Abstract
Objective
To determine whether controlling maternal gestational weight gain (GWG) influences adipose tissue distribution one-year postpartum.
Methods
Women with overweight/obesity (N=210, BMI ≥25/≥30) were randomized to a lifestyle intervention (LI) to control GWG or to usual obstetrical care (UC). Measures included anthropometry, whole-body MRI for visceral (VAT), intermuscular (IMAT) and subcutaneous (SAT) adipose tissue, and cardiometabolic risk factors (CMRF) in pregnancy (15 and 35 weeks) and post-partum (15 and 59 weeks).
Results
Baseline (15 weeks) characteristics were similar (mean±SD): age 33.8±4.3 years, weight 81.9±13.7kg, BMI 30.4±4.5kg/m2, gestational age at randomization 14.9±0.8 wk. LI had less GWG (1.79kg, p=0.003) and SAT gain at 35 weeks (p<0.01). UC postpartum weight (2.92kg) was higher at 15 weeks but not different from baseline or LI at 59 weeks. Postpartum VAT increased from baseline in LI by 0.23kg at 15 weeks and 0.55kg at 59 weeks and in UC, 0.34kg at 15 and 59 weeks, and IMAT remained elevated in LI (0.22kg) at 59 weeks. VAT was associated with several CMRF at 59 weeks.
Conclusion
Despite no weight retention at 59 weeks postpartum, women had increased VAT by ~30%. Postpartum modifiable behaviors are warranted to lower the risk of VAT retention.
Keywords: Adipose tissue, postpartum central fat, body composition, lifestyle modifications, pregnancy, cardiometabolic risk factors
Introduction
Postpartum weight retention contributes to the risk for obesity and its co-morbidities (1,2). This excess weight includes central fat (3,4) which, if maintained, is associated with an increased later risk of cardiovascular disease (CVD) (5,6). Few studies have obtained serial measurements from early pregnancy to one year postpartum to assess fat gain or loss. CARDIA (4) reported a threefold greater increase in visceral adipose tissue (VAT), from preconception to five years postpartum compared to nulliparous women. In a multiethnic cohort, excessive gestational weight gain (GWG) was associated with a 3.5-cm greater waist circumference and 300% increased risk of abdominal adiposity compared with values for adequate GWG (7). Whether fat mass gained during pregnancy is retained postpartum and whether the fat distribution is different has not been adequately studied.
A major barrier confronting investigations of pregnancy-related adiposity changes has been the lack of validated measures to assess body composition during the pregnant state (8). Available in-vivo methods cannot differentiate between mother and fetus (9) so values for fat and fat-free mass reflect the combined units. Also, total body water increases during pregnancy by about 5–8 liters (10–12) and the composition of lean tissue changes as pregnancy progresses, thereby invalidating a basic assumption that 73% of the adult’s fat-free mass compartment is water (11,13–14). An understanding of pregnancy-induced changes in body composition requires a pre-pregnancy measure followed by one early postpartum yet there are few such studies (15) and none with magnetic resonance imaging (MRI) specific adipose tissue distribution.
The purpose of this study involved secondary LIFT (Lifestyle Intervention For Two) (16) aims investigating the effects of controlling GWG on maternal adipose tissue distribution through one year postpartum and relating changes in adipose tissue depots with changes in cardiometabolic parameters. The intervention was developed to control GWG, not to target fat mass. Yet measuring fat depot changes is clinically insightful, since excess fat tends to be centrally distributed which, if maintained, correlates with an increased metabolic risk (7,17). We present adipose tissue distribution from early pregnancy through one year postpartum.
Methods
Study Design
LIFT is in the LIFE-Moms consortium (18,19) consisting of seven independent randomized controlled trials (RCTs), a Coordinating Unit, and NIH as sponsor, collaborating but with different strategies for reducing GWG in women with overweight or obesity. Described previously (16), LIFT was a parallel-group RCT with women assigned in a 1:1 ratio at the beginning of the second trimester to a lifestyle intervention (LI) or to usual care (UC). A secondary hypothesis of LIFT was that maternal body fat and VAT would be lower for LI compared to UC. The study was approved by the institutional review boards of St. Luke’s-Roosevelt Hospital (SLRH) and Columbia University and registered (ClinicalTrials.gov ).
Participants
Women were recruited from hospital affiliated private and clinic practices from February 2013 to October 2015. Eligibility criteria included age ≥18 years, BMI ≥25 at baseline measurement, singleton pregnancy and gestational age between 9,0 (week, day) and 15,6 confirmed by dating ultrasound, and intention to deliver at SLRH. Exclusion criteria included body size that exceeded the MRI field-of-view, metal implants, and claustrophobia.
Assessment visits
During Pregnancy
The baseline visit immediately preceded randomization and occurred between 12,0 and 15,6 weeks; corresponding approximately to the beginning of the second trimester of pregnancy. The final prenatal core visit occurred between 35,0 – 36,6 weeks at approximately the end of the third trimester.
Postpartum
There were two scheduled visits, at 13,0–15,0 and 48,0–56,6 weeks postpartum.
Measurements
Details on anthropometric measurements are provided in the Appendix 1. Height, weight, waist and hip circumferences were measured, and body mass index (BMI) was calculated.
Magnetic Resonance Imaging
Total adipose tissue (TAT) including total VAT, intermuscular (IMAT), and subcutaneous (SAT), and SAT subdivisions were measured by using whole-body multislice MRI (20,21) with participant in a fasted state during pregnancy and postpartum. See details in Supplement Appendix 1. The assessment of VAT in this study reflects all visible VAT extending from the tip of sacrum/coccyx throughout the abdomen to where the lungs appear. The coefficient of variation (CV) for VAT (1.97%) in our laboratory is from the blind re-analysis or rereading of the same 3 scans by the same MRI analyst. In an individual with 1.5 kg of VAT, a CV of 2.0% translates to a standard deviation of 0.030 kg.
Total body fat and fat-free mass were measured by air displacement plethysmography using the BOD POD (COSMED USA, Inc) (22). Total body fat, lean mass and total body water were measured by quantitative magnetic resonance (QMR, EchoMRI ™) (23). The BOD POD and QMR measures were done only postpartum. See details in Supplement Appendix 1.
Secondary Variables
Additional measures obtained at pregnancy and postpartum included the 2010 Healthy Eating Index (HEI) based on a single day 24 hour recall, validated in pregnant women (24). A wrist-worn ActiGraph GT3X+ (ActiGraph) accelerometer provided estimates total physical activity (25). Blood pressures were measured after the subject had been sitting quietly for five minutes. Clinical Biochemistry involving fasting serum assays for cholesterol, HDL cholesterol, Friedewald LDL cholesterol, glucose, insulin, C-peptide, leptin, adiponectin, albumin, glycated albumin, TNFα, and IL-6 were obtained. See Supplement Appendix 1.
