Abstract
Background and Purpose
Alcohol exposure in utero may lead to a wide range of long‐lasting morphological and behavioural deficiencies known as fetal alcohol spectrum disorders (FASD), associated with a higher risk of later developing neuropsychiatric disorders. However, little is known about the long‐term consequences of cocaine use and abuse in individuals with FASD. This study aimed to investigate the effects of maternal binge alcohol drinking during prenatal and postnatal periods on cocaine reward‐related behaviours in adult offspring.
Experimental Approach
Pregnant C57BL/6 female mice were exposed to an experimental protocol of binge alcohol consumption (drinking‐in‐the‐dark test) from gestation to weaning. Male offspring were subsequently left undisturbed until reaching adulthood and were tested for cocaine‐induced motivational responses (conditioned place preference, behavioural sensitization and operant self‐administration). Protein expression of dopamine‐ and glutamate‐related molecules was assessed following cocaine‐induced reinstatement.
Key Results
The results show that prenatal and postnatal alcohol exposure enhanced the preference for the cocaine‐paired chamber in the conditioned place preference test. Furthermore, early alcohol‐exposed mice displayed attenuated cocaine‐induced behavioural sensitization but also higher cocaine self‐administration. Furthermore, alterations in glutamatergic excitability (GluA1/GluA2 ratio) and ΔFosB expression were found in the prefrontal cortex and the striatum of alcohol‐exposed mice after cocaine‐primed reinstatement.
Conclusion and Implications
Our findings demonstrate that maternal binge‐like alcohol consumption during gestation and lactation alters sensitivity to the reinforcing effects of cocaine in adult offspring mice. Together, such data suggest that prenatal and postnatal alcohol exposure may underlie an enhanced susceptibility of alcohol‐exposed offspring to develop drug addiction later in adulthood.
Abbreviations
- AMPAR
AMPA receptor
- CPP
conditioned place preference
- CREB
cAMP‐response element binding
- DARPP‐32
dopamine‐ and cAMP‐regulated phosphoprotein of 32 kDa
- DAT
dopamine transporter
- DID
drinking in the dark
- FASD
fetal alcohol spectrum disorders
- NAc
nucleus accumbens
- PAE
prenatal alcohol exposure
- PD
postnatal day
- PFC
prefrontal cortex
- PLAE
prenatal and lactation alcohol exposure
- PR
progressive ratio
- SA
self‐administration
- STR
striatum
- VTA
ventral tegmental area
What is already known
Alcohol exposure in utero leads to wide range of behavioural and physical abnormalities.
What this study adds
Prenatal and lactation alcohol exposed mice show glutamatergic alterations associated with increased cocaine consumption.
What is the clinical significance
Children with fetal alcohol spectrum disorders have increased risk to develop cocaine addiction in adulthood.
1. INTRODUCTION
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2299 use during pregnancy interferes with fetal development, resulting in physical and mental abnormalities known as fetal alcohol spectrum disorders (FASD; Dörrie, Föcker, Freunscht, & Hebebrand, 2014). It is estimated that, globally, 9.8% of pregnant women consume alcohol, with the highest prevalence in Europe (25.2%), according to the World Health Organization (Popova, Lange, Probst, Gmel, & Rehm, 2018). Furthermore, approximately 3% of women reported to have engaged in a binge pattern of alcohol consumption during gestation (Popova, Lange, Probst, Parunashvili, & Rehm, 2017).
Previous clinical studies have suggested that alcohol exposure in utero may increase the risk for later alcohol abuse and other drug dependencies (Alati et al., 2006; Baer, Sampson, Barr, Connor, & Streissguth, 2003). Preclinical research also shows that animals prenatally exposed to drugs of abuse are more responsive to such drugs and also to other psychotropic substances, suggesting that the reward system may be altered. Animal studies indicate that intrauterine drug exposure may lead to increased self‐administration (SA; Glantz & Chambers, 2006). Furthermore, perinatal alcohol exposure increases alcohol preference, intake (Brancato et al., 2018; Spear & Molina, 2005) and alcohol‐induced reward later in life (Barbier et al., 2008; Cantacorps, González‐Pardo, Arias, Valverde, & Conejo, 2018; Parker et al., 2016).
Such findings support the hypothesis that prenatal alcohol exposure (PAE) may give rise to an increased risk of drug addiction. Nevertheless, available clinical and preclinical evidence has focused predominantly on the effects of PAE on alcohol abuse, as opposed to other drugs of abuse. In the context of illicit drugs, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2286 is the second most widely consumed after cannabis (UNODC, 2019). Cocaine is a psychostimulant that increases dopaminergic neurotransmission through the blockade of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=927 (DATs) at the synaptic level in the mesocorticolimbic system (Dong & Nestler, 2014; Everitt & Robbins, 2005).
The neurobiological substrates involved in the vulnerability to drug abuse and attributable to early life alcohol exposure, are still not fully understood. The main hypotheses often focus on the dysfunction of mesocorticolimbic neurocircuitry, the brain reinforcement system (Koob & Le Moal, 2001). It has been suggested that impairments in the dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and prefrontal cortex (PFC) may influence drug sensitivity (Pierce & Kumaresan, 2006).
PAE diminished dopaminergic activity in the VTA, which could be restored by psychostimulant administration in rats (Choong & Shen, 2004a). Also, behavioural alterations in response to psychostimulants have been described in rodent models of FASD. An increased sensitivity to cocaine‐induced conditioned place preference (CPP) and augmented cocaine consumption in a free two‐bottle choice paradigm (Barbier et al., 2008), in addition to enhanced locomotor sensitization to cocaine and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4804, were reported in adult male offspring rats exposed to alcohol (Barbier et al., 2009). Recent studies have also shown that PAE facilitates the acquisition of amphetamine induced CPP (Wang et al., 2019).
Molecular mechanisms underlying the behavioural alterations caused by psychostimulants in PAE animals remain to be elucidated. It is known that cocaine may elicit persistent neural adaptive changes in the brain's reward system, potentially leading to heightened drug‐seeking behaviour in rodents, such as by modulating glutamatergic excitatory synapses (Engblom et al., 2008; Lüscher & Bellone, 2008). The ionotropic excitatory glutamate activated https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 (AMPARs) mediate the excitability in neurons. As for their subunit composition, a shift in the AMPAR subunit composition, from calcium‐impermeable GluA2‐containing to calcium‐permeable GluA2‐lacking AMPARs, has been reported to facilitate AMPAR currents (Woodward Hopf & Mangieri, 2018). Changes in GluA1/GluA2 ratio may be associated with addictive‐like conduct, such as the incubation of cocaine‐seeking behaviour (Wolf, 2016). In addition, several drugs of abuse up‐regulate the cAMP pathway and cause cAMP‐response element binding (CREB) protein activation in the NAc, although such changes are generally short. In contrast, ΔFosB levels accumulate following chronic drug exposure and remain high after a long period, regulating complex behaviours related to the addiction process (McClung & Nestler, 2003; Nestler, 2008). Furthermore, the dopamine‐ and cAMP‐regulated phosphoprotein of 32 kDa (DARPP‐32) plays a central role in integrating both dopaminergic and glutamatergic signalling in the striatum (STR) and is required for cocaine actions (Gould & Manji, 2005; Zachariou et al., 2006).
In the present study, we investigated if maternal binge‐like alcohol consumption during gestation and lactation periods could lead to an increased risk of psychostimulant addiction. We particularly focused on cocaine intake, as few preclinical studies had previously addressed this issue. Thus, pregnant female mice were subjected to the drinking‐in‐the‐dark (DID) test to model binge‐like alcohol exposure during developmen, and adult male offspring mice were tested for their behavioural responses to cocaine. Moderate and high doses of cocaine were chosen to study the hyperlocomotion elicited by repeated treatment of this drug in prenatal and lactation alcohol exposed (PLAE) and control mice. Then we assessed whether early alcohol exposure could enhance the rewarding effects of cocaine using the CPP. Next, we evaluated the reinforcing effects on the SA paradigm, which better resembles human drug‐taking behaviour. Hence, different behavioural procedures (sensitization, CPP and SA) were performed to characterize the liability for cocaine abuse in adult PLAE mice. Furthermore, brain areas involved in the processing of drug rewarding effects were extracted after cocaine priming‐induced reinstatement in order to study the underlying mechanisms of behavioural response to cocaine alterations found in PLAE offspring mice. We examined the expression of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=444 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=445 AMPAR subunits, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=214‐ and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=215‐type dopamine receptors (D1R and D2R, respectively), ΔFosB, in addition to changes in phosphorylation of CREB and DARPP‐32, key molecular substrates altered by cocaine, in the PFC and the STR, two main areas of the mesocorticolimbic system.
2. METHODS
2.1. Animals
Twelve‐week‐old male and female C57BL/6 inbred mice were purchased from Charles River (Barcelona, Spain) and shipped to our animal facility (UBIOMEX, PRBB). Upon arrival, they were all housed in standard cages at a constant temperature (21 ± 1°C) and humidity (55 ± 10%), with a reverse light–dark cycle (white lights on 20:00–08:00 hr). After 1 week of acclimatization, breeding pairs were mated, and pregnant females were observed daily for parturition. For each litter, the date of birth was designated as postnatal day (PD) 0. Pups remained with their mothers for 21 days and were then weaned (PD21). After weaning, male offspring were housed in groups of four. Female offspring were used for other experiments. Food and water were provided ad libitum except during the DID procedure, as described below. Every effort was made to minimize the number of animals used and their suffering. All animal care and experimental procedures were approved by the local ethics committee (CEEA‐PRBB) and conducted in accordance with the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology (McGrath & Lilley, 2015).
