Abstract
Introduction
Obstructive sleep apnea (OSA) has been linked with erectile dysfunction (ED), but the relatively independent polysomnography (PSG) outcomes of apnea and nocturnal hypoxia may not effectively assess the physiological impairment of OSA well.
Aim
To propose a new calculation method, the blood oxygen accumulation distribution area index (BOADAI), for evaluating the association between OSA and ED.
Methods
In this study, 502 male participants with suspected OSA were enrolled. Clinical questionnaire, physical measurements, and PSG outcomes were obtained by 2 respiratory physicians. ED was assessed by a urologist using the International Index of Erectile Function-5 (IIEF-5). Whole pulse oxygen saturation curves during the sleep time were compressed into a fixed scale image, and the distribution area of oxygen saturation curves was outlined. We then calculated the value of the outlined area and normalized it by total sleep time. The least absolute shrinkage and selection operator logistic regression model was used for selecting the optimal variable associated with ED and model construction. The clinical net benefit of the BOADAI and its related modules was estimated and compared by decision curve analysis.
Main Outcome Measure
ED and OSA were assessed using the IIEF-5, clinical questionnaire, physical measurements, and PSG outcomes.
Results
The frequency of ED in patients with OSA was significantly greater than that in the no-OSA group. Meanwhile, the new BOADAI was negatively correlated with the IIEF-5 score (r = −0.2525, P = .0000). Moreover, the least absolute shrinkage and selection operator method retained BOADAI but not the other PSG parameters such as respiratory disorder index and lowest SaO2. Finally, logistic regression analysis revealed that older age, lips with cyanochroia, systemic hypertension, and BOADAI were independently associated with ED, and decision curve analysis indicated the clinical usefulness of the BOADAI module.
Conclusion
This study revealed novel evidence that OSA is a risk factor for ED. Meanwhile, the BOADAI could act as a potential clinical characteristic to evaluate ED in patients with OSA and to provide clinical treatment recommendations.
Zheng W, Chen X, Huang J, et al. Blood Oxygen Accumulation Distribution Area Index Is Associated With Erectile Dysfunction in Patients With Sleep Apnea—Results From a Cross-sectional Study. Sex Med 2019; 8:36–44.
Key Words: Erectile Dysfunction, Obstructive Sleep Apnea, Blood Oxygen Accumulation Distribution Area Index, Respiratory Disorder Index, Lowest Oxygen Saturation
Introduction
Obstructive sleep apnea (OSA), the most common type of sleep apnea, is characterized by recurrent cessation of breathing during sleep, which is usually associated with sleep fragmentation and reduction in blood oxygen saturation.1 OSA often coexists with systemic complications, such as abnormal glycometabolism, hypertension, cardiovascular diseases, and sexual dysfunction.2, 3, 4, 5, 6 Epidemiological and clinical studies have shown that erectile dysfunction (ED) shares similar comorbidities with OSA.7 A series of studies conducted with sleep medical center patients as participants have reported that the prevalence of ED in patients with OSA ranges from 41% to 80%.8, 9, 10, 11 In a large population-based cohort study, researchers found that people with OSA exhibited an increased risk of ED; this study reported an ED incidence 9.44-fold higher in the apnea cohort than that in the nonapnea cohort.12 On the other hand, some studies have reported that continuous positive airway pressure (CPAP) treatment of patients with OSA elevated the serum levels of testosterone and ameliorated ED symptoms.13,14 In rodent studies, both spontaneous and contact sexual activity in the mice were suppressed after chronic intermittent hypoxia (CIH), modeling human OSA.15 In addition, CIH markedly attenuated intracavernous pressure by decreasing the expression of constitutive nitric oxide synthase and inducing apoptosis of penile endothelial cells.16
Nocturnal intermittent hypoxemia and its consequential pathological changes in patients with OSA have been postulated as the main mechanisms of systemic complications.17,18 Nocturnal hypoxemia–associated indexes, such as lowest nocturnal pulse oxygen saturation (lowest SaO2), mean nocturnal pulse oxygen saturation (mean SaO2), oxygen desaturation index (ODI), and the time spent with oxygen saturation less than 90% (SIT90%), are frequently studied and of importance to the pathophysiological processes underlying comorbidity.19, 20, 21 In a prospective cross-sectional study based on patients with suspected OSA, Roland Popp et al22 reported that both lowest SaO2 and mean SaO2 in normal erectile function group were significantly higher than those in the group with impaired erectile function. The mean SaO2 of patients undergoing polysomnography (PSG) for suspected OSA was confirmed to be independently associated with ED.11 Additional studies have reported that lowest SaO2 and ODI were significantly correlated with ED.23,24 In a separate study, findings demonstrated that patients with OSA and with an altered bulbocavernosus reflex presented a higher apnea-hypopnea index (AHI) and SIT90% and a lower daytime SpO2.10 Although there is strong evidence that apnea and hypoxemia are significant risk factors for ED in patients with OSA, the studies were limited in part by the presentation of the relationship between traditional indexes and erection function and the lack of integrated and comprehensive analysis, specifically, because apnea events are not always accompanied by a decline in nocturnal SpO2. Therefore, a comprehensive measure of PSG data that combines apnea and SpO2 should be developed to evaluate the coexistence of OSA and ED. Addressing these gaps would be of clinical usefulness because it is currently unclear which evaluation index acts as the most useful guideline for standardizing medical care and providing clinical treatment recommendations for ED in patients with OSA. In addition, conflicting results have been reported. The results of the study by Yung Jin Jeon et al showed that the frequency of ED did not differ significantly according to OSA severity, and there was no significant association of ED with either the respiratory disorder index (RDI) or lowest SaO2.25
Based on these considerations, we used comprehensive PSG data in a large sample of patients suspected to have OSA to assess whether those parameters including RDI (or AHI), lowest SaO2, mean SaO2, SIT90%, ODI, obstructive apnea index (OAI), maximum apnea time, microarousal index, oxygen reduction frequency (ORF), maximum oxygen reduction (MOR), longest oxygen reduction time (LORT), central apnea frequency, and obstructive apnea frequency were related to ED. In addition, we propose a new calculation method, the blood oxygen accumulation distribution area index (BOADAI), which integrates the information of apnea events and nocturnal hypoxemia to evaluate the association between OSA and ED.
