Abstract
Background
Coronary vasculitis is a rare, life-threatening complication of systemic lupus erythematosus (SLE).
Case summary
A 23-year-old woman with SLE presented with typical angina and worsening dyspnoea on exertion. Coronary angiography revealed severe triple vessel disease with a ‘string of beads’ appearance classic for coronary vasculitis. Transthoracic echocardiogram revealed ejection fraction of 25–30% with a severely hypokinetic distal septum and distal anterior wall and an akinetic apical wall. Despite vasculitis treatment with cyclophosphamide and pulse-dose steroids, her coronary vasculitis did not improve. She was refractory to anti-anginal and guideline-directed medical therapy for heart failure and successfully underwent orthotopic heart transplant (OHT).
Discussion
This is the first reported case of OHT in the case of SLE coronary vasculitis. Chronic SLE coronary vasculitis is caused by lymphocyic infiltration leading to inflammation and fibrosis of the major epicardial coronary arteries but can be successfully managed with OHT when refractory to medical SLE and heart failure therapies. It can affect patients of all ages with SLE, emphasizing the importance of thorough history taking and clinical evaluation in young patients presenting with cardiac symptoms to establish an appropriate diagnosis and treatment plan.
Keywords: Systemic lupus erythematosus, Coronary vasculitis, Ischaemic cardiomyopathy, Orthotopic heart transplant, Case report
Learning points
A thorough evaluation of chest pain in systemic lupus erythematosus (SLE) patients with accurate history, electrocardiogram and echocardiogram are of paramount importance to guide further management options.
Systemic lupus erythematosus coronary vasculitis is a diagnosis of exclusion requiring coronary angiography or cardiac computed tomographic angiography with a ‘string of beads’ appearance.
When SLE coronary vasculitis is refractory to medical SLE and heart failure therapy, orthotropic heart transplant may be offered without significant concern for recurrent vasculitis affecting transplanted heart.
Introduction
While accelerated coronary atherosclerosis is a well-known complication of inflammation from systemic lupus erythematosus (SLE), coronary vasculitis is rarely reported in SLE. It is a diagnosis of exclusion made by coronary angiography or coronary computed tomographic angiography showing evidence of coronary ectasia or aneurysms in patients with known SLE presenting with chest pain in the absence of coronary atherosclerosis.1–4 Histopathology from autopsy or biopsies demonstrates fibrinoid degeneration, thrombosis, and immune complex deposition with neutrophilic and lymphocytic infiltration in the intima and media of the coronary arteries.5 Chest pain in SLE is typically caused by pericarditis (20–50%), followed by myocarditis (7–10%), and atherosclerotic coronary artery disease (CAD) in 6–10% of patients.6,7 There are few reports of improvement in SLE-associated coronary vasculitis with pulse-dose methylprednisolone and cyclophosphamide rather than percutaneous intervention.8,9
We describe, herein, the first reported case of orthotopic heart transplant (OHT) in a young Hispanic female with SLE who developed acute myocardial infarction and ischaemic cardiomyopathy (ICM) due to SLE coronary vasculitis.
Timeline
| Two years prior to presentation | Systemic lupus erythematosus diagnosed, patient started on hydroxychloroquine and prednisone |
| Five months prior to presentation | Fatigue, weakness, dyspnoea on exertion |
| One month prior to presentation | Substernal chest pain with exertion |
| Five days prior to presentation | Chest pain persisted at rest |
| Two days prior to presentation | Emergency Department visit with diagnosis of pericarditis, given prednisone |
| Admission after return to Emergency Department | Diagnosed with non-ST-elevation myocardial infarction, underwent urgent coronary angiography with ‘string of beads’ pattern consistent with vasculitis Transthoracic echocardiogram with left ventricular ejection fraction 30% and corresponding wall motion abnormalities Cardiac magnetic resonance imaging consistent with ischaemic cardiomyopathy No response to vasculitis therapy and unable to tolerate guideline-directed medical therapy for heart failure Listed for orthotopic heart transplantation, eventually transplanted on hospital Day 16 |
| Follow-up | Normal functional capacity now managed on hydroxychloroquine, mycophenolate, and tacrolimus |
Case presentation
A 23-year-old Hispanic woman with SLE and atrial septal defect closure as a child presented with a 1-month history of stabbing, substernal, and exertional chest pain. Chest pain did not have any positional component and had been persistent for 5 days prior to presentation. She initially presented to her local hospital and was discharged with a provisional diagnosis of pericarditis 2 days prior to presentation. She also endorsed 5 months of fatigue, weakness, and dyspnoea on exertion. She was diagnosed with SLE 2 years ago with arthritis, non-scarring alopecia, rash, and elevated serum anti-nuclear antibody (ANA) and double-stranded DNA antibody (dsDNA Ab).10 Her SLE was managed by her primary care provider with 10 mg prednisone and 200 mg hydroxychloroquine daily. She was a non-smoker, and had no known family history of CAD or sudden cardiac death.
