Abstract
Purpose:
Optimal radiation therapy (RT) target margins for diffuse intrinsic pontine glioma (DIPG) are unknown. We sought to define disease progression patterns in a contemporary cohort treated with conformal RT using different clinical target volume (CTV) margins.
Methods and Materials:
We reviewed 105 patients with newly diagnosed DIPG treated with conformal conventionally fractionated RT at our institution from 2006 to 2014. CTV margins were classified as standard (1 cm) for 60 patients and extended (2–3 cm) for 45 patients. Survival and cumulative incidence of progression in treatment groups were compared by log-rank and Gray’s tests, respectively. Cox proportional hazard models identified predictors of survival.
Results:
For 97 patients evaluated with magnetic resonance imaging at progression, the cumulative incidences of isolated local, isolated distant, and synchronous disease progression at 1 year were 62.6%, 12.3%, and 7.2%, respectively, and did not differ significantly according to the CTV margin. Central dosimetric progression (Vprogression95% ≥95%) was observed in 80 of 81 evaluable patients. Median progression-free survival and overall survival (OS) were 7.6 months (95% confidence interval, 6.9–8.2) and 11.3 months (95% confidence interval, 10.0–12.8), respectively, and did not differ significantly according to margin status. DIPG survival prediction risk group (standard vs high, P =.02; intermediate vs high, P =.009) and development of distant metastasis (P =.003) were independent predictors of OS. For the 41 patients (39%) with a pathologic diagnosis, H3.3 K27M mutation was associated with shorter OS (hazard ratio [HR], 0.41; P =.02), whereas H3.1 K27M and ACVR1 mutations were associated with longer OS (HR, 3.56; P =.004 and HR, 2.58; P =.04, respectively).
Conclusions:
All patients who experienced local failure showed progression within the high-dose volume, and there was no apparent survival or tumor-control benefit to extending the CTV margins beyond 1 cm. Given the increasing use of reirradiation, standardizing the CTV margin to 1 cm may improve retreatment tolerance.
Summary
There is no consensus on clinical target volume (CTV) recommendations for conformal treatment planning for patients with diffuse intrinsic pontine glioma. We reviewed 105 patients treated with conformal radiation with magnetic resonance imaging guided target volume delineation, 45 of whom were prospectively treated with an extended CTV margin of 2 to 3 cm. Neither survival nor tumor-control outcomes differed significantly according to margin status, suggesting limited utility of extended CTV margins beyond an isotropic 1-cm CTV expansion.
Introduction
Most pediatric brain stem gliomas are of the type referred to as diffuse intrinsic pontine glioma (DIPG). Based on recent molecular profiling of DIPGs of typical appearance,1 the World Health Organization has defined a new pathologic entity, diffuse midline glioma, H3 K27M-mutant, which represents approximately 80% of clinically recognized DIPGs.2 Although significant differences in survival3 and response to treatment4 are observed in patients with DIPG with or without this somatic mutation or with variants thereof, the prognosis remains dismal. Radiation therapy (RT) provides a survival gain of several months5 and neurologic symptom palliation in more than 80% of patients6 but is not curative: the 2-year overall survival (OS) is <10%, and there are virtually no long-term survivors.3
Limited retrospective studies in the 1970s and 1980s found similar outcomes in patients with brain stem glioma when treated with whole-brain RT or more limited approaches.7,8 Consequently, focal irradiation has been the standard for RT field design. However, optimal RT target margins are undefined for DIPG. In the most recently completed cooperative group clinical trials of RT for newly diagnosed DIPG,9,10 the recommended clinical target volume (CTV) was a uniform 1-cm expansion of the tumor volume. Despite the infiltrative growth that defines these tumors and their almost uniform high-grade nature, this “standard” CTV is distinct from the larger CTVs employed in pediatric and adult supratentorial high-grade glioma (HGG), probably based more on toxicity concerns than on recognized anatomic routes of spread.
Based on our preliminary study of 37 patients with DIPG treated with a 1-cm CTV margin, in whom 97% of initial failures occurred within 2 cm of the caudal and transverse margins of the tumor and within 3 cm of its superior borders,11 we employed expanded CTV margins of 3 cm cranially and 2 cm radially and caudally for patients enrolled on 2 recently completed institutional prospective clinical trials of RT for DIPG.12,13 Our objectives were to examine the patterns of disease progression after conformal irradiation in a contemporary series of children with newly diagnosed DIPG treated with a standard CTV (sCTV) or an extended CTV (eCTV) and to assess the effect of these different approaches on time to disease progression and survival.
Methods and Materials
Patients
Between April 2006 and November 2014, 109 pediatric patients were enrolled on our institutional, prospective, phase 1 clinical trials or on individualized treatment plans and received RT for a diagnosis of primary, nonmetastatic, histologically confirmed or suspected diffuse brain stem glioma. Of those patients, 105 underwent focal irradiation with conventional fractionation (1.8 Gy/d) and were evaluable for survival analysis. Ninety-seven patients experienced disease progression, had a brain magnetic resonance imaging (MRI) scan obtained at progression, and were evaluable for radiographic disease progression (CONSORT diagram, Fig. 1). This study was approved by our institutional review board.
Fig. 1.
