Abstract
Chronic itch poses major health care and economic burdens worldwide. In 2013, B‐type natriuretic peptide (BNP) was identified as an itch‐selective neuropeptide and shown to be both necessary and sufficient to produce itch behaviour in mice. Since then, mechanistic studies of itch have increased, not only at central levels of the spinal relay of itch signalling but also in the periphery and skin. In this review, we have critically analysed recent findings from complementary pharmacological and physiological approaches, combined with genetic strategies to examine the role of BNP in itch transduction and modulation of other pruritic proteins. Additionally, potential targets and possible strategies against BNP signalling are discussed for developing novel therapeutics in itch. Overall, we aim to provide insights into drug development by altering BNP signalling to modulate disease symptoms in chronic itch, including conditions for which no approved treatment exists.
Abbreviation
- AD
atopic dermatitis
- ANP
- BNP
B‐type natriuretic peptide
- DRG
dorsal root ganglion
- GC
guanylyl cyclase
- GRP
gastrin releasing peptide
- HDM
house dust mite
- ISH
in situ hybridization
- phKCs
primary human keratinocytes
- S1P
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=911
- scfv
single‐chain variable fragment
- SNAP‐25
synaptosome‐associated protein 25 kDa
- SNARE
soluble N‐ethylmaleimide‐sensitive factor activating protein receptor
- TG
trigeminal ganglia
- TGN
trigeminal ganglion neurons
- VAMP
vesicle‐associated membrane protein
1. INTRODUCTION
Chronic itch is a major symptom of numerous dermatological and systemic diseases. It substantially impairs patients' quality of life resulting in considerable socio‐economic costs. Current treatment options for chronic itch are limited and do not treat the underlying causes of itch, so there is a major need to develop novel effective treatments. Neuropeptides mediating itch are released by dedicated sensory C‐fibres in response to a range of environmental factors and their release elicits a scratching behavioural response rather than withdrawal. Given that itch is a significant clinical problem affecting a large global population, it is necessary to discuss the recent findings about itch‐related neuropeptides and their signalling in itch transmission. Several itch neuropeptides have been discovered in recent years, including https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=gastrin+releasing+peptide+&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database (GRP), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4890 (BNP, also known as natriuretic polypeptide b [Nppb], encoded by the Nppb gene), and neuromedin B (Nmb; Koga et al., 2011; Mishra, Holzman, & Hoon, 2012; Mishra & Hoon, 2013; Sukhtankar & Ko, 2013; Sun et al., 2009; Sun & Chen, 2007). Because BNP is an important itch‐selective neuropeptide in central and peripheral systems, the function of BNP in itch transmission has become an interesting and intensive research field. Selective targeting of the BNP signalling pathway provides a potential for itch treatment.
2. ORIGIN OF BNP AND ITS FUNCTION
BNP is a 32 amino‐acid cyclic peptide that was initially discovered in extracts of porcine brain. It is released predominantly from cardiac ventricles (Minamino, Aburaya, Ueda, Kangawa, & Matsuo, 1988; Minamino, Kangawa, & Matsuo, 1988) and is homologous to, but clearly distinct from, https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ANP&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database (ANP) in terms of the amino acid sequence (Chinkers & Garbers, 1989; Maekawa et al., 1988; Minamino, Kangawa, & Matsuo, 1988; Seilhamer et al., 1989; Sugimoto, Shigemi, Okuno, Yawata, & Morimoto, 1989). As it was later established that cardiomyocytes mainly produce BNP, BNP was renamed “B‐type natriuretic peptide,” from its previous name of “brain natriuretic peptide.” BNP plays significant roles in cardiac embryogenesis, maintaining cardiovascular homeostasis and regulating blood pressure (Potter, Yoder, Flora, Antos, & Dickey, 2009; Woodard & Rosado, 2007). Ablation of Nppb triggers hypertension and cardiovascular dysfunction and results in reduced itch response to many pruritogens (Table 1). Deletion of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1747 gene (NprA) in mice causes hypertension and leads to cardiac hypertrophy with increased cardiac mass, fibrosis, and inflammation (Table 1). In dilated cardiomyopathy, increased BNP levels were significantly related to the disease severity (Noori, Mahjoubifard, Shahramian, Teimouri, & Jahangirifard, 2015; Noori, Teimouri, & Shahramian, 2017). Three receptors (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1747, https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=NPRB&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, and https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=NPRC&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database) have been identified for BNP, with differing selectivity. NPRA binds to https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=ANP&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database > BNP > > https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=CNP&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, whereas NPRB binds to CNP > ANP > BNP, and the clearance receptor, NPRC, binds all natriuretic peptides equally. These three receptors show different distribution in the body (Table 1). NPRA is a homodimeric membrane guanylyl cyclase expressed in a variety of tissues (Table 1). BNP, upon binding to NPRA, leads to an increase of the intracellular secondary messenger, cGMP (Huntley et al., 2006), and further stimulates three known cGMP effector molecules: cGMP‐dependent PKGs, cGMP‐dependent PDEs, and cGMP‐dependent ion channels. These cascade events produce anti‐hypertensive, anti‐hypertrophic, and anti‐fibrotic effects. Thus, analogues of BNP have been designed in order to reproduce the beneficial effects of NPRA activation in patients with chronic heart failure. In sensory neurons, cGMP permits signal modulation, amplication, and encoding, before depolarization (Gao et al., 2015).
Table 1.
Presence of BNP and its receptors in tissue, and phenotypes of their knockout in mice
| Gene transcripts or protein | Expression | Consequence of knockout in mice |
|---|---|---|
| Nppb | Brain (Sudoh, Minamino, Kangawa, & Matsuo, 1988); kidney (Herten, Lenz, Gerzer, & Drummer, 1998); liver (Vollmar, Paumgartner, & Gerbes, 1997); thymus (Vollmar, Wolf, & Schulz, 1995); cardiac tissues (Kambayashi et al., 1990; Mukoyama et al., 1991); sensory ganglia (Goswami et al., 2014; Zhang et al., 2010); not expressed or tracelly expressed in dorsal spinal cord (Goswami et al., 2014; Wheeler, Lascelles, Olivry, & Mishra, 2019); gonadal adipose (Neinast et al., 2015); mucosal epithelium of terminal and respiratory bronchioles, smooth muscle cells in the lamina propria of terminal bronchioles and vascular smooth muscle cells, alveolar type II cells, and macrophages (Oztop et al., 2019; Vollmar et al., 1995); lens epithelial cells (Cammarata, Braun, Dimitrijevich, & Pack, 2010); Müller cells (Cao, Yu, Zhao, & Yang, 2004); retina (Fernandez‐Durango, Nunez, & Brown, 1995; Rollin, Mediero, Roldan‐Pallares, Fernandez‐Cruz, & Fernandez‐Durango, 2004); fat cells (Lafontan et al., 2005); pulmonary metastases (Margalit et al., 2003); proximal tubular cells (Mistry et al., 1998; Mistry et al., 2000) | Nppb −/− mice have vascular complications, and fibrosis (Holditch, Schreiber, Burnett, & Ikeda, 2016; Potter et al., 2009; Tamura et al., 2000). They also exhibit greatly attenuated responses to a range of pruritic agents (Mishra & Hoon, 2013; Mishra & Hoon, 2015) |
| NprA | Kidney, lung, adipose, adrenal, brain, eye, liver, heart, testis, and vascular smooth muscle tissue, thymus (Fernandez‐Durango et al., 1995; Goy et al., 2001; Lowe et al., 1989; Nagase, Katafuchi, Hirose, & Fujita, 1997; Ohsaki, Gross, Le, Oie, & Johnson, 1999; Sarzani et al., 1999; Sekiguchi et al., 2001; Vollmar et al., 1997; Wilcox, Augustine, Goeddel, & Lowe, 1991); granulosa cells (De Cesaro et al., 2018); very low expressed in sensory ganglia (Goswami et al., 2014; Marchenkova et al., 2015; Vilotti et al., 2013; Zhang et al., 2010); gonadal adipose (Neinast et al., 2015); skeletal muscle (Coue et al., 2015); lens epithelial cells (Cammarata et al., 2010); NG108‐15 (Muller, Hildebrand, Lubberstedt, Kuhn, & Middendorff, 2010); retina (Rollin et al., 2004); proximal tubular cells (Mistry et al., 2000; Noubani, Farookhi, & Gutkowska, 2000); decidua vera, chorion laeve, myometrium, and placenta (Itoh et al., 1994) | NprA −/− mice show high blood pressure, salt sensitivity, volume overload, and cardiac hypertrophy, fibrosis, and inflammation. (Kuhn et al., 2002; Lopez et al., 1995; Oliver et al., 1997; Pandey, 2019) |
| NprB | Bone, brain, fibroblasts, heart, kidney, liver, lung, uterine, and vascular smooth muscle tissue; eye (Bryan et al., 2006; Chrisman, Schulz, Potter, & Garbers, 1993; Dickey et al., 2007; Fernandez‐Durango et al., 1995; Herman, Dolgas, Rucker, & Langub, 1996; Langub, Dolgas, Watson, & Herman, 1995; Sarzani et al., 1999; Vollmar et al., 1997); rat thymus (Vollmar et al., 1995); bovine granulosa cells (De Cesaro et al., 2018); sensory ganglia (Goswami et al., 2014; Mishra & Hoon, 2015); gonadal adipose tissue (Neinast et al., 2015); lens epithelial cells (Cammarata et al., 2010); retina (Rollin et al., 2004); proximal tubular cells (Mistry et al., 1998; Mistry et al., 2000); decidua vera, chorion laeve, myometrium, and placenta (Itoh et al., 1994) | NprB −/− mice have near‐100% post‐natal mortality, show dwarfism, seizures, female sterility, and decreased adiposity (Blaser et al., 2018; Pandey, 2019; Tamura et al., 2004) |
| NprC | Endothelial cells (Leitman et al., 1986); granulosa cells (De Cesaro et al., 2018); adrenal, brain, heart, kidney, liver, mesentery, eye, and vascular smooth muscle tissue (Fernandez‐Durango et al., 1995; Nagase et al., 1997; Potter et al., 2006; Vollmar et al., 1997; Wilcox et al., 1991); thymus (Vollmar et al., 1995); DRG and TG (Goswami et al., 2014); white adipose tissue (Smith, Fahrenkrug, Jorgensen, Christoffersen, & Goetze, 2015); skeletal muscle (Coue et al., 2015); lens epithelial cells (Cammarata et al., 2010); α cells (Burgess et al., 2009); retina (Rollin et al., 2004); proximal tubular cells (Mistry et al., 2000); neonatal brain and in isolated primary cortical neurons (Ma & Zhang, 2018) | NprC −/− mice have a progressively reduced ability to concentrate urine, exhibit mild diuresis, and tend to be blood volume depleted, skeletal deformities associated with a considerable increase in bone turnover (Matsukawa et al., 1999; Pandey, 2019) |
Later on, BNP was detected in retina, where it suppresses https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=gaba+a+receptor&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database‐mediated current of ON‐type rod‐dominant bipolar cells in the rat (Cao, Zhou, & Yang, 2008; Yu, Cao, & Yang, 2006; Table 1). Thus far, BNP has been detected in many tissues (Table 1). In embryonic stem cells, BNP signalling leads to the down‐regulation of https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=gaba+a+receptor&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database genes and, in turn, suppresses the phosphorylation of the histone H2AX to γ‐H2AX. As a result, activation of embryonic stem cell proliferation occurs (Abdelalim & Tooyama, 2009; Table 1). In other pathways, BNP activates NPRB and the transcription factor Ets‐1, which may enhance the proliferation and survival of embryonic stem cells (Abdelalim & Tooyama, 2009; Table 1).