Statistical Analysis
Within each group, descriptive statistics, means and standard deviations for continuous variables and percentages for discrete variables were calculated. At each visit, an analysis of covariance tested the null hypothesis that the adjusted mean values for the two groups were equal. The covariates include ethnicity, age, baseline BMI, baseline weight, gestational diabetes mellitus (GDM), and the baseline value of the dependent variable. For each variable, the change from baseline was calculated. At each follow-up visit, the paired t-test tested the null hypothesis that the mean change was equal to zero within each group. A t-test tested the null hypothesis that the mean changes from baseline for the two groups were equal. Correlation, partial correlation, and regression analyses explored relationships among body composition variables and weight, physical activity, and HEI variables. Statistical analyses were performed using SAS version 9.2 and STATA version 12. The level of significance was 0.05, two-tailed.
Results
Study Participants
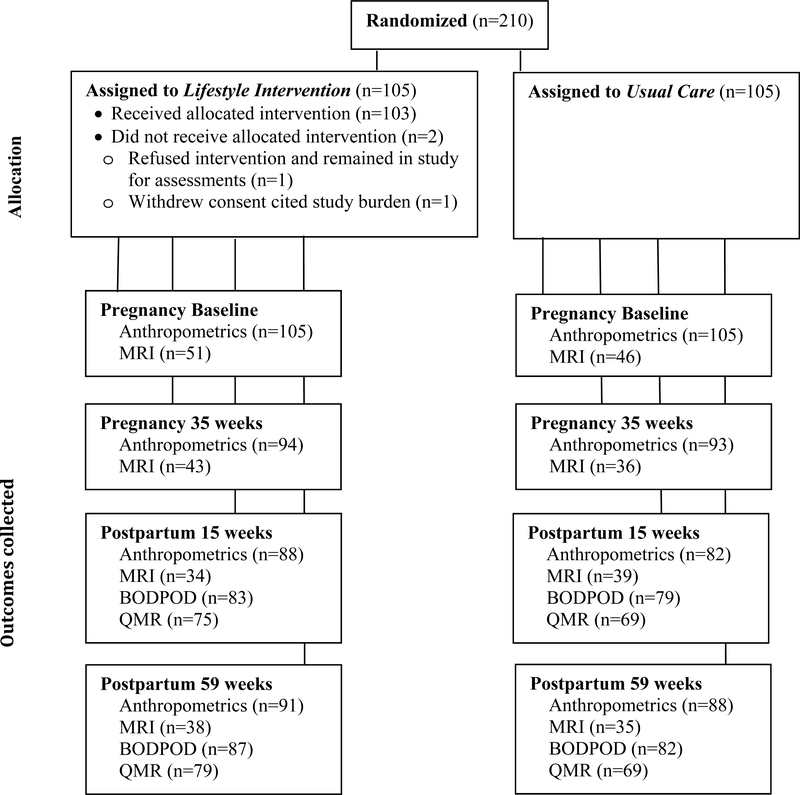
Figure 1 shows enrollment, randomization (n=210) and retention. To maximize retention, the last study window was extended and occurred between 48–184 weeks (59±17 weeks) postpartum. Baseline group characteristics were similar (Table 1). GDM was 10.3% for LI and 6.1% for UC (P=0.28).
Figure 1:
Randomization and follow-up of study participants showing sample size (n) at each time point.
Table 1.
Maternal Baseline Characteristics.
| Lifestyle Intervention | Usual Care | |
|---|---|---|
| Demographic characteristic1 | (n = 105) | (n = 105) |
| Age (years) | 33.8 ± 4.0 | 33.8 ± 4.7 |
| Race | ||
| White | 48 (46%) | 50 (48%) |
| Black | 25 (24%) | 25 (24%) |
| Other | 26 (25%) | 22 (21%) |
| More than one race | 5 (5%) | 8 (8%) |
| Unknown | 1 (1%) | 0 (0%) |
| Ethnicity | ||
| Not Hispanic/Latina | 72 (69%) | 80 (76%) |
| Hispanic | 32 (30%) | 25 (24%) |
| Unknown | 1(1%) | 0 (0%) |
| Height (cm) | 164.3 ± 5.4 | 163.5 ± 7.0 |
| Baseline weight (kg) | 81.5 ± 12.4 | 82.2 ± 15.0 |
| Baseline BMI (kg/m2) | 30.1 ± 4.1 | 30.7 ± 5.0 |
| Baseline BMI Categories | ||
| Overweight (25.0 – 29.9 kg/m2) | 65 (62%) | 60 (57%) |
| Obese (>30.0 kg/m2) | 40 (38%) | 45 (43%) |
| Parity | ||
| 0 | 39 (37%) | 38 (36%) |
| 1 | 30 (29%) | 31 (30%) |
| ≥2 | 36 (34%) | 36 (34%) |
| Gestational age at randomization (wks) | 14.96 ± 0.72 | 14.82 ± 0.78 |
Values are n (%) for categorical variables and mean ± SD for continuous variables.
Differences in baseline characteristics between the treatment groups were not significant.
Intervention Adherence
Adherence was measured by attendance at bi-monthly visits and weekly food and exercise logs. Median attendance of LI women was good at 87.5% of visits through end of second trimester and 72% of visits through end of pregnancy. Adherence to submitting weekly food logs was moderate, with a median rate of 67.5% in the second trimester and 51.1% overall. Adherence to submitting weekly exercise logs had a median rate of 52.5% in the second trimester and 34.2% overall. Exercise class attendance rate was extremely poor at 9.7%.
Intervention effects on GWG
In the entire sample, LI GWG was 1.79 kg (SED=0.59 kg) less than in UC (p<0.003) (16). Among the women with overweight (25.0≥BMI≤29.9 kg/m2), LI GWG was not statistically lower than UC (−1.32 kg, SED=0.70 kg, p=0.06). Among the women with obesity (BMI>30.0 kg/m2), LI weight gain was 2.68 kg (SED=0.96 kg, p=0.007) less than UC. LI GWG in the second trimester compared to UC was 0.97 kg less among women with overweight (SED=0.42 kg, p=0.02) and 2.27 kg (SED=0.57 kg, p<0.001) less among women with obesity. GWG in the third trimester did not differ (0.64 kg, SED=0.38 kg, p=0.09) between groups. Notably, in women with obesity, LI GWG was 69% of UC GWG (6.07 kg, SD=04.24 kg versus 8.75 kg, SD=4.27 kg, SED=0.96 kg, p<0.007) (16). GWG per week above the IOM guidelines was 19% in LI compared to 38% in UC (p=0.002) (16).
Diet components during pregnancy and postpartum
There were no between group differences in the HEI at baseline (Table S1) except for higher total dairy for LI. At 35 weeks, LI compared to UC had a higher total HEI score, higher total fruit, and higher whole fruit reflecting a healthier maternal diet and a higher solid fats, alcohol and added sugar (SOFAAS) score indicating a lower consumption of calories from this category. The change in total HEI by 35 weeks for LI (5.33, p=0.01) was greater (p=0.03) than the change for UC (−1.03, p=0.62). At 59 weeks postpartum, LI continued to have higher total HEI scores, total vegetables, dark-green/organ/legumes, and refined grains compared to UC. The change in HEI from baseline to 59 weeks for UC (−5.57, p=0.02) was less (p=0.03) than the change for LI (1.48, p=0.49) reflecting a less healthy diet in UC at 59 weeks.