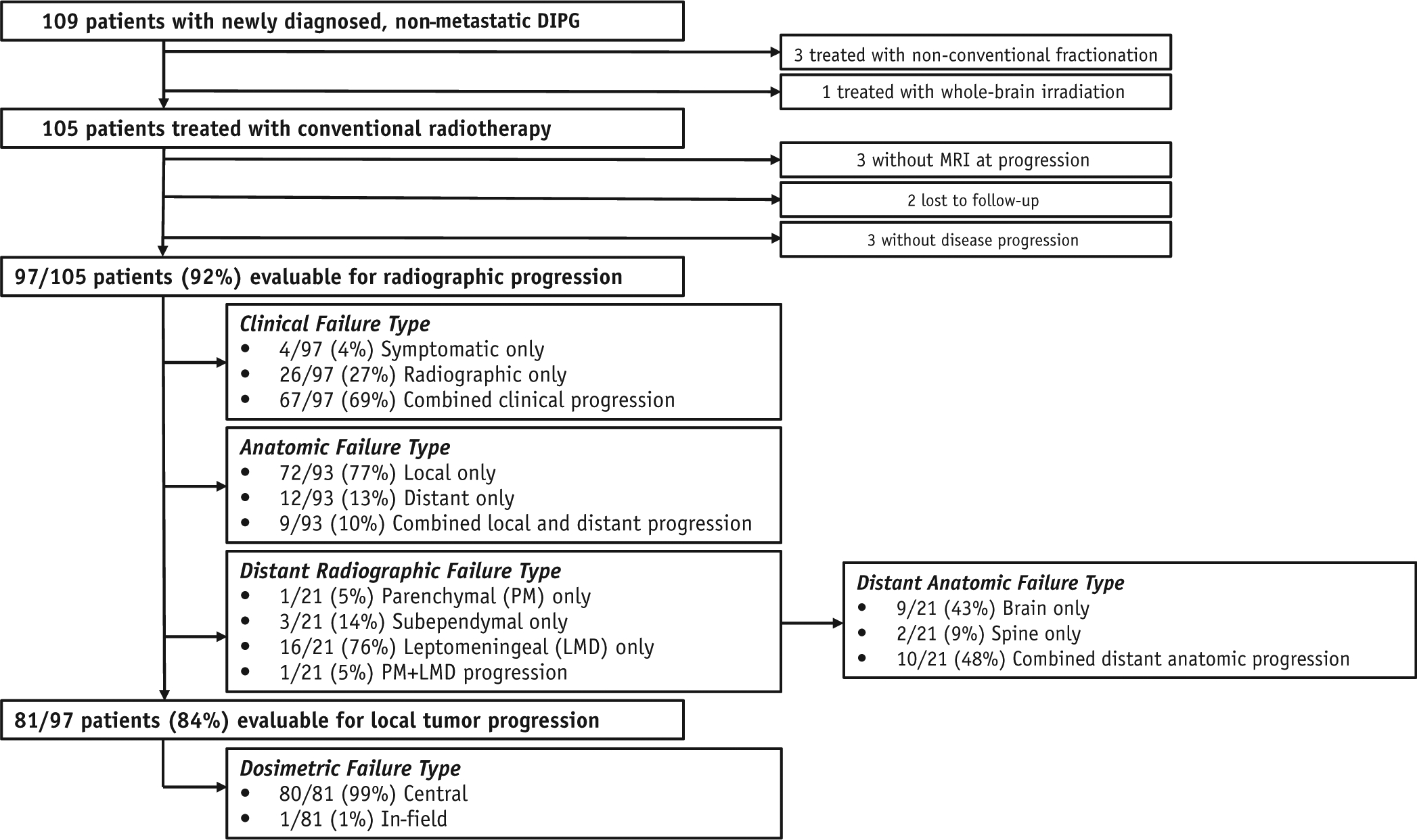
CONSORT diagram of diffuse intrinsic pontine glioma (DIPG) patterns of failure study.
Histology and molecular analysis
Histology and somatic tumor mutation status were previously reported for 25 patients.14 For the remaining 16 patients with evaluable tumor tissue, histologic classification was centrally reviewed (by J.C.H.C.) in accordance with 2016 World Health Organization criteria.2 Assessment of histone H3, ACVR1, and TP53 mutations and polymerase chain reaction primer sequences are detailed in Materials E1 and Table E1 (available online at https://doi.org/10.1016/j.ijrobp.2019.11.020), respectively.
Primary therapy and follow-up
Eighty-nine of the 105 patients (84.7%) were enrolled on 1 of 3 institutional protocols with experimental chemotherapy concurrently and adjuvantly with RT,12,13,15 and 16 were treated with individualized treatment plans that included concurrent and/or adjuvant chemotherapy (9 patients) or RT alone (7 patients). Three-dimensional conformal RT was delivered to 100 patients and intensity modulated RT to 5 patients. Conventional fractionation was delivered to all patients, with a cumulative prescription dose of 54 Gy in all but 2 patients, who were treated to a total dose of 55.8 Gy as a result of treatment interruptions. Target volumes were delineated based on coregistration of computed tomography (CT) and MRI treatment-planning data sets obtained with the patient in the treatment position. An anatomically constrained CTV margin was added to the gross tumor volume (GTV) and consisted of a 1-cm isotropic expansion (sCTV) or a 2-cm (transverse and caudal) and 3-cm (cranial) expansion (eCTV) (Fig. E1, available online at https://doi.org/10.1016/j.ijrobp.2019.11.020). A patient-specific planning target volume margin of 0.3 cm was added to the CTV, with the exception of 2 patients who were treated with a standard CTV and 0.5-cm planning target volume expansions.
A diagnostic brain MRI scan was obtained before the start of (chemo)RT, approximately 4 to 8 weeks after the completion of (chemo)RT, and every 8 to 10 weeks thereafter for the duration of therapy or until disease progression. Radiographic disease progression was defined as a >25% increase in the product of the maximum perpendicular diameters of the tumor lesion or the presence of any new metastatic disease. The date of progression (as determined by clinical or imaging criteria) was coincident with that defined in the protocol for patients enrolled on prospective studies and was determined through chart and imaging review for all others. See Materials E1 (available online at https://doi.org/10.1016/j.ijrobp.2019.11.020) for additional information.