3. DIFFERENTIAL EXPRESSION OF BNP AND ITS RECEPTORS IN PERIPHERAL AND SPINAL CORD NEURONS UNDERLIES THEIR CONTRIBUTIONS TO ITCH
BNP is expressed in the sensory ganglia including DRGs and TGNs but barely expressed in the dorsal horn of the spinal cord (Table 2). Moreover, NPRA, B, C are found in sensory ganglia including DRGs, TGs, and in the spinal cord (Mishra & Hoon, 2013; Zhang et al., 2010; Table 2). However, a more detailed characterization revealed their differential localization, for example, TRPC channels may not express in sensory neurons but it exists in the associated non‐neuronal cells in the ganglia (Table 2).
Table 2.
Differential expression of BNP and its receptors in sensory ganglia and spinal cord
| Gene transcripts or protein | Co‐localized markers | ||
|---|---|---|---|
| Sensory neurons | Spinal cord | ||
| Dorsal horn | Ventral horn | ||
| Nppb | TRPV1+ (Mishra & Hoon, 2013), MrgprA3+ (Usoskin et al., 2015); MrgprC11+ (Mishra & Hoon, 2013), PLCβ (Mishra & Hoon, 2013); CGRP+ and IB4+ neurons (Li et al., 2016); Cysltr2+, Htr1f+, and S1pr1+ (Solinski, Kriegbaum, et al., 2019); somatostatin (Sst)+ (Huang et al., 2018); NMB+ (Mishra & Hoon, 2013); Il31ra+, S100b+, Cpne6+ (Chiu et al., 2014; Li et al., 2016) |
CGRP+ (Abdelalim et al., 2016) |
CGRP+, ChAT+ (Abdelalim et al., 2016) |
| NprA | CGRP+ (Li et al., 2016) | Gastrin releasing peptide (GRP)+ and Sst+ (Chamessian et al., 2018; Kiguchi et al., 2016) | Unknown |
| NprB | Small and medium‐sized CGRP+, IB4+ (Abdelalim, Bellier, & Tooyama, 2013); cGMP‐dependent kinase‐I (cGKI)α + (Schmidt et al., 2007) | CGRP+ (Abdelalim et al., 2013) | Unknown |
| NprC | Not expressed by DRG neurons but localized in cells—most likely Schwann cells or their precursors—associated with the dorsal roots of the spinal cord (Schmidt et al., 2016; Zhao & Ma, 2009) | Neuromedin B receptor (NMBR)+ (Goswami et al., 2014) | Unknown |
The presence of BNP in sensory ganglia has attracted extensive attention especially in itch and pain study. Using the large‐scale single RNA sequencing of mice DRGs, the sensory neurons have been divided into three molecularly different groups, NP1, NP2, and NP3. Among them, NP1 neurons express GPCR https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchWildcard=mrgprd&order=rank&submitWildcard=Do+wildcard+search D (MrgprD) to transmit chemical itch, for example, the itch induced by β‐alanine, and lysophosphatidic acid‐associated cholestatic disorders, as well as light punctate mechanical information. NP2 neurons are characterized by the expression of MrgprA3 and MrgprC11 which also transmit chemical‐induced itch and mediate chloroquine‐ and histamine‐associated acute itch, but not related to pain sensitivity. NP3 neurons are Nppb + neurons, and these neurons express the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1710 (IL‐31Rα) and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2315s, as well as https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=270 (Cysltr2), https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=262 (HRH1), and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=5 (Htr1f) to transduce Il‐31‐, LTD4‐, histamine‐, and 5‐HT‐mediated chronic allergic itch and inflammation (Abdelalim, Bellier, & Tooyama, 2016; Nguyen, Wu, Bonilla, von Buchholtz, & Ryba, 2017; Stantcheva et al., 2016; Usoskin et al., 2015; Table 2). Interestingly, https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=MrgprA3+&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database is not detected in NP3 Nppb + neurons though both NP2 and 3 neurons are partially overlapping populations and express HRH1 as well as IL‐33 receptors. These overlapping neurons also express other neuropeptides and neuropeptide receptors, including https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=2020, neurotensin, and neuropeptide Y. In another study, using flow cytometry in mice models, Nppb was demonstrated to be enriched in a distinct subpopulation with the low levels of Ret and absence of binding to plant isolectin B4, but these neurons express neuropeptides such as somatostatin, along with the NGF receptor, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1817&familyId=326&familyType=CATALYTICRECEPTOR, and multiple transcripts associated with itch. The latter include Cysltr2, IL‐31Rα, and oncostatin M receptor (Table 2). Furthermore, these neurons also express HRH1, Htr1A, Htr1f, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=498, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507, and low levels of MrgprA3 (Stantcheva et al., 2016; Table 2). Interestingly, these neuronal population does not express MrgprC11, endothelin receptors, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5083 receptor transcripts (Il7r and Crlf2), and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485 (Stantcheva et al., 2016). Recently the unique molecular programme associated with Nppb + NP3 neurons was shown to rely on the transcription factor Runx1, as development of NP3 neurons was impaired in Runx1 mutant mice and the impairment resulted in diminished acute and chronic itch response (Qi et al., 2017). Using in situ hybridization (ISH) in sections of human DRGs, the histamine receptor gene, HRH1, was largely co‐expressed with Nppb, and all the Nppb + neurons express HRH1 (Qi et al., 2017). In human DRGs, the neurons positive for the antimalarial drug chloroquine receptor (Mrgprx1)+ express Nppb, although some Nppb + cells are Mrgprx1 −. In addition, ISH also revealed that most of the human Nppb + neurons co‐express IL‐31Rα (Solinski et al., 2019; Table 2). These collective findings are consistent with the involvement of BNP in their agonists‐induced itch behaviours (Mishra & Hoon, 2013; Pitake, Ralph, DeBrecht, & Mishra, 2018). Moreover, the itch responses to subcutaneously injected IL‐31 were significantly attenuated in Nppb‐KO mice and mice treated with Nppb‐saporin, a toxin that ablated 70% BNP receptor positive neurons in the spinal cord (Mishra & Hoon, 2013; Pitake et al., 2018). On the contrary, itch induced by intradermal injection of IL‐31 in mice was not affected by intrathecal injection of Nppb‐saporin and neurokinin B was selectively involved in IL‐31‐induced itch (Sakata et al., 2019). However, the percentage of the neuronal population ablated is lacking in this later study. A similar study is needed using Nppb‐KO mice, to clarify this finding. Moreover, the RNA‐sequence analysis for the overlapping subpopulations of IL‐31RA+ and neurokinin B+ in sensory neurons has not been reported thus far (Chiu et al., 2014; Goswami et al., 2014; Li et al., 2016).
Apart from being the essential neuropeptide in sensory neurons, BNP has been detected in spinal cord motor neurons, and is co‐localized with https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=calcitonin+gene-related+peptide&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=choline+acetyltransferase+&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database in the ventral horn (Abdelalim et al., 2016). Moreover, NPRA was co‐localized with ChAT+ neurons in the brain stem (Abdelalim et al., 2016). Although BNP could play a role in the repair mechanisms following nerve injuries, the exact function of BNP in these neurons has not been established by experiments, so far.
4. ROLE OF BNP IN ITCH TRANSMISSION IN THE SPINAL CORD
There are still debates about the involvement of the BNP located in the spinal cord, in pain or itch. In pain, BNP released from sensory neurons negatively regulates nociceptive transmission through its presynaptic GC receptor NPRA. This results in the activation of intracellular PKG and large‐conductance https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=69 pathways in nociceptive neurons (Abdelalim et al., 2016; Zhang et al., 2010). A previous study showed that rather than generating pain, BNP inhibited the inflammatory pain through activation of NPRA which was expressed in CGRP‐containing neurons, and the BNP as well as NPRA expression levels were increased after peripheral inflammation induced by intraplantar injection of formalin or Freund's complete adjuvant into the adult male rats (Zhang et al., 2010). Moreover, intrathecal injection of BNP inhibited formalin and Freund's complete adjuvant induced pain. Accordingly, intrathecal injection of BNP antibodies into these mice attenuated the recovery from pain (Zhang et al., 2010). Such inhibition of pain in the periphery by BNP was assumed to occur at the spinal cord level. However, these results conflicted with the later findings from Mishra's group showing that BNP‐expressing sensory neurons were not involved in acute, inflammatory, or neuropathic pain, from results with Nppb‐KO mice (Pitake et al., 2018).
The function of BNP as a mediator of itch was first described by Mishra and Hoon (2013). They identified BNP as an itch‐specific neurotransmitter utilized by sensory neurons, and it was both necessary and sufficient to produce itch behaviour in mice. In their results obtained from Nppb‐KO mice, BNP was necessary for both histaminergic and non‐histaminergic itch and was expressed in a small number of TRPV1/MrgprA3/C11+ unmyelinated C‐ and thinly myelinated Aδ‐fibres of primary sensory nerve afferents. They also demonstrated BNP transmitted itch signals to NPRA expressed primarily in lamina I to contribute to spinal processing of itch (Mishra & Hoon, 2013). The BNP released from peripheral sensory neurons stimulates its receptor in NPRA+‐spinal cord neurons to release GRP in the spinal cord to activate canonical https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=39 (Grpr)+‐neurons in order to transmit itch signal to the brain (Hoon, 2015; Kiguchi et al., 2016). In addition, Nppb −/− mice, but not Grpr −/− mice, exhibit scratching behaviour upon intrathecal injection of GRP (Mishra & Hoon, 2013; Sun & Chen, 2007).
Notably, it is still uncertain whether GRP is expressed and acts as a itch neurotransmitter in the sensory ganglia, because several studies were unable to detect its mRNA by ISH (Fleming, Kirby, & Penny, 2012; Mishra & Hoon, 2013; Wada, Way, Lebacq‐Verheyden, & Battey, 1990) or RNA sequencing (Goswami et al., 2014; Usoskin et al., 2015), whereas others claimed it was expressed by primary sensory neurons (Barry et al., 2016). In contrast, GRP was detected predominantly in spinal cord neurons (Goswami et al., 2014; Usoskin et al., 2015). Thus, it is not clear if GRP‐GRPR signalling plays a role in itch in the primary afferents (Liu et al., 2014). Nevertheless, it is possible that GRP expression levels are very low or non‐existent.
To confirm that BNP is selective for itch, Mishra's group also used Nppb‐KO mice in several pain models including chemically induced pain, inflammatory soup‐induced pain, and nerve injury neuropathic pain and demonstrated that there is no any difference detected in perception of pain in these models (Pitake et al., 2018). Moreover, the Nppb + NP3 neurons co‐express several itch‐related receptors including MrgprA3+, MrgprC11+, PLC‐ß, Cysltr2+, Htr1f+, S1pr1+, Sst+, and NMB+ (Table 2).
Recent studies have shown that bile acids accumulated in the tissues of patients with cholestasis induced TRPA1 channel‐dependent release of both GRP and BNP in the dorsal horn of the spinal cord in rats, implicating BNP in the profound pruritus associated with cholestatic disease. Furthermore, this study established that BNP is linked to the disease‐relevant mechanism (Lieu et al., 2014). The significant contribution of spinal BNP to non‐histaminergic itch was confirmed by Kusube et al., (2016), consistent with Mishra's finding that either elimination of BNP or the ablation of spinal interneurons expressing NPRA was enough to profoundly attenuate scratching responses to several pruritogens, including chloroquine, 5‐HT and compound 48/80 in mice (Mishra & Hoon, 2013).