Cardiometabolic parameters during pregnancy and postpartum
Cardiometabolic values during pregnancy and postpartum are presented in Table S2. There were no differences between the groups for any cardiometabolic variable at baseline, 35 weeks, or 59 weeks postpartum. The change scores from baseline to 35 weeks differed between the groups for total cholesterol, HDL-cholesterol, and leptin, reflecting increases in UC compared to LI. The increased leptin in UC was consistent with an increase in total adipose (2.50±3.31kg, p=0.009; Table 3) with no changes in either leptin or adipose tissue observed in LI.
Table 3.
Maternal body composition changes, mean (standard deviation), within group
| Pregnancy | Postpartum | Postpartum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline to 35 weeks | Baseline to 15 weeks post-partum | Baseline to 59 weeks post-partum | |||||||
| LI Change | UC Change | p-value† | LI Change | UC Change | p-value† | LI Change | UC Change | p-value† | |
| N=94 | N=93 | N=88 | N=82 | N=91 | N=87 | ||||
| Weight (kg) | 8.01 (4.01)a | 9.81 (4.20)a | 0.0031 | −0.22 (4.09) | 2.43 (4.62)a | 0.0001 | −0.39 (5.78) | 1.16 (6.02) | 0.0821 |
| Body mass index (kg/m3) | 2.95 (1.47)a | 3.68 (1.60)a | 0.0014 | −0.10 (1.51) | 0.89 (1.75)a | 0.0001 | −0.15 (2.10) | 0.45 (2.23) | 0.0650 |
| Hip circumference (cm) | 1.48 (3.51)a | 2.35 (7.90)b | 0.3354 | −0.92 (3.62)c | 1.32 (4.74)c | 0.0007 | −0.95 (4.64) | −0.10 (5.36) | 0.2553 |
| Waist circumference (cm) | -- | -- | −1.59 (5.52)b | 0.51 (5.43) | 0.0133 | −2.21 (6.70)b | −0.81 (6.69) | 0.1613 | |
| MRI (kg) | N=42 | N=34 | N=33 | N=36 | N=38 | N=30 | |||
| Weight (g) | 7.61 (3.73)a | 10.26(4.70)a | 0.0097 | −0.29 (3.92) | 2.97 (4.85) a | 0.0030 | 0.06 (5.97) | 0.60 (6.22) | 0.7157 |
| Total adipose (kg) | 0.46 (3.22) | 2.50 (3.31)a | 0.0086 | 0.78 (3.25) | 2.91 (3.87)a | 0.0155 | 1.80 (5.81) | 0.61 (5.30) | 0.3834 |
| Subcutaneous (kg) | 0.68 (2.70) | 2.54 (2.93)a | 0.0059 | 0.37 (2.95) | 2.41 (3.55)a | 0.0114 | 1.04 (5.04) | 0.22 (4.74) | 0.4917 |
| Abdominal Deep | - | - | - | 0.03 (0.32) | 0.17 (0.52) | 0.1985 | 0.03 (0.52) | −0.11 (0.59) | 0.3088 |
| Abdominal Superficial | - | - | - | 0.04 (0.42) | 0.31 (0.38)a | 0.0137 | 0.20 (0.52)c | 0.05 (0.50) | 0.2541 |
| Femoral SAT | 0.18 (1.05) | 0.88 (1.58) | 0.0217 | 0.18 (1.05) | 0.88 (1.58)b | 0.0518 | 0.55 (1.61) | −0.04 (1.80) | 0.1826 |
| Perimuscle SAT | −0.01 (12) | 0.00 (0.10) | 0.8508 | −0.01 (0.12) | 0.00 (0.10) | 0.7686 | −0.00 (0.11) | −0.02 (0.11) | 0.6238 |
| Visceral (kg) | - | - | - | 0.23 (0.54)c | 0.34 (0.52)a | 0.4075 | 0.54 (0.71)a | 0.34 (0.54)b | 0.2210 |
| Intermuscular (kg) | 0.13 (0.31)c | 0.18 (0.26)a | 0.4015 | 0.18 (0.41)c | 0.17 (0.34)b | 0.8759 | 0.22 (0.41)b | 0.05 (0.35) | 0.0703 |
| Skeletal muscle (kg) | 0.73 (1.14)a | 1.32 (1.13)a | 0.0262 | −0.59 (1.21)b | −0.06 (1.21) | 0.0771 | −0.05 (1.25) | 0.57 (1.20) | 0.0444 |
Within group changes that are significant are indicated in bold typeface using a paired t-test. Between group p-values were calculated using t-test adjusted for unequal variances.p-value
p<0.001
p<0.01
p<0.05
Body composition at Baseline
There were no between group differences for any anthropometric or MRI derived variable at baseline. Among LI and UC, 49% and 44% respectively, had an MRI scan (see Table 2). Participants who had an MRI scan had a lower body weight (7.2±13.3kg, P<0.0001) compared to those with no scan, among both LI and UC.
Table 2.