Progression pattern analysis
First disease progression was classified by clinical type, anatomic region, radiographic type, dosimetry, and target margins. Clinical progression was defined as symptomatic (based on worsening neurologic status not explained by causes unrelated to tumor progression or increasing dosage of corticosteroids required to maintain stable neurologic status), radiographic, or both. Anatomic progression was defined as local (within the brain stem and/or cerebellar peduncles/cerebellum), distant (other), or both. Radiographic progression was defined as parenchymal, subependymal (originating in the subependymal region of the ventricular system), leptomeningeal (along the central nervous system surface or in the subarachnoid space), or any combination thereof based on MRI of the brain and/or spine.16
For dosimetric assessment, complete CT data sets of RT treatment plans were transferred to MIM software (MIM Software Inc, Cleveland, OH) and composite radiation dose data were assembled for all patients. MRI scans at progression were coregistered to CT data sets with standard vendor-supplied software, and the anatomic tumor extent at progression was manually delineated based on T2-weighted fluid-attenuated inversion recovery and T1 postcontrast abnormalities on MRI. Dose-volume histograms were calculated for the progression volumes, and progression with respect to radiation dose distribution was categorized as previously done for adults with HGG.17 To estimate anisotropic RT target margins based on the extent of tumor progression after conformal RT, we automatically computed failure distances as previously described.18 See Materials E1 and Figure E2 (available online at https://doi.org/10.1016/j.ijrobp.2019.11.020) for additional information.
Statistical analysis
Comparisons across continuous data were made using the 2-sample t test or the Wilcoxon rank-sum test and the Holm correction for multiple comparisons; a χ2 test or Fisher’s exact test was used to compare categorical data. The cumulative incidences of progression types (local, distant, or both) were estimated using the competing-risks method and compared using Gray’s test.19 Competing risks included developing a different progression type. Patients without documented radiographic progression were censored at the last available imaging date. Probability estimates of progression-free survival (PFS) and OS were calculated by the Kaplan–Meier method and compared using the log-rank test. A Cox proportional hazards model was used to identify imaging and clinicopathologic predictors of PFS and OS distributions. Covariates with significance in any pairwise comparison for the categorical variables at the P <.2 level were considered for inclusion in the multivariable model, and a backward variable selection method with P <.2 was used to eliminate the nonsignificant effects in the variable selection process. Risk estimates, estimated by hazard ratios (HRs) and P values, and 95% confidence intervals (CIs) were reported. A 2-sided significance level of P <.05 was considered statistically significant.
Results
Patient and treatment characteristics
Table 1 summarizes the baseline characteristics of our study population. The median duration of follow-up for all patients was 11.3 months (range, 1.8–120.0 months). Stratification of patients according to their 12-month cumulative risk of death by the DIPG survival prediction model20 classified 18.1% as standard risk, 64.8% as intermediate risk, and 17.1% as high risk. All but 7 patients (6.7%) received concurrent and/or adjuvant systemic therapy, in 90% of cases consisting of therapies directed against receptor tyrosine kinases. An eCTV margin (2–3 cm) was employed in 42.9% of patients, whereas 57.1% of patients were treated with an sCTV margin (1 cm). Except for histologic grade, characteristics did not differ significantly between sCTV- and eCTV-treated patients (Table E2, available online at https://doi.org/10.1016/j.ijrobp.2019.11.020).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | n | Percentage or median (IQR) |
|---|---|---|
| Age at diagnosis (y) | 105 | 6.2 (4.4–9.0) |
| <3 | 13 | 12.4% |
| 3–10 | 75 | 71.4% |
| >10 | 17 | 16.2% |
| Sex | ||
| Male | 49 | 46.7% |
| Female | 56 | 53.3% |
| Race | ||
| Black | 20 | 19.1% |
| White | 75 | 71.4% |
| Other | 10 | 9.5% |
| Symptom duration (mo) | 105 | 1 mo (0.5–2) |
| Ring enhancement at diagnosis | ||
| No | 62 | 59.0% |
| Yes | 43 | 41.0% |
| Radiation therapy | ||
| Total dose, Gy | 105 | 54 (range, 54–55.8) |
| 3DCRT | 100 | 95.2% |
| IMRT | 5 | 4.8% |
| Standard CTV | 60 | 57.1% |
| Extended CTV | 45 | 42.9% |
| Any chemotherapy | ||
| No | 7 | 6.7% |
| Yes | 98 | 93.3% |
| Histology (n = 41) | ||
| Grade II | 3 | 7.3% |
| Grade III | 2 | 4.9% |
| Grade IV | 36 | 87.8% |
| Primary biopsy | 8 | 19.5% |
| Progression Biopsy | 1 | 2.4% |
| Spine metastasis | 1 | 2.4% |
| Autopsy | 33 | 80.5% |
| Biopsy + Autopsy | 2 | 4.9% |
| Molecular | ||
| H3 K27M mutation (n = 35) | ||
| Yes | 31 | 88.6% |
| No | 4 | 11.4% |
| H3F3A mutation (n = 33) | ||
| Yes | 21 | 63.6% |
| No | 12 | 36.4% |
| HIST1H3B mutation (n = 33) | ||
| Yes | 8 | 24.2% |
| No | 25 | 75.8% |
| ACVR1 mutation (n = 33) | ||
| Yes | 7 | 21.2% |
| No | 26 | 78.8% |
| TP53 mutation (n = 35) | ||
| Yes | 15 | 42.9% |
| No | 20 | 57.1% |
| DIPG risk stratification | ||
| Standard | 19 | 18.1% |
| Intermediate | 68 | 64.8% |
| High | 18 | 17.1% |
Abbreviations: 3DCRT = three dimensional conformal radiation therapy; CTV = clinical target volume; DIPG = diffuse intrinsic pontine glioma; IMRT = intensity modulated radiation therapy; IQR = interquartile range.