Materials and Methods
Study Population
In this study, we enrolled 551 male patients with suspected OSA who were referred to the sleep medical center between July 2017 and March 2019. The suspicion of apnea was based on clinical symptoms such as snoring, sleepiness, morning dry mouth, and tiredness. A clinical questionnaire, physical measurements, and PSG outcomes were obtained by 2 respiratory physicians; ED was assessed by a urologist using the International Index of Erectile Function-5 (IIEF-5). The exclusion criteria in our study were as follows: (i) historical treatment for OSA, including CPAP and uvulopalatopharyngoplasty (UPPP); (ii) patients had psychiatric disorders or were taking antipsychotics; (iii) not having sex (in the past half year), or a patient was diagnosed with ED and had previously taken medications (influencing erectile function) before the present study; (iv) presence of endocrinological disorders other than diabetes, such as hypogonadism, abnormal pituitary function, Cushing’s syndrome, and so forth; (v) neurological disorders that induce ED or peripheral neuropathy; (vi) presence of non-OSA sleep-disordered breathing conditions; (vii) malignancy (such as prostatic cancer) that could influence sexual function (eg, cancer); and (viii) severe cardiac or pulmonary disease that prevented the completion of PSG monitoring. All participants in this study provided written informed consent. The institutional review board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, approved this study (institutional review board number 2018-[107]).
Clinical Questionnaire and Physical Measurements
The questionnaire in this study was divided into 2 parts: one included full general conditions (age, disease duration, sleepiness, frequency of urination and sweating at night, and so forth), lifestyle (taking sleeping tablets regularly, cigarette smoking, alcohol consumption, and so forth), and OSA-related medical history (CPAP treatment, UPPP treatment, and so forth), and the other one part included male sexual function, which contained sex-related medical history (use of H2 and β-blocker drugs, history of radical prostatectomy, and so forth) and IIEF-5 score (ED was defined as an IIEF-5 score ≤ 21). Daytime sleepiness was evaluated by the Epworth Sleepiness Score (ESS).26,27 Complete physical examination including height, weight, body mass index (BMI), blood pressure, heart rate (HR), clubbing finger, cyanochroia (lips), and mouth opening were recorded by 2 respiratory physicians. Clinically relevant comorbidities including hypertension, diabetes, cardiac and pulmonary disease, malignancy, psychiatric and neurological disorders, endocrinological disorders, and peripheral occlusive disease were assessed by interviewing participants or reviewing medical records.
PSG Measurements
All participants underwent standard PSG monitoring (ALICE 5; Philips Respironics, Pittsburgh, PA), including nose airflow transducers, mouth thermocouple, dynamic pulse oxygen saturation, dynamic electrocardiogram, thoraco-abdominal movement by inductance plethysmography, genioglossus electromyogram, electroencephalography, left and right electrooculogram, acoustic sensor (snore), and body position. In accordance with the American Academic Sleep Medicine criteria,28 respiratory and apnea events (RDI, OAI, maximum apnea time, central apnea frequency, and obstructive apnea frequency), nocturnal hypoxemia (lowest SaO2, mean SaO2, SIT90%, ODI, ORF, MOR, and LORT), sleep quality (total sleep time, rapid eye movement stage sleep %, nonrapid eye movement stage sleep %, slow wave sleep %, and micro-arousal index), and HR were automatically recorded and proofread by an experienced respiratory physician.
Blood Oxygen Accumulation Distribution Area Index
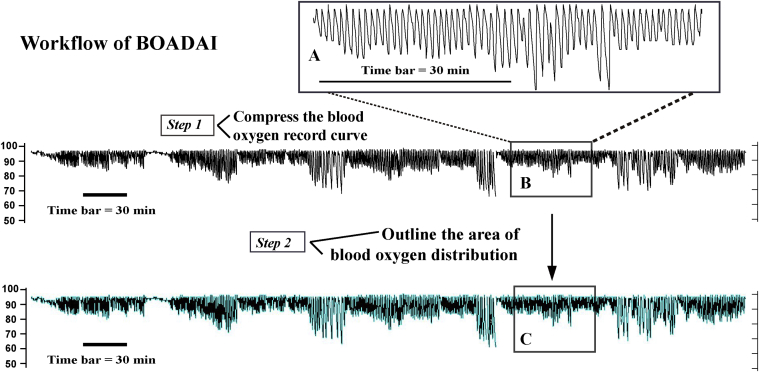
The photochemistry signals of oxygen saturation were recorded automatically and translated into digital image signals (SpO2 curve) by dynamic oxygen monitoring embedded in the ALICE 5 PSG monitoring system (Figure 1A). To calculate the BOADAI, as shown in Figure 1, B and C, we performed the following steps: (i) the whole pulse oxygen saturation curves during sleep time were automatically compressed into a fixed scale image by using the Alice Sleep Wave software (an attached software of ALICE PSG monitor; Figure 1B), and (ii) the distribution of the oxygen saturation curves was outlined (turquoise curve in Figure 1C) and calculated by using the Image Pro Plus software (version 6.0; Media Cybernetics, Rockville, MD). To make each case comparable, we normalized it by dividing the area by the corresponding total sleep time.
Figure 1.
Workflow of Blood oxygen accumulation distribution area index (BOADAI). (A) The photochemistry signals of oxygen saturation were recorded automatically and translated into digital image signals (SpO2 curve); (B) Step 1, the whole pulse oxygen saturation curves during the sleep time were automatically compressed into a fixed scale image; (C) Step 2, The oxygen saturation curves distribution were outlined (turquoise curve) and calculated by Image Pro Plus software; Time bar = 30 min.
Statistical Analysis
Continuously coded variables were reported as mean values and standard deviation, or median values and quartiles, depending on the data distribution which was tested by the Shapiro-Wilk test. Categorical variables were presented as frequencies and proportions. The statistical tests used to compare the ED and no-ED groups included the Student’s t-test for difference in mean values; Mann-Whitney U-test for skewed variables; Pearson chi-square test, Mann-Whitney U-test, and Fisher’s exact test for difference in frequencies and proportions. Pearson (R) and Spearman (Rho) correlation coefficients were used to test the association between different variables. In addition, the comparison of correlation coefficients was performed using the Z statistic.