2.2. DID test
This procedure was conducted as previously reported (Cantacorps et al., 2017, 2018) to model a binge pattern of alcohol drinking, which is defined as the consumption of four or five alcoholic drinks (in women or men, respectively) on a single occasion, resulting in blood alcohol concentrations greater than 0.8 g·L−1 (NIAAA, 2016). Two days after mating, pregnant female mice were randomly assigned to two groups: alcohol and water exposed (control). In short, the food was removed, and the water bottles were replaced with 10‐ml graduated cylinders fitted with sipper tubes containing either 20% (v/v) alcohol in tap water or only tap water 3 hr after the lights were turned off. Following a 2‐hr access period, individual intake was recorded, and the original water drinking bottles and food were returned to the home cage. During this period, female mice were individually housed and each corresponding male breeding pair was removed from the home cage for the DID procedure. This procedure was repeated on Days 2 and 3, and fresh fluids were provided each day (from Monday to Wednesday). On Day 4 (Thursday), alcohol or water cylinders were left for 4 hr, and fluid intakes were recorded. Two empty control cages (water and alcohol) were placed in the rack to measure general liquid loss (leakage/evaporation) and drip values were subtracted from the drinking values. Fluid intakes (g·kg−1 body weight) were calculated on the basis of average 2‐day body weight values, as dams were weighed at 2‐day intervals (Mondays and Wednesdays). The procedure was maintained throughout the gestation and lactation periods. As previously reported (Cantacorps et al., 2017), blood alcohol concentration for dams reached levels of ~0.8 g·L−1 after the last binge drinking session (4‐hr access) of the gestation and lactation periods.
Our model has previously been used to reproduce an episodic pattern of excessive alcohol drinking during pregnancy and lactation periods, mimicking a realistic human situation, as most mothers who drink during pregnancy continue to do so when breastfeeding. As behavioural and neurochemical alterations have been reported in offspring mice, DID was therefore proposed as a useful FASD model (Cantacorps et al., 2017, 2018). The effects of developmental alcohol exposure in the following cocaine‐related behaviours were assessed in different groups of mice, as outlined in Figure 1. The weight of adult offspring mice prior starting the behavioural testing ranged from 21.2 to 25.7 g in control group and from 20.9 to 25.8 g in PLAE group, with no statistical differences between groups.
Figure 1.
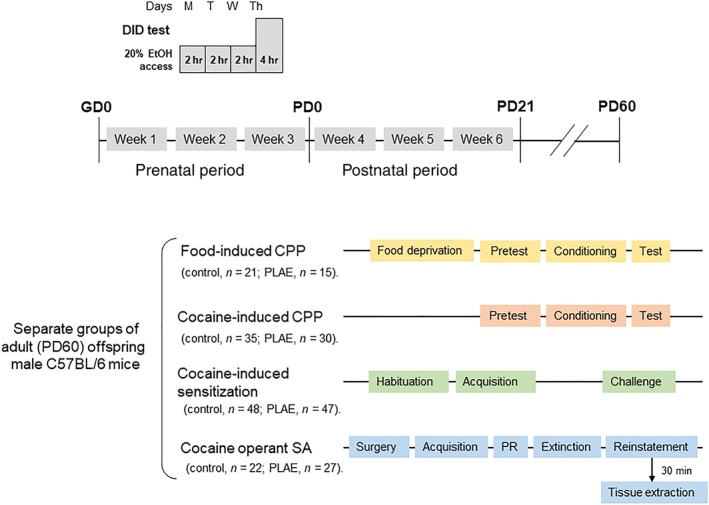
Schematic representation of experimental timeline
2.3. Food‐ or cocaine‐induced CPP
Adult offspring mice (PD60) were tested for the conditioned reinforcement of food or cocaine using an unbiased CPP paradigm. Different sets of animals were used for each experiment. The apparatus consisted of two equally sized compartments (30 × 29 × 35 cm), differing in terms of visual and tactile cues, connected by a corridor (14 × 29 × 35 cm; Cibertec S.A., Madrid, Spain). One compartment had white‐painted walls with prismatic textured flooring, while the other had black walls with a smooth floor. Both compartments were equipped with IR emitter/detector pairs, which allowed us to record the position of the animal and its crossings between compartments.
For the food‐induced CPP, mice were first food deprived to 80–85% of their baseline body weight with limited access to standard chow pellet (2–3 g·day−1 per animal) for 4 days prior to commencing preconditioning and throughout the procedure, as previously described with some minor modifications (Valverde et al., 2004). The CPP procedure consisted of three different phases: preconditioning (one session), conditioning (six sessions), and testing day (one session). During preconditioning, mice could freely explore both compartments for 20 min. Mice showing strong unconditioned aversion (<33% of the session time) or preference (>67%) for either compartment were excluded from the study. Conditioning training began on the following day. Food‐paired mice were confined, for 30 min, to one compartment, in which they had access to palatable food (1 g; Cheerios) on Days 2, 4 and 6. On alternate days (3, 5 and 7), mice were confined to the other chamber with no food available. Food‐unpaired mice were alternatively placed in each compartment without receiving any food. During conditioning, the central area was blocked by guillotine doors. Finally, the testing session (Day 8) was conducted under the same conditions as in the preconditioning phase.
The same procedure was followed for cocaine‐induced CPP, but in this case, mice were not food deprived, as previously described (Luján, Castro‐Zavala, Alegre‐Zurano, & Valverde, 2018). The conditioning phase consisted of four pairings: Mice received an i.p. injection of 5 or 10 mg·kg−1 cocaine immediately prior to confinement to the drug‐paired compartment for 30 min on Days 2, 4, 6 and 8, while on alternate days (3, 5, 7, and 9) mice received physiological saline before being confined to the vehicle‐paired compartment for 30 min. Treatments were counterbalanced between compartments. Non‐drug‐paired mice were administered saline prior to confinement to one of the two compartments every day. The testing session took place on Day 10.
Time spent in each compartment was recorded during the preconditioning and testing sessions. The CPP score for each subject was calculated as the difference between the time spent in the drug‐paired compartment during the test and the preconditioning phase.
2.4. Cocaine‐induced behavioural sensitization
The sensitization to hyperlocomotor responses elicited by cocaine was assessed in adult offspring mice (PD60) as previously described with some minor modifications (Gracia‐Rubio et al., 2016). The sensitization procedure consisted of three phases: habituation, acquisition and challenge. In the habituation phase, mice were placed individually into locomotor activity boxes (24 × 24 × 24 cm; LE881 IR, Panlab s.l.u., Barcelona, Spain) for 30 min immediately after receiving an i.p. saline injection. On the following 5 days (acquisition phase), mice were treated daily with cocaine (7.5 or 10 mg·kg−1, i.p.) or saline immediately prior to confinement in the locomotor activity boxes for 30 min. Following a drug‐free period of 8 days after the last cocaine treatment, mice were injected with cocaine (7.5 or 10 mg·kg−1, i.p.), and the locomotor activity was recorded for 30 min (challenge phase).
2.5. Cocaine operant self‐administration (SA)
2.5.1. Catheter implantation surgery
An indwelling Silastic catheter was surgically implanted into the right jugular vein under https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2505 anaesthesia (1.5–2.0%). During surgery, mice were treated with an analgesic (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7220; Metacam, Boehringer Ingelheim, Barcelona, Spain; 0.5 mg·kg−1 injected in a volume of 0.1 ml·10 g−1, s.c.) and an antibiotic solution (Enrofloxacin; Baytril, Bayer, Barcelona, Spain; 7.5 mg·kg−1 injected in a volume of 0.03 ml·10 g−1, i.p.). Following surgery, mice were individually housed and allowed to recover for at least 3 days prior to commencing SA training. During recovery, mice were treated daily with the analgesic and the antibiotic solutions previously described. The home cages were placed upon thermal blankets to avoid post‐anaesthesia hypothermia.
2.5.2. Acquisition of SA
The SA experiment was carried out in mouse operant chambers (Model ENV‐307A‐CT, Med Associates, Inc. Cibertec S.A.), as previously described with some minor modifications (López‐Arnau et al., 2017; Luján et al., 2018; Soria, Barbano, Maldonado, & Valverde, 2008). The chambers were housed in sound‐ and light‐attenuated boxes and contained two holes, one of which was defined as active and the other as inactive. Nose‐poking into the active hole produced a cocaine infusion (reinforcement) that was paired with a stimulus light placed above the active hole. Nose‐poking into the inactive hole had no consequences. The side on which the active/inactive hole was situated was counterbalanced.