Disease progression patterns
Of the 105 patients, 2 were alive without disease progression at last clinical follow-up, 1 died of influenza-related respiratory failure, and 2 were lost to follow-up. Three others experienced clinical progression without a brain MRI scan within 1 month of progression. The remaining 97 patients were evaluated clinically and underwent MRI of the brain, with or without the spine, at progression (Fig. 1). Local tumor progression predominated (77% of patients), yet isolated and synchronous metastatic progression was observed in 13% and 10% of patients, respectively. The estimated cumulative incidences of isolated local, isolated distant, and synchronous disease progression at 1 year were 62.6% (95% CI, 52.0–71.5), 12.3% (95% CI, 6.7–19.8), and 7.2% (3.1–13.4), respectively, and did not differ significantly according to the CTV margin employed (Fig. 2A–C).
Fig. 2.
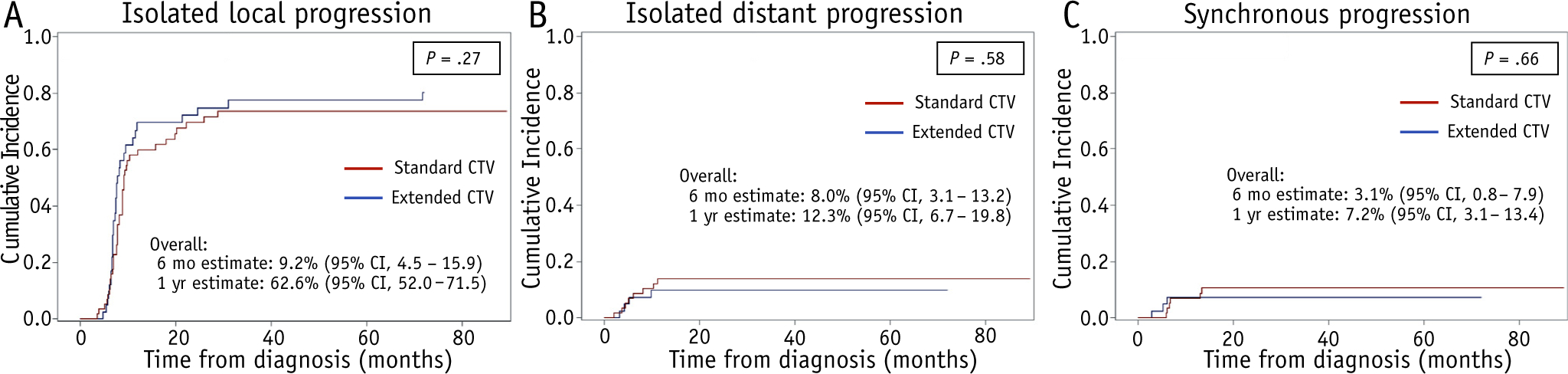
Cumulative incidences of isolated local progression (A), isolated distant progression (B), and synchronous local and distant progression (C) in patients treated with a standard clinical target volume (CTV) (sCTV) (red) or an extended CTV (eCTV) (blue). Abbreviation: CI = confidence interval. (A color version of this figure is available at https://doi.org/10.1016/j.ijrobp.2019.11.020).
The radiographic pattern of neuraxis spread was predominantly leptomeningeal (76%), with frequent spread to the spine (57%) (Fig. 1). Evaluation of the extent of tumor progression relative to the delivered RT dose distribution in the 81 patients who experienced radiographically confirmed local failure revealed central progression (Vprogression95% ≥95%) in all but 1 patient (Fig. 1; Fig. E2, available online at https://doi.org/10.1016/j.ijrobp.2019.11.020). The clinical, anatomic, and dosimetric progression types did not differ significantly in sCTV- versus eCTV-treated patients, whereas the conformity index was significantly larger in eCTV-treated patients (P < .001).
Routes and extent of tumor spread after therapy
In those patients who experienced local tumor progression, the incidence of progression beyond the initial CTV was similar in the sCTV and eCTV groups (39% and 37%, respectively) (Table E3, available online at https://doi.org/10.1016/j.ijrobp.2019.11.020). The proportion of tumor extending beyond the initial CTV at progression was also similar in both groups and was limited in extent (2.7% for sCTV vs 7.8% for eCTV). Spread was predominantly toward the supratentorial compartment in sCTV patients and toward the cerebellum in eCTV patients. Eleven eCTV patients (31%) had any progression beyond a retrospectively applied 1-cm CTV margin, although this too was limited in extent (9.2% of the initial CTV).
Analysis of the component progression vectors representing the distance from the surface of the initial GTV and the surface of the progression volume extending superiorly, inferiorly, and transversely revealed minimal tumor extension beyond the initial GTV (range, 2.7–3.6 mm) (Fig. 3A), consistent with the volumetric assessment. Across the entire cohort, cerebellar (transverse) extension was significantly greater than cranial or caudal extension (Fig. 3B), whereas the net progression vector was significantly longer in the eCTV-treated patients (Fig. 3C).
Fig. 3.