Because of the existence of a high correlation and multicollinearity between nocturnal oxygen saturation features, the least absolute shrinkage and selection operator (LASSO) binary logistic regression model was used to filter the optimal subset of parameters of oxygen with nonzero coefficients using 10-fold cross-validation. The mechanism of the LASSO method was to introduce a tuning parameter to penalize the coefficient of variables entered into the regression model to avoid the multicollinearity and overfitting problems.29 The “glmnet” package of R 3.4.0 was used for LASSO binary logistic regression analysis. Decision curve analysis (DCA) was conducted to evaluate the clinical utility of the BOADAI and its associated models by quantifying the net benefits at different threshold probabilities of models. DCA is a method used for evaluating the clinical benefits of an intervention across a range of patient preferences for accepting risk of overtreatment and undertreatment to facilitate decisions about intervention selection and use. In addition, multiple DCA comparisons help to assess the superiority of the model and the contribution of individual variables to the model. In the DCA curve, the probability thresholds are shown on the horizontal axis and the benefit score on the vertical axis. A curve is drawn for each model or variables that might be taken to establish a diagnosis. Another line is drawn to show what happens when no treatment is given, and another curve is drawn as if all patients receive treatment irrespective of test results. For any given patient’s probability threshold, the DCA distribution curve with the highest benefit score at that threshold is the best choice.
Statistical analyses were carried out on SPSS software (version 25.0; International Business Machines Corporation, Armonk, NY; used for Shapiro-Wilk test, Student’s t-test, Mann-Whitney U-test, Pearson chi-square test, and Fisher's exact test in this study), MedCalc (version 18.11.6; MedCalc Software, Ostend, West Flanders, Belgium, used for Z statistic), R (version 3.4.0; Microsoft, Redmond, WA, Pearson and Spearman correlation coefficients analysis, correlation matrix analysis, and LASSO binary logistic regression model analysis in this study), and STATA (version 15; StataCorp LLC, TX, DCA analysis). All tests in this study were 2-sided, with the significance level set at 0.05 or less.
Results
Subject Characteristics
Among the 551 patients admitted, 14 patients were excluded from erectile function evaluation (3 patients who were not having sex, 4 patients without historical treatment for OSA, 5 patients diagnosed with ED who had previously taken medications, and 2 patients who underwent radical prostatectomy), 32 patients were excluded because of the inability to complete the PSG test, 1 patient did not complete the clinical questionnaire, and 6 patients refused to participate. Of the remaining 502 participants, 408 (81.27%) were diagnosed with OSA (RDI >5), and 325 (64.74%) patients were diagnosed with ED (IIEF-5 < 22). Among the 408 patients with OSA, 81 (19.85%) had mild (RDI 5-15), 88 (21.57%) had moderate (RDI 15-30), and 239 (58.58%) had severe (RDI < 30) apnea. Of the 325 patients with ED, 246 (75.69%) had mild (IIEF-5, 12-21), 46 (14.15%) had moderate (IIEF-5, 8-11), and 33 (10.15%) had severe (IIEF-5, 5-7) ED.
Sexual Dysfunction and OSA-Related Parameters
Patients with or without ED differed with respect to age, ESS, nocturia frequency, snore history, and apnea history (P < .05 or less each, Table 1). The patients with ED also showed higher frequencies of diabetes mellitus (6.21% vs 15.69%), systemic hypertension (26.55% vs 52.92%), cigarette smoking (38.42% vs 52.62%), alcohol consumption (19.77% vs 40.31%), cyanochroia (16.38% vs 50.15%), and clubbing finger (4.52% vs 21.54%; P < .05 or less each, Table 1). As implicated by PSG results, the RDI, longest apnea, lowest SaO2, mean SaO2, ODI, ORF, MOR, SIT90, LORT, and BOADAI in the non-ED group differed significantly from those in the ED groups (P < .05 or less each, Table 2).
Table 1.
Sexual dysfunction and OSA-related parameters (clinical questionnaire and physical measurements)
| Variable | Overall (N = 502) | No ED (N = 177) | ED (N = 325) | P value |
|---|---|---|---|---|
| Age (year)‡ | 43.74 ± 10.87 | 39.21 ± 9.15 | 46.21 ± 10.96 | .0000∗ |
| BMI (kg/m2)‡ | 26.58 ± 3.87 | 26.42 ± 3.55 | 26.66 ± 4.04 | .4766NS |
| Heart rate (times/min)‡ | 69.84 ± 10.86 | 70.41 ± 11.06 | 69.28 ± 10.66 | .2737NS |
| ESS§ | 11 (6, 14) | 10 (5, 13) | 11 (8, 15) | .0019∗ |
| Nocturia frequency (times/night)§ | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | .0029* |
| Sweat frequency (times/week)§ | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | .2001NS |
| Snore history (years)§ | 8 (3, 10) | 5 (2, 10) | 10 (5, 10) | .0000∗ |
| Apnea history (years)§ | 4 (1, 8) | 2 (0, 6) | 5 (2, 10) | .0000∗ |
| Systemic hypertension† | 219 (43.63%) | 47 (26.55%) | 172 (52.92%) | .0000∗ |
| Diabetes mellitus† | 62 (12.35%) | 11 (6.21%) | 51 (15.69%) | .0033∗ |
| Cigarette smoking† | 239 (47.61%) | 68 (38.42%) | 171 (52.62%) | .0032∗ |
| Alcohol consumption† | 131 (26.10%) | 35 (19.77%) | 131 (40.31%) | .0230∗ |
| Cyanochroia (lips)† | 192 (38.25%) | 29 (16.38%) | 163 (50.15%) | .0000∗ |
| Clubbing finger† | 78 (15.54%) | 8 (4.52%) | 70 (21.54%) | .0000∗ |
BMI = body mass index; ED = erectile dysfunction; ESS = Epworth sleepiness score; NS = not significant; OSA = obstructive sleep apnea; SD = standard deviation.
Data are presented as mean ± SD, median (quartile) or %, unless otherwise stated. No ED international index of erectile function-5 ≥ 22, ED international index of erectile function-5 < 22.
P value < 0.05 or less.
Comparison between patients without and with ED by the Pearson Chi-square test.
Comparison between patients without and with ED by the unpaired t-test.
Comparison between patients without and with ED by the Mann-Whitney U-test.
Table 2.