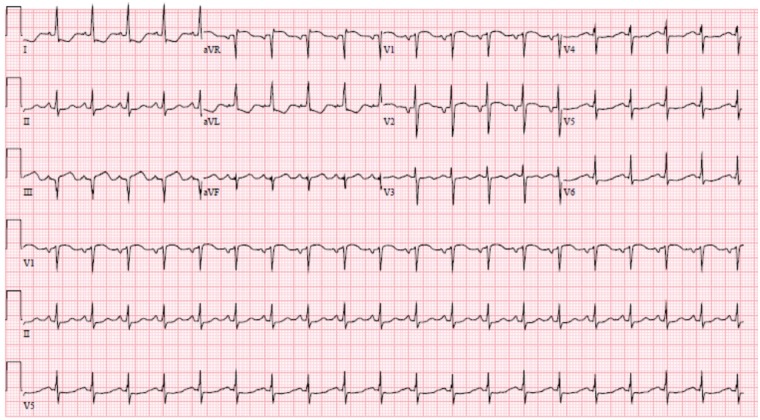
On presentation, she was afebrile, normotensive at 113/72, tachycardic to 106 b.p.m., saturating 95% on room air, but had significant dyspnoea during speech. Her examination revealed an S3 gallop, an 8-cm jugular venous distension, with mild bi-basilar pulmonary crackles. Initial complete blood count and basic metabolic panel were unremarkable, suggesting no bone marrow or renal involvement, respectively, related to SLE. Electrocardiogram (ECG) demonstrated sinus tachycardia, ST depressions in I, aVL, and poor R-wave progression in the lateral precordial leads (Figure 1). Chest X-ray demonstrated mild pulmonary artery congestion without Kerley B lines or pleural effusion and a normal cardiac silhouette. Total cholesterol was 113 mg/dL and triglycerides were 75 mg/dL. The B-type natriuretic peptide level 85.8 pg/mL (normal < 100 pg/mL). The serum estimated sedimentation rate (ESR) and C-reactive protein (CRP) were both within normal limits. Serum ANA titer was 1:640 with a homogenous pattern, dsDNA Ab was elevated, and both C-3 and C-4 complements were decreased, consistent with serologically active SLE. Troponin T was initially 0.3 ng/mL (normal <0.03 ng/mL) and peaked at 4.6 ng/mL within 8 h of initial presentation.
Figure 1.
Initial electrocardiogram on presentation. The electrocardiogram on presentation demonstrates sinus tachycardia with ST-segment elevation in aVR and V1 and diffuse ST-segment depression, most evident in the lateral leads (I, aVL, and V5–V6).
Given her persistent angina, ECG changes and troponin rise, an urgent coronary angiography was performed. It revealed 90–95% stenosis of proximal left anterior descending artery, 100% occlusion of the left circumflex artery, and 100% occlusion of the right posterior lateral branch with a ‘string of beads’ appearance concerning for coronary vasculitis (Figures 2 and 3). There were no targets for percutaneous intervention; a transthoracic echocardiogram revealed an ejection fraction of 25–30%, with a severely hypokinetic mid inferoseptal, inferolateral, anteroseptal and anterior walls, and an akinetic apex. There was mild to moderate aortic regurgitation. No pericardial effusion was noted. After discussion with rheumatology for treatment of coronary vasculitis, the patient was given intravenous methylprednisolone 1000 mg daily for 3 days followed by 1 mg/kg/day prednisone equivalent in addition to a single dose of intravenous cyclophosphamide 860 mg and oral hydroxychloroquine 400 mg daily.
