Abstract
Background: Previous studies had been published to explore the association about carbohydrate intake on esophageal cancer risk, with inconsistent results. This meta-analysis aimed to assess the association between dietary carbohydrate intake and the risk of esophageal cancer.
Methods: Suitable studies were carefully searched with the databases of PubMed, Embase, the Cochrane Library, and Wanfang Database. A random-effects model was used for combined odds ratio (OR) and 95% confidence interval (CI). Stata software 14.0 was adopted for the analysis.
Results: At the end, 13 publications were included in our study. Pooled results suggested that highest category versus lowest category of carbohydrate intake could reduce the risk of esophageal cancer (summarized OR = 0.627, 95% CI = 0.505–0.778, I2 = 59.9%, P for heterogeneity = 0.001). The results for carbohydrate intake on the risk of esophageal adenocarcinoma (summarized OR = 0.569, 95% CI = 0.417–0.777) and esophageal squamous cell carcinoma (summarized OR = 0.665, 95% CI = 0.453–0.975) were consistent with the overall result. A positive association was found in European, Asian, North American populations, instead of South American populations.
Conclusions: In conclusions, dietary carbohydrate intake may have a protective effect against the risk of esophageal cancer.
Keywords: Carbohydrate, Dietary, Esophageal cancer, Meta-analysis
Introduction
Cancer is a crucial health problem on a global scale that has become one of the primary causes of death. According to Globocan estimates in the year 2018, an estimated of 9.6 million were deaths from cancer [1]. Esophageal cancer remained an indispensable cause of cancer-related deaths and had shown a dramatic increase in global morbidity by more than six times [2]. Efforts to identify lifestyle factors [3] that may affect the risk of esophageal cancer had been ongoing, as well as some dietary factors, such as dietary vitamins [4,5], dietary fiber intake [6], dietary folate intake [7,8], total iron and zinc intake [9] and so on, may affect the development of esophageal cancer. Previous studies had been published to assess carbohydrate intake and some cancers risk, such as colorectal cancer [10], breast cancer [11], prostate cancer [12], but no meta-analysis was performed between carbohydrate intake and the risk of esophageal cancer. So far, numerous researchers explored dietary carbohydrate intake on the potential effects of esophageal cancer, but existing epidemiological data were inconsistent. Hence, we aimed to evaluate results from previous studies systematically and carefully by conducting a meta-analysis of observational studies to find: (1) whether highest versus lowest category of dietary carbohydrate intake could reduce the risk of esophageal cancer; (2) whether between-study heterogeneity or publication bias exited in our study.
Method
We used the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines in the present study [13].
Data source and search strategy
Two authors independently performed a literature search in databases of PubMed, Embase, the Cochrane Library, and Wanfang Database. All suitable studies published from beginning to July 1, 2019 were considered to be included. The associated medical subject headings and terms were ‘diet’ OR ‘dietary’ AND ‘carbohydrate’ OR ‘sugar’ AND ‘esophageal cancer’ OR ‘esophageal tumor’ OR ‘esophageal carcinoma’ OR ‘esophageal adenocarcinoma’ OR ‘esophageal squamous cell carcinoma’. Divergence in the search results was resolved by discussion.
Inclusion criteria
The studies were included in our meta-analysis if they met the following criteria: (1) the studies were with case–control design or cohort design or cross-sectional study; (2) studies assessing the associations between dietary carbohydrate intake and the risk of esophageal cancer; (3) studies reporting in humans; (4) available odds ratio (OR) in case–control studies or relative risk (RR) in cohort studies and 95% confidence interval (CI) for highest category versus lowest category of dietary carbohydrate intake.
Exclusion criteria
Overlapped studies or populations, conference reports, editor comments, reviews, case reports, and academic dissertations were excluded for the analysis.
Data extraction
Two authors independently extracted the following data from each eligible study: first author’s name, publication year, research location, sample size, average cases age, disease type, study design, OR or RR and 95% CI of dietary carbohydrate intake, assessment of intake, adjusted or matched for factors. Divergence in the extraction was resolved by discussion.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale was used to assess the quality of the included studies [14].