Sexual dysfunction and OSA-related parameters (PSG measurements)
| Variable | Overall (N = 502) | No ED (N = 177) | ED (N = 325) | P value |
|---|---|---|---|---|
| Hypopnea (n)‡ | 45 (15, 86) | 36 (15, 75) | 48 (15, 88) | .1309NS |
| RDI‡ | 27.9 (8.4, 57.4) | 19.1 (6.8, 52.9) | 31.6 (10, 58.5) | .0324∗ |
| OAI‡ | 2.5 (0.7, 8.4) | 2.1 (0.5, 8.5) | 2.9 (0.8, 8.3) | .2166NS |
| Longest apnea (s)‡ | 49.5 (29.5, 65) | 41.5 (23, 63.5) | 52 (32.5, 66.5) | .0359∗ |
| Micro-arousal index (MI)‡ | 22.4 (12.5, 43.5) | 20.9 (11.4, 40.8) | 23.6 (13.3, 45.8) | .0977NS |
| SaO2, lowest (%)‡ | 81 (68, 88) | 84 (74, 89) | 79 (66, 86) | .0000∗ |
| SaO2, mean (%)‡ | 95 (93, 96) | 95 (93, 96) | 95 (93, 96) | .0184∗ |
| ODI (/h)‡ | 26.1 (7.2, 56.9) | 16.4 (5.2, 53.6) | 28.9 (8.8, 58.2) | .0103∗ |
| ORF (/h)‡ | 10.2 (2.7, 30.4) | 6.6 (1.7, 24.4) | 12.2 (3.6, 31) | .0053∗ |
| BOADAI (/min)‡ | 6.7 (5.9, 7.4) | 6.3 (5.5, 7.2) | 6.9 (6.1, 7.6) | .0000∗ |
| MOR %‡ | 16 (9, 28.8) | 14.0 (7, 25) | 17 (10, 31) | .0030∗ |
| SIT90‡ | 3.1 (0.2, 21.4) | 1.4 (0, 18.1) | 4 (0.3, 23.2) | .0053∗ |
| Total sleep time (min)† | 270.4 ± 76.24 | 276.54 ± 69.40 | 276.02 ± 79.62 | .1648NS |
| REM/sleep period time (%)‡ | 8.8 (2.6, 16.3) | 8.6 (3.1, 16.2) | 8.8 (2.2, 16.5) | .9735NS |
| NREM/sleep period time (%)‡ | 90.8 (83, 97.3) | 91.2 (83.3, 96.8) | 90.5 (81.9, 97.6) | .9817NS |
| SWS/sleep period time (%)‡ | 13.9 (5.6, 23.5) | 13.7 (6.1, 22.3) | 13.9 (5.4, 24.1) | .9956NS |
| Heart rate (times/min)† | 69.36 ± 12.98 | 69.80 ± 14.40 | 69.12 ± 12.16 | .5950NS |
| LORT (s)‡ | 52 (40, 71) | 49 (38, 62) | 54 (42, 73) | .0036∗ |
BOADAI = blood oxygen accumulation distribution area index; ED = erectile dysfunction; LORT = longest oxygen reduction time; MOR = maximum oxygen reduction; NREM = no-rapid eye movement stage sleep; NS = not significant; OAI = obstructive apnea index; ODI = oxygen decrease index; ORF = oxygen reduction frequency; OSA = obstructive sleep apnea; PSG = polysomnography; RDI = respiratory disorder index; REM = rapid eye movement stage sleep; SIT90% = oxygen saturation less than 90%; SWS = slow wave sleep.
Data are presented as mean ± SD or median (quartile), unless otherwise stated. No ED international index of erectile function-5 ≥ 22, ED international index of erectile function-5 < 22.
P value < 0.05 or less.
Comparison between patients without and with ED by the unpaired t-test.
Comparison between patients without and with ED by the Mann-Whitney U-test.
ED Frequencies According to the Presence of OSA
Patients in the OSA group showed higher frequencies of ED (66.91%) than those in the non-OSA group (55.32%; Fisher's exact test, P = .042, Table 3). However, the severity of ED in this section was not associated with the presence of OSA as defined by RDI (5 was served as the cutoff value; Mann-Whitney U test, P = .07, Table 3). We then analyzed the distribution characteristics of ED, as shown in Table 3: among the 408 patients with OSA, 50.74% patients had mild, 9.8% had moderate, and 10.15% had severe ED. In addition, both the non-OSA group and all participants showed the highest frequency of mild ED (41.49% and 49%, respectively). On the other hand, patients in the OSA group showed higher frequencies of systemic hypertension and higher BMI than those in the non-OSA group (Supplementary Table S1).
Table 3.
ED severity according to the presence of OSA
| Variable | No OSA (RDI < 5) | OSA (RDI ≥ 5) | All patients | P value |
|---|---|---|---|---|
| No ED | 42 (44.68%) | 135 (33.09%) | 177 (35.26%) | |
| ED† | 52 (55.32%) | 273 (66.91%) | 325 (64.74%) | .042∗ |
| Mild ED‡ | 39 (41.49%) | 207 (50.74%) | 246 (49.00%) | .106NS |
| Moderate ED‡ | 6 (6.38%) | 40 (9.8%) | 46 (9.16%) | .3NS |
| Severe ED‡ | 7 (7.45%) | 26 (6.37%) | 33 (6.57%) | .705NS |
| All patients§ | 94 (100%) | 408 (100%) | 502 (100%) | .07NS |
ED = erectile dysfunction; NS = not significant; OSA = obstructive sleep apnea; RDI = respiratory disorder index.
Data are presented as frequency and %. No ED, international index of erectile function-5 ≥ 22; ED, international index of erectile function-5 < 22.
P value < 0.05 or less.
Comparison between patients without and with OSA by the Fisher's exact test.
Comparison between patients without and with ED by the Pearson chi-square test.
Comparison between patients without and with OSA by the Mann-Whitney U-test.
Correlation Analysis Between OSA-Related Parameters and IIEF-5
The correlations in the whole cohort were significantly identified, although weak, between IIEF-5 and age (r = –0.39, P = .0000), nocturia frequency (r = –0.20, P = .0000), ESS (r = –0.17, P = .0001), snoring (r = –0.24, P = .0000), and apnea history (r = –0.27, P = .0000; Supplementary Table S2). However, the sweat frequency, BMI, and HR were not significantly correlated with the IIEF-5 score (Supplementary Table S2). In terms of PSG variables, significant correlations were found between IIEF-5 and RDI (r = –0.13, P = .0021), central apnea (r = –0.12, P = .0063), longest apnea (r = –0.13, P = .0084), mean SaO2 (r = –0.17, P = .0002), lowest SaO2 (r = 0.22, P = .0000), ODI (r = –0.15, P = .0008), ORF (r = –0.17, P = .0002), MOR (r = –0.17, P = .0000), LORT (r = –0.17, P = .0001), SIT90 (r = –0.15, P = .0005), and BOADAI (r = –0.25, P = .0000; Supplementary Table S3). No correlation was found between stage-related parameters, such as rapid eye movement stage sleep %, nonrapid eye movement stage sleep %, and slow wave sleep %, and IIEF-5 score (Supplementary Table S4).