Mice were trained for 2 hr·day−1 to nose‐poke in order to receive a cocaine infusion (0.75 mg·kg−1) under a fixed ratio 1 schedule of reinforcement for 10 consecutive days (acquisition phase). When mice responded on the active hole, cocaine was delivered in a 20‐μl infusion over 2 s via a syringe mounted on a microinfusion pump (PHM‐100A, Med‐Associates, Georgia, VT, USA) connected via Tygon tubing (0.96‐mm outer diameter, Portex Fine Bore Polythene Tubing, Portex Limited, UK) to a single‐channel liquid swivel (375/25, Instech Laboratories, Plymouth Meeting, PA, USA) and to the intravenous catheter implanted in the mice. Each infusion was followed by a 15‐s time‐out period during which nose‐poking on the active hole had no consequences. Each acquisition‐phase session began with a priming infusion of the drug, with the box light turned on for 3 s and then deactivated for the rest of the session.
Acquisition criteria were met when (a) mice performed at least five cocaine infusions per session, (b) ≥65% of responses were received at the active hole, and (c) the number of reinforcements deviated less than 20% from the mean number of reinforcements in two consecutive days. After 10 days of training, mice achieving the acquisition criteria were moved to a progressive ratio (PR) session. In the PR session (2 hr), the response requirement to earn an injection escalated throughout the following series: 1–2–3–5–12–18–27–40–60–90–135–200–300–450–675–1,000.
The number of cocaine infusions received on the last day of the acquisition phase was used as the baseline to determine extinction criteria in each mouse. Non‐acquiring animals were excluded from the study.
2.5.3. Extinction and reinstatement
Once acquisition criteria were met, the cocaine was removed and mice were tested for latency to extinguish nose‐poking behaviour over successive once‐daily 2‐hr sessions. During the extinction phase, nose‐pokes into the active hole produced neither cocaine infusion nor stimulus light presentation. Extinction criteria were met when response levels decreased to ≤40% of the acquisition baseline levels in two consecutive days for each mouse. Twenty‐four hours after achieving the extinction criteria, mice underwent a cocaine‐primed reinstatement session. Mice were confined to the operant chambers for 2 hr immediately after being administered cocaine (10 mg·kg−1, i.p.). Nose‐poking had no consequences in any of the holes.
2.6. Tissue sample preparation and western blotting
Animals were killed 30 min after the cocaine‐primed reinstatement session, and PFC and STR were extracted, quickly frozen in dry ice and stored at −80°C until used for western blotting, as previously described (Moscoso‐Castro, López‐Cano, Gracia‐Rubio, Ciruela, & Valverde, 2017). In addition, PFC and STR were extracted in cocaine‐naïve animals to analyse GluA1 and GluA2 levels at basal conditions (see Supporting Information). Tissue was first homogenized in 30 μl of lysis buffer (0.15 NaCl, 1% TX‐100, 10% glycerol, 1‐mM EDTA, 50‐mM Tris, pH = 7.4, and a phosphatase and protease inhibitor cocktail [complete ULTRA Protease Inhibitor Cocktail Tablets and PhosSTOP Inhibitor Cocktail Tablets, respectively; Roche, Basel, Switzerland]) per milligrams (wet weight). Homogenates were centrifuged at 1,000× g for 20 min at 4°C, and the resulting supernatants were collected for protein quantification. The lysate protein concentration was determined using a stock solution of 5 mg·ml−1 BSA as a protein standard. Equal amounts of protein (16 μg) for each sample were mixed with loading buffer (153‐mM Tris, pH = 6.8, 7.5% SDS, 40% glycerol, 5‐mM EDTA, 12.5% 2‐β‐mercaptoethanol, and 0.025% bromophenol blue), loaded onto 10% polyacrylamide gels and transferred to PVDF sheets (Immobilion‐P, MERCK, Burlington, USA). Membranes were blocked with 5% BSA in Tris‐buffered saline (100 mmol·L−1 NaCl, 10 mmol·L−1 Tris, pH = 7.4) and 0.1% Tris‐buffered saline Tween‐20 for 1 hr and then immunoblotted using the primary antibodies listed in Table 1 overnight at 4°C. Finally, membranes were incubated for 1 hr with their respective secondary fluorescent antibodies: anti‐mouse (1:2,500, IRDye 800, Rockland, Cat# 610‐132‐121, RRID: AB_10703265) and anti‐rabbit (1:2,500, IRDye 680, Rockland, Cat# 611‐144‐002, RRID: AB_2713919). Protein expression was quantified using a LI‐COR Odyssey scanner and software (LI‐COR Biosciences, Lincoln, USA). Protein densities were normalized to the detection of the housekeeping control gene in the same samples and expressed as a fold change of the control group. The Immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
Table 1.
Primary antibodies
| Primary antibody | Description | Host | Dilution | Company | Catalogue number | RRID |
|---|---|---|---|---|---|---|
| ΔFosB | Delta FosB | Mouse | 1:250 | Abcam | #ab11959 | AB_298732 |
| CREB | cAMP‐response element binding protein | Rabbit | 1:500 | MERCK | #04‐767 | AB_1586959 |
| D1R | Dopamine receptor 1 | Rabbit | 1:500 | Abcam | #ab20066 | AB_445306 |
| D2R | Dopamine receptor 2 | Rabbit | 1:500 | MERCK | #324393 | AB_10681601 |
| DARPP‐32 | Dopamine‐ and cAMP‐regulated phosphoprotein | Rabbit | 1:1,000 | Cell Signaling | #2306 | AB_823479 |
| GAPDH | Glyceraldehyde‐3‐phosphate dehydrogenase | Mouse | 1:2,500 | Santa Cruz Biotechnology | #sc‐365062 | AB_10847862 |
| GluA1 | AMPA receptor subunit 1 | Rabbit | 1:1,000 | MERCK | #ABN241 | AB_2721164 |
| GluA2 | AMPA receptor subunit 2 | Rabbit | 1:1,000 | MERCK | #AB1768‐I | AB_2313802 |
| pCREB | Phosphorylated CREB | Rabbit | 1:1,000 | MERCK | #06‐519 | AB_310153 |
| pDARPP‐32(Thr34) | Phosphorylated DARPP‐32(Thr34) | Rabbit | 1:1,000 | MERCK | #ab9206 | AB_347689 |
| β‐Tubulin | β‐Tubulin | Mouse | 1:5,000 | BD PharMingen | #556321 | AB_396360 |
| β‐Tubulin | Class III β‐tubulin | Rabbit | 1:1,000 | Abcam | #ab6046 | AB_2210370 |
2.7. Data and analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Statistical analysis was performed when group size was at least n = 5. The declared group size is the number of independent values. Animals were randomly assigned to an experimental group, and during the behavioural manipulations, researchers were not aware of the pretreatment that each animal had received.
Data obtained from the DID test and maternal body weight were analysed using two‐way ANOVA with repeated measures analysis. We analysed the results of the CPP using two‐way ANOVA with group (control and PLAE) and treatment (0, 5, or 10 mg·kg−1 cocaine; presence or absence of food) as between‐subject factors. We calculated a three‐way ANOVA with repeated measures for the locomotor sensitization with group and treatment as between‐subject factors and day of test as a within‐subject factor. To analyse the acquisition, extinction, and reinstatement of the SA, a three‐way ANOVA with repeated measures was performed with group as a between‐subject factor and day of training and nose‐poke (active vs. inactive holes) as within‐subject factors. When F values reached the levels of statistical significance required and no significant variance in homogeneity was observed, ANOVA analyses were followed by Bonferroni post hoc tests. The unpaired two‐tailed Student's t ‐‐test was used to analyse the differences between groups in the total of active nose‐pokes, total of cocaine consumption, day of acquisition, PR test, and day of extinction. The AUC was calculated for extinction curve and compared between groups using the Student's t‐test. Protein expression was also analysed by the Student's t‐test. Technical replicates in the western blot were used to ensure the reliability of single values.
Statistical analyses were performed using SPSS Statistics v23. Data were expressed as mean ± SEM. The α level of statistical significance was set at P < .05. The exact group size for the individual experiments is shown in the corresponding figure legends.
2.8. Materials
Ethyl alcohol was purchased from Merck Chemicals (Darmstadt, Germany) and diluted in tap water in order to obtain a 20% (v/v) alcohol solution. Cocaine hydrochloride was provided by the Spanish National Institute of Toxicology and prepared in sterile 0.9% NaCl (physiological saline, pH = 7.4) for injection immediately before administration.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Maternal alcohol consumption and body weight
Dams were randomly assigned to either water or alcohol experimental groups and were given access to either water or 20% alcohol, following the DID weekly schedule. Daily water and alcohol consumption were recorded throughout the gestation and lactation periods. Maternal alcohol intake during the 6‐week DID procedure is represented in Figure 2a. Analysis of averaged daily alcohol intake during 2‐ and 4‐hr sessions showed a significant effect of the session duration, revealing a significantly greater oral intake during the 4‐hr sessions (Figure 2b). Also, an increase in voluntary alcohol consumption was observed during the last 3 weeks (lactation) compared with the first 3 weeks (gestation) of exposure.
Figure 2.