Statistical analysis
Association analysis between dietary carbohydrate intake and the risk of esophageal cancer was performed using a random-effects model. The effect size was estimated by calculating the summarized OR or RR and its 95% CI [15]. The I2 statistic was used to estimate the degree of heterogeneity among the studies [16]. Meta-regression was performed to interpret the between-group heterogeneity [17]. Furthermore, sensitivity analyses and publication biases by Egger’s test [18] and Begg’s funnel plots [19] were performed. All tests were two-tailed, and a P value less than 0.05 were considered statistically significant.
Results
Characteristic of included studies
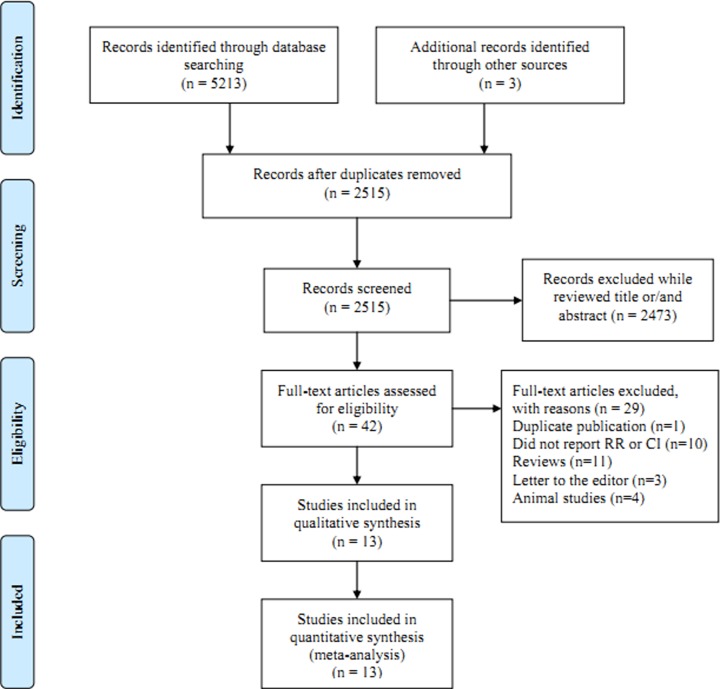
Our research returned 5213 articles from the above mentioned databases. Three articles were identified from the references of the relevant articles. After removing the duplicates from the different databases, 2515 articles were reviewed with titles and abstract. Then, 2473 articles were excluded due to not suitable for our analysis while reviewed the titles and/or abstract. The full texts of 42 articles were assessed. Twenty-nine articles were further excluded with reasons, which showed in the Figure 1. Finally, we included 13 articles [20–32] that assessed a total of 3033 patients in our meta-analysis. The quality evaluation scores (Table 1) of each study ranged from 6 to 9 and the methodological quality was higher. The characteristics of the included studies are shown in Table 1. Since all the included studies were with case–control design, we used the pooling OR instead of RR.
Figure 1. Flow chart of meta-analysis for exclusion/inclusion of studies.
Table 1. Characteristics of the included studies.
| Study, year | Design | Age | Participants, Cases | Country | Disease type | Assessment of intake | Quality score | OR (95%CI) Highest versus lowest | Adjusted for or matched for |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2002 | PBCC | 62.3 ± 12.4 | 573, 124 | United States | Esophageal adenocarcinoma | HHHQ | 7 | 0.4(0.2–0.9) | Age, age squared, sex, respondent type, BMI, alcohol use, tobacco use, education, family history of cancers, and vitamin supplement use |
| De Stefani et al., 2006 | HBCC | 40–89 | 1170, 234 | Uruguay | Esophageal squamous cell carcinoma | FFQ | 8 | 0.74(0.47–1.17) | Age, sex, residence, urban/rural status, birthplace, education, body mass index, smoking status, years since quit smoking, number of cigarettes smoked per day, alcohol drinking, mate consumption, and total energy intake. |
| De Stefani et al., 1999 | HBCC | NA | 459, 66 | Uruguay | Esophageal cancer | FFQ | 6 | 0.8(0.5–1.1) | Age, sex, residence, urban/rural status, education, BMI, tobacco smoking, total alcohol intake, and total energy intake |
| Jessri et al., 2011 | HBCC | 40–75 | 143, 47 | Iran | Esophageal squamous cell carcinoma | FFQ | 8 | 0.22(0.05–0.84) | Age, sex, reflux, BMI, smoking, physical activity, and education |
| Lagergren et al., 2013 | PBCC | <80 | 1008, 188 | Sweden | Esophageal adenocarcinoma | FFQ | 9 | 0.68(0.40–1.16) | Age, sex, reflux, BMI, smoking, alcohol consumption, education grade, and total energy intake |