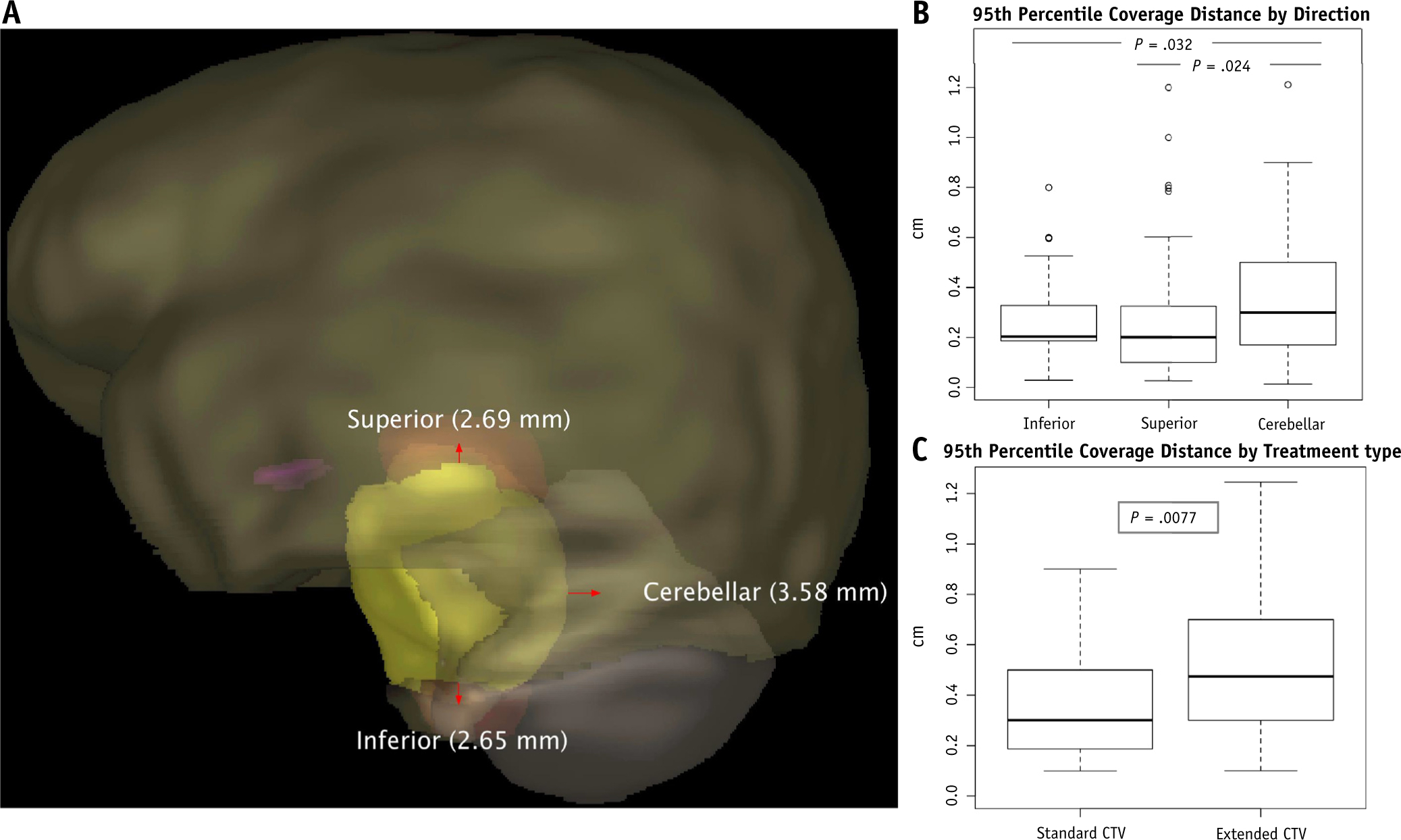
(A) Superior, cerebellar (transverse), and inferior net progression vectors (in mm) beyond the gross tumor volume (GTV) projected on an exemplar baseline magnetic resonance imaging with volumetric delineation of the initial GTV (yellow), brain stem (orange), cerebellum (gray), optic chiasm (purple), and brain (brown) for context. Box plots show the 95th percentile of progression vectors from the initial GTV surface by the 3 cardinal directions of diffuse intrinsic pontine glioma tumor spread (B) and by applied clinical target volume margins (C). (A color version of this figure is available at https://doi.org/10.1016/j.ijrobp.2019.11.020).
Survival outcomes and associated prognostic factors
Median PFS and OS for the overall cohort were 7.6 months (95% CI, 6.9–8.2 months) and 11.3 months (95% CI, 10.0–12.8 months), respectively (Fig. 4A, 4B). Survival outcomes did not differ significantly for the sCTV and eCTV groups (PFS,P =.61; OS, P =.44). OS was significantly worse in patients with any distant metastasis after treatment (1-year OS was 19.1% with and 50.6% without distant progression; P =.003) (Fig. 4C). OS differed significantly according to the DIPG survival predication risk group (1-year OS was 47.4% for standard risk, 48.5% for intermediate risk, 23.5% for high risk; P = .03) (Fig. 4D), histone H3 mutation (1-year OS was 19.1% for H3.3 K27M, 50.0% for H3 WT, 87.5% for H3.1 K27M mutant; P= .01) (Fig. 4E), and ACVR1 mutation status (1-year OS was 85.7% with ACVR1 mutation vs 23.1% without; P= .03) (Fig. 4F).
Fig. 4.