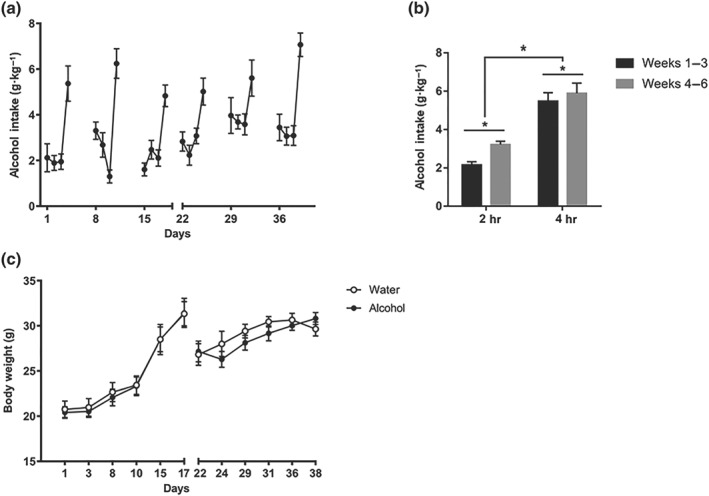
Maternal alcohol intake. (a) Representation of daily alcohol intake during DID procedure throughout gestation and lactation periods. (b) Average alcohol intake during 2‐hr sessions (first to third day of each week) and 4‐hr sessions (fourth day of each week). (c) Body weight of dams was measured twice weekly during the 6‐week‐long procedure (n = 14 per group)
Analysis of maternal body weight (Figure 2c) revealed a significant effect of time, but no significant effect of alcohol exposure. Thus, in agreement with previous studies (Cantacorps et al., 2017; Wilcox et al., 2014), our results show that alcohol consumption during the DID procedure does not affect maternal body weight.
3.2. Effects of prenatal and postnatal alcohol exposure on cocaine‐induced CPP in adulthood
Results for cocaine‐ and food‐induced CPP are presented in Figure 3. Analysis showed a significant effect of group, cocaine treatment and interaction between these factors. There was a significant difference between 5 and 10 mg·kg−1 cocaine‐treated mice and saline‐treated mice in both groups. Also, an enhanced preference towards the 5 mg·kg−1 cocaine‐paired compartment in PLAE mice compared with the control group was revealed (Figure 3a).
Figure 3.
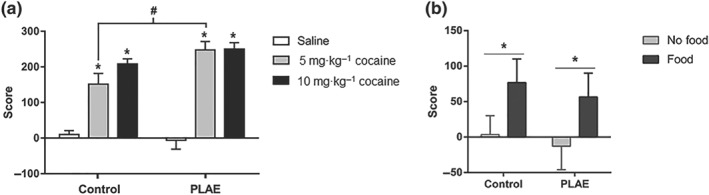
Cocaine‐induced conditioned place preference (CPP) increased in PLAE offspring mice. (a) Cocaine‐induced CPP score (control saline‐treated group, n = 10; control 5 mg·kg−1 cocaine‐treated group, n = 12; control 10 mg·kg−1 cocaine‐treated group, n = 13; PLAE saline‐treated group, n = 9; PLAE 5 mg·kg−1 cocaine‐treated group, n = 9; PLAE 10 mg·kg−1 cocaine‐treated group, n = 12). Bonferroni * P < .05 treatment comparisons; # P < .05 control versus PLAE mice treated with 5 mg·kg−1 cocaine. (b) Food‐induced CPP score (control no‐food conditioning group, n = 10; control food‐conditioned group, n = 11; PLAE no‐food conditioning group, n = 7; PLAE food‐conditioned group, n = 8). * P < .05 treatment effect
Such effects were cocaine specific, since no between‐group differences were found in food‐induced CPP (Figure 3b). There was a significant effect of treatment, but no significant effect of group and no interaction between these factors.
3.3. Cocaine‐induced behavioural sensitization is attenuated in PLAE mice
3.3.1. 10 mg·kg−1 cocaine‐induced behavioural sensitization
The effects of prenatal and postnatal alcohol exposure on cocaine‐induced behavioural sensitization were tested on locomotor activity boxes following the experimental procedure shown in Figure 4. Following saline administration on the habituation day, no significant between‐group differences were found in basal locomotor activity (data not shown). Cocaine (10 mg·kg−1) induced sensitization acquisition (Figure 4b) showed a significant effect of day and treatment,with significant interaction between Day × Treatment factors, but no significant Day × Group interaction, Group × Treatment interaction or Day × Group × Treatment interaction. There were significant differences between Day 2, Day 3, Day 4 and Day 5 versus Day 1 in cocaine‐treated mice, whereas there was no significant differences between Day 2, Day 3, Day 4 and Day 5 versus Day 1 in mice receiving saline.
Figure 4.
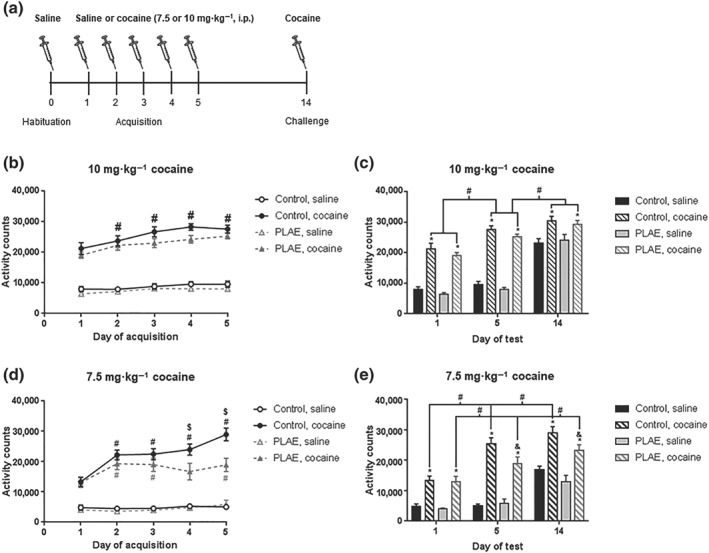
Effects of early alcohol exposure on cocaine‐induced locomotor sensitization. (a) Schematic representation of experimental procedure. (b) Five‐day acquisition of 10 mg·kg−1 cocaine‐induced locomotor sensitization (control saline‐treated group, n = 16; control 10 mg·kg−1 cocaine‐treated group, n = 15; PLAE saline‐treated group, n = 18; PLAE 10 mg·kg−1 cocaine‐treated group, n = 17). (c) Three‐day comparison of 10 mg·kg−1 cocaine‐induced locomotor sensitization (acute cocaine [Day 1], repeated cocaine [Day 5] and cocaine challenge [Day 14]). (d) Five‐day acquisition of 7.5 mg·kg−1 cocaine‐induced locomotor sensitization (control saline‐treated group, n = 8; control 7.5 mg·kg−1 cocaine‐treated group, n = 9; PLAE group, n = 6). (e) Three‐day comparison of 7.5 mg·kg−1 cocaine‐induced locomotor sensitization (acute cocaine [Day 1], repeated cocaine [Day 5] and cocaine challenge [Day 14]). Data are presented as mean ± SEM of cumulative breaks per 30 min in locomotor activity boxes. Bonferroni post hoc comparisons: # P < .05 comparison of each day versus Day 1 (in panels b and d) or day effects as indicated by lines (in panels c and e); & P < .05 comparison between groups (control vs. PLAE) on the same day; * P < .05 treatment effect (saline vs. cocaine) on the same day within group; $ P < .05 comparison between control and PLAE mice receiving cocaine
Comparison of Days 1, 5 and 14 of 10 mg·kg−1 cocaine‐induced sensitization (Figure 4c) revealed a significant effect of day and treatment, but no significant effect of group. Also, there was a significant interaction between Day × Treatment factors, but no significant interactions between Day × Group factors, Group × Treatment factor or interaction Day × Group × Treatment were found. Locomotor activity due to cocaine treatment in control mice was significantly increase on Days 1, 5 and 14. Likewise, an increase on Days 1, 5, and 14 in locomotor activity after cocaine treatment in PLAE mice was osserved. Furthermore, a significant increase in locomotor activity was observed between Days 1 and 5 in both the control group and the PLAE group, which also showed a significant increase on Day 14 compared with Day 5.
3.3.2. 7.5 mg·kg−1 cocaine‐induced behavioural sensitization
Cocaine (7.5 mg·kg−1) induced sensitization (Figure 4d) showed a significant effect of day and treatment, but no significant effect of group. In addition, significant interactions between Day × Group, Day × Treatment and Day × Group × Treatment factors were observed. However, no significant interaction between Group × Treatment factors was found. Subsequent post hoc comparisons indicated significant differences between Day 2, Day 3, Day 4 and Day 5 versus Day 1 within the control group, in addition to differences between Day 2, Day 3 and Day 4 versus Day 1 within the PLAE group. Moreover, significant differences between control and PLAE cocaine‐treated mice were found on Days 4 and 5, suggesting that PLAE mice show an attenuated acquisition of 7.5 mg·kg−1 cocaine‐induced sensitization.
Again cocaine (7.5 mg·kg−1) induced sensitization comparing the first and last day of acquisition with the challenge day (Figure 4e) showed a significant effect of day and treatment, but no significant effect of group. Furthermore, significant interactions between Day × Group factors, Day × Treatment and triple interaction Day × Group × Treatment were also observed. Interaction between Group × Treatment factors was not significant. Cocaine treatment increased locomotor activity on Days 1, 5 and 14 in both groups of mice. Also, a significant increase in locomotor activity was found between Day 1 and Day 5 in both the control group and the PLAE group. In addition, there was a significant increase on Day 14 compared with the final day of acquisition in control and PLAE mice. Moreover, PLAE mice showed an attenuated locomotor sensitization compared with the control group on both the last day of acquisition and the challenge day.