In human psychophysical studies, most chemical‐induced itch sensations are accompanied by weaker nociceptive sensations, such as burning, pricking or stinging (LaMotte, Dong, & Ringkamp, 2014; Liu et al., 2012; Sikand, Dong, & LaMotte, 2011; Sikand, Shimada, Green, & LaMotte, 2009). This raises an interesting question whether BNP is also involved in pain generation in humans. These mixed sensations also question the “selectivity” of itch pathways (Sun et al., 2017). Thus, an elegant study concluded that Grp +‐neurons positively code for itch while negatively regulating pain transmission with a “leaky gate” (Sun, Xu, et al., 2017). In addition, substance P (encoded by Tac1 gene) has been identified as the transmitter used by Grpr + neurons in the activation of the last step in the spinal cord itch circuit and the stimulation of spinothalamic projection interneurons (Sakai et al., 2016). There are several studies on BNP in induction of the delayed modulation of TRPV1 channels and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=480&familyId=77&familyType=ICs in mouse TGNs (Marchenkova, van den Maagdenberg, & Nistri, 2016; Marchenkova, Vilotti, Fabbretti, & Nistri, 2015; Marchenkova, Vilotti, Ntamati, van den Maagdenberg, & Nistri, 2016; Vilotti, Marchenkova, Ntamati, & Nistri, 2013). But these studies lack further linkage of this function with the involvement of BNP in diseases. In‐depth investigations are needed to investigate if BNP is involved in potentiation of these channel proteins to enhance the itch sensitization. It alsonot clear how the BNP‐dependent P2X3 receptor modulation are initiated in trigeminal neurons because NPRA barely exists in sensory neurons (Table 2).
At the spinal cord level, scratching behaviour was significantly diminished upon intrathecal injection of either BNP or GRP in R7bp KO mice, suggesting that the BNP‐GRP pathway is dependent on the Gai/o‐directed GAP activity in sensory systems (Pandey et al., 2017). The inhibitory spinal GABAA receptors mainly contain α1, α2, α3, or α5 subunits together with β2/3 subunits and a γ subunit. The antipruritic action of TPA023B, a selective ligand of the pentameric channel α2/α3GABAA receptor, has been demonstrated to reduce scratching behaviours in the acute model elicited by intrathecal injection of BNP or GRP and the chronic oxazolone models in mice, as well as HDM‐induced atopic dermatitis (AD) model in dogs (Ralvenius et al., 2018). This finding is consistent with an itch inhibitory effect occurring primarily via intrinsic dorsal horn neurons, involving GABA pathways in BNP‐GRP itch transmission (Ralvenius et al., 2018; Figure 1). It is also in agreement with the notion that pain neurons and touch neurons can inhibit ascending itch signals via GABAergic interneurons (Steinhoff, Oaklander, Szabo, Stander, & Schmelz, 2019; Steinhoff, Schmelz, Szabo, & Oaklander, 2018). In relation to the touch‐induced inhibition of both pain and itch, it would also be interesting to investigate if excitation of glycinergic inhibitory neurons in the spinal dorsal horn could also reduce itch inhibition by targeting postsynaptic BNP pathways.
Figure 1.
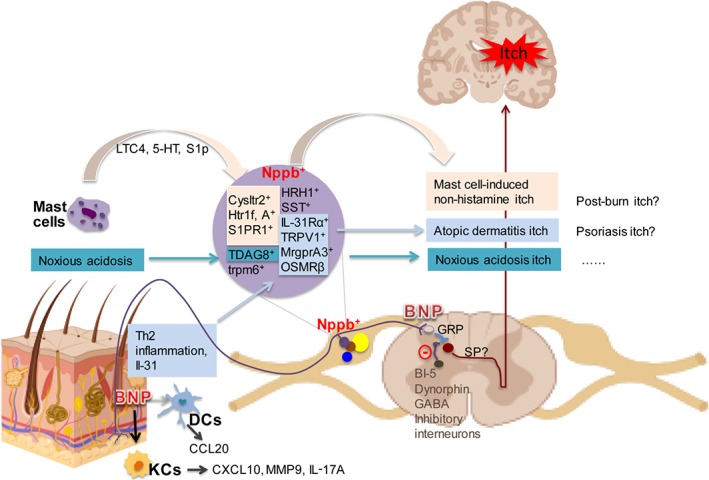
BNP plays critical roles in itch transmission. Mast cell activation elicits the release of LTC4, 5‐HT, and S1P. These mediators subsequently bind to their respective receptors expressed in Nppb +‐sensory neurons. Noxious acidosis activates TDAG8 in Nppb + neurons. Th2 inflammation results in IL‐31 release which activates sensory neurons to release BNP. The elevated BNP level in skin promotes the release from KCs and dendritic cells of several inflammatory and pruritic cytokines including CCL20, CXCL10, IL‐17A, and MMP9 (as indicated). Activation of Nppb +‐sensory neurons also results in BNP release at their central terminals, followed by binding to GRP+ spinal cord neurons. This BNP‐GRP itch pathway is under the regulation by BI‐5, dynorphin, and GABA releasing inhibitory interneurons. In addition, inhibition of BNP receptors by antagonists or inducing GABAA receptor expression on GRP neurons can lead to block of the pruritus. [Colour figure can be viewed at http://wileyonlinelibrary.com]
5. PERIPHERAL ROLE OF BNP IN ITCHY SKIN
Nppb + NP3 neurons innervate the border area between dermis and epidermis in both hairy and glabrous skin (Stantcheva et al., 2016), and NPRA and NPRB are expressed in both epidermis and dermis (Meng et al., 2018). In the periphery, somatostatin is exclusively expressed in Nppb + neurons (Table 2). In these neurons, somatostatin potentiates itch by inhibiting inhibitory dynorphin subset B5‐I neurons, and this results in disinhibition of Grpr +‐neurons (Huang et al., 2018). Recently, our group found that BNP is also expressed in chronic pruritic AD skin and its innervated sensory neurons (Meng et al., 2018). Additionally, the signal from immuno‐reactive BNP is enhanced in skin from AD patients or from HDM‐treated mice (Meng et al., 2018; Figure 1). Likewise, levels of BNP in the skin were increased in animal models of itch (Ewald et al., 2017; Liu et al., 2016; Meng et al., 2018), as well as in diseased skins with AD (Meng et al., 2018; Figure 2a) or psoriatic conditions (Meng et al., 2018; Nattkemper et al., 2018). We also observed that BNP level was increased in human prurigo nodularis skin (Figure 2a). All of these findings implicated BNP in persistent itch in skin, although peripheral injection of BNP is unable to elicit itch behaviours in mice (Mishra & Hoon, 2013).
Figure 2.
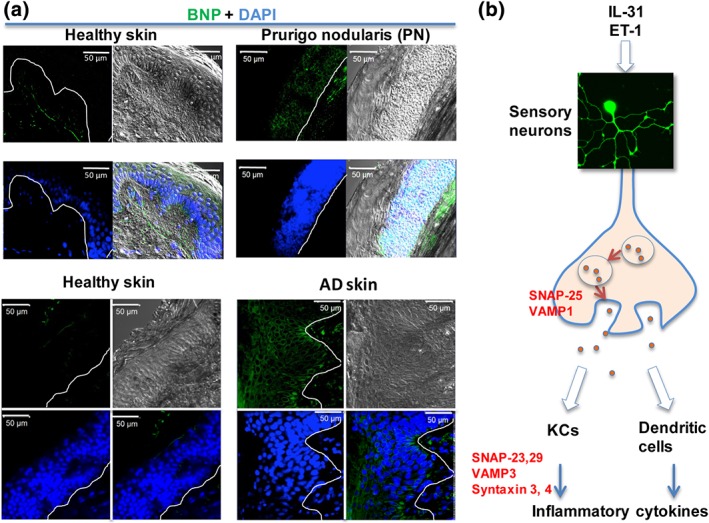
BNP immuno‐reactive signals are increased in human itchy skin and its release from sensory neurons is via a SNARE‐dependent mechanism. (a) Skin BNP is up‐regulated in itchy human skin, including AD and prurigo nodularis (PN); green, BNP; and blue, DAPI. (b) IL‐31‐ and ET‐1‐induced BNP release from sensory neurons is through SNARE‐dependent mechanisms. In addition, different isoforms of SNARE proteins are also required by BNP‐induced pruritic and inflammatory cytokine release from phKCs, including ET‐1. The latter can further stimulate BNP release from sensory neurons. Thus, it might result in amplification of itch circuits. Immunohistochemistry was performed as in Meng et al. (2018). All the clinical samples were purchased from Tissue Solutions Ltd (Glasgow) [Colour figure can be viewed at http://wileyonlinelibrary.com]
We have shown that BNP is an essential messenger molecule for Th2 cell‐mediated itch transduction in skin (Meng et al., 2018). In primary human keratinocytes (phKCs), several key intracellular phospho‐kinases were activated by BNP, and this elicited several cytokines (Figure 2b), involved in itchy skin (Meng et al., 2018). The effect of BNP in skin cells is closely correlated with dermatological diseases and conditions (Meng, Wang, Buddenkotte, Buhl, & Steinhoff, 2019). To reveal the connections between the shared differentially regulated genes (Figure 3a), we constructed the biomolecular networks in BNP‐activated phKCs (Figure 3b), and the established and putative interactions of cytokines were shown using IPA analysis (Figure 3b). The differentially regulated genes were associated with one another directly or indirectly. Validating these signalling pathways may aid in identification of biomarkers for chronic itch or potential therapeutic targets.
Figure 3.
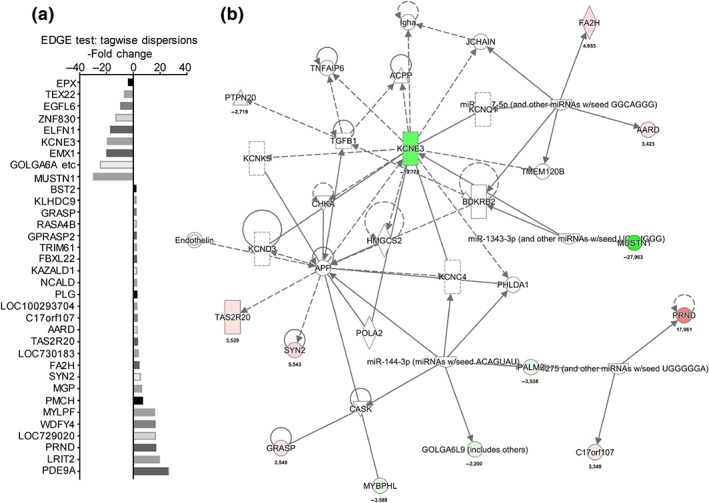
Transcriptome analysis of BNP‐treated phKCs showed changes of gene transcriptions which generated de novo discovery of pathways in phKCs. (a) Genes affected by BNP in phKCs. (b) Highly interconnected networks illustrate the relationship between the regulated gene profiles identified by RNAseq from BNP‐treated phKCs. Hot and cold coloured genes are up‐ and down‐regulated respectively. Transcriptome experiments and analysis was carried as described in Meng et al. (2019). IPA analysis was performed using QIAGEN's Ingenuity Pathway Analysis (QIAGEN Bioinformatics) software [Colour figure can be viewed at http://wileyonlinelibrary.com]
Within AD, different TRP channels constitute a large family of ion channels activated in response to distinct itch stimuli at skin, peripheral, and central sensory neuronal levels. Importantly, BNP was demonstrated by ISH to be expressed in a fraction of both mouse and human TRPV1 +‐neurons (Solinski, Dranchak, et al., 2019). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4995 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=989 (ET‐1) released from Th2 cells initiate a closed itch circuit between sensory neurons and skin cells, as well as immune cells, through BNP. Thus, BNP acts as an itch relay centre (Meng et al., 2018; Meng et al., 2019). In addition, BNP further augments AD‐related cytokine release from skin cells, through its specific receptors elevated or activated in diseased epidermal skin. Notably, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1753s mediate ET‐1 release from keratinocytes, which might activate DRGs, leading to the release of BNP. In this scheme, the toll‐like receptor represents an “innate biosensor” to bridge skin and central itch (Szollosi et al., 2019). Thus, BNP plays a bidirectional part on intercellular networks to initiate itch at the level of the spinal cord and simultaneously to amplify itch‐related inflammatory signals in peripheral skin.