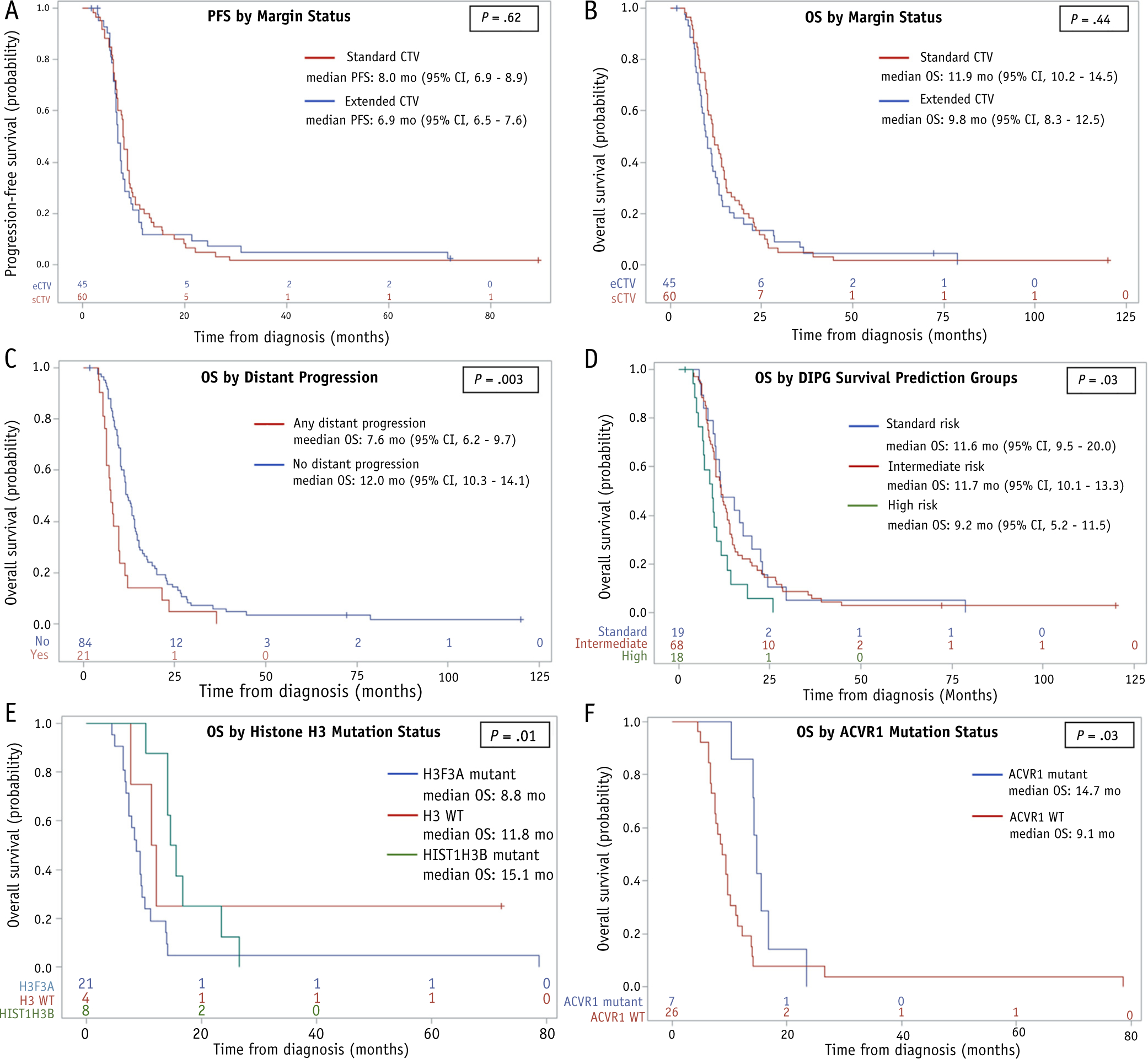
Progression-free (A) and overall survival (OS) (B) estimates for patients treated with a standard clinical target volume (CTV) (sCTV) (red) and an extended CTV (eCTV) (blue). (C-F) OS estimates with respect to development of any distant progression (C), diffuse intrinsic pontine glioma survival prediction model risk groups (D), histone mutation status (E), and ACVR1 mutation status (F). +, censored patients. Abbreviation: CI = confidence interval. (A color version of this figure is available at https://doi.org/10.1016/j.ijrobp.2019.11.020).
Univariate Cox proportional hazards analysis identified several predictors of survival outcomes (Table 2). Patients classified as standard- or intermediate-risk by the DIPG survival prediction model had a significantly decreased hazard of progression or death compared with high-risk patients (HR range, 0.41–0.52), whereas patients who experienced any distant progression after treatment had a significantly increased hazard for death (HR = 2.04; P =.003). H3.3 K27M mutation was associated with significantly shorter OS (HR = 0.41 for patients without H3.3 K27M–mutant tumors vs those with H3.3 K27M–mutant tumors; P=.02), whereas H3.1 K27M andACVR1 mutations were associated with significantly longer OS (HR = 3.56, P = .004 and HR=2.58,P = .04, respectively). H3.3 or H3.1 K27M mutation status did not significantly correlate with the incidence of distant metastasis after treatment (P=.67). RT margins, as well as individual variables incorporated into the DIPG survival predication model, did not significantly affect survival outcomes. Multivariate regression analysis (without incorporating the tumor mutation status, given the low number of observations) confirmed these variables as independent predictors of progression or death and identified a marginal effect of sex on PFS (HR = 0.67; P=.07) (Table 2).
Table 2.
Cox proportional hazards modeling
| Univariable analysis | ||
|---|---|---|
| Covariate | PFS | OS |
| HR (95% CI) P | HR (95% CI) P | |
| Age at diagnosis (y) | 1.00 (0.95–1.05) .90 | 0.99 (0.94–1.04) .73 |
| Age groups | ||
| <3 y vs 3–10 y | 0.72 (0.39–1.34) .30 | 0.69 (0.38–1.26) .23 |
| >10 y vs 3–10 y | 1.02 (0.61–1.70) .94 | 1.06 (0.63–1.77) .84 |
| Sex (male vs female) | 0.75 (0.50–1.11) .15 | 0.82 (0.55–1.22) .33 |
| Race (white vs other) | 0.88 (0.57–1.36) .57 | 0.82 (0.53–1.27) .38 |
| Symptom duration (mo) | 0.93 (0.83–1.04) .18 | 0.93 (0.82–1.04) .22 |
| Ring enhancement (n vs y) | 1.14 (0.76–1.70) .52 | 1.27 (0.85–1.90) .24 |
| RT margin (sCTV vs eCTV) | 0.90 (0.60–1.35) .62 | 0.86 (0.58–1.27) .44 |
| Any distant failure | — | 2.04 (1.25–3.32) .004 |
| Any chemotherapy | 0.82 (0.38–1.78) .62 | 0.68 (0.31–1.47) .32 |
| H3 K27M mutation (n vs y) | 0.75 (0.22–2.51) .64 | 1.29 (0.38–4.36) .68 |
| H3F3A mutation (n vs y) | 0.46 (0.21–0.97) .04 | 0.41 (0.19–0.88) .02 |
| HIST1H3B mutation (n vs y) | 2.27 (0.99–5.18) .051 | 3.56 (1.49–8.49) .004 |
| ACVR1 mutation (n vs y) | 1.73 (0.74–4.05) .21 | 2.58 (1.07–6.22) .04 |
| TP53 mutation (n vs y) | 0.84 (0.41–1.71) .62 | 0.76 (0.37–1.54) .44 |
| DIPG risk stratification | ||
| Standard vs intermediate | 0.92 (0.55–1.56) .77 | 0.90 (0.54–1.50) .69 |
| Standard vs high | 0.49 (0.25–0.96) .04 | 0.47 (0.24–0.91) .02 |
| Intermediate vs high | 0.53 (0.31–0.91) .02 | 0.52 (0.30–0.89) .02 |
| Multivariable analysis | ||
| Covariate | PFS | OS |
| HR (95% CI) P | HR (95% CI) P | |
| Any distant failure | — | 2.12 (1.30–3.48) .003 |
| Sex (male vs female) | 0.67 (0.20–0.83) .07 | — |
| DIPG risk stratification | ||
| Standard vs high | 0.41 (0.20–0.83) .01 | 0.47 (0.24–0.90) .02 |
| Intermediate vs high | 0.52 (0.30–0.90) .02 | 0.48 (0.28–0.83) .009 |
Abbreviations: CI = confidence interval; CTV = clinical target volume; eCTV = extended CTV; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; sCTV = standard CTV.
