who participated in the Nurture study.
References
- 1. Fryar CD, Carrol MD, Ogden CL. Prevalence of high weight‐for‐recumbent length among infants and toddlers from birth to 24 months of age: United States, 1971‐1974 through 2015‐2016. Health E-Stats. Hyattsville, MD: National Center for Health Statistics; 2018. [Google Scholar]
- 2. Belfort MB, Rifas‐Shiman SL, Rich‐Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr 2007;151:670‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shibli R, Rubin L, Akons H, Shaoul R. Morbidity of overweight (>or=85th percentile) in the first 2 years of life. Pediatrics 2008;122:267‐272. [DOI] [PubMed] [Google Scholar]
- 4. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929. doi: 10.1136/bmj.38586.411273.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obes Rev 2005;6:143‐154. [DOI] [PubMed] [Google Scholar]
- 6. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783‐787. [DOI] [PubMed] [Google Scholar]
- 7. Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med 2009;163:344‐348. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta‐regression analysis. Epidemiol Rev 2007;29:6‐28. [DOI] [PubMed] [Google Scholar]
- 9. Kuh D, Ben‐Shlomo Y, eds. A Life Course Approach to Chronic Disease Epidemiology. 2nd ed London: Oxford University Press; 2004. [Google Scholar]
- 10. Wells JC, Cole TJ, Davies PS. Total energy expenditure and body composition in early infancy. Arch Dis Child 1996;75:423‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells JC, Stanley M, Laidlaw AS, Day JM, Davies PS. The relationship between components of infant energy expenditure and childhood body fatness. Int J Obes Relat Metab Disord 1996;20:848‐853. [PubMed] [Google Scholar]
- 12. Li R, O'Connor L, Buckley D, Specker B. Relation of activity levels to body fat in infants 6 to 12 months of age. J Pediatrics 1995;126:353‐357. [DOI] [PubMed] [Google Scholar]
- 13. Prioreschi A, Brage S, Westgate K, Micklesfield LK. Describing the diurnal relationships between objectively measured mother and infant physical activity. Int J Behav Nutr Phys Activ 2018;15:59. doi: 10.1186/s12966-018-0692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hesketh KD, Downing KL, Campbell K, Crawford D, Salmon J, Hnatiuk JA. Proportion of infants meeting the Australian 24‐hour Movement Guidelines for the Early Years: data from the Melbourne InFANT Program. BMC Public Health 2017;17(suppl 5):856. doi: 10.1186/s12889-017-4856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borkhoff CM, Heale LD, Anderson LN, et al. Objectively measured physical activity of young Canadian children using accelerometry. Appl Physiol Nutr Metab 2015;40:1302‐1308. [DOI] [PubMed] [Google Scholar]
- 16. Trost SG, Fees BS, Haar SJ, Murray AD, Crowe LK. Identification and validity of accelerometer cut‐points for toddlers. Obesity (Silver Spring) 2012;20:2317‐2319. [DOI] [PubMed] [Google Scholar]
- 17. Van Cauwenberghe E, Gubbels J, De Bourdeaudhuij I, Cardon G. Feasibility and validity of accelerometer measurements to assess physical activity in toddlers. Int J Behav Nutr Phys Act 2011;8:67. doi: 10.1186/1479-5868-8-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costa S, Barber SE, Cameron N, Clemes SA. The objective measurement of physical activity and sedentary behaviour in 2–3 year olds and their parents: a cross‐sectional feasibility study in the bi‐ethnic Born in Bradford cohort. BMC Public Health 2015;15:1109. doi: 10.1186/s12889-015-2481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson E, Ekelund U, Nero H, Marcus C, Hagstromer M. Calibration and cross‐validation of a wrist‐worn Actigraph in young preschoolers. Pediatr Obes 2015;10:1‐6. [DOI] [PubMed] [Google Scholar]
- 20. Prioreschi A, Micklesfield LK. A scoping review examining physical activity measurement and levels in the first 2 years of life. Child Care Health Dev 2016;42:775‐783. [DOI] [PubMed] [Google Scholar]
- 21. Cliff DP, Reilly JJ, Okely AD. Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0–5 years. J Sci Med Sport 2009;12:557‐567. [DOI] [PubMed] [Google Scholar]
- 22. Prioreschi A, Brage S, Hesketh KD, Hnatiuk J, Westgate K, Micklesfield LK. Describing objectively measured physical activity levels, patterns, and correlates in a cross sectional sample of infants and toddlers from South Africa. Int J Behav Nutr Phys Act 2017;14:176. doi: 10.1186/s12966-017-0633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benjamin Neelon SE, Ostbye T, Bennett GG, et al. Cohort profile for the Nurture Observational Study examining associations of multiple caregivers on infant growth in the Southeastern USA. BMJ Open 2017;7:e013939. doi: 10.1136/bmjopen-2016-013939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181‐188. [DOI] [PubMed] [Google Scholar]