| Lagergren et al., 2013 | PBCC | <80 | 987, 167 | Sweden | Esophageal squamous cell carcinoma | FFQ | 9 | 1.05(0.61–1.80) | Age, sex, reflux, BMI, smoking, alcohol consumption, education grade, and total energy intake |
| Lahmann et al., 2014 | PBCC | 18–79 | 1778, 88 | Australia | Esophageal adenocarcinoma | FFQ | 8 | 0.79(0.49–1.25) | Age, sex, education, BMI, smoking, physical activity, alcohol intake, NSAID, diabetes, total fruit intake (except for fiber), red meat, processed meat, and total energy |
| Lahmann et al., 2014 | PBCC | 18–79 | 1717, 227 | Australia | Esophageal squamous cell carcinoma | FFQ | 8 | 0.46(0.28–0.75) | Age, sex, education, BMI, smoking, physical activity, alcohol intake, NSAID, diabetes, total fruit intake (except for fiber), red meat, processed meat, and total energy |
| Li et al., 2017 | PBCC | 30–79 | 2527, 500 | United States | Esophageal adenocarcinoma | FFQ | 8 | 0.93(0.56–1.54) | Age, sex, race, study indicator, BMI, fruits and vegetables intake, cigarette smoking, GERD frequency, and total energy intake |
| Mayne et al., 2001 | PBCC | 30–80 | 969, 282 | United States | Esophageal adenocarcinoma | FFQ | 7 | 0.34(0.20–0.58) | Age, site, sex, race, proxy status, BMI, income, education, smoking, and alcohol consumption |
| Mayne et al., 2001 | PBCC | 30–80 | 893, 206 | United States | Esophageal squamous cell carcinoma | FFQ | 7 | 0.68(0.37–1.25) | Age, site, sex, race, proxy status, BMI, income, education, smoking, and alcohol consumption |
| Mulholland et al., 2009 | PBCC | 64 ± 11 | 480, 224 | Ireland | Esophageal adenocarcinoma | FFQ | 8 | 0.39(0.16–0.98) | Age, sex, energy intake, smoking, BMI, education, occupation, alcohol, regular NSAID use, location, and H. pylori |
| Tzonou et al., 1996 | HBCC | NA | 256, 56 | Greece | Esophageal adenocarcinoma | FFQ | 6 | 0.84(0.59–1.19) | Age, sex, birth place, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake |
| Tzonou et al., 1996 | HBCC | NA | 243, 43 | Greece | Esophageal squamous cell carcinoma | FFQ | 6 | 1.12(0.75–1.69) | Age, sex, birth place, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake |
| Wolfgarten et al., 2001 | PBCC | 62.2 ± 1.9 | 140, 40 | Germany | Esophageal adenocarcinoma | FFQ | 8 | 0.07(0.03–0.40) | Age, gender, height, weight, BMI and socioeconomic data such as marital status and earning capacity |
| Wolfgarten et al., 2001 | PBCC | 58.1 ± 1.2 | 145, 45 | Germany | Esophageal squamous cell carcinoma | FFQ | 8 | 0.16(0.03–0.59) | Age, gender, height, weight, BMI and socioeconomic data such as marital status and earning capacity |
| Wu et al., 2007 | PBCC | 30–74 | 1514, 206 | United States | Esophageal adenocarcinoma | FFQ | 7 | 0.66(0.40–1.10) | Age, sex, race, birthplace, education, smoking, BMI, reflux, use of vitamins, total calories, and fat |
| Zhang et al., 1997 | HBCC | NA | 214, 90 | United States | Esophageal adenocarcinoma | HHHQ | 7 | 0.7(0.3–1.8) | Age, sex, race, education, smoking, alcohol intake, BMI, and total dietary intake in calories |
Abbreviation: OR: odds ratio; CI: Confidence Intervals; PBCC: Population-based case–control study; HBCC: Hospital-based case–control study; NA: Not available; HHHQ: Health habits and history questionnaire; FFQ: Food frequency questionnaire; BMI: Body mass index.