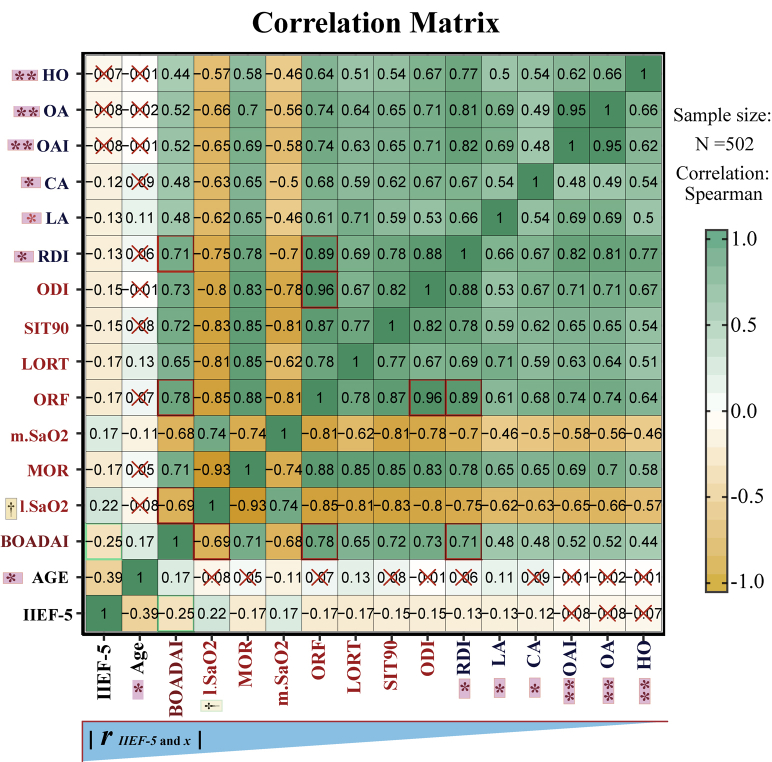
To acquire more thorough cognition, a correlation matrix analysis including apnea-hypoxia–related parameters and IIEF-5 was performed (Supplementary Figure 1). The main results of the correlation matrix analysis are as follows: (i) From the absolute value of the correlation coefficient, the correlation coefficient between the BOADAI and sexual function (IIEF-5) was the highest. (ii) The correlation coefficient between the BOADAI and IIEF-5 was significantly higher than the coefficients of most apnea-related parameters such as LA, CA, OAI, and IIEF-5 (P < .05 or less). However, there was no significant difference between the correlation coefficient of BOADAI and IIEF-5 and the correlation coefficients of hypoxia-related parameters (lowest SaO2, MOR, mean SaO2, ORF, LORT, SIT90, and ODI) and IIEF-5 (P > .05). Interestingly, the coefficient between lowest SaO2 and IIEF-5 showed no significant difference from the coefficient between DRI and IIEF-5 (P > .05). These results supposed that the 2 most commonly used indicators of PSG results (RDI and lowest SaO2) have no significant difference in reflecting ED conditions, but the new BOADAI can better reflect sexual function than RDI. (iii) Stronger correlations were detected between the BOADAI and lowest SaO2 (r = –0.69, P = .0000) and RDI (r = 0.71, P = .0000), which indicated that BOADAI effectively integrated the information from apnea (RDI) and hypoxia (lowest SaO2) events. (iv) The internal parameters of PSG results showed a strong correlation, which indicated that there is strong collinearity between these parameters. Therefore, LASSO regression can be used as an effective method for variable screening in this study.
Relationship Between BOADAI and ED
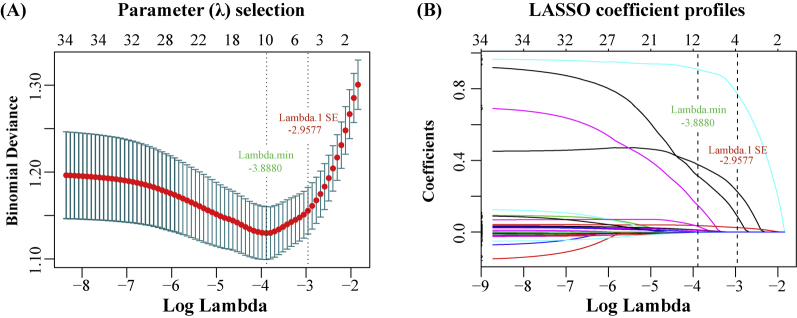
Supplementary Figure 2 A and B show, respectively, that as the parameter log (λ) changes from –10 to 0, the absolute values of the coefficients of the variables shrink toward zero, and the number of variables that enter into the model is also reduced. When log (λ.1SE) (−2.96) was selected as the optimal fit criteria, nonzero coefficients were detected only in systemic hypertension (coefficient = 0.2252), cyanochroia (coefficient = 0.7712), age (coefficient = 0.0254), and BOADAI (coefficient = 0.0876), whereas coefficients of lowest SaO2 and apnea-hypoxia–related parameters were reduced to zero in the LASSO logistic regression model (Supplementary Table S5). Table 4 shows that systemic hypertension (odds ratio [OR] = 1.70, P = .0190), cyanochroia of the lips (OR = 3.27, P = .0000), older age (OR = 1.04, P = .0000), and BOADAI value (OR = 1.67, P = .0290) were independently associated with ED. In the DCA (Supplementary Figure 3), both full (including hypertension, cyanochroia, age, and BOADAI) and one-parameter models (BOADAI) showed a better cost-effectiveness than treating all and treating none, and the full model exhibited an ideal net benefit.
Table 4.
Results of multivariate logistic regression analysis
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Cyanochroia (lips) | 3.27 | (2.02, 5.29) | .0000∗ |
| Systemic hypertension | 1.70 | (1.09, 2.66) | .0190∗ |
| Age | 1.04 | (1.02, 1.07) | .0000∗ |
| BOADAI | 1.67 | (1.05, 2.65) | .0290∗ |
| Intercept | 0.04 | (0.01, 0.18) | .0000∗ |
BOADAI = blood oxygen accumulation distribution area index; CI = confidence interval.
Akaike information criterion (AIC) = 565.4, Bayesian information criterion (BIC) = 586.50, Pseudo R2 = 0.1477.