Although BNP is known to be the key molecule in Th2‐initiated itch circuits in AD through IL‐31‐regulated mechanisms, it is not known if BNP could modulate TRP, the non‐selective cation channels, in the sensory neurons and the skin to contribute to itch, even though Nppb + and MrgprA3 + neuronal cells co‐express the TRPV1 (Liu et al., 2009; Mishra & Hoon, 2013) and TRPA1 channels (Lieu et al., 2014). The TRPV1 channels respond to many different noxious stimuli and were involved in IL‐31‐mediated itch in mice (Cevikbas et al., 2014). Thus BNP modulation of TRPV1 channels might underlie hypersensitivity to noxious stimulation in neurogenic inflammation.
Another significant role for BNP in skin‐derived itch is likely because Nppb +‐neurons are the sensors of mast cell‐induced itch (Figure 1). Skin‐resident mast cells are key players in the histamine‐dependent, type 1 hypersensitivity itch. Mast cells also release 5‐HT, LTC4, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=911 (S1P) to induce scratch responses via GRP signalling in spinal cord. Interestingly, these non‐histamine pruriception from mast cells are dependent on Nppb +‐sensory neurons activation because 5‐HT1F, CysLT2 and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=135 were expressed in Nppb +‐neurons (Solinski et al., 2019). In addition, noxious acidosis‐induced itch upon intradermal injection into mice, also activates Nppb +‐sensory neurons via a TRPV1 channel‐dependent mechanism, and the proton‐sensing GPCR, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=113, is highly expressed on Nppb +‐neurons (Lin et al., 2017; Figure 1).
6. POTENTIAL THERAPEUTIC TARGETS FOR BNP SIGNALLING IN ITCH
Insight into the functionality of BNP in modulation of itch signal transmission, pruritic channel function, and mediating the release of itch mediators certainly aids in developing novel effective therapies to treat this debilitating and widespread condition. As a result, targeting BNP signalling is a logical approach to new therapeutic agents. Although BNP signalling has been effectively targeted in animal models of acute and chronic itch, there is still a long way to go for clinically useful compounds.
Thus far, many antagonists have been synthesized to inhibit the BNP signalling pathway. These classic pharmacological inhibitors against BNP signalling include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4952, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4866 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4856. HS‐142‐1 is a potent non‐peptide microbial polysaccharide antagonizing natriuretic peptide receptors (Morishita et al., 1991) and anantin is an NPR antagonist extracted from fungi to reduce the increase of intracellular cGMP produced by ANP or BNP (Wyss, Lahm, Manneberg, & Labhardt, 1991). A71915 is a synthetic derivative of ANP and can inhibit NPRA competitively (Delporte, Winand, Poloczek, Von Geldern, & Christophe, 1992). Among them, only A71915 has been tested for itch relief in animal models (Solinski, Dranchak, et al., 2019).
6.1. Current achievement in targeting BNP signalling for itch relief
Initially, Kiguchi et al. observed that the onset and magnitude of intrathecal BNP‐induced scratching are slower and smaller than that induced by intrathecal GRP (Kiguchi et al., 2016), thus raising the hypothesis that BNP initiates itch indirectly through activation of a GRP pathway. They showed that intrathecal administration of BNP antagonist A71915 in mice had no effect on intrathecal GRP‐induced scratching, whereas the GRP antagonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6183 inhibited BNP‐induced scratching (Kiguchi et al., 2016), confirming that the BNP‐NPRA system may act upstream of GRP‐GRPR to regulate itch in the mouse spinal cord (Kiguchi et al., 2016). In their study, A71915 also had little effect on peripherally elicited scratching upon intradermal injection of ET‐1, thromboxane A2 analogue, https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=BAM8-22&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database (activator of MrgprC11), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3741 (a https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=348 agonist) in mice, thus concluding that there was a minimal role for the spinal BNP‐NPRA system in regulating peripheral itch (Kiguchi et al., 2016). Their finding was later challenged by Mishra & Hoon (2013), who found that elimination of Nppb or the ablation of spinal interneurons expressing NPRA profoundly attenuated scratching response to intradermal administration of histamine, chloroquine, ET‐1, 5‐HT, SLIGRL, and compound 48/80. Quite recently, the effectiveness of A71915 in blocking NPRA activity by competing for binding sites with BNP, was re‐examined. They showed that A71915, in fact, acts partly as a strong agonist at NPRA (Solinski, Dranchak, et al., 2019). Further high‐throughput screening on a stable cGMP sensory cell line expressing human NPRA and NPRB to screen the candidate molecules from a large chemical library, identified a specific antagonist JS‐11 and two other promising ligands for human NPRA and NPRB (Solinski, Dranchak, et al., 2019). Upon intraperitoneal or intrathecal administration, JS‐11 was effective in eliminating pruritic behaviours in acute and contact dermatitis mice models as well as relief of IL‐31‐dependent hapten‐induced itch, but not skin inflammation, in mice (Solinski, Dranchak, et al., 2019). This is a breakthrough finding that has great potential in finding a direct inhibitor for the BNP‐NPR pathways, with good prospects for alleviating itch. However, as rightly recognised by the authors, new compounds such as JS‐11 still have limitations because of relatively low affinities, cross‐reactivities, possible side effects and inadequate physiocochemical properties.
6.2. Antibody targeting of BNP pathways
BNP can be used as diagnostic and prognostic marker for patients with heart failure. It is noteworthy that so far the screening of anti‐BNP single‐chain variable fragment (scFv) from phage display, and its production in Escherichia coli or in methylotrophic yeast Pichia pastoris, have been successful (Bu, Zhou, Tang, Jing, & Zhang, 2013; Maeng, Choi, Sa, Shin, & Kim, 2012; Maeng, Nam, & Kim, 2011; Zhang, 2013). However, these antibodies have not yet been used as therapeutic agents to inhibit BNP‐NPRA signalling, despite being highly specific. Most of them were designed to measure BNP levels in the analytical diagnosis of heart failure, with the advantage of their small size compared with that of the whole antibody.
Most recently, a therapeutically relevant, intact full‐length anti‐NPRA IgG4‐humanized monoclonal antibody has been created and identified to specifically target the NPRA extracellular domain bound to its natural cyclic natriuretic peptide ligands (Blech et al., 2019). However, this antibody was created to stabilize the receptor‐peptide complex and potentiate BNP‐dependent cGMP production in NPRA‐presenting HEK cells.
It might be worthwhile to develop the functional anti‐BNP ScFv, or the full length antibody, to reduce levels of BNP in skin or to antagonize the biological action of NPRA by interrupting the BNP‐NPRA binding, in order to interfere with itch transmission.
6.3. Targeting exocytotic proteins involved in BNP release
For targeting purposes, one of the interesting findings is that the synthesis and release of BNP from pruritic sensory neurons differ from other neurotransmitters, such as https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=Substance+P&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database and CGRP. Release of BNP could be elicited by IL‐31 (Meng et al., 2019; Pitake et al., 2018) and ET‐1 (Meng et al., 2019), the key itch mediators that play important roles in the pathogenesis of AD. In contrast, IL‐31 did not elicit synthesis and release of CGRP or substance P. The enhanced BNP release, but not its resting level, from pruritic sensory neurons requires a particular exocytotic process involving the soluble N‐ethylmaleimide‐sensitive factor activating protein receptor (SNARE) (Meng & Wang, 2015). We found that vesicle‐associated membrane protein 1 (VAMP1) and synaptosome‐associated protein 25 kDa (SNAP‐25), but not VAMP7, mediated BNP release (Meng et al., 2018). These exocytotic proteins might serve as promising targets for blocking BNP‐mediated itch (Figure 2b). In addition, different SNARE isoforms also regulate the release of ET‐1, thymic stromal lymphopoietin, and many other itch‐related cytokine/chemokines, from skin cells that play important roles in immune‐neuron communication (Meng et al., 2019; Figure 2b). Thus, targeting these SNARE proteins should break the crosstalk link between sensory neurons and skin cells. In this perspective, SNARE‐inactivating agents, such as botulinum neurotoxins, have shown promising in certain itchy diseases which include lichen simplex chronicus, psoriasis and rosacea, meralgia paresthetica, and post‐burn (Akhtar & Brooks, 2012; Boozalis, Sheu, Selph, & Kwatra, 2018; Heckmann, Heyer, Brunner, & Plewig, 2002; Perez‐Perez et al., 2014; Salardini, Richardson, & Jabbari, 2008; Wallengren & Bartosik, 2010; Weinfeld, 2007; Zanchi et al., 2008). Altogether, these findings highlight the importance of SNARE as a therapeutic target in the treatment of chronic itch diseases.
6.4. Targeting BNP‐activated intracellular kinases
Intracellular kinases including https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=518, and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=509 activated by BNP in phKCs, might also be potential targets (Meng et al., 2018). Upon their activation, BNP induced release of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=835, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1633, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4982. Inhibition of glycogen synthase kinase 3 abolished IL‐17A release. IL‐17A is a critical cytokine implicated in itchy atopic and psoriatic skin where it feeds back to KCs to activate further inflammatory response. In addition, BNP also activated c‐Jun in cultured human dendritic cells, and meantime, it induced CCL20 release. This neuron‐immuno‐modulatory mechanism might result in the activation of immature dendritic cells, which could contribute to the persistent itch (Meng et al., 2019). Selective inhibition of these kinases might benefit the recovery of itch and its associated inflammatory skin conditions. As these intracellular kinases are also needed by other cell types or tissues, local application of these inhibitors is necessary to avoid the potential side effects.
6.5. Targeting upstream regulators of BNP
In phKCs, BNP also induced transcription changes of many other genes including WD repeat‐ and FYVE domain‐containing protein 4 (Meng et al., 2019; Figure 3a). Some of these genes were shown be linked to certain types of skin diseases, for example, WD repeat‐ and FYVE domain‐containing protein 4 is known to be associated with the autoimmune disease, systemic lupus erythematosus (Yang et al., 2010; Zhang, Bo, Zhang, Zhuang, & Liu, 2014). Further validation of these genes might identify molecules that are pivotal in regulating BNP signalling. Importantly, in relation to the predicted BNP‐induced network in phKCs (Figure 3b), their upstream regulators might include key itch mediators such as IL‐27 and plasminogen activator, urokinase (PLAU) or receptor (PLAUR), or endothelin receptor (Table 3). These upstream regulators need to be validated before being considered as potential targets for BNP‐mediated itch signalling.
Table 3.