Maternal body composition, mean (standard deviation), during early and late pregnancy, and postpartum
| ----------------------------------------Pregnancy--------------------------------- | -----------------------------------Postpartum------------------------------ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LI Baseline N=105 | UC Baseline N=105 | Adj Diff (SED1) | LI 35 wk N=94 | UC 35 wk N=93 | Adj Diff (SED) | LI 15.3 wk N=88 | UC 15.4 wk N=82 | Adj Diff (SED) | LI 58 wk N=91 | UC 59.34 wk N=88 | Adj Diff (SED) | |
| Gestational age (weeks) | 15.0 (0.72) | 14.8 (0.78) | 35.7 (0.72) | 35.9 (0.92) | -- | -- | -- | -- | ||||
| Post-delivery (weeks) | - | - | - | - | - | - | 15.1 (2.9) | 15.2 (3.5) | −0.02 (0.51) | 57.7 (13.4) | 59.3 (20.2) | −2.37 (2.47) |
| Weight (kg) | 81.5 (12.4) | 82.2 (15.0) | −0.58 (1.89) | 89.6 (12.0) | 92.1 (14.6) | −1.87 (0.58)b | 81.8 (13.3) | 84.6 (15.0) | −2.81 (0.69)a | 81.8 (14.8) | 83.7 (17.0) | −1.44 (0.92) |
| Body mass index (kg/m3) | 30.1 (4.1) | 30.7 (5.0) | −0.48 (0.62) | 33.0 (3.8) | 34.4 (4.9) | −0.73 (0.22)a | 30.3 (4.3) | 31.5 (5.1) | −1.06 (0.26)a | 30.2 (5.0) | 31.2 (5.9) | −0.57 (0.34) |
| Circumferences | ||||||||||||
| Hip (cm) | 112.0 (8.2) | 112.7 (8.6) | −0.14 (0.50) | 113.4 (8.3) | 115.3 (11.4) | −1.37 (0.93) | 111.3 (9.1) | 114.0 (10.1) | −2.61 (0.66)a | 111.4 (10.2) | 112.9 (110) | −0.97 (0.77) |
| Waist (cm) | 95.1 (8.7) | 95.4 (10.4) | 0.48 (0.67) | - | - | - | 94.0 (9.5) | 95.7 (10.7) | −1.77 (0.84)c | 93.5 (10.8) | 94.8 (12.8) | −1.15 (1.02) |
| BODPOD | N=83 | N=79 | N=87 | N=82 | ||||||||
| Weeks post-delivery | - | - | - | - | - | - | 15.3 (3.9) | 15.4 (4.6) | −0.13 (0.70) | 57.8 (13.4) | 57.6 (14.4) | −1.07 (2.01) |
| Percentage fat (%) | - | - | - | - | - | - | 37.95 (6.03) | 40.05 (6.84) | −1.99 (0.82)c | 37.60 (8.55) | 38.41 (7.77) | −0.53 (0.94) |
| Fat mass (kg) | - | - | - | - | - | - | 31.57 (9.00) | 34.61 (11.53) | −2.96 (0.87)c | 31.94 (12.16) | 33.25 (12.95) | −0.95 (1.02) |
| Fat-free mass (kg) | - | - | - | - | - | - | 50.34 (6.15) | 50.04(5.93) | 0.26 (0.56) | 50.37 (5.39) | 50.75 (5.80) | −0.32 (0.51) |
| QMR | N=75 | N=69 | N=79 | N=69 | ||||||||
| Weeks post-delivery | - | - | - | - | - | - | 15.3 (3.9) | 16.3 (5.5) | −1.03 (0.84) | 58.6 (13.9) | 58.5 (15.5) | −1.31 (2.27) |
| Fat mass (kg) | - | - | - | - | - | - | 31.22 (8.54) | 34.61 (10.99) | −3.14 (0.90)a | 31.92 (11.35) | 33.85 (13.19) | −1.56 (1.09) |
| Lean mass (kg) | - | - | - | - | - | - | 40.91 (6.14) | 42.02 (5.6) | −0.56 (0.86) | 42.10 (6.67) | 43.06 (5.59) | −0.69 (0.82) |
| TBW (kg) | - | - | - | - | - | - | 33.93 (6.38) | 34.25 (7.03) | −0.02 (1.08) | 33.95 (7.44) | 34.27 (7.45) | −0.38 (1.19) |
| MRI (kg) | N=51 | N=46 | N=43 | N=36 | N=34 | N=39 | N=38 | N=35 | ||||
| Gestational age (wks) | 14.98 (0.74) | 15.03 (0.57) | −0.02 (0.13) | 35.66 (0.67) | 35.8 (0.70) | −0.00 (0.16) | - | - | - | - | ||
| Post-delivery (wks) | - | - | - | - | - | - | 14.34 (1.1) | 15.4 (3.8) | −1.30 (0.79) | 57.03 (110) | 61.8 (29.0) | −7.67 (6.22) |
| Weight (kg) | 78.6 (9.6) | 78.8 (9.5) | −0.70 (1.98) | 85.8 (9.1) | 88.9 (9.8) | −2.32 (0.99)c | 77.2 (10.4) | 82.0 (9.7) | −2.97 (1.25)c | 78.4 (13.1) | 79.8 (10.3) | −0.42 (1.67) |
| Total adipose (kg) | 32.8 (7.6) | 33.3 (6.0) | −0.13 (0.73) | 32.8 (7.2) | 36.0 (5.9) | −2.01 (0.80)c | 32.6 (8.0) | 36.1 (7.0) | −1.86 (0.99) | 34.0 (11.7) | 34.2 (8.3) | 1.58 (1.50) |
| Subcutaneous (kg) | 30.0 (7.1) | 30.6 (5.4) | −0.09 (0.63) | 30.3 (6.7) | 33.4 (5.7) | −1.85 (0.69)b | 29.7 (7.4) | 32.8 (6.4) | −1.83 (0.94) | 30.5 (10.5) | 30.9 (7.4) | 0.98 (1.32) |
| Abdominal Deep | 2.92 (0.81) | 3.11 (0.68) | −0.08 (0.15) | - | - | - | 2.95 (0.91) | 3.28 (0.79) | −0.10 (0.13) | 2.96 (1.12) | 2.99 (0.77) | 0.17 (0.17) |
| Abdominal Superficial | 2.91 (0.96) | 2.99 (0.75) | 0.04 (0.14) | - | - | - | 2.95 (0.88) | 3.29 (0.82) | −0.37 (0.11)a | 3.11 (1.20) | 3.03 (0.93) | 0.08 (0.14) |
| Femoral SAT | 10.50 (2.39) | 11.49 (1.93) | −0.68 (0.43) | 10.69 (2.73) | 12.37 (2.78) | −0.76 (0.42) | 10.69 (2.73) | 12.37(2.78) | −0.67 (0.43) | 11.06 (3.36) | 11.44 (2.88) | 0.68 (0.50) |
| Perimuscle SAT | 0.50 (0.13) | 0.48 (0.10) | 0.03 (0.03) | 0.49 (0.12) | 0.48 (0.10) | −0.01 (0.03) | 0.49 (0.12) | 0.48 (0.10) | −0.00 (0.03) | 0.49 (0.12) | 0.47 (0.11) | 0.05 (0.03) |
| Visceral (kg) | 1.45 (0.84) | 1.45 (0.99) | −0.05 (0.17) | - | - | - | 1.57 (0.74) | 1.78 (1.08) | −0.05 (0.12) | 1.99 (1.11) | 1.94 (1.26) | 0.31 (0.15)c |
| Intermuscular (kg) | 1.27 (0.54) | 1.28 (0.47) | 0.001 (0.10) | 1.42 (0.54) | 1.43 (0.43) | −0.03 (0.07) | 1.38 (0.56) | 1.48(0.51) | 0.04 (0.09) | 1.46 (0.67) | 1.38 (0.52) | 0.17 (0.09) |
| Skeletal muscle (kg) | 20.4 (3.1) | 20.2 (3.1) | 0.21 (0.44) | 21.1 (2.6) | 21.3 (3.1) | −0.39 (0.26) | 19.8 (2.9) | 20.8 (3.0) | −0.38 (0.29) | 20.5 (2.9) | 20.8 (2.8) | −0.37 (0.33) |
SED=standard error difference; SAT=subcutaneous adipose tissue. The adjusted difference at each time-point was calculated using the covariates: variable’s baseline value, mother’s baseline age, weight, BMI, and ethnicity. For 35 week analysis, additional covariates included GDM and gestational age. For the post-partum visit, additional covariates included weeks since delivery and GDM.
p-value in bold
p<0.001
p<0.01
p<0.05
Body composition in late pregnancy
Measurements were acquired at 35 weeks in LI (n=94) and UC (n=93) (see Table 2). Among the MRI measurement subgroup, body weight was 2.49 kg (SED=0.96 kg) lower in LI (n=43) compared to UC (n=36) which was reflected in less SAT (1.78 kg, SED=0.66 kg) in LI in late pregnancy. There were no between group differences in IMAT or skeletal muscle at this time.