3.4. PLAE mice show increased drug‐seeking and drug‐taking behaviour during cocaine SA acquisition
3.4.1. Acquisition and PR
Adult offspring were tested for 0.75 mg·kg−1 cocaine infusions SA in operant boxes on 10 consecutive days (Figure 5). Infusions during the acquisition phase (Figure 5b) yielded significant effects of group, indicating an effect of developmental alcohol exposure on the acquisition of cocaine SA behaviour. Both groups of mice acquired as indicated by an effect of the day of training and they were able to discriminate between active and inactive nose‐pokes as revealed by the nose‐poke factor. There were also significant interactions between Day of training × Nose‐poke and Group × Nose‐poke factors . No other interactions were found between Day × Group factors or triple interaction Day × Group × Nose‐poke. However there was a significant discrimination between active and inactive holes from Day 3 onwards and then each day until Day 10. Furthermore, differences were detected between the PLAE and control groups in terms of active nose‐poking during the 10‐day training phase.
Figure 5.
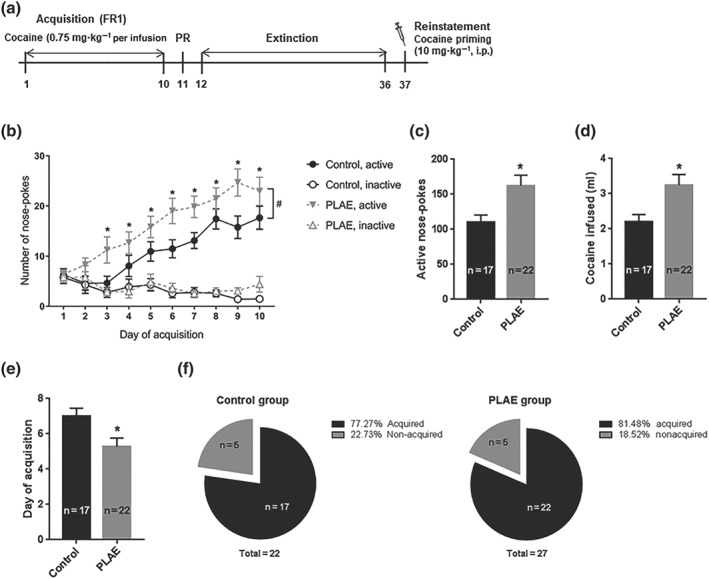
PLAE mice show enhanced cocaine‐seeking behaviour in the SA paradigm. (a) Schematic representation of the behavioural procedures carried out. (b) Acquisition of cocaine (0.75 mg·kg−1 per infusion, i.v.) SA in control and PLAE offspring adult mice. A number of active (infusions) and inactive nose‐pokes in 2‐hr sessions over 10 consecutive days of animals reaching acquisition criteria are represented. Bonferroni post hoc comparisons: * P < .05 active versus inactive nose‐pokes; # P < .05 control versus PLAE group on active nose‐pokes. (c) Total number of active nose‐pokes throughout the acquisition phase. (d) Total amount of cocaine infused during the whole acquisition phase. (e) Average day in which animals reached acquisition criteria. (f) Proportion of animals reaching acquisition criteria in control and PLAE groups. Student's t test, * P < .05 control versus PLAE group (control group, n = 17 and PLAE group, n = 22). FR1, fixed ratio 1
In addition, a significant increase in the total number of active nose‐pokes (Figure 5c) was found in the PLAE group and also in terms of total cocaine consumption (Figure 5d) during the acquisition phase. Furthermore, PLAE mice acquired faster than their control littermates, as the average day of acquisition was lower in the PLAE group (Figure 5e), while the percentage of animals reaching acquisition criteria was similar in both groups, with 77.27% recorded in the control group and 81.48% in the PLAE group (Figure 5f). However, no significant between‐group differences were found in the breaking point achieved in the PR session (data not shown).
3.4.2. Extinction and cocaine‐primed reinstatement
As shown in Figure 6, nose‐poking behaviour was extinguished by both groups of mice. Although the AUC (Figure 6b) of the extinction phase was reduced in the PLAE group, no significant differences were found in the proportion of animals, in either of the groups, reaching extinction criteria (53.33% in the control group and 59.09% of PLAE mice; Figure 6c) or on the day of extinction (Figure 6d). Analysis of nose‐poking behaviour on the first and final days of extinction and in the reinstatement session (Figure 6e), showed a significant effect of day, group and nose‐poke. Also, significant interactions between Day × Group, Day × Nose‐poke and interaction Day × Group × Nose‐poke were observed. No significant interaction between Group × Nose‐poke factors were found. There was also significant discrimination between active and inactive nose‐pokes during both the first day of extinction and reinstatement in both groups of mice. Moreover, significant differences were appreciated in terms of active nose‐pokes of the last day of extinction compared with the first day of extinction and reinstatement. Furthermore, PLAE mice showed a reduction of active nose‐pokes compared with the control group on the first day of the extinction phase and in the reinstatement session.
Figure 6.
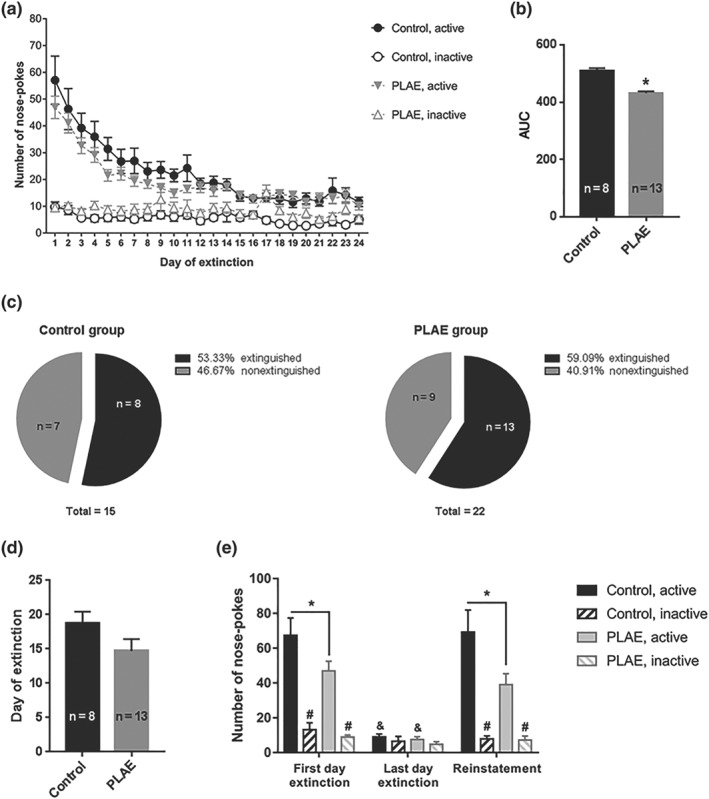
PLAE mice show attenuated extinction of cocaine‐seeking behaviour and cocaine‐primed reinstatement. (a) Nose‐poke activations during the extinction training sessions. All animals are represented, regardless of achieving extinction criteria (n = 15–22 per group). (b) AUC of the active nose‐pokes made throughout the extinction phase, showing only mice that extinguished. Student's t‐test, * P < .05 control versus PLAE group (control group, n = 8 and PLAE group, n = 13). (c) Proportion of animals reaching extinction criteria in control and PLAE groups. (d) Average of the day of extinction. (e) Cocaine‐induced (10 mg·kg−1, i.p.) reinstatement of self‐administration behaviour in a 2‐hr session, 24 hr after reaching extinction criteria. Only mice reaching extinction criteria are represented (control group, n = 8 and PLAE group, n = 13). Three‐way ANOVA and Bonferroni post hoc analysis. * P < .05 group comparisons in active nose‐pokes; # P < .05 active versus inactive nose‐pokes; & P < .05 day effect
3.5. Altered expression of GluA1/GluA2 ratio is found in PLAE mice after cocaine‐induced reinstatement in the self‐administration (SA)
PFC and STR brain areas were extracted 30 min after cocaine‐induced reinstatement in the SA procedure. GluA1/GluA2 ratio expression (Figure 7a) in the PFC was greater in PLAE mice compared with the control group, while it decreased in the STR of PLAE mice, No changes were found in the phosphorylation of CREB (Figure 7b) in the PFC or the STR either in the phosphorylation of DARPP‐32 (Figure 7c) in the PFC or in the STR.
Figure 7.