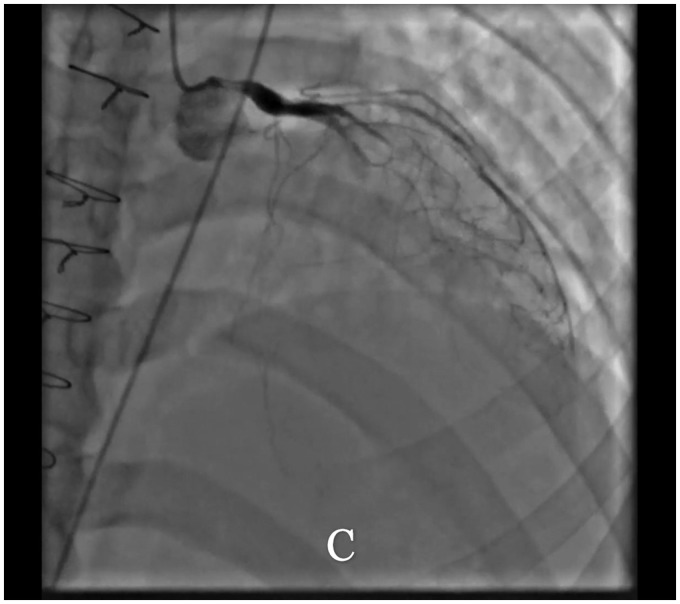
Figure 2.
Initial and 2-week post-treatment coronary angiography. Patient's initial coronary angiogram with left anterior oblique cranial (A) of the left coronary system and a left anterior oblique view of the right coronary system (B). The left main coronary artery shows an ostial and proximal 70% stenosis, the left anterior descending demonstrated a proximal left anterior descending 90–95% stenosis, severe diffuse disease and a ‘string of beads’ appearance of the remainder of the vessel. The mid-left circumflex demonstrates a 100% occlusion, and a small right posterior descending artery is totally occluded ostially. The right coronary artery demonstrates diffuse disease and right-to-left collaterals. On her second angiogram, the left anterior descending is 100% stenosed in the mid-segment (C). The first diagonal and obtuse marginal arteries remain severely diseased. The right coronary artery remains diffusely diseased with an ostially occluded R-PDA.
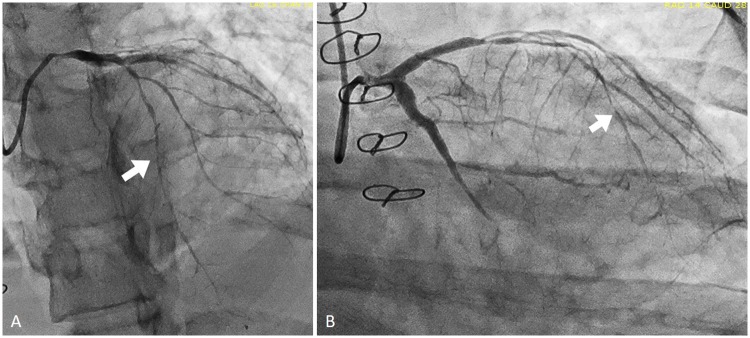
Figure 3.
Still images of coronary angiography. These still images of the initial angiography demonstrate a ‘string of beads’ pattern consistent with coronary vasculitis. The coronary anatomy is described in Figure 2. Arrows indicate the ‘string of beads’ or pruned appearance of the coronary arteries consistent with vasculitis.