Pregnancy Changes
Within group changes for weight, BMI and hip circumference from baseline to late pregnancy are in Table 3.
MRI changes
Results are presented for LI (n=42) and UC (n=34) participants who had MRI at baseline and 35 weeks (see Table 3). Within LI, increases occurred in IMAT (0.13±0.31 kg) and skeletal muscle (0.73±1.14 kg) but not for TAT. Within UC, increases occurred in TAT (2.50±3.31 kg), including SAT (2.54±2.93 kg) and IMAT (0.18±0.26 kg), and in skeletal muscle (1.32±1.13 kg). We deemed our efforts to quantify VAT measures at 35 weeks to be unreliable and results are not included. Late in pregnancy, the heavily distended uterus compresses the surrounding maternal tissues including the intra-abdominal VAT tissue, abdominal SAT and presumably abdominal skeletal muscle, preventing an accurate quantification of the tissues in the abdomen at this visit.
Body composition postpartum – 15 weeks
The first postpartum measurements were acquired at 15 weeks gestation in LI (n=88) and UC (n=82) (see Table 2). LI had lower body weight (−2.92±0.68 kg), BMI, circumferences of hip, and waist than UC. LI had lower fat mass and %fat by BODPOD (−3.00±0.86 kg; −2.02±0.81%) and lower fat mass by QMR (−3.18±0.88 kg) than UC, and there were no between group differences in fat-free mass or lean mass. Among the MRI subgroups, LI weight was less (3.80±1.14 kg) than UC, reflected in less total SAT (−2.24 kg). Among the SAT depots, LI had less abdominal superficial SAT (−0.41±0.11 kg) than UC. There were no between group differences in VAT, IMAT or skeletal muscle.
Changes from baseline
Within group changes in body composition variables from baseline to 15 weeks postpartum are presented in Table 3. In LI, body weight was not different to baseline body weight at 15 weeks postpartum. However, waist and hip circumferences were significantly lower than baseline. In UC, body weight (2.43±4.62 kg), BMI and hip circumference remained above baseline values.
MRI adipose tissue changes
Results are presented for LI (n=33) and UC (n=36) participants who had MRI at baseline and 15 weeks postpartum (see Table 3). Within LI, body weight was not different from baseline. VAT (0.23±0.54 kg; Figure 2) and IMAT (0.18±0.41 kg) were increased and skeletal muscle mass was lower (−0.59±1.21 kg) compared to baseline. In UC, body weight and adipose tissue subdivisions, with the exception of abdominal deep SAT and femoral perimuscular SAT, continued to be above baseline.
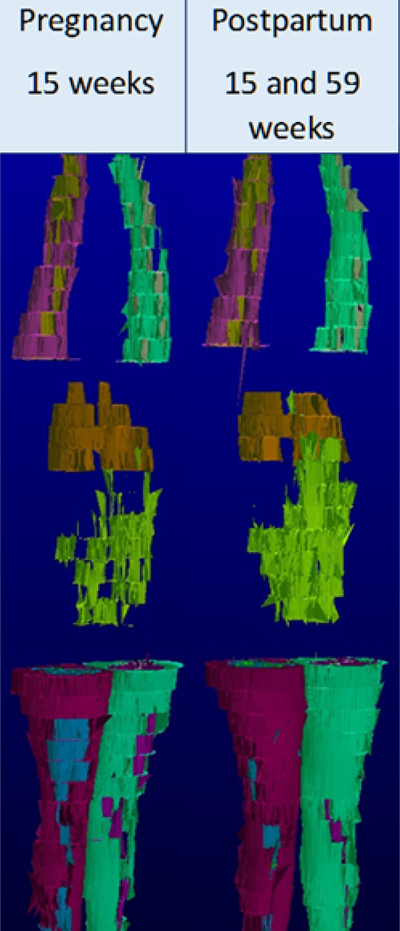
Figure 2:
A processed 3-dimensional volume rendering of visceral adipose tissue mass (green) in a single study participant at 15 weeks gestation (Weight 84.7 kg, VAT 1.64 kg) and at 15 weeks postpartum (Weight 83.5kg, VAT 2.93 kg). The participant is positioned in the MRI scanner with arms extended above head.
Body composition postpartum – 59 weeks
Measurements were acquired at 59 weeks GA in LI (n=91) and UC (n=88) (see Table 2). There were no statistically significant between group differences for any variable.
Changes from baseline
Within group changes in body composition variables from baseline to 59 weeks postpartum are presented in Table 3. In LI, waist remained below baseline. In UC, body weight was not different from baseline. The overall mean group changes (baseline to 59 weeks) did not differ between groups for any anthropometric measure.
MRI changes from baseline
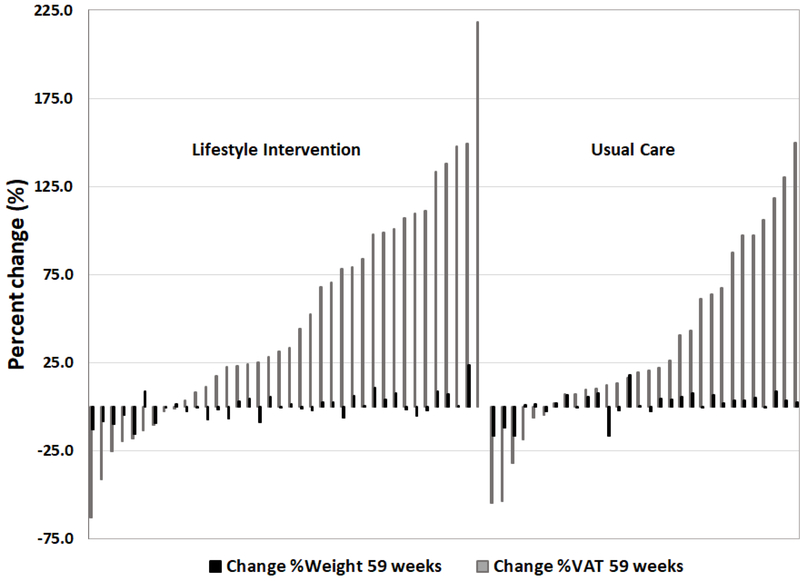
Results are presented for LI (n=38) and UC (n=30) participants who had MRI at baseline and 59 weeks (Table 4). VAT remained above baseline in LI (0.54±0.71 kg) and UC (0.34±0.54 kg) whereas body weight was not different from baseline for either group. Only in LI were abdominal superficial SAT (0.20 kg; p<0.05) and IMAT (0.22 kg; p=0.05) marginally elevated compared to baseline. Presented in Figure 3 are the individual participant data, showing percentage change in VAT and corresponding percentage change in weight, for the period from 15 weeks gestation (our baseline pregnancy time point) to 59 weeks postpartum.