In our included articles, there are five texts (Lagergren et al. 2013, Lahmann et al. 2014, Mayne et al. 2001, Tzonou et al. 1996, and Wolfgarten et al. 2001) reported both esophageal adenocarcinoma and esophageal squamous cell carcinoma about dietary carbohydrate intake. Therefore, 13 articles with 18 studies were used for the analysis.
Main results
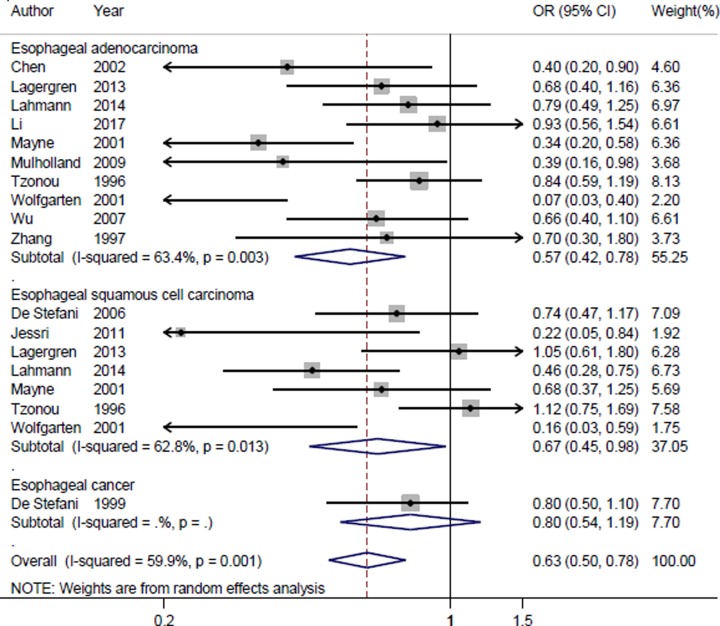
In the results of the overall analysis, highest category versus lowest category of dietary carbohydrate intake could significantly reduce the risk of esophageal cancer (summarized OR = 0.627, 95% CI = 0.505–0.778, I2 = 59.9%, P for heterogeneity = 0.001) (Figure 2). The results in the subgroup of esophageal adenocarcinoma (summarized OR = 0.569, 95% CI = 0.417–0.777) and esophageal squamous cell carcinoma (summarized OR = 0.665, 95% CI = 0.453–0.975) were consistent with the overall result. A positive association was found in European, Asian, North American populations, instead of South American populations. When we conducted a subgroup analysis by study design, the association was significant in population-based case–control studies (PBCC), but not in the hospital-based case–control studies (HBCC). The detailed results are shown in Table 2.
Figure 2. The forest plot of the association between dietary carbohydrate intake and esophageal cancer risk.
Table 2. Summary results about the association between dietary carbohydrate intake and esophageal cancer risk.
| Subgroups | Number of studies | Number of cases | OR(95% CI) | P for trend | Heterogeneity test | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Total | 18 | 3033 | 0.627(0.505–0.778) | <0.001 | 59.9 | 0.001 |
| Disease type | ||||||
| Esophageal adenocarcinoma | 10 | 1998 | 0.569(0.417–0.777) | <0.001 | 63.4 | 0.003 |
| Esophageal squamous cell carcinoma | 7 | 969 | 0.665(0.453–0.975) | 0.037 | 62.8 | 0.013 |
| Study design | ||||||
| PBCC | 12 | 2497 | 0.541(0.401–0.729) | <0.001 | 63.2 | 0.002 |
| HBCC | 6 | 536 | 0.831(0.669–1.030) | 0.091 | 15.3 | 0.316 |
| Geographic locations | ||||||
| Europe | 7 | 763 | 0.586(0.364–0.943) | 0.028 | 75.5 | <0.001 |
| Asia | 3 | 562 | 0.534(0.308–0.927) | 0.026 | 53.9 | 0.114 |
| North America | 6 | 1408 | 0.590(0.425–0.820) | 0.002 | 43.1 | 0.118 |
| South America | 2 | 300 | 0.774(0.574–1.043) | 0.092 | 0.0 | 0.800 |
OR: odds ratio; CI: confidence interval; PBCC: population-based case–control studies; HBCC: hospital-based case–control studies
Publication bias and sensitivity analysis
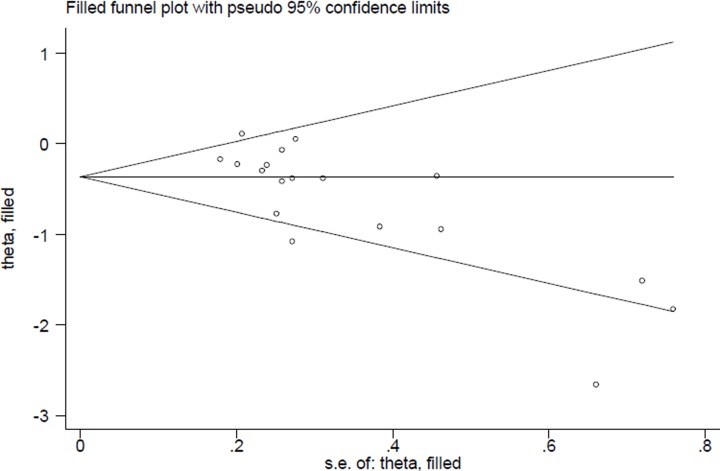
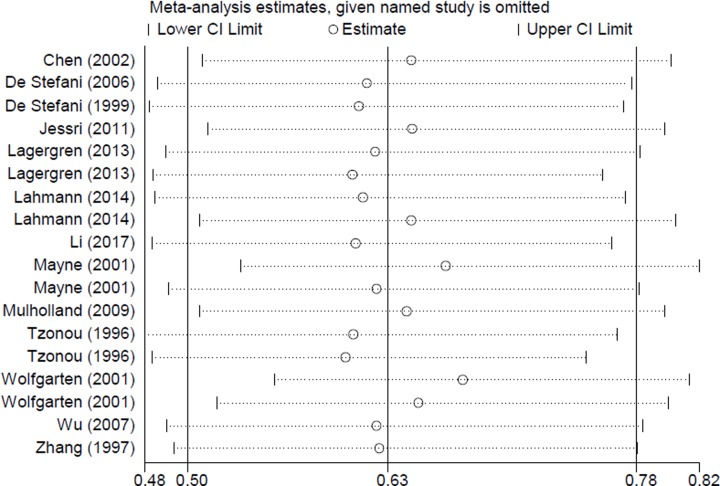