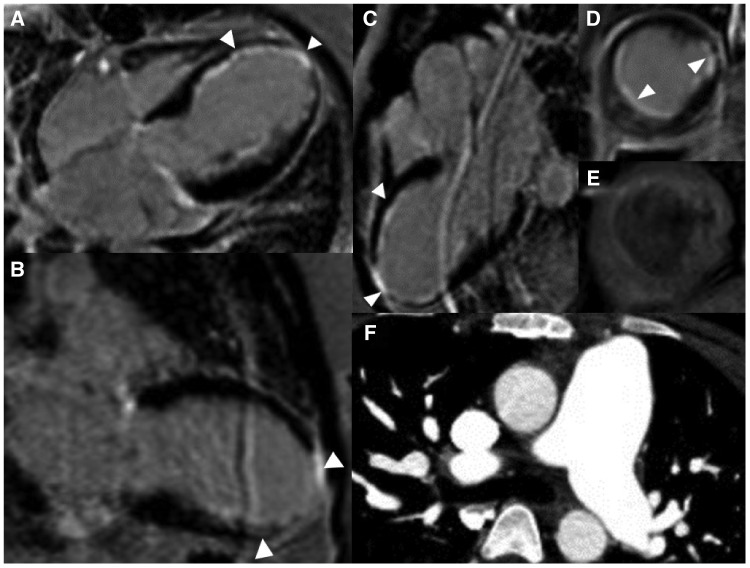
Cardiac magnetic resonance imaging (CMRI) was performed to determine the aetiology of cardiomyopathy and to exclude a secondary process such as SLE myocarditis. Cardiac magnetic resonance imaging revealed left ventricular ejection fraction of 30% (Figure 5) and a transmural delayed enhancement (DE) in the apex without any viability, subendocardial DE in mid-septal, and inferolateral walls with significant viable myocardium (Figure 4). There was no evidence of myocarditis based on T2-weighted magnetic resonance imaging sequences.11 The CMRI findings favoured myocardial infarction and ICM. Computed tomographic angiography of the chest did not demonstrate aortic or other large vessel vasculitis (Figure 4).
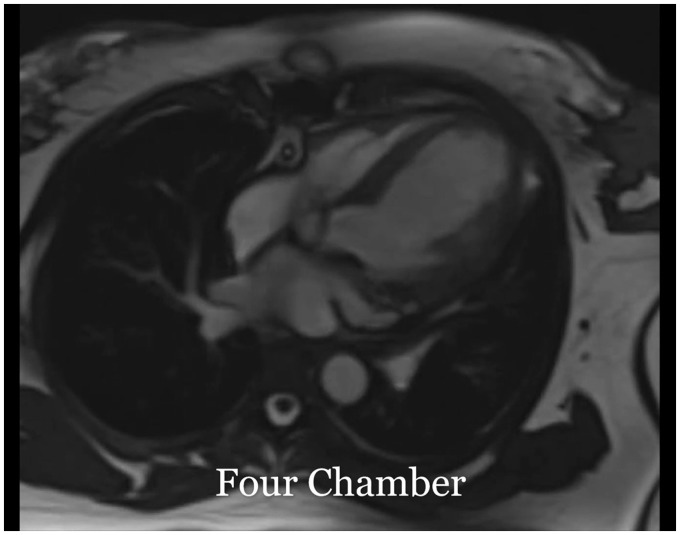
Figure 5.
Steady-state free procession. Cine imaging of left ventricle four-chamber and short-axis view: left ventricle is mildly dilated without any filling defect or mass. There is thinning and akinesis of the entire apex and moderate to severe hypokinesis of the mid anterior, anteroseptal, inferoseptal, and inferolateral segments. The right ventricle demonstrates mild global hypokinesis. There is mild to moderate aortic regurgitation.
Figure 4.
Cardiac magnetic resonance imaging and coronary computed tomographic angiography images. (A) Cardiac magnetic resonance imaging four-chamber late gadolinium enhancement. About 25–50% mural thickness late gadolinium enhancement in the mid to apical inferoseptal wall and both papillary muscles, and full-thickness late gadolinium enhancement of the apex is seen. (B) Cardiac magnetic resonance imaging showing two-chamber late gadolinium enhancement of left ventricle. About 25–50% mural thickness late gadolinium enhancement of the mid to apical inferior wall and full-thickness late gadolinium enhancement of apex is seen. (C) Cardiac magnetic resonance imaging three-chamber late gadolinium enhancement. About 25–50% mural thickness late gadolinium enhancement of mid to distal anteroseptal and 25–50% of mid to apical inferolateral wall and full-thickness late gadolinium enhancement of apex. (D) Cardiac magnetic resonance imaging short-axis late gadolinium enhancement of left ventricle at the mid-ventricular level. About 25–50% inferolateral, inferoseptal, and anterolateral wall late gadolinium enhancement is seen. (E) Cardiac magnetic resonance imaging short-axis T2-weighted turbo spin-echo imaging of the left ventricle at the mid-ventricular level with fat saturation (matching level as image D) demonstrates faint subendocardial enhancement in the area of late gadolinium enhancement. (F) Coronary computed tomographic angiography does not demonstrate evidence of arterial wall inflammation of the great vessels. Pulmonary artery measured to be 2.6 cm, thought to be mildly dilated due to history of atrial septal defect repair.