Table 4.
Regression coefficient (standard error) of VAT (kg) at 15 weeks in pregnancy and at 59 weeks postpartum and Spearman correlation between VAT (kg) and cardiometabolic risk factors at 59 weeks postpartum.
| -----Pregnancy---- | -----Postpartum---- | -----Postpartum---- | ||||
|---|---|---|---|---|---|---|
| 15 weeks | 59 weeks | 59 weeks | ||||
| ‡β (SE) | p-value | ‡β (SE) | p-value | †r | p-value | |
| DBP (mm Hg) | 0.775 (0.796) | 0.3330 | 0.089 (0.568) | 0.8761 | −0.10 | 0.4151 |
| SBP (mm Hg) | 1.022 (1.073) | 0.3433 | 1.011 (0.969) | 0.3011 | −0.06 | 0.5938 |
| Triglycerides (mg/dL) | 14.396 (4.415) | 0.0016 | 11.769 (6.124) | 0.0596 | 0.41 | 0.0003 |
| Chol (mg/dL) | 3.971 (3.986) | 0.3219 | −0.872 (3.411) | 0.7992 | 0.20 | 0.0912 |
| HDL (mg/dL) | −2.775 (1.966) | 0.1618 | −3.209 (1.328) | 0.0189 | −0.26 | 0.0280 |
| CHOL/HDL (ratio) | 0.183 (0.082) | 0.0279 | 0.159 (0.084) | 0.0625 | 0.37 | 0.0012 |
| Friedewalk LDL Chol (mg/dL) | 3.889 (3.433) | 0.2604 | 0.205 (2.898) | 0.9439 | 0.28 | 0.0151 |
| Insulin (uU/mL) | 1.962 (0.570) | 0.0009 | 0.495 (0.726) | 0.4978 | 0.32 | 0.0051 |
| Glucose (mg/dL) | 1.933 (0.860) | 0.0272 | 0.494 (1.075) | 0.6475 | 0.29 | 0.0116 |
| HOMA-IR | 9.404 (2.719) | 0.0008 | 2.910 (3.387) | 0.3939 | 0.36 | 0.0017 |
| C-Peptide (ng/mL) | 0.223 (0.046) | 0.0001 | 0.074 (0.078) | 0.3507 | 0.52 | 0.0001 |
| Glycated serum protein (umol/L) | - | - | −3.487 (4.184) | 0.4079 | −0.34 | 0.0033 |
| Glycated albumin (%) | - | - | −0.159 (0.150) | 0.2947 | −0.38 | 0.0010 |
| Albumin (g/dL) | - | - | 0.003 (0.027) | 0.9187 | −0.03 | 0.8004 |
| Leptin (ug/L) | 3.640 (1.928) | 0.0624 | 8.777 (4.273) | 0.0446 | 0.40 | 0.0005 |
| Adiponectin HMW (mg/dL) | −543.58 (278.91) | 0.0546 | −195.96 (222.21) | 0.3815 | −0.17 | 0.1539 |
| TNF-α (pg/mL) | 0.099 | 0.0526 | - | - | - | - |
| IL-6 (pg/mL) | 0.34 | 0.0014 | - | - | - | - |
Regression model included VAT (kg), baseline metabolic variable, group, ethnicity, baseline age, baseline weight, baseline BMI, and weeks postpartum. GDM was an additional covariate for the 59 week regression models. Statistically significant p-values are in bold.
Spearman correlations between VAT (kg) and cardiometabolic risk factors at 59 weeks postpartum
Figure 3:
Change in percentage VAT and corresponding change in percentage weight in individual participants from 15 weeks gestation to 59 weeks postpartum. LI=lifestyle intervention; UC=Usual Care.
Relationship with GWG
The partial correlations of total GWG with maternal body composition variables during pregnancy (35 weeks) and postpartum (Table S3) for combined groups were calculated, adjusted for time (35 wks GA, 15 and 59 wks postpartum) and baseline values for age, weight, BMI. Total GWG was positively correlated with all 35 week body composition variables and with changes in body composition variables from baseline to 35 weeks of pregnancy (Table S4). At 15 weeks postpartum, GWG was positively correlated with waist and hip circumferences, total and percentage body fat, abdominal deep SAT and femoral SAT, but not VAT. There were significant positive correlations between GWG and changes in waist, hip circumferences, total SAT including femoral SAT (all p<0.001), and skeletal muscle mass (p<0.02). At 59 weeks postpartum, GWG was positively correlated with QMR fat mass (p<0.05) and changes in waist circumference (p<0.04) and skeletal muscle (p<0.03) from baseline to 59 wk.
Relationship with HEI
The HEI total score at the concurrent visit was included as an additional independent variable in regression models containing group, ethnicity, baseline values for age, weight, BMI, time to measurement, and the baseline value of the variable, to predict body composition variables (Table S5). HEI total score was a significant predictor of hip circumference (p<0.04) and VAT (p<0.03) only at 15 weeks postpartum, i.e., an increase in HEI score was associated with an increase in VAT, although we do not consider this relationship to be biologically significant.
Relationship with physical activity
Total physical activity (MAD) was included as an additional independent variable in regression models to predict the maternal body composition variables (Table S6). At 35 weeks GA, the MAD coefficient was significant for three models, total adipose tissue (p=0.02), total subcutaneous tissue (p=0.03), and total intermuscular tissue (p=0.01); i.e., less physical activity was associated with greater TAT, SAT, and IMAT. At 59 weeks, the MAD coefficient was significant for BODPOD fat mass (p=0.02); i.e., less physical activity was associated with great BODPOD fat mass.
Relationship of change in VAT to metabolic parameters
At 59 weeks postpartum (Table 4), VAT was positively associated with 59 week triglycerides (p=0.04) and Chol/HDL ratio (p=0.02), and negatively associated with HDL (p=0.03).
Safety Events
Through end of pregnancy, 13.3% of women in LI (n=14) and 14.3% (n=15) in UC (p=0.84) reported serious adverse events. Maternal hospitalizations accounted for 79.3% of these. None were considered related to the study intervention.
Discussion
These prospective data are among the first to report on the effects of pregnancy and early postpartum on adipose tissue distribution, including VAT mass by MRI. VAT mass was elevated by 30% at one-year postpartum despite body weight having returned to baseline/first trimester levels in both LI and UC. The LI promoting healthy diet and physical activity during the second and third trimesters in women with overweight or obesity, designed to restrict excessive GWG, had no effect on preventing or lessening the increase in VAT.