Discussion
Our evaluation of a relatively large subset of patients prospectively treated with an eCTV margin and our assessment of progressive disease in the context of radiation dosimetry have provided unique insights into optimal target margins for conformal RT for DIPG. Neither survival outcomes nor tumor-control outcomes in patients treated with a 1-cm CTV margin differed from those in patients treated with a CTV margin of 2 to 3 cm. We observed no marginal dosimetric local failures, and the extent of progressive disease beyond the high-dose irradiated volume was minimal, representing <10% of the progressive tumor volume irrespective of the CTV margin employed. We also confirmed the predominance of local tumor progression, the notable posttreatment craniospinal dissemination in a subset of patients, and the relevance of several factors associated with survival.8,16,21
In a seminal report, Halperin8 demonstrated a crude infield recurrence rate of 88%, primarily by using CT-based assessment, and no significant differences in relapse-free survival or OS in pediatric patients with brain stem glioma treated with focal or whole-brain RT. Subsequent studies incorporating MRI have also supported the findings that the vast majority of treatment failures in pediatric brain stem glioma occur within focal irradiation ports22 and that dose escalation through hyperfractionation does not significantly alter this progression pattern.23 In our current analysis, using extended margins of 1 to 2 cm beyond the more traditional 1-cm CTV margins had no significant effect on the rate or kinetics of local tumor control, did not shift the pattern of dosimetric or anatomically defined progression, and did not improve survival outcomes. In conjunction with our recent report demonstrating noncontiguous treatment-related radiologic abnormalities in a subset of these patients treated with extended margins, approximately half of whom experienced new or worsening clinical symptoms,24 this suggests that there is limited utility and perhaps increased risk of treatment-related morbidity in extending CTV margins beyond 1 cm for patients with DIPG.
Despite the predominance of local failure after (chemo) RT, we observed radiographic signs of craniospinal dissemination as a component of first progression in 23% of patients and noted spinal metastasis in more than half of them, which is consistent with previous reports.16,22 This rate is probably an underestimate, considering only 12% of patients underwent complete spine MRI at progression. Indeed, a prospective trial evaluating routine complete neuraxis MRI surveillance in patients with DIPG showed craniospinal dissemination in 56% of patients with progressive disease after therapy.25 Although local contiguous spread through the brain stem and cerebellum has been most frequently observed in autopsy studies of patients with DIPG,26,27 skip areas of spread and distant central nervous system seeding have been increasingly recognized.28–31 Accordingly, several authors have suggested re-examining RT fields with consideration of extended, whole-brain, or even complete neuraxis irradiation.29,31 Although the feasibility of craniospinal irradiation (with concurrent temozolomide) has been demonstrated in a limited number of patients with newly diagnosed metastatic HGG or DIPG, significant hematopoietic toxicity limited systemic therapy delivery and PFS was limited to 4.0 months.32 Concomitant local tumor progression was observed in nearly half of our patients with distant progression, and histologic progression restricted to areas outside the focal high-dose irradiated tissue is rarely observed,33 suggesting that tumor growth confined to areas outside a limited RT field is not a major cause of mortality for these patients.
To identify anatomic routes of spread that may inform anisotropic CTV margin design, we evaluated the incidence and extent of cranial, caudal, and transverse disease progression beyond the initially employed target volumes. The incidence of progression beyond the initial CTV was similar in the sCTV and eCTV groups and was limited in extent relative to the total progression volume. Using a custom algorithm to estimate the component progression vectors of these cardinal directions of tumor spread, we found minimal tumor extension beyond the initial GTV across the entire cohort (range, 2.7–3.6 mm), substantially larger cerebellar extension, and, intriguingly, a significantly longer net progression vector in eCTV-treated patients. Although this latter finding is not yet fully understood, the difference of only 2 mm calls into question its clinical significance. Given the relatively small extension beyond the initial GTV, one might speculate that smaller margins can be used without compromising local control.
In an evaluation of 316 patients with DIPG, Jansen et al identified 4 prognostic variables at diagnosis, namely patient age, symptom duration, ring enhancement on MRI, and use of chemotherapy, which were used to develop a DIPG survival model to distinguish patients at standard, intermediate, and high risk of death at 1 year.20 We observed a significant decrease in the hazard of progression or death in standard- or intermediate-risk patients compared with high-risk patients (HR range, 0.47–0.52). The lack of a significant difference in the survival of standard- versus intermediate-risk patients may reflect the subjective nature of the clinical diagnosis of DIPG, for which our practice has been to recommend confirmatory biopsy to demonstrate grade II-IV glioma for atypical-appearing DIPG, the clinicoradiologic findings of which may have fulfilled the common criteria used by Jansen et al. We also confirmed the influence of metastatic evolution on DIPG survival,4 with multivariable analysis showing a significant hazard for the risk of death in patients who developed metastasis after treatment. Building on initial genomic characterization efforts,14,34,35 large-scale integrated genomic reports have demonstrated inferior outcomes in patients with DIPG who harbor tumors with a histone H3.3 mutation, compared with an H3.1 mutation or WT H3,3,21 and our study recapitulated these results. Interestingly, in contrast to a previous report,4 we observed no significant correlation between H3 K27M mutation and the incidence of metastasis, although caution must be exercised here because the number of evaluable patients was low, precluding an evaluation based on distinct H3 mutations.