Log likelihood = −277.71.
P value < 0.05 or less.
Discussion
This study demonstrated that the prevalence rate of ED in patients with OSA was significantly higher than that of the control group. Moreover, the severity of ED according to the IIEF-5 was linked to nocturnal hypoxemia but not to apnea frequency. Correlation matrix analysis and LASSO screening results showed that the new BOADAI is most closely related to ED in PSG indicators. In addition, binary logistic regression analyses confirmed established factors (including hypertension, cyanochroia, and age) for sexual dysfunction but, most importantly, demonstrated that even in the presence of these risk factors, the new BOADAI was independently associated with ED. The likely reason that the BOADAI was superior to other apnea/hypoxemia indicators, such as RDI and lowest SaO2, is that the BOADAI not only effectively integrated the information from apnea and hypoxia events but also showed a time accumulation effect at the same time. Although RDI can reflect apnea frequency and time accumulation effects, it does not effectively indicate the degree of hypoxia. Similarly, the lowest SaO2 can explain the degree of hypoxia well, but it does not reflect apnea frequency or time effects.
These results seem to have clinical usefulness because both the BOADAI and its related model could act as guidelines for standardizing medical care and providing clinical recommendations for the treatment of ED in patients with OSA. As depicted in the DCA, the full BOADAI model performed better in terms of cost-effectiveness than strategies according to which “treat no patients” or “treat all patients” are screened for ED, when examined within the threshold probability of 20% to 95%. Both CPAP and UPPP treatment are effective ways to improve OSA and its complications.30, 31, 32, 33 Moreover, previous meta-analyses have reported that CPAP as a treatment for patients with OSA and coexisting ED is associated with a significant improvement in sexual function in most of the studies.34 Therefore, the BOADAI and its model may be important for guiding CPAP treatment in patients with ED. In this study, systemic hypertension was also independently associated with ED after adjusting for multiple factors. Actually, systemic hypertension and ED are closely intertwined diseases, as the arteriolar smooth muscle cells of the corpora cavernosa are greatly associated with blood pressure, and they share common risk factors such as atherosclerosis and vascular endothelial dysfunction.35, 36, 37, 38 Reportedly, the prevalence of ED was significantly higher for hypertensive men than for normotensive men.39,40 Furthermore, antihypertensive therapy for patients with hypertension and ED is an important step in the effective management of patients with ED.41 However, from the perspective of this research, what we need to pay attention to is that OSA and its nocturnal hypoxemia are independent risk factors for hypertension.42,43 Therefore, treatment of OSA not only can relieve hypertension and ED but it may also prevent and treat ED during the treatment of hypertension. Interestingly, to our knowledge, this is the first study to report cyanochroia of the lips, a physical sign of hypoxaemia,44 as an independent risk factor for ED which further suggests that hypoxia is closely related to ED. In addition, many studies have investigated the underlying mechanisms of OSA triggering or enhancing ED, most of them suggesting a dominant role of CIH.16 In line with this, a previous study in as mice model to determine the role of intermittent hypoxia for erectile function showed that erection frequencies were significantly reduced by CIH. Meanwhile, the expression intensity of endothelial nitric oxide synthase in cavernous endothelial cells was decreased.15
The present findings are in agreement with some previous studies, which reported that nocturnal hypoxemia was associated with ED. The results from the study by Goncalves et al45 showed that older age and nocturnal lowest SaO2, but not the RDI or AHI, are the predictors of ED. Moreover, Budweiser et al11 found that mean SaO2 was independently correlated with ED even in the presence of these confounding factors. On the other hand, some studies reported that OSA or nocturnal hypoxemia was not associated with ED.25,46 Yung et al25 analyzed data from 713 male patients who underwent full-night PSG monitoring and found that the frequency of ED did not differ significantly according to OSA severity and there was no significant association of ED with either the AHI or lowest SaO2. The possible reasons for the discrepancies between our findings and some previous studies are as follows: (i) The exclusion criteria were different. For example, because not all asexual life is caused by abnormal erectile function, we therefore excluded participants who were sexually free for 6 months from the study, but Yang et al.'s study research did not pay attention to or explain this point; (ii) the clinical questionnaire, physical measurements, and PSG outcomes in this study were obtained by respiratory physicians, and ED was assessed by urologists, which means that more accurate and more reliable information can be extracted from the data; (iii) the cultures of different ethnic groups have different content and features. The Han people are relatively conservative about sexual issues. Because the results of a meta-analysis based on previous studies and animal experiments are consistent with our report, we therefore believe that OSA and nocturnal hypoxia are closely related to ED.
To our knowledge, this is the first study to integrate the information of apnea events and nocturnal hypoxemia based on oxygen accumulation distribution area for evaluating the association between OSA and ED. Our study has some strengths, including objective PSG data rather than self-reported evaluations and robust LASSO-Logistic regression model, increasing the quality of the evidence. Meanwhile, our present study is not devoid of limitations. First, this hospital-based observational study lacked a community-based prospective design. Second, because this study was cross-sectional, temporality was unclear; therefore, causality cannot be further inferred. However, it should be noted that ED, as a special clinical symptom, seems unlikely to cause OSA. In addition, this shortcoming does not affect the importance of the BOADAI as a marker of undiagnosed ED risk factors for early intervention. Third, as ED assessed by the IIEF-5 questionnaire is subjective and free, further accurate and objective measures, such as nocturnal penile tumescence and rigidity monitoring with RigiScan and penile doppler ultrasonography,47 are needed to obtain more clinical evidence. Finally, nearly 10% of participants were excluded because of incomplete questionnaires or because they were unable to complete PSG monitoring, which would impair real-world evidence and potentially increase the possibility of selection bias.
Conclusion
In conclusion, this study revealed novel evidence that OSA is associated with ED. Meanwhile, higher BOADAI values, older age, cyanochroia of the lips, and systemic hypertension status emerged to be independently associated with ED, and they could be considered as useful indicators to implement early clinical preventive interventions in those male populations at risk of the consequences from missing apnea-hypoxemia treatment and effective blood pressure control. Further community-based prospective cohort studies are needed to validate the relationship between the BOADAI and ED. In addition, developing a BOADAI computing module embedded in a PSG monitor system will help to complement and refine the PSG report.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Jican Dai; Qingyun Li
-
(b)Acquisition of Data
- Wenzhong Zheng; Jingwen Huang; Xiang Chen; Liu Zhang
-
(c)Analysis and Interpretation of Data
- Tao chen; Xianxin Li
Category 2
-
(a)Drafting the Article
- Wenzhong Zheng
-
(b)Revising It for Intellectual Content
- Jican Dai; Qingyun Li
Category 3
-
(a)Final Approval of the Completed Article
- Wenzhong Zheng; Xiang Chen; Jingwen Huang; Shengxiong Zhang; Tao chen; Liu Zhang; Xianxin Li; Qingyun Li; Jican Dai
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This study was supported by the National Natural Science Foundation of China (No. 814701813).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.esxm.2019.11.001.