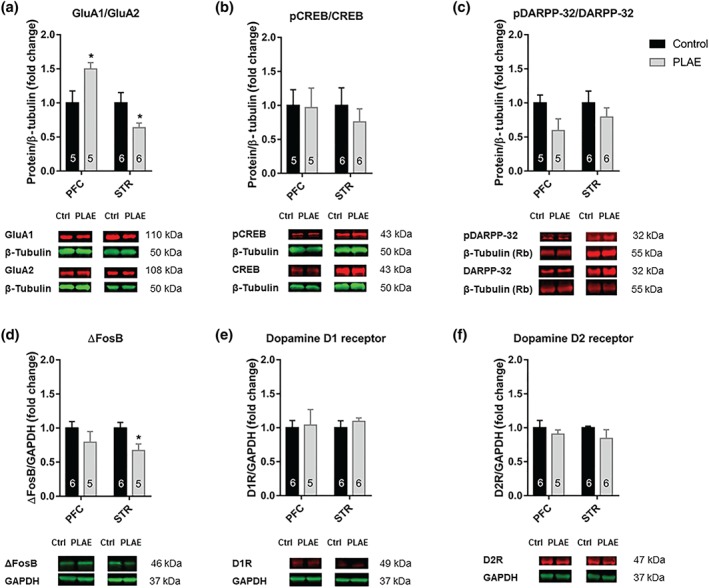
GluA1/GluA2 ratio and striatal ΔFosB expression were altered in PLAE mice after cocaine‐primed reinstatement session. (a–f) Western blot analyses of GluA1/GluA2, pCREB/CREB, pDARPP‐32/DARPP‐32, ΔFosB, D1R, and D2R protein expression in the PFC and STR of PLAE and control mice following cocaine‐induced reinstatement. Student's t test, * P < .05 control versus PLAE group (n = 5–6 per group). The numbers in the bars represent the amount of individuals in the group. The lower panels show representative fluorescent immunoblots
Although the expression of ΔFosB (Figure 7d) was unchanged in the PFC, a significant decrease was observed in the STR of PLAE mice. We also measured the protein expression of D1R (Figure 7e) in both brain structures, but no changes were found, either in the PFC or in the STR, as a result of developmental alcohol exposure. Similar results were obtained for D2R expression (Figure 7f) in the PFC and the STR.
4. DISCUSSION
Fetal alcohol spectrum disorders (FASD) can be a risk factor for the later use and abuse of drugs in life, such as cocaine or other psychostimulants. In this study, we evaluated the long‐term effects of maternal binge‐like alcohol drinking during gestation and lactation on the reinforcing effects of cocaine in adult offspring. To our knowledge, this is the first study to demonstrate increased vulnerability to cocaine‐taking behaviour in an FASD mouse model.
Our results demonstrate increased cocaine‐induced CPP in PLAE animals when compared to their counterparts, based on the lowest dosage of cocaine assessed (5 mg·kg−1), whereas no differences were found in 10 mg·kg−1 cocaine‐induced CPP, since it is a highly effective rewarding dose. Instead, the 5 mg·kg−1 dose of cocaine elicits a lower rewarding effect, thus allowing differences in the conditioned response between groups to be observed. Hence, the degree by which cocaine produces approaching behaviours towards conditioned contexts was affected by developmental alcohol exposure, while it did not alter food induced CPP, which is a natural reward. Such results would indicate that PLAE mice do not show anhedonia‐like behaviour, as, like their counterparts, they are capable of displaying an enhanced motivation towards the food‐paired compartment. Furthermore, we also observed differences in cocaine‐induced locomotor sensitization in terms of the PLAE mice. An attenuated behavioural sensitization to the lowest dose of cocaine used (7.5 mg·kg−1) was also observed in animals exposed to alcohol during early development, while both groups of animals were similarly sensitized to a repeated treatment of 10 mg·kg−1 cocaine. The findings obtained in the CPP paradigm demonstrate that developmental alcohol exposure induces a long‐lasting increase in the conditioned rewarding effects of cocaine. Thus, we subsequently evaluated an operant response in the cocaine SA paradigm, allowing us to study drug‐seeking and drug‐taking behaviour, hallmarks of addictive behaviour in humans. In the cocaine SA procedure, we appreciated increased drug‐seeking and drug‐taking behaviour during the acquisition phase in PLAE mice receiving cocaine infusions of 0.75 mg·kg−1. However, no differences were found in the PR session, which is an indicator of the effort level each subject is willing to exert in order to gain another drug infusion. PLAE animals extinguished drug‐seeking behaviour and relapsed after a 10 mg·kg−1 cocaine priming, similarly to the control group. Nevertheless, the number of active nose‐pokes on the first day of extinction and in the reinstatement session was lower in the PLAE mice than the controls.
Our results would therefore suggest that alcohol exposure during the gestation and lactation periods increases the reinforcing effects of cocaine in adulthood but diminishes cocaine‐induced locomotor sensitization. Such findings are not contradictory, given that different neural substrates are involved in the drug‐induced behavioural sensitization and associative learning processes that lead to CPP or drug SA (Runegaard et al., 2018). Therefore, the drug's rewarding properties are not associated with the sensitivity to its locomotor stimulation effects (Carr, Phillips, & Fibiger, 1988). In agreement with our results, previous research has shown increased cocaine intake and cocaine‐induced CPP in adult offspring rats exposed to alcohol during gestation and lactation periods (Barbier et al., 2008). Likewise, increased sensitivity in the CPP, induced by a psychostimulant drug such as amphetamine, was observed in PAE rats upon reaching adulthood (Wang et al., 2019). Moreover, rats receiving alcohol during gestation self‐administered more amphetamine and exerted greater effort to obtain the drug under a PR schedule (Hausknecht et al., 2015; Wang et al., 2018). In contrast to our findings, increased locomotor sensitization to psychostimulants, such as cocaine and amphetamine, was described in adult PLAE offspring rats (Barbier et al., 2009).
In order to elucidate the neural mechanisms underlying the behavioural alterations found in response to cocaine in PLAE mice, molecular changes in the PFC and the STR, brain areas involved in the mesocorticolimbic dopamine (DA) system, were assessed after cocaine‐induced reinstatement. Our findings revealed an increased GluA1/GluA2 ratio in the PFC of PLAE mice, suggesting an enhanced glutamatergic excitability, and a reduction was found in the STR, indicating diminished neuronal excitability therein. There is good evidence regarding glutamatergic synaptic plasticity evoked by drug exposure in the brain's reward circuitry (Cooper, Robison, & Mazei‐Robison, 2017; Lüscher, 2016). Indeed, neuroadaptations in the PFC to enhance excitatory output have been related with drug‐seeking behaviour (Van den Oever, Spijker, Smit, & De Vries, 2010). Glutamatergic neurons in the mPFC have been linked with cocaine‐associated memories (Zhang et al., 2019). Also, GluA2‐lacking AMPARs in the NAc regulate the incubation of cocaine craving after prolonged withdrawal (Conrad et al., 2008). In an FASD rat model, Hausknecht et al. (2015) reported a persistent augmentation of calcium‐permeable AMPAR expression in VTA DA neurons, which could lead to an enhanced excitatory synaptic strength. Also, binge alcohol exposure during the third trimester‐equivalent period to human pregnancy increased the frequency of spontaneous excitatory glutamatergic postsynaptic currents in the basolateral amygdala of offspring rats (Baculis, Diaz, & Fernando Valenzuela, 2015).
In our study, no changes in the phosphorylation of CREB were found in PLAE mice, but surprisingly, a decreased protein expression of ΔFosB in the STR was revealed. Drugs of abuse induce a short‐term CREB activation, whereas ΔFosB induction after chronic drug treatment in the NAc persists after weeks of withdrawal (McClung & Nestler, 2003). We speculated that alcohol exposure during development would induce the accumulation of ΔFosB in the NAc, thus resulting in greater cocaine‐induced reward and intake. Nevertheless, we found a decreased ΔFosB expression in the STR in terms of PLAE mice. However, we should consider that brain areas were extracted after cocaine‐induced reinstatement, and this was lower in PLAE mice. We cannot therefore conclude that ΔFosB is not involved in the heightened sensitivity to cocaine reinforcing effects in PLAE mice, even though other mechanisms might well be involved.
Furthermore, addictive drugs stimulate striatal dopaminergic receptors inducing changes in intracellular pathways that may underlie functional and structural neuroplasticity (Philibin, Hernandez, Self, & Bibb, 2011). Here, no significant differences were found in the protein expression of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=214 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=215 receptors or in the phosphorylation of DARPP‐32 protein between PLAE and control mice. Notwithstanding, other dopaminergic receptors, such as https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=216 receptors which is involved in drug reward (Kong, Kuang, Li, & Xu, 2011; Leggio et al., 2019), might be playing a role in the altered drug sensitivity in PLAE mice.