During the hospital course, the patient’s angina was poorly controlled with isosorbide dinitrate 30 mg daily and ranolazine 500 mg twice a day due to significant hypotension and QTc prolongation respectively. She also developed clinical symptoms consistent with heart failure requiring aggressive diuresis. Once euvolemic, she underwent right heart catheterization which revealed mean pulmonary artery (PA) pressure of 21 mmHg, right atrial pressure of 2 mmHg, pulmonary capillary wedge pressure 7 mmHg, thermodilution cardiac index of 0.89 (L/min/m2), pulmonary vascular resistance of 3 Woods Units, and PA oxygen saturation of 51%. She could not ambulate more than 10 feet on a flat surface at a slow pace without significant dyspnoea. On hospital Day 14, a second coronary angiography revealed disease progression despite ongoing CAD and SLE management (Figure 2).
During a multi-disciplinary meeting between cardiology, cardiothoracic surgery, and rheumatology, it was decided that her myocardium, cardiac output, and functional status were not likely to recover due to rapidly progressive CAD unresponsive to immunosuppression and medical therapy, not amenable to percutaneous or surgical revascularization. Her heart failure and chest pain were refractory to conservative measures putting her at significant risk of sudden cardiac death,12 therefore, the option of OHT was offered. On hospital Day 14, she was listed as Status 3 for OHT on United Network for Organ Sharing. She underwent OHT on hospital Day 16.
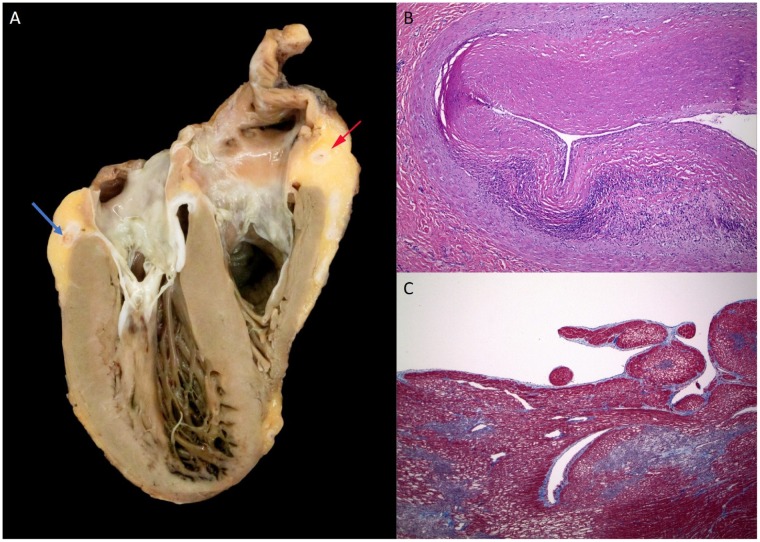
Pathology of the explanted heart (Figure 6) demonstrated concentric intimal fibrosis with associated moderate lymphocytic inflammation of the thickened intima of all major epicardial coronary arteries with sparing of the small branch coronary arteries. Acute multifocal microinfarction of the left ventricle was seen. There was no significant myocarditis, pericarditis, or vacuolization of the myocytes to suggest hydroxychloroquine toxicity.13
Figure 6.
Pathology of explanted heart. (A) Ventral half of the explanted heart, showing a dilated left ventricle (on the left), with small areas of myocardial discolouration near the apex, and a markedly stenotic cross section of the right coronary artery (red arrow), and the left circumflex artery (blue arrow). (B) Image of the right coronary artery, near the area of the arrow in A, showing marked intimal fibrosis that markedly narrows the lumen of the artery, with moderate chronic inflammation deep in the intima. (C) Trichrome stain of discoloured myocardium near the left ventricular apex, showing area of subendocardial ischaemia (pale) with surrounding early fibrosis (blue). Normal myocardium stains red.