- 25. Varma VR, Dey D, Leroux A, et al. Re‐evaluating the effect of age on physical activity over the lifespan. Prev Med 2017;101:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organizatin . WHO Child Growth Standards: Length/height‐for‐age, Weight‐for‐age, Weight‐for‐length, Weight‐for‐Height, and Body Mass Index‐for‐age: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 27. National Household Survey Capability Programme . How to Weigh and Measure Children: Assessing the Nutritional Status of Young Children in Household Surveys. New York, NY: United Nations; 1986. https://unstats.un.org/unsd/publication/unint/dp_un_int_81_041_6E.pdf. Accessed July 16, 2019. [Google Scholar]
- 28. Benjamin Neelon SE, Oken E, Taveras EM, Rifas‐Shiman SL, Gillman MW. Age of achievement of gross motor milestones in infancy and adiposity at age 3 years. Matern Child Health J 2012;16:1015‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benjamin SE, Rifas‐Shiman SL, Taveras EM, et al. Early child care and adiposity at ages 1 and 3 years. Pediatrics 2009;124:555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oken E, Huh SY, Taveras EM, Rich‐Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res 2005;13:2021‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villar J, Altman DG, Purwar M, et al. The objectives, design and implementation of the INTERGROWTH‐21st Project. BJOG 2013;120(suppl 2):9‐26. [DOI] [PubMed] [Google Scholar]
- 32. Sijtsma A, Sauer PJ, Stolk RP, Corpeleijn E. Infant movement opportunities are related to early growth–GECKO Drenthe cohort. Early Hum Dev 2013;89:457‐461. [DOI] [PubMed] [Google Scholar]
- 33. Wells JC, Ritz P. Physical activity at 9–12 months and fatness at 2 years of age. Am J Hum Biol 2001;13:384‐389. [DOI] [PubMed] [Google Scholar]
- 34. Chen LW, Tint MT, Fortier MV, et al. Which anthropometric measures best reflect neonatal adiposity? Int J Obes (Lond) 2018;42:501‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos S, Gaillard R, Oliveira A, et al. Associations of infant subcutaneous fat mass with total and abdominal fat mass at school‐age: the Generation R Study. Paediatr Perinat Epidemiol 2016;30:511‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ortega FB, Ruiz JR, Sjostrom M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Activity 2007;4:61. doi: 10.1186/1479-5868-4-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konkel L. Racial and ethnic disparities in research studies: the challenge of creating more diverse cohorts. Environ Health Perspect 2015;123:A297‐A302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He Q, Horlick M, Thornton J, et al. Sex and race differences in fat distribution among Asian, African‐American, and Caucasian prepubertal children. J Clin Endocrinol Metab 2002;87:2164‐2170. [DOI] [PubMed] [Google Scholar]
- 39. Hoyo C, Daltveit AK, Iversen E, et al. Erythrocyte folate concentrations, CpG methylation at genomically imprinted domains, and birth weight in a multiethnic newborn cohort. Epigenetics 2014;9:1120‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pitchford EA, Ketcheson LR, Kwon HJ, Ulrich DA. Minimum accelerometer wear time in infants: a generalizability study. J Phys Act Health 2017;14:421‐428. [DOI] [PubMed] [Google Scholar]
- 41. Ricardo LIC, Da Silva ICM, Martins RC et al. Protocol for objective measurement of infants' physical activity using accelerometry. Med Sci Sports Exerc 2018;50:1084‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evenson KR, Wen F, Hales D, Herring AH. National youth sedentary behavior and physical activity daily patterns using latent class analysis applied to accelerometry. Int J Behav Nutr Phys Act 2016;13:55. doi: 10.1186/s12966-016-0382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pate RR, Almeida MJ, McIver KL, Pfeiffer KA, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14:2000‐2006. [DOI] [PubMed] [Google Scholar]
- 44. van Cauwenberghe E, Labarque V, Trost SG, de Bourdeaudhuij I, Cardon G. Calibration and comparison of accelerometer cut points in preschool children. Int J Pediatr Obes 2011;6:e582‐e589. [DOI] [PubMed] [Google Scholar]
- 45. Costa S, Barber SE, Cameron N, Clemes SA. Calibration and validation of the ActiGraph GT3X+ in 2–3 year olds. J Sci Med Sport 2014;17:617‐622. [DOI] [PubMed] [Google Scholar]
- 46. Bai J, Di C, Xiao L, et al. An activity index for raw accelerometry data and its comparison with other activity metrics. PLoS One 2016;11:e0160644. doi: 10.1371/journal.pone.0160644 [DOI] [PMC free article] [PubMed] [Google Scholar]