In the publication bias assessment, the results from funnel plots (Figure 3) and Egger’s test (P = 0.107) detected no publication bias. Sensitivity analyses (Figure 4) showed no single study had essential effect on the overall result.
Figure 3. Funnel plot for the analysis of publication bias between dietary carbohydrate intake and esophageal cancer risk.
Figure 4. Sensitivity analyses between dietary carbohydrate intake and esophageal cancer risk.
Discussion
Numerous of studies about dietary carbohydrate intake and esophageal cancer had been published, with conflicting results. However, no meta-analysis was performed to obtain a definitive conclusion. Therefore, we conducted this study to clarify whether dietary carbohydrate intake had some inverse effects on the development of esophageal cancer. In total, our results suggested that dietary carbohydrate intake had significant association on the lower development of esophageal cancer.
In the current meta-analysis, people with higher carbohydrate intake may reduce the risk of esophageal cancer. On the one hand, carbohydrate intake was negatively correlated with fat intakes. Therefore, people who were with higher carbohydrate intake may also have lower intake of fat, then explained its inverse association with esophageal cancer [23]. On the other hand, people who were with higher intake of carbohydrate could be reflection of more plant-based food intakes, and especially fruit and vegetable, which had been confirmed having a relationship with esophageal cancer [33].
We found significant between-study heterogeneity in the whole pooled results of dietary carbohydrate intake and esophageal cancer risk. As introduced in the methods, we used meta-regression to explore the causes of heterogeneity for covariates of publication year, disease type, study design, geographic locations, assessment of intake and number of cases. Results from meta-regression suggested that no covariates increased the high between-study heterogeneity. Moreover, between-study heterogeneity also exited in the subgroup analyses by disease type, study design and geographic locations. We then used leave-one-out analysis to reduce the between-study heterogeneity. The I2 was reduced to 47.1% when we leaved one study by Wolfgarten et al. 2001 [30] (about the esophageal adenocarcinoma study). And the pooled result about the remaining 17 studies was not changed (summarized OR = 0.673, 95% CI = 0.558–0.811). Meanwhile, all the pooling results in our analysis are based on adjusted OR in each individual study, and thereby could control some between-study heterogeneity.