Her post-OHT course was uncomplicated and she was discharged home. She continues to be followed by a transplant cardiologist and rheumatologist at our institution. Her post-transplant biopsies have not demonstrated evidence of rejection or vasculitis, and five months after transplantation, she no longer reports any symptom-limiting activities.
Discussion
We describe a case of chronic coronary vasculitis leading to ICM requiring OHT in a young patient with undertreated SLE. First and foremost, this case highlights the importance of detailed history taking. Unfortunately for several months, the patient’s chest pain of typical angina and dyspnoea on exertion were not recognized as anything more sinister than simple pericarditis without further cardiac workup. In a young female patient with SLE and chest pain, pericarditis and myocarditis are indeed more common than CAD or coronary vasculitis, though accelerated CAD has been reported to be 50 times more likely in young females and more so in females with SLE.7,14 We suspect that, due to our patient’s young age, clinical evaluation of her condition was incomplete for some time and she remained underdiagnosed until she presented to our institution. There have been at least two reported cases of coronary vasculitis with active SLE in the literature that showed improvement in symptoms of chest pain with immunosuppression, however, in our case, the disease process was likely recognized too late and months of chronic inflammation from undertreated SLE left the patient with irreversible coronary vasculitis.8,9
Coronary vasculitis in SLE remains a diagnosis of exclusion without established diagnostic criteria. Systemic lupus erythematosus is an independent predictor of early atherosclerotic CAD, hence the distinction between atherosclerotic CAD and coronary vasculitis in SLE patients is paramount for appropriate management.14 The classic ‘string of beads’ pattern on angiography, similar to that of cardiac allograft vasculopathy (CAV), suggestive of vasculitis rather than atherosclerotic disease.15 Although serologically active SLE is not necessary to diagnose coronary vasculitis, this patient had decreased serum complements and elevated dsDNA Ab, consistent with an active SLE flare. Our diagnosis was confirmed with explant heart pathology consistent with chronic coronary artery wall inflammation with fibrosis, without an evidence of lipid deposition which may explain the lack of response to the medical therapy.1,2
The pathophysiology of SLE coronary vasculitis is poorly understood. Histologic findings in prior case reports describe neutrophilic and lymphocytic infiltration, and immune-complex deposition on the coronary artery intima.5 The inflammation results in segmental stenoses leading to ectasia or aneurysms.16 In this case, pathology demonstrated coronary artery intimal lymphocytic infiltration with severe diffuse sclerosis of all major epicardial coronary arteries mimicking the diffuse intimal thickening seen in chronic epicardial coronary vasculopathy of transplantation.15 However, the small branch coronary arteries, which often show severe disease in CAV, were generally normal in this heart.15 The coronary vasculopathy lacked the calcification and atheromatous changes of ordinary CAD. Furthermore, this concentric thickening is a classic finding of coronary vasculitis.17 In the absence of another obvious source, the vasculitis was likely the result of a chronic inflammatory process from SLE.
There is no established therapy for SLE coronary vasculitis, though symptomatic and serological improvement has been reported with intravenous pulse-dose methylprednisolone for global immunosuppression and intravenous cyclophosphamide for B- and T-cell suppression in patients with active SLE flares.1,8,9,18 Unfortunately, this approach did not improve our patient’s CAD, cardiomyopathy or functional capacity. Active vasculitis can take up to 6 months to fully induce remission, however, the imaging findings and otherwise normal ESR and CRP, in this case, suggested chronic, severe coronary vasculitis.18 Additionally, the lack of significant response to medical therapy along with low ejection fraction and delayed enhancement without any evidence of myocardial oedema on CMRI was thought to be due to chronic vasculitis.