BNP‐induced transcription changes in phKCs predicted the upstream regulators that might serve as potential targets for blocking BNP‐mediated, skin‐derived, itch signalling
| Top upstream regulators | Molecule type | Activation z‐score | P value of overlap | Target molecules in dataset |
|---|---|---|---|---|
| miR‐1254 (and other miRNAs w/seed GCCUGGA) | Mature microrna | 1,122 | 1,61E‐03 | EMX1, PTPN20, TAS2R20, TEX22 |
| IL27 | Cytokine | 1,70E‐03 | BST2, LAG3, TNFSF13 | |
| SNAP91 | Other | 1,85E‐03 | BST2 | |
| PLAUR | Transmembrane receptor | 2,02E‐03 | C5AR1, PLG | |
| miR‐296‐5p (miRNAs w/seed GGGCCCC) | Mature microrna | −0,020 | 2,36E‐03 | BMP8A, GRASP, KAZALD1, NCALD, TEX22, TNFSF13 |
| miR‐4515 (miRNAs w/seed GGACUGG) | Mature microrna | 2,73E‐03 | EMX1, GOLGA6L9 (includes others), LAG3 | |
| KCNE2 | Ion channel | 3,69E‐03 | KCNE3 | |
| miR‐4660 (miRNAs w/seed GCAGCUC) | Mature microrna | 5,50E‐03 | GOLGA6A (includes others), NCALD, SYN2 | |
| INPP1 | Phosphatase | 5,53E‐03 | MYLPF | |
| EPZ004777 | Chemical reagent | 5,53E‐03 | HOXA10 | |
| DNM2 | Enzyme | 5,53E‐03 | BST2 | |
| Lanthanum chloride | Chemical reagent | 5,53E‐03 | MGP | |
| PLAU | Peptidase | 6,43E‐03 | C5AR1, PLG | |
| Endothelin receptor | Group | 7,36E‐03 | MYLPF | |
| RGL2 | Other | 7,36E‐03 | MYLPF | |
| miR‐4661‐3p (and other miRNAs w/seed AGGAUCC) | Mature microrna | 8,48E‐03 | AARD, BMP8A | |
| CCL15 | Cytokine | 9,20E‐03 | EPX | |
| miR‐1299 (miRNAs w/seed UCUGGAA) | Mature microrna | 1,05E‐02 | GOLGA6A (includes others), NCALD | |
| CAMP | Other | 1,05E‐02 | BMP8A, C5AR1 | |
| AP2M1 | Transporter | 1,10E‐02 | BST2 |
7. FUTURE DIRECTIONS
The recent advances in our understanding of the molecular mechanism of BNP action in itch communication between immune cells, sensory neurons, and spinal cord central neurons highlight its importance as a therapeutic target to block itch transmission. It facilitates drug development with biomarkers of chronic itch across a range of skin diseases. Future studies will focus on evaluating the utility of BNP as the biomarker for chronic itch diagnosis and evaluation of effectiveness of targeted therapeutics; investigate if any of the established antagonists, such as HS‐142‐1 or anantin, would act as itch relief agents; and create antibodies interrupting BNP‐NPRA pathways. Moreover, experiments will also involve the confirmation of lack of side effects on other tissues expressing NPRA, including the kidney and the vasculature (Potter, Abbey‐Hosch, & Dickey, 2006). For example, it would be worth investigating if interfering with BNP signalling or NPRA pathways might have effects on blood pressure and hypertension, sodium excretion, vascular complication, salt sensitivity, volume overload, and cardiac hypertrophy, inflammation, and fibrosis, as described and observed in mice after ablation of BNP or NPRA (Table 1).
Considering that the BNP‐NPRA itch signalling pathway is conserved between mice and humans, defining JS‐11 or developing other more potent and selective NPRA antagonists would be beneficial in relief of itch in humans. Such relief would be valuable in a range of conditions including AD, cholestatic liver disease, opioid therapy and also nervous system disorders (e.g., multiple sclerosis), as well as infection and end stage kidney disease (Lieu et al., 2014). Although BNP has only recently been discovered, inhibition of its signalling pathways provides a real prospect for the treatment of chronic itch, a condition that is mostly resistant to treatment with traditional medicines. There is a real need for newly designed inhibitors for NPRA, for patient‐reported outcomes and for clinical assessments.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a; Alexander, Fabbro et al., 2019b; Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENTS
The authors' research that is relevant for this review is and has been supported by Science Foundation Ireland (SFI) Investigators' Awards: Grant 15/SIRG/3508T to J.M. and Grants 13/CDA/2093 and 17/TIDA/4977 to J.W.
Meng J, Chen W, Wang J. Interventions in the B‐type natriuretic peptide signalling pathway as a means of controlling chronic itch. Br J Pharmacol. 2020;177:1025–1040. 10.1111/bph.14952
Contributor Information
Jianghui Meng, Email: jianghui.meng@dcu.ie.
Jiafu Wang, Email: jiafu.wang@dcu.ie.
REFERENCES
- Abdelalim, E. M. , Bellier, J. P. , & Tooyama, I. (2013). Expression of NPR‐B in neurons of the dorsal root ganglia of the rat. Peptides, 43, 56–61. 10.1016/j.peptides.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Abdelalim, E. M. , Bellier, J. P. , & Tooyama, I. (2016). Localization of brain natriuretic peptide immunoreactivity in rat spinal cord. Frontiers in Neuroanatomy, 10, 116 10.3389/fnana.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelalim, E. M. , & Tooyama, I. (2009). BNP signaling is crucial for embryonic stem cell proliferation. PLoS ONE, 4, e5341 10.1371/journal.pone.0005341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar, N. , & Brooks, P. (2012). The use of botulinum toxin in the management of burns itching: Preliminary results. Burns, 38, 1119–1123. 10.1016/j.burns.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, D. M. , Li, H. , Liu, X. Y. , Shen, K. F. , Liu, X. T. , Wu, Z. Y. , … Chen, Z. F. (2016). Critical evaluation of the expression of gastrin‐releasing peptide in dorsal root ganglia and spinal cord. Molecular Pain, 12, 174480691664372 10.1177/1744806916643724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser, M. C. , Wei, K. , Adams, R. L. E. , Zhou, Y. Q. , Caruso, L. L. , Mirzaei, Z. , … Simmons, C. A. (2018). Deficiency of natriuretic peptide receptor 2 promotes bicuspid aortic valves, aortic valve disease, left ventricular dysfunction, and ascending aortic dilatations in mice. Circulation Research, 122, 405–416. 10.1161/CIRCRESAHA.117.311194 [DOI] [PubMed] [Google Scholar]
- Blech, M. , Horer, S. , Kuhn, A. B. , Kube, S. , Goddeke, H. , Kiefer, H. , … Tully, M. D. (2019). Structure of a therapeutic full‐length anti‐NPRA IgG4 antibody: Dissecting conformational diversity. Biophysical Journal, 116, 1637–1649. 10.1016/j.bpj.2019.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozalis, E. , Sheu, M. , Selph, J. , & Kwatra, S. G. (2018). Botulinum toxin type A for the treatment of localized recalcitrant chronic pruritus. Journal of the American Academy of Dermatology, 78, 192–194. 10.1016/j.jaad.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Bryan, P. M. , Smirnov, D. , Smolenski, A. , Feil, S. , Feil, R. , Hofmann, F. , … Potter, L. R. (2006). A sensitive method for determining the phosphorylation status of natriuretic peptide receptors: cGK‐Iα does not regulate NPR‐A. Biochemistry, 45, 1295–1303. 10.1021/bi051253d [DOI] [PubMed] [Google Scholar]
- Bu, D. , Zhou, Y. , Tang, J. , Jing, F. , & Zhang, W. (2013). Expression and purification of a novel therapeutic single‐chain variable fragment antibody against BNP from inclusion bodies of Escherichia coli. Protein Expression and Purification, 92, 203–207. 10.1016/j.pep.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Burgess, M. D. , Moore, K. D. , Carter, G. M. , Alli, A. A. , Granda, C. S. , Ichii, H. , … Gower WR Jr (2009). C‐type natriuretic peptide receptor expression in pancreatic α cells. Histochemistry and Cell Biology, 132, 95–103. 10.1007/s00418-009-0591-3 [DOI] [PubMed] [Google Scholar]
- Cammarata, P. R. , Braun, B. , Dimitrijevich, S. D. , & Pack, J. (2010). Characterization and functional expression of the natriuretic peptide system in human lens epithelial cells. Molecular Vision, 16, 630–638. [PMC free article] [PubMed] [Google Scholar]
- Cao, L. H. , Yu, Y. C. , Zhao, J. W. , & Yang, X. L. (2004). Expression of natriuretic peptides in rat Muller cells. Neuroscience Letters, 365, 176–179. 10.1016/j.neulet.2004.04.090 [DOI] [PubMed] [Google Scholar]
- Cao, L. H. , Zhou, B. , & Yang, X. L. (2008). Modulation by BNP of GABA receptors on ON‐type rod bipolar cells is dependent on subcellular sites. Brain Research, 1216, 46–52. 10.1016/j.brainres.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Cevikbas, F. , Wang, X. , Akiyama, T. , Kempkes, C. , Savinko, T. , Antal, A. , … Steinhoff, M. (2014). A sensory neuron‐expressed IL‐31 receptor mediates T helper cell‐dependent itch: Involvement of TRPV1 and TRPA1. The Journal of Allergy and Clinical Immunology, 133, 448–460. 10.1016/j.jaci.2013.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamessian, A. , Young, M. , Qadri, Y. , Berta, T. , Ji, R. R. , & Van de Ven, T. (2018). Transcriptional profiling of somatostatin interneurons in the spinal dorsal horn. Scientific Reports, 8, 6809 10.1038/s41598-018-25110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers, M. , & Garbers, D. L. (1989). The protein kinase domain of the ANP receptor is required for signaling. Science, 245, 1392–1394. 10.1126/science.2571188 [DOI] [PubMed] [Google Scholar]
- Chiu, I. M. , Barrett, L. B. , Williams, E. K. , Strochlic, D. E. , Lee, S. , Weyer, A. D. , … Woolf, C. J. (2014). Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife, 3 10.7554/eLife.04660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman, T. D. , Schulz, S. , Potter, L. R. , & Garbers, D. L. (1993). Seminal plasma factors that cause large elevations in cellular cyclic GMP are C‐type natriuretic peptides. The Journal of Biological Chemistry, 268, 3698–3703. [PubMed] [Google Scholar]
- Coue, M. , Badin, P. M. , Vila, I. K. , Laurens, C. , Louche, K. , Marques, M. A. , … Galgani, J. E. (2015). Defective natriuretic peptide receptor signaling in skeletal muscle links obesity to type 2 diabetes. Diabetes, 64, 4033–4045. 10.2337/db15-0305 [DOI] [PubMed] [Google Scholar]
- De Cesaro, M. P. , Dos Santos, J. T. , Ferst, J. G. , Nobrega, J. E. Jr. , Rosa, P. , Rovani, M. T. , … Bordignon, V. (2018). Natriuretic peptide system regulation in granulosa cells during follicle deviation and ovulation in cattle. Reproduction in Domestic Animals, 53, 710–717. 10.1111/rda.13161 [DOI] [PubMed] [Google Scholar]
- Delporte, C. , Winand, J. , Poloczek, P. , Von Geldern, T. , & Christophe, J. (1992). Discovery of a potent atrial natriuretic peptide antagonist for ANPA receptors in the human neuroblastoma NB‐OK‐1 cell line. European Journal of Pharmacology, 224, 183–188. 10.1016/0014-2999(92)90803-c [DOI] [PubMed] [Google Scholar]
- Dickey, D. M. , Flora, D. R. , Bryan, P. M. , Xu, X. , Chen, Y. , & Potter, L. R. (2007). Differential regulation of membrane guanylyl cyclases in congestive heart failure: Natriuretic peptide receptor (NPR)‐B, not NPR‐A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology, 148, 3518–3522. 10.1210/en.2007-0081 [DOI] [PubMed] [Google Scholar]
- Dong, W. , Wu, D. F. , Li, G. S. , Wu, D. W. , & Wang, Z. C. (2018). Next‐generation sequencing from bulked segregant analysis identifies a dwarfism gene in watermelon. Scientific Reports, 8(1), 290–298. 10.1038/s41598-018-21293-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald, D. A. , Noda, S. , Oliva, M. , Litman, T. , Nakajima, S. , Li, X. , … Guttman‐Yassky, E. (2017). Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. The Journal of Allergy and Clinical Immunology, 139, 562–571. 10.1016/j.jaci.2016.08.029 [DOI] [PubMed] [Google Scholar]