The present study still had several limitations. First, all the included studies were case–control studies. As well as known, the selection bias, recall bias and some other confounding factors cannot be excluded in the case–control studies. Hence, it is requirement for evidence from prospective cohort studies. Second, all the included studies were with English language and this may omit other languages studies. Meanwhile, the papers which had been published in the journal or online were searched and included in our analysis. Those papers which published in the meetings or unpublished were not searched. However, we did not detect any publication bias in our meta-analysis. Third, we only assessed the association between dietary total carbohydrate intake and the risk of esophageal cancer, and did not assess the association between carbohydrate type and esophageal cancer risk due to the limitation data provided in all the included original articles. Hence, more articles with detailed carbohydrate type are warranted to further assess the risk of esophageal cancer.
Conclusions
In summary, our results indicated that dietary intake of carbohydrate may contribute to the lower development of esophageal cancer. As some limitations existed in our analysis, large scale prospective studies with detailed type of dietary carbohydrate intake are needed to verify our results.
Abbreviations
- CI
confidence interval
- HBCC
hospital-based case–control studies
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- OR
odds ratio
- PBCC
population-based case–control studies
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
F.X., W.L., and X.Q.G. participated in the design of the study, acquisition of data; X.Q.G. performed the statistical analysis; F.X. and C.Y.L. draft the manuscript; C.Y.L. reviewed and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Globocan (2018) Estimated cancer incidence, mortality and prevalence worldwide in 2018. Int. Agency Res. Cancer WHOAvailable online: http://gco.iarc.fr/ [Google Scholar]
- 2.Simard E.P., Ward E.M., Siegel R. and Jemal A. (2012) Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 62, 118–128 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 3.Sardana R.K., Chhikara N., Tanwar B. and Panghal A. (2018) Dietary impact on esophageal cancer in humans: a review. Food Funct. 9, 1967–1977 10.1039/C7FO01908D [DOI] [PubMed] [Google Scholar]
- 4.Ma J.L., Zhao Y., Guo C.Y., Hu H.T., Zheng L., Zhao E.J. et al. (2018) Dietary vitamin B intake and the risk of esophageal cancer: a meta-analysis. Cancer Manag Res. 10, 5395–5410 10.2147/CMAR.S168413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui L., Li L., Tian Y., Xu F. and Qiao T. (2018) Association between Dietary Vitamin E Intake and Esophageal Cancer Risk: An Updated Meta-Analysis. Nutrients 10, pil: E801 10.3390/nu10070801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McRae M.P. (2018) The Benefits of Dietary Fiber Intake on Reducing the Risk of Cancer: An Umbrella Review of Meta-analyses. J. Chiropr Med. 17, 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Zhou H., Zhu Y. and Tie C. (2017) Associations between dietary folate intake and risks of esophageal, gastric and pancreatic cancers: an overall and dose-response meta-analysis. Oncotarget 8, 86828–86842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Guo C., Hu H., Zheng L., Ma J., Jiang L. et al. (2017) Folate intake, serum folate levels and esophageal cancer risk: an overall and dose-response meta-analysis. Oncotarget 8, 10458–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J., Li Q., Fang X., Chen L., Qiang Y., Wang J. et al. (2018) Increased total iron and zinc intake and lower heme iron intake reduce the risk of esophageal cancer: A dose-response meta-analysis. Nutr. Res. 59, 16–28 10.1016/j.nutres.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Pan G., Jiang H., Li W., Dong J., Zhang H. et al. (2017) A meta-analysis between dietary carbohydrate intake and colorectal cancer risk: evidence from 17 observational studies. Biosci. Rep. 37, pii: BSR20160553 10.1042/BSR20160553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlesinger S., Chan D.S.M., Vingeliene S., Vieira A.R., Abar L., Polemiti E. et al. (2017) Carbohydrates, glycemic index, glycemic load, and breast cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Nutr. Rev. 75, 420–441 10.1093/nutrit/nux010 [DOI] [PubMed] [Google Scholar]