The first postpartum measure showed no weight retention but there was increased VAT (0.23 kg; 16%) and IMAT in LI compared to baseline values, whereas UC retained weight (3.0 kg), VAT (23%), and total SAT that was reflected in abdominal superficial SAT and femoral SAT sub-divisions. The earlier return to baseline weight in LI may reflect less total GWG (~2.0 kg) and therefore less excess weight to shed compared to UC. By 59 weeks postpartum, however, neither group showed weight retention compared to baseline yet VAT remained elevated by 37% and 23% in LI and UC, and only in LI, IMAT remained elevated (~6%). A study with follow-up at seven years of changes in VAT, found that having 1 vs. 0 births was associated with a greater increase in VAT and concluded that childbearing was associated with a threefold greater increase in VAT deposition from preconception to postpartum compared to nulliparous women (4). An increase in VAT by 0.30 to 0.50 kg (Table 3) from a single pregnancy and in women who are relatively early in their lifecourse sets them on a negative health trajectory. In women (BMI≥30 kg/m2) in the Netherlands Epidemiology of Obesity Study, a 1 SD higher VAT was associated with an OR of 5.77 (3.02, 11.01) on having at least one cardiometabolic risk factor compared with individuals without any risk factors (26). In the presence of no change in body weight, increased VAT with no change in subcutaneous AT is comparable to the adipose tissue distribution profile of aging older adults.
We found no evidence of changes in abdominal deep SAT or perimuscular femoral SAT during pregnancy and postpartum. In previous studies, deep abdominal SAT correlated strongly with insulin resistance yet superficial SAT did not, in non-pregnant adults with overweight and obesity (27); and perimuscular adipose tissue adjacent to skeletal muscle was associated with insulin resistance in women with polycystic ovary syndrome (28).
Implications/Clinical Relevance
An expanded VAT is associated with hypertrophic adipocytes and a migration of inflammatory macrophages into the VAT depot (29,30). Intra-abdominal adipose tissue and resident macrophages within this tissue are considered the primary source of cytokines relevant to metabolic disease. Adipose tissue is a source of pro-inflammatory cytokines and chronic inflammation in VAT and is importantly associated with the development of T2DM, dyslipidemia, metabolic syndrome and CVD in individuals with overweight and obesity (31–33). The observed between group difference in leptin was consistent with increased total adipose tissue (+2.56kg, p=0.009; Table 3) in UC, with no changes in either leptin or adipose tissue observed in LI. The between groups differences for change (baseline to 35 weeks GA; Table S2) in total cholesterol (−10.7 mg/dl, p=0.019), HDL-cholesterol (−3.76 mg/dl, p=0.017), and leptin (−6.34 ug/L, p=0.05) were reflective of stable values in LI during the intervention period compared to positive and non-significant increases in UC.
In the combined cohort of women with pre-pregnancy overweight and obesity, while acknowledging that hormonal changes that occur in pregnancy influence the levels of many cardiometabolic risk factors, nonetheless, after adjusting for baseline metabolic variable, age, weight, and BMI, group, and ethnicity, VAT was a significant predictor of five cardiometabolic risk factors, triglycerides, Chol/HDL ratio, insulin, glucose, HOMA-IR, c-peptide, and the Il-6 marker of inflammation (Table 4) at baseline. At 59 weeks postpartum, VAT was a significant predictor of triglycerides, Chol/HDL ratio and a negative predictor of HDL-cholesterol (inflammatory markers not measured). Moreover, at 59 weeks postpartum, VAT was unfavorably correlated with four established CVD (triglycerides, HDL, Chol/HDL ratio, LDL) and six metabolic risk factors (insulin, glucose, HOMA-IR, C-Peptide, glycated serum protein, and glycated albumin).
At 15 weeks postpartum in LI, waist and hip circumferences were lower than baseline (15 weeks GA) suggesting that baseline values may have been elevated compared to pre-pregnancy values and the 15 week postpartum circumferences are reflecting pre-pregnancy values. LI gained less skeletal muscle compared to UC during pregnancy and LI showed an unexplained 3% loss of skeletal muscle mass at 15 weeks postpartum compared to baseline that had normalized at 59 weeks.
GWG was associated with increases in weight and AT; however, by 15 weeks postpartum, these effects were dissipating and by one year, the only persistent effects of GWG was on fat mass by QMR (Table S3). GWG was correlated with changes in waist circumference and skeletal muscle mass at 59 weeks postpartum (Table S4). Total physical activity by accelerometry (MAD) was negatively associated with total adipose tissue including sub-divisions SAT and IMAT at 35 weeks of pregnancy, i.e, greater physical activity was associated with less adipose tissue. At 59 weeks, the MAD negative coefficient was associated with BODPOD fat mass (p=0.02) only; i.e, greater physical activity was associated with less fat mass.
Study entry measures collected at 15 weeks gestation cannot be assumed to reflect pre-pregnancy values due to changes in weight and shape. Due to the RCT design, first trimester weight gain and body composition changes could not be determined. Waist and hip circumference measures at 15 weeks postpartum were significantly lower than values recorded at baseline (15 weeks gestation), which could possibly suggest a return to pre-pregnancy values.
Limitations
A significant percentage of our cohort was larger than could be accommodated by MRI, which impacts the extrapolation of our findings to persons larger than the current MRI cohort. Quantifying VAT late in pregnancy was unsuccessful due to compression effects of the highly distended uterus on the VAT. We cannot address the confounding influences of lifestyle factors (e.g, physical activity, diet, medications, and lactation) on changes in body composition and cardiometabolic risk measures between childbirth and 15 weeks and again between 15 and 59 weeks. Cytokines (TNF-α and IL-6) were unavailable for analysis relative to VAT at 59 weeks.
Next Steps
Observational studies have shown that breastfeeding for >3 months is associated with reduced VAT at 7 years postpartum (7,34), breastfeeding women have less abdominal adiposity through to age of menopause (35–37), and breastfeeding is associated with lower post-partum weight retention in all categories of prepregnancy BMI (38).The mechanism may be through the additional energy cost of lactation. We did not collect information on lactation in LIFT.
There is an established and growing body of literature supporting the superior and independent effects of aerobic exercise over caloric restriction on reducing VAT in persons with overweight and obesity (39). Even in the absence of weight loss following exercise training, VAT loss can occur (40). No studies however, have been conducted in postpartum populations, and appropriately, designed studies involving exercise interventions, factored on lactation, are needed to document efficacy to reduce VAT. Findings could guide health promotion practices in women both during their reproductive years and longer-term.
Conclusion
In women with preconception overweight and obesity, pregnancy resulted in ~30% increase in VAT that persisted at 59 weeks postpartum despite a return to baseline weight. Moreover, VAT was significantly associated with several cardiometabolic risk factors at 59 weeks postpartum. Finally, women enrolled in a lifestyle intervention to control excess GWG during the second and third trimesters had returned to early pregnancy body weight by 15 weeks postpartum compared to the non-intervention group who had not.
Supplementary Material
What is already known about this subject.
Pregnancy is associated with a central deposition of fat.
Pregnancy weight gain and weight retention postpartum are associated with later maternal obesity and co-morbidities.
What this study adds.
Strong evidence of pregnancy induced increase in visceral adipose tissue, on the order of thirty percent at 59 weeks postpartum, despite a return to baseline weight.
Visceral adipose tissue mass is associated with elevated cardiometabolic risk factors at 59 weeks postpartum, implying a negative health effect.