Supplementary Data
Supplementary Figure 1.
Correlation matrix analysis. IIEF-5 represent International Erectile Function Score 5; RDI represent Respiratory disorder index; OAI represent Obstructive apnea index; ODI represent Oxygen decrease index; LORT represent Longest oxygen reduction time; ORF represent oxygen reduction frequency; MOR represent Maximum oxygen reduction; SIT90% represent Oxygen saturation less than 90%; BOADAI represent Blood oxygen accumulation distribution area index. × represent the P value of Spearman (Rho) correlation coefficient > 0.05; The inverted triangle below the matrix indicates the direction of correlation coefficient (absolute value) decreases. * represent the correlation coefficient between BOADAI and IIEF-5 was significantly higher than the coefficients of most apnea-related parameters including LA, CA, OAI, OA and HO, and IIEF-5 (*represent P < .05, ** represent P < .01). † represent the coefficient between lowest SaO2 and IIEF-5 was not significantly different with coefficient between DRI and IIEF-5.
Supplementary Figure 2.
Feature selection for the LASSO logistic regression model. (A) Tuning parameter (Lambda) selection by 10-fold cross-validation with minimum criteria. Binomial deviance (y-axis) was plotted against log (Lambda) (x-axis). (b) LASSO coefficient profiles for the 33 OSA-related features. The dotted log (Lambda 1SE) criteria vertical line was plotted at the value selected with 10-fold cross-validation, where 4 optimal features with nonzero coefficients are indicated in the plot.
Supplementary Figure 3.
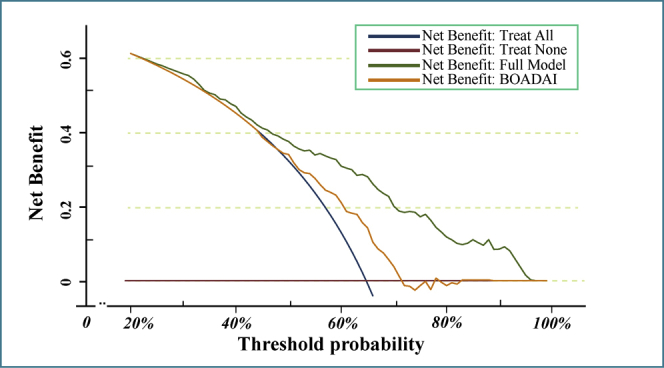
Decision curve analysis (DCA) assessing net benefit for prediction of ED status. The y-axis measures the net benefit. The x-axis represent the threshold probability. The green line represents the full module (including systemic hypertension, cyanochroia, age and BOADAI). Orange line represents single BOADAI module. The blue line represents the “treat all” strategy. The claret line represent the “treat none” strategy.
References
- 1.Malhotra A., White D.P. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Huang T., Lin B.M., Stampfer M.J. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care. 2018;41:2111–2119. doi: 10.2337/dc18-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson D.A., Thomas S.J., Abdalla M. Association between sleep apnea and blood pressure control among blacks. Circulation. 2019;139:1275–1284. doi: 10.1161/CIRCULATIONAHA.118.036675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floras J.S. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122:1741–1764. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 5.Petersen M., Kristensen E., Berg S. Sexual function in female patients with obstructive sleep apnea. J Sex Med. 2011;8:2560–2568. doi: 10.1111/j.1743-6109.2011.02358.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalejaiye O., Raheem A.A., Moubasher A. Sleep disorders in patients with erectile dysfunction. BJU Int. 2017;120:855–860. doi: 10.1111/bju.13961. [DOI] [PubMed] [Google Scholar]
- 7.Chen C.M., Tsai M.J., Wei P.J. Erectile dysfunction in patients with sleep apnea--a Nationwide Population-Based Study. PloS one. 2015;10:e0132510. doi: 10.1371/journal.pone.0132510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouloukaki I., Papadimitriou V., Sofras F. Abnormal cytokine profile in patients with obstructive sleep apnea-hypopnea syndrome and erectile dysfunction. Mediators Inflamm. 2014;2014:568951. doi: 10.1155/2014/568951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teloken P.E., Smith E.B., Lodowsky C. Defining association between sleep apnea syndrome and erectile dysfunction. Urology. 2006;67:1033–1037. doi: 10.1016/j.urology.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Fanfulla F., Malaguti S., Montagna T. Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep. 2000;23:775–781. [PubMed] [Google Scholar]
- 11.Budweiser S., Enderlein S., Jorres R.A. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med. 2009;6:3147–3157. doi: 10.1111/j.1743-6109.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen K.F., Liang S.J., Lin C.L. Sleep disorders increase risk of subsequent erectile dysfunction in individuals without sleep apnea: a nationwide population-base cohort study. Sleep Med. 2016;17:64–68. doi: 10.1016/j.sleep.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Tang T., Wu W. Efficacy of nasal continuous positive airway pressure on patients with OSA with erectile dysfunction and low sex hormone levels. Respir Med. 2016;119:130–134. doi: 10.1016/j.rmed.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Budweiser S., Luigart R., Jorres R.A. Long-term changes of sexual function in men with obstructive sleep apnea after initiation of continuous positive airway pressure. J Sex Med. 2013;10:524–531. doi: 10.1111/j.1743-6109.2012.02968.x. [DOI] [PubMed] [Google Scholar]
- 15.Soukhova-O'Hare G.K., Shah Z.A., Lei Z. Erectile dysfunction in a murine model of sleep apnea. Am J Respir Crit Care Med. 2008;178:644–650. doi: 10.1164/rccm.200801-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu D., Deng Y., Pan Y. N-acetylcysteine ameliorates the erectile dysfunction caused by chronic intermittent hypoxia in rats: partly involvement of endoplasmic reticulum stress. Urology. 2015;86 doi: 10.1016/j.urology.2015.07.013. 844.e7-e14. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-de-la-Torre M., Campos-Rodriguez F., Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 18.Baguet J.P., Barone-Rochette G., Tamisier R. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688. doi: 10.1038/nrcardio.2012.141. [DOI] [PubMed] [Google Scholar]
