Abstract
Background
Subcutaneous implantable cardioverter-defibrillators (S-ICDs) are increasingly used in patients at risk of fatal cardiac arrhythmias. Twiddler’s syndrome is a condition in which a device is manipulated by the patient after implantation leading to lead twisting and retraction. Device manipulation has been reported multiple times in transvenous pacing systems and occasionally leads to inappropriate discharges from implanted defibrillators. However, little has been reported about device manipulation in S-ICD devices.
Case summary
We present the case of a 16-year-old who underwent insertion of an S-ICD for idiopathic dilated cardiomyopathy. He represented for a pacing check following a discharge from the device. This showed a significant change in the sensed vectors. Chest radiographs confirmed lead retraction and suggested device manipulation. The device was turned off to prevent further inappropriate shocks. The patient underwent successful reimplantation of a S-ICD device.
Discussion
This case highlights that twiddler’s syndrome can occur in those with an S-ICD and lead to an inappropriate device discharge. The patient in this case had a number of risk factors that have been previously associated with twiddler’s syndrome.
Keywords: Case report, Twiddler’s syndrome, Subcutaneous ICD
Learning points
Twiddler’s syndrome should be considered prior to implantation in subcutaneous implantable cardioverter-defibrillator (S-ICD) cases as well as transvenous cases.
Twiddler’s syndrome can lead to a significant change in sensed vectors and result in inappropriate device discharge in S-ICDs.
Introduction
Twiddler’s syndrome is an uncommon condition in which an implanted device, a pacemaker or implantable cardioverter-defibrillator (ICD), is manipulated by the patient, resulting in lead displacement. This has been recognized in transvenous pacemakers, ICDs, deep brain stimulation devices,1 and implanted pump devices.2 This condition was first recognized by Bayliss et al. in 1968.3 Lead macrodisplacement (LMD) has been reported as an uncommon complication in patients with transvenous pacemakers and ICDs.4,5 In the case of pacemakers, the condition often presents with failure of the device; in ICDs it can lead to inappropriate shocks.4
Subcutaneous ICDs (S-ICDs) are composed of a subcutaneous electrode placed along the sternum and a generator placed below the left axilla.6 Patient selection is important prior to S-ICD implantation to prevent inappropriate shocks through T-wave oversensing.7 It has been suggested that the use of S-ICDs may reduce systemic infections and other complications that occur with repeated replacement of transvenous ICDs and specifically avoid the complications related to venous access and having leads in the vascular system; this is currently being investigated in a randomized controlled trial.8 We present a case of twiddler’s syndrome occurring in a patient with a S-ICD, presenting with an inappropriate shock.
Timeline
| Date | Events |
|---|---|
| Day 0 | The patient was admitted to local hospital with signs and symptoms consistent with congestive cardiac failure |
| Day 1 | The patient was transferred to a specialist paediatric cardiology centre where he underwent a transthoracic echocardiogram showing a left ventricular ejection fraction (LVEF) 10% |
| The patient was diagnosed with dilated cardiomyopathy and an associated left ventricular apical thrombus | |
| Day 151 | Follow-up transthoracic echocardiogram was performed showing an LVEF 10% and no evidence of thrombus |
| Day 166 | A decision was made with the patient to implant an subcutaneous implantable cardioverter-defibrillator (S-ICD) |
| Referral made to advanced heart failure service for monitoring and consideration of heart transplantation if the patient deteriorated | |
| Referral to genetic screening service made | |
| Day 199 | Insertion of a primary prevention Boston Scientific S-ICD under general anaesthetic |
| 12 months | Routine review in heart function clinic |
| No implantable cardioverter-defibrillator (ICD) therapies had occurred during this time | |
| 18 months | Attended pacing clinic for review following inappropriate ICD shock |
| Retraction of subcutaneous lead noted on chest radiograph and change in sensing vectors apparent on interrogation of the device | |
| 21 months | Reimplantation of an S-ICD under general anaesthetic |
Case presentation
The male patient initially presented to clinical care at the age of 16 years with increasing shortness of breath, peripheral oedema, and orthopnoea. Past medical history included pathological hyperphagia and obesity. Clinical examination on initial presentation revealed body mass index (BMI) of 33.4 kg/m2, bi-basal crackles on respiratory auscultation, and pitting oedema to the knees. Cardiac auscultation demonstrated normal heart sounds without any murmurs. During his initial admission, he was transferred to a specialist paediatric cardiology centre for ongoing care. An echocardiogram demonstrated a dilated left ventricle with an ejection fraction (LVEF) 10% and a left ventricular thrombus. He was initiated on warfarin (target INR 2–3), bisoprolol 2.5 mg o.d., ramipril 2.5 mg b.i.d., and spironolactone 25 mg o.d. He underwent psychiatric assessment whilst an inpatient and was commenced on sertraline 100 mg, principally to assist with appetite control.