Pregnant women with overweight and obesity enrolled in a lifestyle intervention to control excess gestational weight gain during the second and third trimesters had returned to early pregnancy body weight by 15 weeks postpartum compared to a non-intervention group who had not.
Acknowledgments
We thank: LIFE-Moms consortium members for their contributions to the development and oversight of common measures and procedures across the trials; LIFT women and infants for enrolling in this study; LIFT staff for their herculean efforts: Kasey Faulkner, Maryanne Holowaty, Jill Johnson, Kim Kelly, Rachel Koletsky, Jennifer Patricio, Julie Roman, and Wen Yu; Rebecca Gersnoviez Clifton, Ph.D., at The George Washington University Biostatistics Center for guidance specific to LIFE-Moms consortium outcomes and definitions. All data generated from this study will be administered in accordance with University and NIH policies, including the most current NIH data release and resource sharing policy. The results from this work will be presented at scientific meetings, published in scientific journals, and made freely available to the public according to NIH guidelines.
Funding: National Institutes of Health Grants U01-DK094463; U01-DK094463-Supplement (Supplement to promote diversity, T. Toro-Ramos, PhD); P30-DK026687; T32-DK007559 (Toro-Ramos, Widen); T32DK091227 (Widen); K99/R00HD086304 to Dr. Widen. Research reported in this publication was supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, U01 DK094418, U01 DK094463, U01 DK094416, 5U01 DK094466 (RCU)), the National Heart, Lung, and Blood Institute (NHLBI, U01 HL114344, U01 HL114377), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U01 HD072834), the National Center for Complementary and Integrative Health (NCCIH), the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the NIH Office of Disease Prevention (ODP), the Indian Health Service, the Intramural Research Program of the NIDDK, and the Office of the Director, National Institutes of Health (OD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the LIFE-Moms consortium members for their contributions to the development and oversight of the common measures and procedures shared across the trials.
Footnotes
Disclosure: The authors declare no conflict of interest.
Clinical Trial Registry ; www.ClinicalTrials.gov
References
- 1.Vesco KK, Dietz PM, Rizzo J, et al. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol. 2009;114(5):1069–75. doi: 10.1097/AOG.0b013e3181baeacf. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine/National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: 2009. Committee to Reexamine IOM Pregnancy Weight Guidelines, Food and Nutrition Board and Board on Children, Youth, and Families. [Google Scholar]
- 3.Cho GJ, Yoon HJ, Kim EJ, Oh MJ, Seo HS, Kim HJ. Postpartum changes in body composition. Obesity (Silver Spring). 2011;19(12):2425–8. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring). 2008;16(5):1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CD, Jacobs DR Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86(1):48–54. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. [DOI] [PubMed] [Google Scholar]
- 7.McClure CK, Catov JM, Ness R, Bodnar LM. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am J Clin Nutr. 2013;98(5):1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widen EM, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr. 2014;68(6):643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes GB. Human Body Composition: Growth, Aging, Nutrition and Activity. New York, NY, USA: Springer-Verlag; 1987. pp. 199–205. [Google Scholar]
- 10.Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942–7. [DOI] [PubMed] [Google Scholar]
- 11.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48(1):24–9. [DOI] [PubMed] [Google Scholar]
- 12.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–8 [DOI] [PubMed] [Google Scholar]
- 13.Fidanza F The density of fat-free body mass during pregnancy. Int J Vitam Nutr Res. 1987;57:104. [PubMed] [Google Scholar]
- 14.Catalano PM, Wong WW, Drago NM, Amini SB. Estimating body composition in late gestation: a new hydration constant for body density and total body water. Am J Physiol. 1995;268:E153–E158. [DOI] [PubMed] [Google Scholar]
- 15.Sohlström A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61(2):287–95. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher D, Rosenn B, Toro-Ramos T, et al. Greater Neonatal Fat-Free Mass and Similar Fat Mass Following a Randomized Trial to Control Excess Gestational Weight Gain. Obesity (Silver Spring). 2018;26(3):578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puhkala J, Raitanen J, Kolu P, et al. Metabolic syndrome in Finnish women 7 years after a gestational diabetes prevention trial. BMJ Open 2017;7:e014565. doi: 10.1136/bmjopen-2016-014565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clifton RG, Evans M, Cahill AG, Franks PW, Gallagher D, Phelan S, Pomeroy J, Redman LM, Van Horn L; LIFE-Moms Research Group. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring). 2016;24(2):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peaceman AM, Clifton RG, Phelan S, et al. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis. Obesity (Silver Spring). 2018;26(9):1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher D, Kelley DE, Yim JE, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 2004;79: 874–880. [DOI] [PubMed] [Google Scholar]
- 22.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc 1995;27:1686–91. [PubMed] [Google Scholar]
- 23.Gallagher D, Thornton JC, He Q, et al. Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased. Obesity (Silver Spring). 2010;18(10):2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pick ME, Edwards M, Moreau D, Ryan EA. Assessment of diet quality in pregnant women using the Healthy Eating Index. J Am Diet Assoc. 2005;105(2):240–6. [DOI] [PubMed] [Google Scholar]
- 25.Vähä-Ypyä H, Vasankari T, Husu P, et al. Validation of Cut-Points for Evaluating the Intensity of Physical Activity with Accelerometry-Based Mean Amplitude Deviation (MAD). PLoS One. 2015;20;10(8):e0134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elffers TW, de Mutsert R, Lamb HJ, de Roos A, Willems van Dijk K, Rosendaal FR, Jukema JW, Trompet S. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS One. 2017. September 28;12(9):e0185403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278(5):E941–8. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SA, Goss AM, Azziz R, Raju DA, Gower BA. Peri-muscular adipose tissue may play a unique role in determining insulin sensitivity/resistance in women with polycystic ovary syndrome. Hum Reprod. 2017;32(1):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin Invest 2003;112: 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa K, Endo J, Katsumata Y, et al. Negative legacy of obesity. PLoS One. 2017;12(10):e0186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116: 39–48. [DOI] [PubMed] [Google Scholar]
- 33.Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, et al. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes 2005;54: 672–678. [DOI] [PubMed] [Google Scholar]
- 34.McClure CK, Catov J, Ness R, Schwarz EB. Maternal visceral adiposity by consistency of lactation. Matern Child Health J. 2012;16(2):316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton-Tyrrell KC. Breastfeeding and subsequent maternal visceral adiposity. Obesity (Silver Spring). 2011;19(11):2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tørris C, Thune I, Emaus A, et al. Duration of lactation, maternal metabolic profile, and body composition in the Norwegian EBBA I-study. Breastfeed Med. 2013;8(1):8–15. [DOI] [PubMed] [Google Scholar]
- 37.Kirkegaard H, Stovring H, Rasmussen KM, Abrams B, Sørensen TI, Nohr EA. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr. 2014;99(2):312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sørensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88(6):1543–51. [DOI] [PubMed] [Google Scholar]
- 39.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheggen RJ, Maessen MF, Green DJ, et al. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev 2016;17:664–90.doi: 10.1111/obr.12406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.