Conclusion
To our knowledge, this is the first reported case of OHT of a female patient with ICM due to chronic SLE coronary vasculitis as diagnosed by serologic markers of SLE, ‘string of beads’ appearance on coronary angiography and lymphocytic infiltration with fibrosis of the intima on histology. Chronic SLE coronary vasculitis is caused by chronic inflammation of the major epicardial coronary arteries but can be successfully managed with OHT when refractory to medical SLE and heart failure therapies. It can affect patients of all ages with SLE, emphasizing the importance of thorough history taking and clinical evaluation in young patients presenting with cardiac symptoms to establish an appropriate diagnosis and treatment plan.
Lead author biography

Shuktika Nandkeolyar is a cardiology fellow in training at Loma Linda University Medical Center. She earned Bachelors in Science from the University of California Los Angeles where she graduated with honours, attending medical school at the Medical College of Wisconsin, and completed Internal Medicine residency at the University of California Irvine. She ultimately wishes to train and practice as an advanced heart failure and transplant cardiologist.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the help and support of David Farchadi during the writing of this manuscript.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Caracciolo EA, Marcu CB, Ghantous A, Donohue TJ, Hutchinson G.. Coronary vasculitis with acute myocardial infarction in a young woman with systemic lupus erythematosus. J Clin Rheumatol 2004;10:66.. [DOI] [PubMed] [Google Scholar]
- 2. Matayoshi AH, Dhond MR, Laslett LJ.. Multiple coronary aneurysms in a case of systemic lupus erythematosus. Chest 1999;116:1116–1118. [DOI] [PubMed] [Google Scholar]
- 3. Heibel RH, O'Toole JD, Curtiss EI, Medsger TA, Reddy SP, Shaver JA.. Coronary arteritis in systemic lupus erythematosus. Chest 1976;69:700–703. [DOI] [PubMed] [Google Scholar]
- 4. Bonfiglio TA, Botti RE, Hagstrom J.. Coronary arteritis, occlusion, and myocardial infarction due to lupus erythematosus. Am Heart J 1972;83:153–158. [DOI] [PubMed] [Google Scholar]
- 5. M. Korbet S, M. Schwartz M, J. Lewis E.. Immune complex deposition and coronary vasculitis in systemic lupus erythematosus: report of two cases. Am J Med 1984;77:141–146. [DOI] [PubMed] [Google Scholar]
- 6. Prasad M, Hermann J, Gabriel SE, Weyand CM, Mulvagh S, Mankad R, Oh JK, Matteson EL, Lerman A.. Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat Rev Cardiol 2015;12:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M.. Cardiac involvement in systemic lupus erythematosus. Lupus 2005;14:683–686. [DOI] [PubMed] [Google Scholar]
- 8. Shriki J, Shinbane JS, Azadi N, Su T-IK, Hirschbein J, Quismorio FP, Bhargava P.. Systemic lupus erythematosus coronary vasculitis demonstrated on cardiac computed tomography. Curr Prob Diagn Radiol 2014;43:294–297. [DOI] [PubMed] [Google Scholar]
- 9. Saparia T, Lundstrom RJ.. Severe coronary vasculitis during a systemic lupus erythematosus flare improved angiographically with immune-suppressant therapy. Circulation 2016;133:1048. [DOI] [PubMed] [Google Scholar]
- 10. Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae S-C, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Magder LS.. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy J-P, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P.. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossi JS, Flaherty JD, Fonarow GC, Nunez E, Stough WG, Abraham WT.. Influence of coronary artery disease and coronary revascularization status on outcomes in patients with acute heart failure syndromes: a report from OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure)*. Eur J Heart Fail 2008;10:1215–1223. [DOI] [PubMed] [Google Scholar]
- 13. Joyce E, Fabre A, Mahon N.. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen-McWilliams L, D'Agostino RB, Kuller LH.. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–415. [DOI] [PubMed] [Google Scholar]
- 15. Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands R.. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol 2016;68:80–91. [DOI] [PubMed] [Google Scholar]
- 16. Jain D, Halushka MK.. Cardiac pathology of systemic lupus erythematosus. J Clin Pathol 2009;62:584–592. [DOI] [PubMed] [Google Scholar]
- 17. James TN. De subitaneis mortibus. VIII. Coronary arteries and conduction system in scleroderma heart disease. Circulation 1974;50:844–856. [DOI] [PubMed] [Google Scholar]
- 18. Luqmani RA. State of the art in the treatment of systemic vasculitides. Front Immunol 2014;5:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.