Following discharge, the patient was reviewed in the local heart function clinic. A repeat echocardiogram confirmed an LVEF of 10% and an electrocardiogram showed a narrow QRS complex. A decision was made with the patient and his mother for him to have a S-ICD fitted for primary prevention.
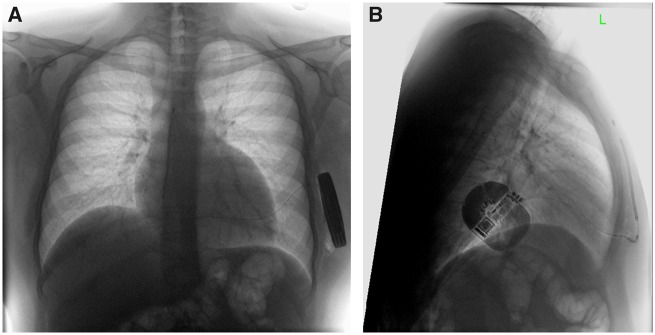
The patient underwent screening investigations to ensure he was suitable for S-ICD implantation. The implantation was performed under general anaesthetic and was uncomplicated. The S-ICD was implanted using an intermuscular approach with the device placed between the anterior surface of serratus anterior and the posterior surface of latissimus dorsi.9 A ventricular fibrillation induction test was performed at implant, with successful cardioversion. A chest radiograph confirmed appropriate positioning of the lead and generator (Figure 1) and appropriate sensing vectors were confirmed post-implant (Figure 2).
Figure 1.
Chest radiograph following device implantation showing correct lead position. (A) PA projection. (B) Lateral projection.
Figure 2.
Sensing vectors after initial implant.
He was reviewed 6 months later and reported a general improvement in symptoms. No therapies were recorded from the device in this timeframe. Thirteen months after device implant (October 2018), the patient presented emergently to the pacing clinic, having experienced a shock. On interrogation, it was noted that the vectors on the device had changed significantly (Figure 3); this prompted a repeat chest radiograph (Figure 4). It was noted that there had been retraction of the subcutaneous lead and coiling of the lead around the generator. Given the presentation with an inappropriate shock, the device was switched off.
Figure 3.
Sensing vectors after the patient presented for an inappropriate shock.
Figure 4.
Chest radiograph after an inappropriate shock showing retraction of the chest lead back towards the subcutaneous implantable cardioverter-defibrillator generator. (A) PA projection. (B) Lateral projection.
On direct questioning, the patient stated that he was not aware of any conscious manipulation of the device. It was agreed that repositioning of the S-ICD lead was in his best interest, and this was undertaken successfully in December 2018 with the device again placed intermuscularly between serratus anterior and latissimus dorsi. Routine device follow-up in February 2019 has revealed normal sensing vectors (Figure 5) and no further shocks.
Figure 5.
Sensing vectors after lead repositioning.
Discussion
This case highlights the risk of twiddler’s syndrome in the S-ICD population and the potential for this to result in inappropriate therapies. In a review of the literature, only one other case of twiddler’s syndrome in association with an S-ICD has been reported. Unlike the case described, that patient presented with chest pain and a loss of sensing from the device.10
The risk of inappropriate therapies has previously been recognized to be higher in patients with S-ICDs compared to those with transvenous ICDs; however, a recent meta-analysis has shown similar rates of total inappropriate therapy between S-ICD and transvenous systems.11 The higher rate of inappropriate therapies previously seen in S-ICD patients is largely due to subcutaneous electrodes, rather than an intracardiac electrogram, interpreting the cardiac rhythm.12 Prescreening prior to S-ICD insertion attempts to reduce the likelihood of a patient experiencing an inappropriate shock, particularly due to T-wave oversensing. Twiddler’s syndrome causing lead retraction in patients with an S-ICD will significantly alter the sensed vectors, predisposing to inappropriate device discharge.
Twiddler’s syndrome is a form of LMD. A recent retrospective cohort analysis of transvenous cardiac devices revealed that LMD occurred in 1.8% of cases (total cohort = 1074)4; however, only one case was reported as twiddler’s syndrome. Of 19 cases with LMD, eight were ICDs, of these two presented with inappropriate shocks. Increasing pacing thresholds/lead impendence are commonly noted in twiddler’s syndrome.13 In this case, the dramatic alteration in sensing across all three vectors prompted radiographic re-evaluation of lead positioning, and established the diagnosis.
A number of risk factors have previously been described for the development of twiddler’s syndrome. Those most commonly quoted include: female gender,4,13 increased BMI,4,13 paediatric patient,14 elderly patient,13 past mental health history,14 and device-pocket size mismatch.15 In retrospect, the patient described fulfils a number of these risk factors (increased BMI, paediatric patient, and history of psychiatric disorder). The S-ICD device and leads are secured with sutures, but recognition of the higher risk twiddler patient might necessitate extra sutures for example. Use of the three incision technique should be considered to reduce risk of lead dislocation.