- 19.Gleadhill I.C., McCrum E.E., Patterson C.C. Sleep related hypoxaemia in hypertensive and normotensive men. Thorax. 1993;48:534–536. doi: 10.1136/thx.48.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shawon M.S., Perret J.L., Senaratna C.V. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med Rev. 2017;32:58–68. doi: 10.1016/j.smrv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Suen C., Ryan C.M., Mubashir T. Sleep study and oximetry parameters for predicting postoperative complications in patients with OSA. Chest. 2019;155:855–867. doi: 10.1016/j.chest.2018.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popp R., Kleemann Y., Burger M. Impaired vigilance is associated with erectile dysfunction in patients with sleep apnea. J Sex Med. 2015;12:405–415. doi: 10.1111/jsm.12789. [DOI] [PubMed] [Google Scholar]
- 23.Shin H.W., Rha Y.C., Han D.H. Erectile dysfunction and disease-specific quality of life in patients with obstructive sleep apnea. Int J Impot Res. 2008;20:549–553. doi: 10.1038/ijir.2008.39. [DOI] [PubMed] [Google Scholar]
- 24.Martin S.A., Appleton S.L., Adams R.J. Erectile dysfunction is independently associated with apnea-hypopnea index and oxygen desaturation index in elderly, but not younger, community-dwelling men. Sleep Health. 2017;3:250–256. doi: 10.1016/j.sleh.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Jeon Y.J., Yoon D.W., Han D.H. Low quality of life and depressive symptoms as an independent risk factor for erectile dysfunction in patients with obstructive sleep apnea. J Sex Med. 2015;12:2168–2177. doi: 10.1111/jsm.13021. [DOI] [PubMed] [Google Scholar]
- 26.Bloch K.E., Schoch O.D., Zhang J.N. German version of the Epworth Sleepiness Scale. Respiration. 1999;66:440–447. doi: 10.1159/000029408. [DOI] [PubMed] [Google Scholar]
- 27.Johns M.W. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Berry R.B., Budhiraja R., Gottlieb D.J. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid S., Tibshirani R. Regularization paths for conditional logistic regression: the clogitL1 package. J Stat Softw. 2014;58 [PMC free article] [PubMed] [Google Scholar]
- 30.Shim C.Y., Kim D., Park S. Effects of continuous positive airway pressure therapy on left ventricular diastolic function: a randomised, sham-controlled clinical trial. Eur Respir J. 2018;51 doi: 10.1183/13993003.01774-2017. [DOI] [PubMed] [Google Scholar]
- 31.Lee H.M., Kim H.Y., Suh J.D. Uvulopalatopharyngoplasty reduces the incidence of cardiovascular complications caused by obstructive sleep apnea: results from the National Insurance Service Survey 2007-2014. Sleep Med. 2018;45:11–16. doi: 10.1016/j.sleep.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Ortega A., Manas E., Lopez-Reyes R. Obstructive sleep apnoea and venous thromboembolism: pathophysiological links and clinical implications. Eur Respir J. 2019;53 doi: 10.1183/13993003.00893-2018. [DOI] [PubMed] [Google Scholar]
- 33.Melehan K.L., Hoyos C.M., Hamilton G.S. Randomized trial of CPAP and vardenafil on erectile and arterial function in men with obstructive sleep apnea and erectile dysfunction. J Clin Endocrinol Metab. 2018;103:1601–1611. doi: 10.1210/jc.2017-02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campos-Juanatey F., Fernandez-Barriales M., Gonzalez M. Effects of obstructive sleep apnea and its treatment over the erectile function: a systematic review. Asian J Androl. 2017;19:303–310. doi: 10.4103/1008-682X.170440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes K.P., Labazi H., Webb R.C. New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens. 2012;21:163–170. doi: 10.1097/MNH.0b013e32835021bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun P., Swindle R. Are men with erectile dysfunction more likely to have hypertension than men without erectile dysfunction? A naturalistic national cohort study. J Urol. 2005;174:244–248. doi: 10.1097/01.ju.0000162050.84946.86. [DOI] [PubMed] [Google Scholar]
- 37.Castela A., Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13:266–274. doi: 10.1038/nrurol.2016.23. [DOI] [PubMed] [Google Scholar]
- 38.Luscher T.F. Heterogeneity of endothelial dysfunction in hypertension. Eur Heart J. 1992;13 Suppl D:50–55. doi: 10.1093/eurheartj/13.suppl_d.50. [DOI] [PubMed] [Google Scholar]
- 39.Hirshkowitz M., Karacan I., Gurakar A. Hypertension, erectile dysfunction, and occult sleep apnea. Sleep. 1989;12:223–232. doi: 10.1093/sleep/12.3.223. [DOI] [PubMed] [Google Scholar]
- 40.Burchardt M., Burchardt T., Baer L. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164:1188–1191. [PubMed] [Google Scholar]
- 41.Papatsoris A.G., Korantzopoulos P.G. Hypertension, antihypertensive therapy, and erectile dysfunction. Angiology. 2006;57:47–52. doi: 10.1177/000331970605700107. [DOI] [PubMed] [Google Scholar]
- 42.Hla K.M., Young T.B., Bidwell T. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki N., Nagai M., Mizuno H. Associations between characteristics of obstructive sleep apnea and nocturnal blood pressure surge. Hypertension. 2018;72:1133–1140. doi: 10.1161/HYPERTENSIONAHA.118.11794. [DOI] [PubMed] [Google Scholar]
- 44.Maitre B., Similowski T., Derenne J.P. Physical examination of the adult patient with respiratory diseases: inspection and palpation. Eur Respir J. 1995;8:1584–1593. [PubMed] [Google Scholar]
- 45.Goncalves M.A., Guilleminault C., Ramos E., Palha A., Paiva T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005;6:333–339. doi: 10.1016/j.sleep.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Santos T., Drummond M., Botelho F. Erectile dysfunction in obstructive sleep apnea syndrome--prevalence and determinants. Rev Port Pneumol. 2012;18:64–71. doi: 10.1016/j.rppneu.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Elhanbly S., Elkholy A. Nocturnal penile erections: the role of RigiScan in the diagnosis of vascular erectile dysfunction. J Sex Med. 2012;9:3219–3226. doi: 10.1111/j.1743-6109.2012.02954.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.