- Fernandez‐Durango, R. , Nunez, D. J. , & Brown, M. J. (1995). Messenger RNAs encoding the natriuretic peptides and their receptors are expressed in the eye. Experimental Eye Research, 61, 723–729. 10.1016/s0014-4835(05)80023-5 [DOI] [PubMed] [Google Scholar]
- Fleming, M. , Kirby, B. , & Penny, K. I. (2012). Record linkage in Scotland and its applications to health research. Journal of Clinical Nursing, 21, 2711–2721. 10.1111/j.1365-2702.2011.04021.x [DOI] [PubMed] [Google Scholar]
- Gao, S. , Nagpal, J. , Schneider, M. W. , Kozjak‐Pavlovic, V. , Nagel, G. , & Gottschalk, A. (2015). Optogenetic manipulation of cGMP in cells and animals by the tightly light‐regulated guanylyl‐cyclase opsin CyclOp. Nature Communications, 6, 8046 10.1038/ncomms9046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, S. C. , Thierry‐Mieg, D. , Thierry‐Mieg, J. , Mishra, S. , Hoon, M. A. , Mannes, A. J. , & Iadarola, M. J. (2014). Itch‐associated peptides: RNA‐Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Molecular Pain, 10, 44 10.1186/1744-8069-10-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy, M. F. , Oliver, P. M. , Purdy, K. E. , Knowles, J. W. , Fox, J. E. , Mohler, P. J. , … Maeda, N. (2001). Evidence for a novel natriuretic peptide receptor that prefers brain natriuretic peptide over atrial natriuretic peptide. The Biochemical Journal, 358, 379–387. 10.1042/0264-6021:3580379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann, M. , Heyer, G. , Brunner, B. , & Plewig, G. (2002). Botulinum toxin type A injection in the treatment of lichen simplex: An open pilot study. Journal of the American Academy of Dermatology, 46, 617–619. 10.1067/mjd.2002.120455 [DOI] [PubMed] [Google Scholar]
- Herman, J. P. , Dolgas, C. M. , Rucker, D. , & Langub, M. C. Jr. (1996). Localization of natriuretic peptide‐activated guanylate cyclase mRNAs in the rat brain. The Journal of Comparative Neurology, 369, 165–187. [DOI] [PubMed] [Google Scholar]
- Herten, M. , Lenz, W. , Gerzer, R. , & Drummer, C. (1998). The renal natriuretic peptide urodilatin is present in human kidney. Nephrology, Dialysis, Transplantation, 13, 2529–2535. 10.1093/ndt/13.10.2529 [DOI] [PubMed] [Google Scholar]
- Holditch, S. J. , Schreiber, C. A. , Burnett, J. C. , & Ikeda, Y. (2016). Arterial remodeling in B‐type natriuretic peptide knock‐out females. Scientific Reports, 6, 25623 10.1038/srep25623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon, M. A. (2015). Molecular dissection of itch. Current Opinion in Neurobiology, 34, 61–66. 10.1016/j.conb.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Polgar, E. , Solinski, H. J. , Mishra, S. K. , Tseng, P. Y. , Iwagaki, N. , … Zeilhofer, H. U. (2018). Circuit dissection of the role of somatostatin in itch and pain. Nature Neuroscience, 21, 707–716. 10.1038/s41593-018-0119-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley, B. K. , Sandberg, S. M. , Noser, J. A. , Cataliotti, A. , Redfield, M. M. , Matsuda, Y. , & Burnett, J. C. (2006). BNP‐induced activation of cGMP in human cardiac fibroblasts: Interactions with fibronectin and natriuretic peptide receptors. Journal of Cellular Physiology, 209, 943–949. 10.1002/jcp.20793 [DOI] [PubMed] [Google Scholar]
- Itoh, H. , Sagawa, N. , Hasegawa, M. , Nanno, H. , Kobayashi, F. , Ihara, Y. , … Yoshimasa, T. (1994). Expression of biologically active receptors for natriuretic peptides in the human uterus during pregnancy. Biochemical and Biophysical Research Communications, 203, 602–607. 10.1006/bbrc.1994.2225 [DOI] [PubMed] [Google Scholar]
- Kambayashi, Y. , Nakao, K. , Mukoyama, M. , Saito, Y. , Ogawa, Y. , Shiono, S. , … Imura, H. (1990). Isolation and sequence determination of human brain natriuretic peptide in human atrium. FEBS Letters, 259, 341–345. 10.1016/0014-5793(90)80043-i [DOI] [PubMed] [Google Scholar]
- Kiguchi, N. , Sukhtankar, D. D. , Ding, H. , Tanaka, K. , Kishioka, S. , Peters, C. M. , & Ko, M. C. (2016). Spinal functions of B‐type natriuretic peptide, gastrin‐releasing peptide, and their cognate receptors for regulating itch in mice. The Journal of Pharmacology and Experimental Therapeutics, 356, 596–603. 10.1124/jpet.115.229997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga, K. , Chen, T. , Li, X. Y. , Descalzi, G. , Ling, J. , Gu, J. , & Zhuo, M. (2011). Glutamate acts as a neurotransmitter for gastrin releasing peptide‐sensitive and insensitive itch‐related synaptic transmission in mammalian spinal cord. Molecular Pain, 7, 47 10.1186/1744-8069-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, M. , Holtwick, R. , Baba, H. A. , Perriard, J. C. , Schmitz, W. , & Ehler, E. (2002). Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC‐A) deficient mice. Heart, 87, 368–374. 10.1136/heart.87.4.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusube, F. , Tominaga, M. , Kawasaki, H. , Yamakura, F. , Naito, H. , Ogawa, H. , … Takamori, K. (2016). Electrophysiological properties of brain‐natriuretic peptide‐ and gastrin‐releasing peptide‐responsive dorsal horn neurons in spinal itch transmission. Neuroscience Letters, 627, 51–60. 10.1016/j.neulet.2016.05.051 [DOI] [PubMed] [Google Scholar]
- Lafontan, M. , Moro, C. , Sengenes, C. , Galitzky, J. , Crampes, F. , & Berlan, M. (2005). An unsuspected metabolic role for atrial natriuretic peptides: The control of lipolysis, lipid mobilization, and systemic nonesterified fatty acids levels in humans. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 2032–2042. 10.1161/01.ATV.0000183728.14712.d8 [DOI] [PubMed] [Google Scholar]
- LaMotte, R. H. , Dong, X. , & Ringkamp, M. (2014). Sensory neurons and circuits mediating itch. Nature Reviews. Neuroscience, 15, 19–31. 10.1038/nrn3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langub, M. C. Jr. , Dolgas, C. M. , Watson, R. E. Jr. , & Herman, J. P. (1995). The C‐type natriuretic peptide receptor is the predominant natriuretic peptide receptor mRNA expressed in rat hypothalamus. Journal of Neuroendocrinology, 7, 305–309. 10.1111/j.1365-2826.1995.tb00762.x [DOI] [PubMed] [Google Scholar]
- Leitman, D. C. , Andresen, J. W. , Kuno, T. , Kamisaki, Y. , Chang, J. K. , & Murad, F. (1986). Identification of multiple binding sites for atrial natriuretic factor by affinity cross‐linking in cultured endothelial cells. The Journal of Biological Chemistry, 261, 11650–11655. [PubMed] [Google Scholar]
- Li, C. L. , Li, K. C. , Wu, D. , Chen, Y. , Luo, H. , Zhao, J. R. , … Zhang, X. (2016). Somatosensory neuron types identified by high‐coverage single‐cell RNA‐sequencing and functional heterogeneity. Cell Research, 26, 83–102. 10.1038/cr.2015.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu, T. , Jayaweera, G. , Zhao, P. , Poole, D. P. , Jensen, D. , Grace, M. , … Bunnett, N. W. (2014). The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology, 147, 1417–1428. 10.1053/j.gastro.2014.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. H. , Steinhoff, M. , Ikoma, A. , Chang, Y. C. , Cheng, Y. R. , Chandra Kopparaju, R. , … Chen, C. C. (2017). Involvement of TRPV1 and TDAG8 in pruriception associated with noxious acidosis. The Journal of Investigative Dermatology, 137, 170–178. 10.1016/j.jid.2016.07.037 [DOI] [PubMed] [Google Scholar]
- Liu, B. , Tai, Y. , Achanta, S. , Kaelberer, M. M. , Caceres, A. I. , Shao, X. , … Jordt, S. E. (2016). IL‐33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proceedings of the National Academy of Sciences of the United States of America, 113, E7572–E7579. 10.1073/pnas.1606608113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Sikand, P. , Ma, C. , Tang, Z. , Han, L. , Li, Z. , … Dong, X. (2012). Mechanisms of itch evoked by β‐alanine. The Journal of Neuroscience, 32, 14532–14537. 10.1523/JNEUROSCI.3509-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Tang, Z. , Surdenikova, L. , Kim, S. , Patel, K. N. , Kim, A. , … Dong, X. (2009). Sensory neuron‐specific GPCR Mrgprs are itch receptors mediating chloroquine‐induced pruritus. Cell, 139, 1353–1365. 10.1016/j.cell.2009.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. Y. , Wan, L. , Huo, F. Q. , Barry, D. M. , Li, H. , Zhao, Z. Q. , & Chen, Z. F. (2014). B‐type natriuretic peptide is neither itch‐specific nor functions upstream of the GRP‐GRPR signaling pathway. Molecular Pain, 10, 4 10.1186/1744-8069-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, M. J. , Wong, S. K. , Kishimoto, I. , Dubois, S. , Mach, V. , Friesen, J. , … Beuve, A. (1995). Salt‐resistant hypertension in mice lacking the guanylyl cyclase‐A receptor for atrial natriuretic peptide. Nature, 378, 65–68. 10.1038/378065a0 [DOI] [PubMed] [Google Scholar]
- Lowe, D. G. , Chang, M. S. , Hellmiss, R. , Chen, E. , Singh, S. , Garbers, D. L. , & Goeddel, D. V. (1989). Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. The EMBO Journal, 8, 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. , & Zhang, L. (2018). C‐type natriuretic peptide functions as an innate neuroprotectant in neonatal hypoxic‐ischemic brain injury in mouse via natriuretic peptide receptor 2. Experimental Neurology, 304, 58–66. 10.1016/j.expneurol.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa, K. , Sudoh, T. , Furusawa, M. , Minamino, N. , Kangawa, K. , Ohkubo, H. , … Matsuo, H. (1988). Cloning and sequence analysis of cDNA encoding a precursor for porcine brain natriuretic peptide. Biochemical and Biophysical Research Communications, 157, 410–416. 10.1016/s0006-291x(88)80062-7 [DOI] [PubMed] [Google Scholar]
- Maeng, B. H. , Choi, J. , Sa, Y. S. , Shin, J. H. , & Kim, Y. H. (2012). Functional expression of recombinant anti‐BNP scFv in methylotrophic yeast Pichia pastoris and application as a recognition molecule in electrochemical sensors. World Journal of Microbiology and Biotechnology, 28, 1027–1034. 10.1007/s11274-011-0901-5 [DOI] [PubMed] [Google Scholar]
- Maeng, B. H. , Nam, D. H. , & Kim, Y. H. (2011). Coexpression of molecular chaperones to enhance functional expression of anti‐BNP scFv in the cytoplasm of Escherichia coli for the detection of B‐type natriuretic peptide. World Journal of Microbiology and Biotechnology, 27, 1391–1398. 10.1007/s11274-010-0590-5 [DOI] [PubMed] [Google Scholar]
- Marchenkova, A. , van den Maagdenberg, A. M. , & Nistri, A. (2016). Loss of inhibition by brain natriuretic peptide over P2X3 receptors contributes to enhanced spike firing of trigeminal ganglion neurons in a mouse model of familial hemiplegic migraine type‐1. Neuroscience, 331, 197–205. 10.1016/j.neuroscience.2016.06.034 [DOI] [PubMed] [Google Scholar]