Supplementary Material
Acknowledgments—
The authors would like to thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript; Melissa Gargone, MHS, RT(T), CMD, for assistance with radiation dosimetry; and Brandon Bianski, BS, for assistance with data extraction.
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), National Cancer Institute grant P30 CA021765 (St. Jude Cancer Center Support Grant), and the Dunagan MD Medical Education Fund (to K.C.).
Footnotes
Disclosures: none.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.11.020.
References
- 1.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 2012;44:251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 3.Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017;32:520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 2015;130:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langmoen IA, Lundar T, Storm-Mathisen I, et al. Management of pediatric pontine gliomas. Childs Nerv Syst 1991;7:13–15. [DOI] [PubMed] [Google Scholar]
- 6.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet Oncol 2006;7: 241–248. [DOI] [PubMed] [Google Scholar]
- 7.Greenberger JS, Cassady JR, Levene MB. Radiation therapy of thalamic, midbrain and brain stem gliomas. Radiology 1977;122:463–468. [DOI] [PubMed] [Google Scholar]
- 8.Halperin EC. Pediatric brain stem tumors: Patterns of treatment failure and their implications for radiotherapy. Int J Radiat Oncol Biol Phys 1985;11:1293–1298. [DOI] [PubMed] [Google Scholar]
- 9.Baxter P. Veliparib, radiation therapy, and temozolomide in treating younger patients with newly diagnosed diffuse pontine gliomas. https://clinicaltrials.gov/ct2/show/NCT01514201?termZ01514201&rankZ1. Accessed November 11, 2019.
- 10.Su J. Vorinostat and radiation therapy followed by maintenance therapy with vorinostat in treating younger patients with newly diagnosed diffuse intrinsic pontine glioma. https://clinicaltrials.gov/ct2/show/NCT01189266. Accessed November 11, 2019.
- 11.Chang A, Merchant T. Patterns of failure for diffuse infiltrating brainstem glioma: new guidelines for radiotherapy planning. Neuro Oncology 2008;10:392; (abstract BSG4). [Google Scholar]
- 12.Broniscer A, Baker SD, Wetmore C, et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 2013;19:3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wetmore C, Broniscer A, Turner D, et al. First-in-pediatrics phase I study of crenolanib besylate (CP-868,596–26) administered during and after radiation therapy (RT) in newly diagnosed diffuse intrinsic pontine glioma (DIPG) and recurrent high-grade glioma (HGG). J Clin Oncol 2014;32(15 suppl):10064. [Google Scholar]
- 14.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 2014;46:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol 2010;28:4762–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gururangan S, McLaughlin CA, Brashears J, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol 2006;77:207–212. [DOI] [PubMed] [Google Scholar]
- 17.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol 2002;20:1635–1642. [DOI] [PubMed] [Google Scholar]
- 18.Becksfort JW, Williams N, Tsang DSC, et al. A novel methodology for anatomically and biologically determined clinical target volume margin estimation in pediatric high grade glioma. Int J Radiat Oncol Biol Phys 2017;99:S175–S176. [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154. [Google Scholar]
- 20.Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, et al. Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol 2015;17:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): A collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J Clin Oncol 2018;36:1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue B, Allen J, Siffert J, et al. Patterns of recurrence in brain stem gliomas: Evidence for craniospinal dissemination. Int J Radiat Oncol Biol Phys 1998;40:677–680. [DOI] [PubMed] [Google Scholar]
- 23.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: Results of a pediatric oncology group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 1999;43: 959–964. [DOI] [PubMed] [Google Scholar]
- 24.Patay Z, Merchant TE, Nguyen R, et al. Treatment-related noncontiguous radiologic changes in children with diffuse intrinsic pontine glioma treated with expanded irradiation fields and antiangiogenic therapy. Int J Radiat Oncol Biol Phys 2017;99:1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi R, Allen J, Donahue B, et al. Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J Neurooncol 2011;102:121–127. [DOI] [PubMed] [Google Scholar]
- 26.Mantravadi RV, Phatak R, Bellur S, et al. Brain stem gliomas: An autopsy study of 25 cases. Cancer 1982;49:1294–1296. [DOI] [PubMed] [Google Scholar]
- 27.Nishio S, Fukui M, Tateishi J. Brain stem gliomas: A clinicopathological analysis of 23 histologically proven cases. J Neurooncol 1988;6:245–250. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura J, Onda K, Tanaka R, et al. Clinicopathological study of diffuse type brainstem gliomas: Analysis of 40 autopsy cases. Neurol Med Chir (Tokyo) 2003;43:375–382. [DOI] [PubMed] [Google Scholar]
- 29.Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol 2014;128:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikbakht H, Panditharatna E, Mikael LG, et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun 2016;7:11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 2014;128: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller K, Schlamann A, Guckenberger M, et al. Craniospinal irradiation with concurrent temozolomide for primary metastatic pediatric high-grade or diffuse intrinsic pontine gliomas. A first report from the GPOH-HIT-HGG Study Group. Strahlenther Onkol 2014;190: 377–381. [DOI] [PubMed] [Google Scholar]
- 33.Grigsby PW, Garcia DM, Ghiselli R. Analysis of autopsy findings in patients treated with irradiation for thalamic and brain stem tumors. Am J Clin Oncol 1989;12:255–258. [DOI] [PubMed] [Google Scholar]
- 34.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 2014;46:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 2014;46:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