- 12.Zhai L., Cheng S. and Zhang D. (2015) Dietary carbohydrate and prostate cancer risk: a meta-analysis. Nutr. Cancer 67, 594–602 10.1080/01635581.2015.1019639 [DOI] [PubMed] [Google Scholar]
- 13.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283, 2008–2012 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P. and Thompson S.G. (2004) Controlling the risk of spurious findings from meta-regression. Stat. Med. 23, 1663–1682 10.1002/sim.1752 [DOI] [PubMed] [Google Scholar]
- 18.Egger M., Davey Smith G., Schneider M. and Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Tucker K.L., Graubard B.I., Heineman E.F., Markin R.S., Potischman N.A. et al. (2002) Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer 42, 33–40 10.1207/S15327914NC421_5 [DOI] [PubMed] [Google Scholar]
- 21.De Stefani E., Ronco A.L., Boffetta P., Deneo-Pellegrini H., Acosta G., Correa P. et al. (2006) Nutrient intake and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr. Cancer 56, 149–157 10.1207/s15327914nc5602_5 [DOI] [PubMed] [Google Scholar]
- 22.De Stefani E., Ronco A., Mendilaharsu M. and Deneo-Pellegrini H. (1999) Diet and risk of cancer of the upper aerodigestive tract–II. Nutrients Oral Oncol. 35, 22–26 10.1016/S1368-8375(98)00061-X [DOI] [PubMed] [Google Scholar]
- 23.Jessri M., Rashidkhani B., Hajizadeh B., Jessri M. and Gotay C. (2011) Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: a case-control study in Iran. Nutr. J. 10, 137 10.1186/1475-2891-10-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagergren K., Lindam A. and Lagergren J. (2013) Dietary proportions of carbohydrates, fat, and protein and risk of oesophageal cancer by histological type. PLoS One 8, e54913 10.1371/journal.pone.0054913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahmann P.H., Ibiebele T.I., Webb P.M., Nagle C.M., Whiteman D.C. and Australian Cancer S. (2014) A case-control study of glycemic index, glycemic load and dietary fiber intake and risk of adenocarcinomas and squamous cell carcinomas of the esophagus: the Australian Cancer Study. BMC Cancer 14, 877 10.1186/1471-2407-14-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N., Petrick J.L., Steck S.E., Bradshaw P.T., McClain K.M., Niehoff N.M. et al. (2017) A pooled analysis of dietary sugar/carbohydrate intake and esophageal and gastric cardia adenocarcinoma incidence and survival in the USA. Int. J. Epidemiol. 46, 1836–1846 10.1093/ije/dyx203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayne S.T., Risch H.A., Dubrow R., Chow W.H., Gammon M.D., Vaughan T.L. et al. (2001) Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 10, 1055–1062 [PubMed] [Google Scholar]
- 28.Mulholland H.G., Cantwell M.M., Anderson L.A., Johnston B.T., Watson R.G., Murphy S.J. et al. (2009) Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 20, 279–288 10.1007/s10552-008-9242-6 [DOI] [PubMed] [Google Scholar]
- 29.Tzonou A., Lipworth L., Garidou A., Signorello L.B., Lagiou P., Hsieh C. et al. (1996) Diet and risk of esophageal cancer by histologic type in a low-risk population. Int. J. Cancer 68, 300–304 [DOI] [PubMed] [Google Scholar]
- 30.Wolfgarten E., Rosendahl U., Nowroth T., Leers J., Metzger R., Holscher A.H. et al. (2001) Coincidence of nutritional habits and esophageal cancer in Germany. Onkologie 24, 546–551 [DOI] [PubMed] [Google Scholar]
- 31.Wu A.H., Tseng C.C., Hankin J. and Bernstein L. (2007) Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control 18, 713–722 10.1007/s10552-007-9014-8 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z.F., Kurtz R.C., Yu G.P., Sun M., Gargon N., Karpeh M. Jr et al. (1997) Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr. Cancer 27, 298–309 10.1080/01635589709514541 [DOI] [PubMed] [Google Scholar]
- 33.Li B., Jiang G., Zhang G., Xue Q., Zhang H., Wang C. et al. (2014) Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur. J. Nutr. 53, 1511–1521 10.1007/s00394-014-0656-5 [DOI] [PubMed] [Google Scholar]