As for molecular changes in the DA system, previous studies show that PAE causes a persistent reduction in the spontaneous electrical activity of midbrain DA neurons in adult mice (Choong & Shen, 2004b) and a lower frequency of evoked action potentials in VTA DA neurons, which could lead to decreased excitability (Wang, Haj‐Dahmane, & Shen, 2006). Furthermore, an imbalance between D1 and D2 receptors has been found in the dorsolateral STR of PAE rats, impairing the development and maturation of corticostriatal synaptic plasticity (Zhou, Wang, & Zhu, 2012). However, inconsistent findings have been described regarding D1 and D2 receptors expression and activity. On one hand, Boggan, Xu, Shepherd, and Middaugh (1996) reported an elevation of D1 receptror binding in adolescent PAE mice, but not in adulthood, while D2 receptor binding was not affected. On the other hand, decreased D1 binding in the STR and increased D2 receptor mRNA levels were observed in adult PLAE rats (Barbier et al., 2008). Furthermore, a report in rhesus monkeys showed that moderate alcohol exposure during early gestation reduced the striatal D2 receptor binding to DA synthesis ratio in adulthood, whereas middle‐to‐late alcohol gestation exposure heightened dopaminergic function, suggesting a timing‐dependent effect (Schneider et al., 2005). An increased expression of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=176 in the PFC and STR of PAE mice has also been reported (Kim et al., 2013), while a decrease in DAT binding in the STR was found in PAE rats (Barbier et al., 2009). Interestingly, higher DA levels in the NAc were found in rats treated prenatally with alcohol when compared to controls, which could be due to a down‐regulation of postsynaptic receptors or desensitization of presynaptic receptors (Muñoz‐Villegas, Rodríguez, Giordano, & Juárez, 2017). Adolescent PAE rats also displayed greater dopaminergic activity in the VTA after a postnatal alcohol challenge than controls (Fabio, Vivas, & Pautassi, 2015). Moreover, a lower baseline level of striatal dopaminergic activity has been reported in other models of FASD (Carneiro et al., 2005). Thus, as Fabio et al. (2015) stated, it is probable that animals exposed to alcohol during gestation and breastfeeding have a similar or lower dopaminergic function compared to controls and, when re‐exposed to the drug, they would exhibit heightened DA activity.
In summary, we have demonstrated that alcohol exposure during pregnancy and breastfeeding in a mouse model leads to increased sensitivity to the reinforcing effects of cocaine and increased drug‐seeking and drug‐taking behaviour, as assessed in the cocaine‐induced CPP and cocaine SA paradigms, respectively. Furthermore, PLAE mice showed an attenuation of the locomotor sensitization induced by repeated cocaine treatment. We have also shown adaptations in glutamatergic neurotransmission related with the behavioural alterations found in PLAE mice.
Taken together, our results demonstrate that binge‐like alcohol exposure during critical periods for brain development alters the response of the mesocorticolimbic pathway increasing the vulnerability to later cocaine addiction.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1. GluA1/GluA2 ratio is not altered at basal conditions in PLAE and control mice. Western Blot analysis of GluA1/GluA2 protein ratio expression in the PFC and STR of PLAE and control mice (n = 4–5 per group). The numbers in the bars represent the amount of individuals in the group. The lower panel show representative fluorescent immunoblots.
ACKNOWLEDGEMENTS
This study was supported by the European Union's Horizon 2020 research and innovation program 2014–2020 under Grant Agreement 634143, Ministerio de Economía y Competitividad (SAF2016‐75347‐R), and Ministerio de Sanidad of the Spanish government (Retic‐ISCIII‐RD/16/0017/0010‐FEDER and Plan Nacional sobre Drogas 2018/007). L.C. received a FPI grant from the Ministerio de Economía y Competitividad (BES‐2014‐070657), M.A.L. received an FPU fellowship from the Ministerio de Educación, Cultura y Deporte (15/02492), and S.M.R. received an APOSTD/2017/102 European Social fund grant from the Generalitat Valenciana. The Department of Experimental and Health Sciences (UPF) is an “Unidad de Excelencia María de Maeztu” funded by the MINECO (Ref. MDM‐2014‐0370). The authors thank Gerald‐Patrick Fannon for his English proof reading and editing of the manuscript.
Cantacorps L, Montagud‐Romero S, Luján Miguel Ángel, Valverde O. Prenatal and postnatal alcohol exposure increases vulnerability to cocaine addiction in adult mice. Br J Pharmacol. 2020;177:1090–1105. 10.1111/bph.14901
REFERENCES
- Alati, R. , Al Mamun, A. , Williams, G. M. , O'Callaghan, M. , Najman, J. M. , Bor, W. , et al. (2006). In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Archives of General Psychiatry, 63, 1009–1016. 10.1001/archpsyc.63.9.1009 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. M. , Striessnig, J. , Kelly, E. , et al. (2019). The coincise guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. A. , et al. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis, B. C. , Diaz, M. R. , & Fernando Valenzuela, C. (2015). Third trimester‐equivalent ethanol exposure increases anxiety‐like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacology, Biochemistry, and Behavior, 137, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer, J. S. , Sampson, P. D. , Barr, H. M. , Connor, P. D. , & Streissguth, A. P. (2003). A 21‐year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Archives of General Psychiatry, 60, 377–385. [DOI] [PubMed] [Google Scholar]
- Barbier, E. , Houchi, H. , Warnault, V. , Pierrefiche, O. , Daoust, M. , & Naassila, M. (2009). Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience, 161, 427–440. [DOI] [PubMed] [Google Scholar]
- Barbier, E. , Pierrefiche, O. , Vaudry, D. , Vaudry, H. , Daoust, M. , & Naassila, M. (2008). Long‐term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure. Neuropharmacology, 55, 1199–1211. [DOI] [PubMed] [Google Scholar]
- Boggan, W. O. , Xu, W. , Shepherd, C. L. , & Middaugh, L. D. (1996). Effects of prenatal ethanol exposure on dopamine systems in C57BL/6J mice. Neurotoxicology and Teratology, 18, 41–48. [DOI] [PubMed] [Google Scholar]
- Brancato, A. , Castelli, V. , Cavallaro, A. , Lavanco, G. , Plescia, F. , & Cannizzaro, C. (2018). Pre‐conceptional and peri‐gestational maternal binge alcohol drinking produces inheritance of mood disturbances and alcohol vulnerability in the adolescent offspring. Frontiers in Psychiatry, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantacorps, L. , Alfonso‐Loeches, S. , Moscoso‐Castro, M. , Cuitavi, J. , Gracia‐Rubio, I. , López‐Arnau, R. , … Valverde, O. (2017). Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice. Neuropharmacology, 123, 368–384. [DOI] [PubMed] [Google Scholar]
- Cantacorps, L. , González‐Pardo, H. , Arias, J. L. , Valverde, O. , & Conejo, N. M. (2018). Altered brain functional connectivity and behaviour in a mouse model of maternal alcohol binge‐drinking. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 84, 237–249. [DOI] [PubMed] [Google Scholar]
- Carneiro, L. M. V. , Diógenes, J. P. L. , Vasconcelos, S. M. M. , Aragão, G. F. , Noronha, E. C. , Gomes, P. B. , & Viana, G. S. (2005). Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicology and Teratology, 27, 585–592. [DOI] [PubMed] [Google Scholar]
- Carr, G. D. , Phillips, A. G. , & Fibiger, H. C. (1988). Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology, 94, 221–226. [DOI] [PubMed] [Google Scholar]
- Choong, K. , & Shen, R. (2004a). Methylphenidate restores ventral tegmental area dopamine neuron activity in prenatal ethanol‐exposed rats by augmenting dopamine neurotransmission. The Journal of Pharmacology and Experimental Therapeutics, 309, 444–451. [DOI] [PubMed] [Google Scholar]
- Choong, K. , & Shen, R. (2004b). Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience, 126, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Conrad, K. L. , Tseng, K. Y. , Uejima, J. L. , Reimers, J. M. , Heng, L.‐J. , Shaham, Y. , … Wolf, M. E. (2008). Formation of accumbens GluR2‐lacking AMPA receptors mediates incubation of cocaine craving. Nature, 454, 118–121. 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, S. , Robison, A. J. , & Mazei‐Robison, M. S. (2017). Reward circuitry in addiction. Neurotherapeutics, 14, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , & Nestler, E. J. (2014). The neural rejuvenation hypothesis of cocaine addiction. Trends in Pharmacological Sciences, 35, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörrie, N. , Föcker, M. , Freunscht, I. , & Hebebrand, J. (2014). Fetal alcohol spectrum disorders. European Child & Adolescent Psychiatry, 23, 863–875. [DOI] [PubMed] [Google Scholar]
- Engblom, D. , Bilbao, A. , Sanchis‐Segura, C. , Dahan, L. , Perreau‐Lenz, S. , Balland, B. , … Spanagel, R. (2008). Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron, 59, 497–508. 10.1016/j.neuron.2008.07.010 [DOI] [PubMed] [Google Scholar]
- Everitt, B. J. , & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Fabio, M. C. , Vivas, L. M. , & Pautassi, R. M. (2015). Prenatal ethanol exposure alters ethanol‐induced Fos immunoreactivity and dopaminergic activity in the mesocorticolimbic pathway of the adolescent brain. Neuroscience, 301, 221–234. [DOI] [PubMed] [Google Scholar]