Conclusion
Lead displacement due to manipulation of the generator in patients with S-ICD can result in inappropriate shocks. Possible risk factors for this include obesity and a history of psychiatric illness. Careful assessment of the balance in favour of S-ICD over transvenous ICD should be made in patients with these conditions.
Lead author biography

C. Fielder Camm is a cardiology registrar working in the Cardiology Department of the Royal Berkshire Hospital. He has a clinical interest in cardiac devices and electrophysiology. He is currently the Assistant Editor of European Heart Journal - Case Reports.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: C.F.C. is the Assistant Editor of European Heart Journal - Case Reports.
Supplementary Material
References
- 1. Sobstyl MR, Ząbek M, Brzuszkiewicz-Kuźmicka G, Pasterski T.. Dual anchor internal pulse generator technique may lower risk of twiddler's syndrome: a case series and literature review. Neuromodulation 2017;20:606–612. [DOI] [PubMed] [Google Scholar]
- 2. Shao J, Frizon L, Machado AG, McKee K, Bethoux F, Hartman J, Nagel SJ.. Occlusion of the Ascenda catheter in a patient with pump twiddler's syndrome: a case report. Anesth Pain Med 2018;8:e65312.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayliss CE, Beanlands DS, Baird RJ.. The pacemaker-twiddler's syndrome: a new complication of implantable transvenous pacemakers. Can Med Assoc J 1968;99:371–373. [PMC free article] [PubMed] [Google Scholar]
- 4. Tseng AS, Shipman JN, Lee JZ, Mi L, Amin M, Killu AM, Deshmukh AJ, Madhavan M, McLeod CJ, Srivathsan KK, Shen W-K, Osborn MJ, Cha Y-M, Asirvatham SJ, Friedman PA, Mulpuru SK.. Incidence, patterns, and outcomes after transvenous cardiac device lead macrodislodgment: insights from a population-based study. Heart Rhythm 2019;16:140–147. [DOI] [PubMed] [Google Scholar]
- 5. Morales JL, Nava S, Márquez MF, González J, Gómez-Flores J, Colín L, Martínez-Ríos MA, Iturralde P.. Idiopathic lead migration: concept and variants of an uncommon cause of cardiac implantable electronic device dysfunction. JACC Clin Electrophysiol 2017;3:1321–1329. [DOI] [PubMed] [Google Scholar]
- 6. McLeod CJ, Boersma L, Okamura H, Friedman PA.. The subcutaneous implantable cardioverter defibrillator: state-of-the-art review. Eur Heart J 2017;38:247–257. [DOI] [PubMed] [Google Scholar]
- 7. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DAMJ, Boersma LVA, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD.. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 8. Olde Nordkamp LR, Knops RE, Bardy GH, Blaauw Y, Boersma LV, Bos JS, Delnoy PP, van Dessel PF, Driessen AH, de Groot JR, Herrman JP, Jordaens LJ, Kooiman KM, Maass AH, Meine M, Mizusawa Y, Molhoek SG, van Opstal J, Tijssen JG, Wilde AA.. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J 2012;163:753–760.e2. [DOI] [PubMed] [Google Scholar]
- 9. Ferrari P, Giofre F, De Filippo P.. Intermuscular pocket for subcutaneous implantable cardioverter defibrillator: single-center experience. J Arrhythm 2016;32:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kooiman KM, Brouwer TF, Van Halm VP, Knops RE.. Subcutaneous implantable cardioverter defibrillator lead failure due to twiddler syndrome. Pacing Clin Electrophysiol 2015;38:1369–1371. [DOI] [PubMed] [Google Scholar]
- 11. Basu-Ray I, Liu J, Jia X, Gold M, Ellenbogen K, DiNicolantonio J, Komócsi A, Vorobcsuk A, Kim J, Afshar H, Lam W, Mathuria N, Razavi M, Rasekh A, Saeed M.. Subcutaneous versus transvenous implantable defibrillator therapy: a meta-analysis of case-control studies. JACC Clin Electrophysiol 2017;3:1475–1483. [DOI] [PubMed] [Google Scholar]
- 12. Brisben A. How the S-ICD (subcutaneous implantable cardiac defibrillator) senses cardiac signals to minimize cardiac over-sensing and maximize rhythm discrimination. J Electrocardiol 2018;51:S38–S43. [DOI] [PubMed] [Google Scholar]
- 13. Boyle NG, Anselme F, Monahan KM, Beswick P, Schuger CD, Zebede J, Josephson ME.. Twiddler's syndrome variants in ICD patients. Pacing Clin Electrophysiol 1998;21:2685–2687. [DOI] [PubMed] [Google Scholar]
- 14. Tahirovic E, Haxhibeqiri-Karabdic I.. Twiddler's syndrome: case report and literature review. Heart Views 2018;19:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spencker S, Poppelbaum A, Muller D.. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008;129:e24–e26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.