- Marchenkova, A. , Vilotti, S. , Fabbretti, E. , & Nistri, A. (2015). Brain natriuretic peptide constitutively downregulates P2X3 receptors by controlling their phosphorylation state and membrane localization. Molecular Pain, 11, 71 10.1186/s12990-015-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenkova, A. , Vilotti, S. , Ntamati, N. , van den Maagdenberg, A. M. , & Nistri, A. (2016). Inefficient constitutive inhibition of P2X3 receptors by brain natriuretic peptide system contributes to sensitization of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine. Molecular Pain, 12, 174480691664611 10.1177/1744806916646110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit, O. , Eisenbach, L. , Amariglio, N. , Kaminski, N. , Harmelin, A. , Pfeffer, R. , … Berger, R. (2003). Overexpression of a set of genes, including WISP‐1, common to pulmonary metastases of both mouse D122 Lewis lung carcinoma and B16‐F10.9 melanoma cell lines. British Journal of Cancer, 89, 314–319. 10.1038/sj.bjc.6600977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa, N. , Grzesik, W. J. , Takahashi, N. , Pandey, K. N. , Pang, S. , Yamauchi, M. , & Smithies, O. (1999). The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proceedings of the National Academy of Sciences of the United States of America, 96, 7403–7408. 10.1073/pnas.96.13.7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, J. , Moriyama, M. , Feld, M. , Buddenkotte, J. , Buhl, T. , Szollosi, A. , … Reeh, P. W. (2018). New mechanism underlying IL‐31‐induced atopic dermatitis. The Journal of Allergy and Clinical Immunology, 141(1677–1689), e1678 10.1016/j.jaci.2017.12.1002 [DOI] [PubMed] [Google Scholar]
- Meng, J. , & Wang, J. (2015). Role of SNARE proteins in tumourigenesis and their potential as targets for novel anti‐cancer therapeutics. Biochimica et Biophysica Acta, 1856, 1–12. 10.1016/j.bbcan.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Meng, J. , Wang, J. , Buddenkotte, J. , Buhl, T. , & Steinhoff, M. (2019). Role of SNAREs in the atopic dermatitis‐related cytokine secretion and skin‐nerve communication. The Journal of Investigative Dermatology, 139, 2324–2333. 10.1016/j.jid.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Minamino, N. , Aburaya, M. , Ueda, S. , Kangawa, K. , & Matsuo, H. (1988). The presence of brain natriuretic peptide of 12,000 daltons in porcine heart. Biochemical and Biophysical Research Communications, 155, 740–746. 10.1016/s0006-291x(88)80557-6 [DOI] [PubMed] [Google Scholar]
- Minamino, N. , Kangawa, K. , & Matsuo, H. (1988). Isolation and identification of a high molecular weight brain natriuretic peptide in porcine cardiac atrium. Biochemical and Biophysical Research Communications, 157, 402–409. 10.1016/S0006-291X(88)80061-5 [DOI] [PubMed] [Google Scholar]
- Mishra, S. K. , Holzman, S. , & Hoon, M. A. (2012). A nociceptive signaling role for neuromedin B. The Journal of Neuroscience, 32, 8686–8695. 10.1523/JNEUROSCI.1533-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S. K. , & Hoon, M. A. (2013). The cells and circuitry for itch responses in mice. Science, 340, 968–971. 10.1126/science.1233765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S. K. , & Hoon, M. A. (2015). Transmission of pruriceptive signals. Handbook of Experimental Pharmacology, 226, 151–162. 10.1007/978-3-662-44605-8_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry, S. , Lussert, B. , Stewart, K. , Hawksworth, G. M. , Struthers, A. , & McLay, J. S. (2000). The expression and secretion of atrial natriuretic factor and brain natriuretic peptide by rat proximal tubular cells. Biochemical Pharmacology, 59, 783–790. 10.1016/s0006-2952(99)00379-2 [DOI] [PubMed] [Google Scholar]
- Mistry, S. K. , Chatterjee, P. K. , Weerackody, R. P. , Hawksworth, G. M. , Knott, R. M. , & McLay, J. S. (1998). Evidence for atrial natriuretic factor induced natriuretic peptide receptor subtype switching in rat proximal tubular cells during culture. Experimental Nephrology, 6, 104–111. 10.1159/000020512 [DOI] [PubMed] [Google Scholar]
- Morishita, Y. , Sano, T. , Ando, K. , Saitoh, Y. , Kase, H. , Yamada, K. , & Matsuda, Y. (1991). Microbial polysaccharide, HS‐142‐1, competitively and selectively inhibits ANP binding to its guanylyl cyclase‐containing receptor. Biochemical and Biophysical Research Communications, 176, 949–957. 10.1016/0006-291x(91)90374-g [DOI] [PubMed] [Google Scholar]
- Mukoyama, M. , Nakao, K. , Hosoda, K. , Suga, S. , Saito, Y. , Ogawa, Y. , … Yasue, H. (1991). Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. The Journal of Clinical Investigation, 87, 1402–1412. 10.1172/JCI115146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, D. , Hildebrand, M. , Lubberstedt, J. , Kuhn, M. , & Middendorff, R. (2010). The membrane receptors guanylyl cyclase‐A and ‐B undergo distinctive changes in post‐translational modification during brain development. Journal of Neurochemistry, 115, 1024–1034. 10.1111/j.1471-4159.2010.06985.x [DOI] [PubMed] [Google Scholar]
- Nagase, M. , Katafuchi, T. , Hirose, S. , & Fujita, T. (1997). Tissue distribution and localization of natriuretic peptide receptor subtypes in stroke‐prone spontaneously hypertensive rats. Journal of Hypertension, 15, 1235–1243. 10.1097/00004872-199715110-00007 [DOI] [PubMed] [Google Scholar]
- Nattkemper, L. A. , Tey, H. L. , Valdes‐Rodriguez, R. , Lee, H. , Mollanazar, N. K. , Albornoz, C. , … Yosipovitch, G. (2018). The genetics of chronic itch: Gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. The Journal of Investigative Dermatology, 138, 1311–1317. 10.1016/j.jid.2017.12.029 [DOI] [PubMed] [Google Scholar]
- Neinast, M. D. , Frank, A. P. , Zechner, J. F. , Li, Q. , Vishvanath, L. , Palmer, B. F. , … Clegg, D. J. (2015). Activation of natriuretic peptides and the sympathetic nervous system following Roux‐en‐Y gastric bypass is associated with gonadal adipose tissues browning. Molecular Metabolism, 4, 427–436. 10.1016/j.molmet.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, M. Q. , Wu, Y. , Bonilla, L. S. , von Buchholtz, L. J. , & Ryba, N. J. P. (2017). Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS ONE, 12, e0185543 10.1371/journal.pone.0185543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori, N. M. , Mahjoubifard, M. , Shahramian, I. , Teimouri, A. , & Jahangirifard, A. (2015). Comparison between procalcitonin, brain natriuretic peptide, and uric acid in children with cardiomyopathy and controls. BioMed Research International, 2015, 510450 10.1155/2015/510450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori, N. M. , Teimouri, A. , & Shahramian, I. (2017). Comparison between brain natriuretic peptide and calcitonin gene‐related peptide in children with dilated cardiomyopathy and controls. Nigerian Medical Journal, 58, 37–43. 10.4103/0300-1652.218413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubani, A. , Farookhi, R. , & Gutkowska, J. (2000). B‐type natriuretic peptide receptor expression and activity are hormonally regulated in rat ovarian cells. Endocrinology, 141, 551–559. 10.1210/endo.141.2.7305 [DOI] [PubMed] [Google Scholar]
- Ohsaki, Y. , Gross, A. J. , Le, P. T. , Oie, H. , & Johnson, B. E. (1999). Human small cell lung cancer cells produce brain natriuretic peptide. Oncology, 56, 155–159. 10.1159/000011957 [DOI] [PubMed] [Google Scholar]
- Oliver, P. M. , Fox, J. E. , Kim, R. , Rockman, H. A. , Kim, H. S. , Reddick, R. L. , … Maeda, N. (1997). Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proceedings of the National Academy of Sciences of the United States of America, 94, 14730–14735. 10.1073/pnas.94.26.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop, M. , Ozbek, M. , Liman, N. , Beyaz, F. , Ergun, E. , Ergun, L. , … Ergen, E. (2019). Expression patterns of natriuretic peptides in pre‐hibernating and hibernating anatolian ground squirrel (Spermophilus xanthoprymnus) lung. Acta Histochemica, 121, 852–865. 10.1016/j.acthis.2019.08.003 [DOI] [PubMed] [Google Scholar]
- Pandey, K. N. (2019). Genetic ablation and guanylyl cyclase/natriuretic peptide receptor‐A: Impact on the pathophysiology of cardiovascular dysfunction. International Journal of Molecular Sciences, 20 10.3390/ijms20163946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, M. , Zhang, J. H. , Mishra, S. K. , Adikaram, P. R. , Harris, B. , Kahler, J. F. , … Simonds, W. F. (2017). A central role for R7bp in the regulation of itch sensation. Pain, 158, 931–944. 10.1097/j.pain.0000000000000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Perez, L. , Garcia‐Gavin, J. , Allegue, F. , Caeiro, J. L. , Fabeiro, J. M. , & Zulaica, A. (2014). Notalgia paresthetica: Treatment using intradermal botulinum toxin A. Actas Dermo‐Sifiliográficas, 105, 74–77. 10.1016/j.adengl.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Pitake, S. , Ralph, P. C. , DeBrecht, J. , & Mishra, S. K. (2018). Atopic dermatitis linked cytokine interleukin‐31 induced itch mediated via a neuropeptide natriuretic polypeptide B. Acta Dermato‐Venereologica, 98, 795–796. 10.2340/00015555-2977 [DOI] [PubMed] [Google Scholar]
- Potter, L. R. , Abbey‐Hosch, S. , & Dickey, D. M. (2006). Natriuretic peptides, their receptors, and cyclic guanosine monophosphate‐dependent signaling functions. Endocrine Reviews, 27, 47–72. 10.1210/er.2005-0014 [DOI] [PubMed] [Google Scholar]
- Potter, L. R. , Yoder, A. R. , Flora, D. R. , Antos, L. K. , & Dickey, D. M. (2009). Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handbook of Experimental Pharmacology, 341–366. 10.1007/978-3-540-68964-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. , Huang, C. , Wu, X. , Tao, Y. , Yan, J. , Shi, T. , … Liu, Y. (2017). Hierarchical specification of pruriceptors by runt‐domain transcription factor Runx1. The Journal of Neuroscience, 37, 5549–5561. 10.1523/JNEUROSCI.0094-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralvenius, W. T. , Neumann, E. , Pagani, M. , Acuna, M. A. , Wildner, H. , Benke, D. , … Frauenknecht, K. (2018). Itch suppression in mice and dogs by modulation of spinal α2 and α3GABAA receptors. Nature Communications, 9, 3230 10.1038/s41467-018-05709-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin, R. , Mediero, A. , Roldan‐Pallares, M. , Fernandez‐Cruz, A. , & Fernandez‐Durango, R. (2004). Natriuretic peptide system in the human retina. Molecular Vision, 10, 15–22. [PubMed] [Google Scholar]
- Sakai, K. , Sanders, K. M. , Youssef, M. R. , Yanushefski, K. M. , Jensen, L. , Yosipovitch, G. , & Akiyama, T. (2016). Mouse model of imiquimod‐induced psoriatic itch. Pain, 157, 2536–2543. 10.1097/j.pain.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata, D. , Uruno, T. , Matsubara, K. , Andoh, T. , Yamamura, K. , Magoshi, Y. , … Fukui, Y. (2019). Selective role of neurokinin B in IL‐31‐induced itch response in mice. The Journal of Allergy and Clinical Immunology, 144, 1130–1133.e8. 10.1016/j.jaci.2019.06.031 [DOI] [PubMed] [Google Scholar]
- Salardini, A. , Richardson, D. , & Jabbari, B. (2008). Relief of intractable pruritus after administration of botulinum toxin A (botox): A case report. Clinical Neuropharmacology, 31, 303–306. 10.1097/WNF.0b013e3181672225 [DOI] [PubMed] [Google Scholar]
- Sarzani, R. , Opocher, G. , Paci, M. V. , Belloni, A. S. , Mantero, F. , Dessi‐Fulgheri, P. , & Rappelli, A. (1999). Natriuretic peptides receptors in human aldosterone‐secreting adenomas. Journal of Endocrinological Investigation, 22, 514–518. 10.1007/BF03343602 [DOI] [PubMed] [Google Scholar]
- Schmidt, H. , Peters, S. , Frank, K. , Wen, L. , Feil, R. , & Rathjen, F. G. (2016). Dorsal root ganglion axon bifurcation tolerates increased cyclic GMP levels: The role of phosphodiesterase 2A and scavenger receptor Npr3. The European Journal of Neuroscience, 44, 2991–3000. 10.1111/ejn.13434 [DOI] [PubMed] [Google Scholar]