- Glantz, M. D. , & Chambers, J. C. (2006). Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Development and Psychopathology, 18, 893–922. [DOI] [PubMed] [Google Scholar]
- Gould, T. D. , & Manji, H. K. (2005). DARPP‐32: A molecular switch at the nexus of reward pathway plasticity. Proceedings of the National Academy of Sciences, 102, 253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia‐Rubio, I. , Martinez‐Laorden, E. , Moscoso‐Castro, M. , Milanés, M. V. , Laorden, M. L. , et al. (2016). Maternal separation impairs cocaine‐induced behavioural sensitization in adolescent mice. PLoS ONE, 11, e0167483 10.1371/journal.pone.0167483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht, K. , Haj‐Dahmane, S. , Shen, Y.‐L. , Vezina, P. , Dlugos, C. , & Shen, R.‐Y. (2015). Excitatory synaptic function and plasticity is persistently altered in ventral tegmental area dopamine neurons after prenatal ethanol exposure. Neuropsychopharmacology, 40, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, P. , Park, J. H. , Choi, C. S. , Choi, I. , Joo, S. H. , Kim, M. K. , … Shin, C. Y. (2013). Effects of ethanol exposure during early pregnancy in hyperactive, inattentive and impulsive behaviors and MeCP2 expression in rodent offspring. Neurochemical Research, 38, 620–631. 10.1007/s11064-012-0960-5 [DOI] [PubMed] [Google Scholar]
- Kong, H. , Kuang, W. , Li, S. , & Xu, M. (2011). Activation of dopamine D3 receptors inhibits reward‐related learning induced by cocaine. Neuroscience, 176, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob, G. F. , & Le Moal, M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24, 97–129. [DOI] [PubMed] [Google Scholar]
- Leggio, G. M. , Di Marco, R. , Gulisano, W. , D'Ascenzo, M. , Torrisi, S. A. , Geraci, F. , … Salomone, S. (2019). Dopaminergic‐GABAergic interplay and alcohol binge drinking. Pharmacological Research, 141, 384–391. 10.1016/j.phrs.2019.01.022 [DOI] [PubMed] [Google Scholar]
- López‐Arnau, R. , Luján, M. A. , Duart‐Castells, L. , Pubill, D. , Camarasa, J. , Valverde, O. , & Escubedo, E. (2017). Exposure of adolescent mice to 3,4‐methylenedioxypyrovalerone increases the psychostimulant, rewarding and reinforcing effects of cocaine in adulthood. British Journal of Pharmacology, 174, 1161–1173. 10.1111/bph.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján, M. Á. , Castro‐Zavala, A. , Alegre‐Zurano, L. , & Valverde, O. (2018). Repeated Cannabidiol treatment reduces cocaine seeking and modulates neural proliferation and CB1R expression in the hippocampus. Neuropharmacology, 143, 163–175. [DOI] [PubMed] [Google Scholar]
- Lüscher, C. (2016). The emergence of a circuit model for addiction. Annual Review of Neuroscience, 39, 257–276. [DOI] [PubMed] [Google Scholar]
- Lüscher, C. , & Bellone, C. (2008). Cocaine‐evoked synaptic plasticity: A key to addiction? Nature Neuroscience, 11, 737–738. [DOI] [PubMed] [Google Scholar]
- McClung, C. A. , & Nestler, E. J. (2003). Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nature Neuroscience, 6, 1208–1215. [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. British Journal of Pharmacology, 172, 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso‐Castro, M. , López‐Cano, M. , Gracia‐Rubio, I. , Ciruela, F. , & Valverde, O. (2017). Cognitive impairments associated with alterations in synaptic proteins induced by the genetic loss of adenosine A2Areceptors in mice. Neuropharmacology, 126, 48–57. [DOI] [PubMed] [Google Scholar]
- Muñoz‐Villegas, P. , Rodríguez, V. M. , Giordano, M. , & Juárez, J. (2017). Risk‐taking, locomotor activity and dopamine levels in the nucleus accumbens and medial prefrontal cortex in male rats treated prenatally with alcohol. Pharmacology, Biochemistry, and Behavior, 153, 88–96. [DOI] [PubMed] [Google Scholar]
- Nestler, E. J. (2008). Transcriptional mechanisms of addiction: Role of FosB. Philos. Trans. R. Soc. B Biol. Sci., 363, 3245–3255. 10.1098/rstb.2008.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2016). Drinking levels defined. [Google Scholar]
- Parker, M. O. , Evans, A. M.‐D. , Brock, A. J. , Combe, F. J. , Teh, M.‐T. , & Brennan, C. H. (2016). Moderate alcohol exposure during early brain development increases stimulus‐response habits in adulthood. Addiction Biology, 21, 49–60. [DOI] [PubMed] [Google Scholar]
- Philibin, S. D. , Hernandez, A. , Self, D. W. , & Bibb, J. A. (2011). Striatal signal transduction and drug addiction. Frontiers in Neuroanatomy, 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, R. C. , & Kumaresan, V. (2006). The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and Biobehavioral Reviews, 30, 215–238. [DOI] [PubMed] [Google Scholar]
- Popova, S. , Lange, S. , Probst, C. , Gmel, G. , & Rehm, J. (2018). Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder. Biochemistry and Cell Biology, 96, 237–240. [DOI] [PubMed] [Google Scholar]
- Popova, S. , Lange, S. , Probst, C. , Parunashvili, N. , & Rehm, J. (2017). Prevalence of alcohol consumption during pregnancy and Fetal Alcohol Spectrum Disorders among the general and Aboriginal populations in Canada and the United States. European Journal of Medical Genetics, 60, 32–48. [DOI] [PubMed] [Google Scholar]
- Runegaard, A. H. , Sørensen, A. T. , Fitzpatrick, C. M. , Jørgensen, S. H. , Petersen, A. V. , Hansen, N. W. , … Gether, U. (2018). Locomotor‐ and reward‐enhancing effects of cocaine are differentially regulated by chemogenetic stimulation of Gi‐signaling in dopaminergic neurons. Eneuro, 5, ENEURO.0345–17.2018. 10.1523/ENEURO.0345-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, M. L. , Moore, C. F. , Barnhart, T. E. , Larson, J. a. , DeJesus, O. T. , Mukherjee, J. , … Kraemer, G. W. (2005). Moderate‐level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcoholism, Clinical and Experimental Research, 29, 1685–1697. 10.1097/01.alc.0000179409.80370.25 [DOI] [PubMed] [Google Scholar]
- Soria, G. , Barbano, M. F. , Maldonado, R. , & Valverde, O. (2008). A reliable method to study cue‐, priming‐, and stress‐induced reinstatement of cocaine self‐administration in mice. Psychopharmacology, 199, 593–603. [DOI] [PubMed] [Google Scholar]
- Spear, N. E. , & Molina, J. C. (2005). Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcoholism, Clinical and Experimental Research, 29, 909–929. [DOI] [PubMed] [Google Scholar]
- UNODC (2019). World drug report. [Google Scholar]
- Valverde, O. , Mantamadiotis, T. , Torrecilla, M. , Ugedo, L. , Pineda, J. , Bleckmann, S. , … Maldonado, R. (2004). Modulation of anxiety‐like behavior and morphine dependence in CREB‐deficient mice. Neuropsychopharmacology, 29, 1122–1133. 10.1038/sj.npp.1300416 [DOI] [PubMed] [Google Scholar]
- Van den Oever, M. C. , Spijker, S. , Smit, A. B. , & De Vries, T. J. (2010). Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neuroscience and Biobehavioral Reviews, 35, 276–284. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Haj‐Dahmane, S. , & Shen, R.‐Y. (2006). Effects of prenatal ethanol exposure on the excitability of ventral tegmental area dopamine neurons in vitro. The Journal of Pharmacology and Experimental Therapeutics, 319, 857–863. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Hausknecht, K. A. , Shen, Y.‐L. , Haj‐Dahmane, S. , Vezina, P. , & Shen, R.‐Y. (2018). Environmental enrichment reverses increased addiction risk caused by prenatal ethanol exposure. Drug and Alcohol Dependence, 191, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Shen, Y.‐L. , Hausknecht, K. A. , Chang, L. , Haj‐Dahmane, S. , Vezina, P. , & Shen, R. Y. (2019). Prenatal ethanol exposure increases risk of psychostimulant addiction. Behavioural Brain Research, 356, 51–61. 10.1016/j.bbr.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, M. V. , Cuzon Carlson, V. C. , Sherazee, N. , Sprow, G. M. , Bock, R. , Thiele, T. E. , … Alvarez, V. A. (2014). Repeated binge‐like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology, 39, 579–594. 10.1038/npp.2013.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, M. E. (2016). Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews. Neuroscience, 17, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward Hopf, F. , & Mangieri, R. A. (2018). Do alcohol‐related AMPA‐type glutamate receptor adaptations promote intake? Handbook of Experimental Pharmacology, 248, 157–186. [DOI] [PubMed] [Google Scholar]
- Zachariou, V. , Sgambato‐Faure, V. , Sasaki, T. , Svenningsson, P. , Berton, O. , Fienberg, A. A. , … Nestler, E. J. (2006). Phosphorylation of DARPP‐32 at threonine‐34 is required for cocaine action. Neuropsychopharmacology, 31, 555–562. 10.1038/sj.npp.1300832 [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Yanagida, J. , Kamii, H. , Wada, S. , Domoto, M. , Sasase, H. , … Kaneda, K. (2019). Glutamatergic neurons in the medial prefrontal cortex mediate the formation and retrieval of cocaine‐associated memories in mice. Addiction Biology, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhou, R. , Wang, S. , & Zhu, X. (2012). Prenatal ethanol exposure alters synaptic plasticity in the dorsolateral striatum of rat offspring via changing the reactivity of dopamine receptor. PLoS ONE, 7, e42443 10.1371/journal.pone.0042443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. GluA1/GluA2 ratio is not altered at basal conditions in PLAE and control mice. Western Blot analysis of GluA1/GluA2 protein ratio expression in the PFC and STR of PLAE and control mice (n = 4–5 per group). The numbers in the bars represent the amount of individuals in the group. The lower panel show representative fluorescent immunoblots.