- Schmidt, H. , Stonkute, A. , Juttner, R. , Schaffer, S. , Buttgereit, J. , Feil, R. , … Rathjen, F. G. (2007). The receptor guanylyl cyclase Npr2 is essential for sensory axon bifurcation within the spinal cord. The Journal of Cell Biology, 179, 331–340. 10.1083/jcb.200707176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhamer, J. J. , Arfsten, A. , Miller, J. A. , Lundquist, P. , Scarborough, R. M. , Lewicki, J. A. , & Porter, J. G. (1989). Human and canine gene homologs of porcine brain natriuretic peptide. Biochemical and Biophysical Research Communications, 165, 650–658. 10.1016/s0006-291x(89)80015-4 [DOI] [PubMed] [Google Scholar]
- Sekiguchi, T. , Miyamoto, K. , Mizutani, T. , Yamada, K. , Yazawa, T. , Yoshino, M. , … Kojima, M. (2001). Molecular cloning of natriuretic peptide receptor A from bullfrog (Rana catesbeiana) brain and its functional expression. Gene, 273, 251–257. 10.1016/s0378-1119(01)00585-6 [DOI] [PubMed] [Google Scholar]
- Sikand, P. , Dong, X. , & LaMotte, R. H. (2011). BAM8‐22 peptide produces itch and nociceptive sensations in humans independent of histamine release. The Journal of Neuroscience, 31, 7563–7567. 10.1523/JNEUROSCI.1192-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand, P. , Shimada, S. G. , Green, B. G. , & LaMotte, R. H. (2009). Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain, 144, 66–75. 10.1016/j.pain.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. , Fahrenkrug, J. , Jorgensen, H. L. , Christoffersen, C. , & Goetze, J. P. (2015). Diurnal gene expression of lipolytic natriuretic peptide receptors in white adipose tissue. Endocrine Connections, 4, 206–214. 10.1530/EC-15-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinski, H. J. , Dranchak, P. , Oliphant, E. , Gu, X. , Earnest, T. W. , Braisted, J. , … Hoon, M. A. (2019). Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Science Translational Medicine, 11, eaav5464 10.1126/scitranslmed.aav5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinski, H. J. , Kriegbaum, M. C. , Tseng, P. Y. , Earnest, T. W. , Gu, X. , Barik, A. , … Hoon, M. A. (2019). Nppb neurons are sensors of mast cell‐induced itch. Cell Reports, 26(3561–3573), e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stantcheva, K. K. , Iovino, L. , Dhandapani, R. , Martinez, C. , Castaldi, L. , Nocchi, L. , … Heppenstall, P. A. (2016). A subpopulation of itch‐sensing neurons marked by Ret and somatostatin expression. EMBO Reports, 17, 585–600. 10.15252/embr.201540983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff, M. , Oaklander, A. L. , Szabo, I. L. , Stander, S. , & Schmelz, M. (2019). Neuropathic itch. Pain, 160(Suppl 1), S11–S16. 10.1097/j.pain.0000000000001551 [DOI] [PubMed] [Google Scholar]
- Steinhoff, M. , Schmelz, M. , Szabo, I. L. , & Oaklander, A. L. (2018). Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurology, 17, 709–720. 10.1016/S1474-4422(18)30217-5 [DOI] [PubMed] [Google Scholar]
- Sudoh, T. , Minamino, N. , Kangawa, K. , & Matsuo, H. (1988). Brain natriuretic peptide‐32: N‐terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochemical and Biophysical Research Communications, 155, 726–732. 10.1016/s0006-291x(88)80555-2 [DOI] [PubMed] [Google Scholar]
- Sugimoto, E. , Shigemi, K. , Okuno, T. , Yawata, T. , & Morimoto, T. (1989). Effect of ANP on circulating blood volume. The American Journal of Physiology, 257, R127–R131. 10.1152/ajpregu.1989.257.1.R127 [DOI] [PubMed] [Google Scholar]
- Sukhtankar, D. D. , & Ko, M. C. (2013). Physiological function of gastrin‐releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS ONE, 8, e67422 10.1371/journal.pone.0067422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Wang, G. H. , Zhang, X. , Zhang, X. R. , Qiao, P. , Long, L. , … Cai, Y. (2017). Genome‐wide identification of the TIFY gene family in three cultivated Gossypium species and the expression of JAZ genes. Scientific Reports, 7, 9 10.1038/srep42418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S. , Xu, Q. , Guo, C. , Guan, Y. , Liu, Q. , & Dong, X. (2017). Leaky gate model: Intensity‐dependent coding of pain and itch in the spinal cord. Neuron, 93(4), 840–853, e845 10.1016/j.neuron.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. G. , & Chen, Z. F. (2007). A gastrin‐releasing peptide receptor mediates the itch sensation in the spinal cord. Nature, 448, 700–703. 10.1038/nature06029 [DOI] [PubMed] [Google Scholar]
- Sun, Y. G. , Zhao, Z. Q. , Meng, X. L. , Yin, J. , Liu, X. Y. , & Chen, Z. F. (2009). Cellular basis of itch sensation. Science, 325, 1531–1534. 10.1126/science.1174868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi, A. G. , McDonald, I. , Szabo, I. L. , Meng, J. , van den Bogaard, E. , & Steinhoff, M. (2019). TLR3 in chronic human itch: A keratinocyte‐associated mechanism of peripheral itch sensitization. The Journal of Investigative Dermatology, 139(11), 2393–2396, e2396 10.1016/j.jid.2019.04.018 [DOI] [PubMed] [Google Scholar]
- Tamura, N. , Doolittle, L. K. , Hammer, R. E. , Shelton, J. M. , Richardson, J. A. , & Garbers, D. L. (2004). Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proceedings of the National Academy of Sciences of the United States of America, 101, 17300–17305. 10.1073/pnas.0407894101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, N. , Ogawa, Y. , Chusho, H. , Nakamura, K. , Nakao, K. , Suda, M. , … Katsuki, M. (2000). Cardiac fibrosis in mice lacking brain natriuretic peptide. Proceedings of the National Academy of Sciences of the United States of America, 97, 4239–4244. 10.1073/pnas.070371497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin, D. , Furlan, A. , Islam, S. , Abdo, H. , Lonnerberg, P. , Lou, D. , … Linnarsson, S. (2015). Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nature Neuroscience, 18, 145–153. 10.1038/nn.3881 [DOI] [PubMed] [Google Scholar]
- Vilotti, S. , Marchenkova, A. , Ntamati, N. , & Nistri, A. (2013). B‐type natriuretic peptide‐induced delayed modulation of TRPV1 and P2X3 receptors of mouse trigeminal sensory neurons. PLoS ONE, 8, e81138 10.1371/journal.pone.0081138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar, A. M. , Paumgartner, G. , & Gerbes, A. L. (1997). Differential gene expression of the three natriuretic peptides and natriuretic peptide receptor subtypes in human liver. Gut, 40, 145–150. 10.1136/gut.40.1.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar, A. M. , Wolf, R. , & Schulz, R. (1995). Co‐expression of the natriuretic peptides (ANP, BNP, CNP) and their receptors in normal and acutely involuted rat thymus. Journal of Neuroimmunology, 57, 117–127. 10.1016/0165-5728(94)00171-j [DOI] [PubMed] [Google Scholar]
- Wada, E. , Way, J. , Lebacq‐Verheyden, A. M. , & Battey, J. F. (1990). Neuromedin B and gastrin‐releasing peptide mRNAs are differentially distributed in the rat nervous system. The Journal of Neuroscience, 10, 2917–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallengren, J. , & Bartosik, J. (2010). Botulinum toxin type A for neuropathic itch. The British Journal of Dermatology, 163, 424–426. 10.1111/j.1365-2133.2010.09783.x [DOI] [PubMed] [Google Scholar]
- Wang, D. J. , Yang, C. L. , Dong, L. , Zhu, J. C. , Wang, J. P. , & Zhang, S. F. (2015). Comparative transcriptome analyses of drought‐resistant and ‐ susceptible Brassica napus L. and development of EST‐SSR markers by RNA‐Seq. Journal of Plant Biology, 58, 259–269. 10.1007/s12374-015-0113-x [DOI] [Google Scholar]
- Weinfeld, P. K. (2007). Successful treatment of notalgia paresthetica with botulinum toxin type A. Archives of Dermatology, 143, 980–982. 10.1001/archderm.143.8.980 [DOI] [PubMed] [Google Scholar]
- Wheeler, J. J. , Lascelles, B. D. , Olivry, T. , & Mishra, S. K. (2019). Itch‐associated neuropeptides and their receptor expression in dog dorsal root ganglia and spinal cord. Acta Dermato‐Venereologica, 99, 1131–1135. 10.2340/00015555-3297 [DOI] [PubMed] [Google Scholar]
- Wilcox, J. N. , Augustine, A. , Goeddel, D. V. , & Lowe, D. G. (1991). Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Molecular and Cellular Biology, 11, 3454–3462. 10.1128/mcb.11.7.3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard, G. E. , & Rosado, J. A. (2007). Recent advances in natriuretic peptide research. Journal of Cellular and Molecular Medicine, 11, 1263–1271. 10.1111/j.1582-4934.2007.00125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss, D. F. , Lahm, H. W. , Manneberg, M. , & Labhardt, A. M. (1991). Anantin—A peptide antagonist of the atrial natriuretic factor (ANF). II. Determination of the primary sequence by NMR on the basis of proton assignments. Journal of Antibiotics (Tokyo), 44, 172–180. 10.7164/antibiotics.44.172 [DOI] [PubMed] [Google Scholar]
- Yang, W. , Shen, N. , Ye, D. Q. , Liu, Q. , Zhang, Y. , Qian, X. X. , … Asian Lupus Genetics Consortium (ALGC) (2010). Genome‐wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genetics, 6, e1000841 10.1371/journal.pgen.1000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. C. , Cao, L. H. , & Yang, X. L. (2006). Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON‐type bipolar cells. The Journal of Neuroscience, 26, 696–707. 10.1523/JNEUROSCI.3653-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi, M. , Favot, F. , Bizzarini, M. , Piai, M. , Donini, M. , & Sedona, P. (2008). Botulinum toxin type‐A for the treatment of inverse psoriasis. Journal of the European Academy of Dermatology and Venereology, 22, 431–436. 10.1111/j.1468-3083.2007.02457.x [DOI] [PubMed] [Google Scholar]
- Zhang, F. X. , Liu, X. J. , Gong, L. Q. , Yao, J. R. , Li, K. C. , Li, Z. Y. , … Zhang, X. (2010). Inhibition of inflammatory pain by activating B‐type natriuretic peptide signal pathway in nociceptive sensory neurons. The Journal of Neuroscience, 30, 10927–10938. 10.1523/JNEUROSCI.0657-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. F. , Lu, T. T. , Mia, W. W. , Sun, L. R. , Tian, M. , Wang, J. , & Hao, F. (2017). Genome‐wide identification of ABA receptor PYL family and expression analysis of PYLs in response to ABA and osmotic stress in Gossypium. PeerJ, 5, 30, e4126 10.7717/peerj.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. (2013). Phage display generation of a novel humanized single‐chain antibody against brain natriuretic peptide with potent neutralizing activity. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 32, 187–192. 10.1089/mab.2012.0125 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Bo, L. , Zhang, H. , Zhuang, C. , & Liu, R. (2014). E26 transformation‐specific‐1 (ETS1) and WDFY family member 4 (WDFY4) polymorphisms in Chinese patients with rheumatoid arthritis. International Journal of Molecular Sciences, 15, 2712–2721. 10.3390/ijms15022712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , & Ma, L. (2009). Regulation of axonal development by natriuretic peptide hormones. Proceedings of the National Academy of Sciences of the United States of America, 106, 18016–18021. 10.1073/pnas.0906880106 [DOI] [PMC free article] [PubMed] [Google Scholar]


