Abstract
Selective decontamination of the digestive tract (SDD) is an infection prevention measure for intensive care unit (ICU) patients that was proposed more than 30 years ago, and that is currently considered standard of care in the Netherlands, but only used sporadically in ICUs in other countries. In this narrative review, we first describe the rationale of the individual components of SDD and then review the evidence base for patient-centered outcomes, where we distinguish ICUs with low prevalence of antibiotic resistance from ICUs with moderate–high prevalence of resistance. In settings with low prevalence of antibiotic resistance, SDD has been associated with improved patient outcome in three cluster-randomized studies. These benefits were not confirmed in a large international cluster-randomized study in settings with moderate-to-high prevalence of antibiotic resistance. There is no evidence that SDD increases antibiotic resistance. We end with future directions for research.
Electronic supplementary material
The online version of this article (10.1007/s00134-019-05883-9) contains supplementary material, which is available to authorized users.
Keywords: Selective decontamination, Infection prevention, Antibiotic resistance
Take-home message
| In settings with low prevalence of antibiotic resistance, SDD is consistently associated with less antibiotic resistance and with improved patient outcome. In settings with moderate-to-high prevalence of antibiotic resistance, benefits of SDD on clinically relevant patient outcomes remain to be demonstrated. |
Selective decontamination of the digestive tract (SDD) is an infection prevention measure for intensive care unit (ICU) patients, which was proposed more than 30 years ago [1], and that is currently considered standard of care in the Netherlands [2], but only used sporadically in ICUs in other countries [3]. In this narrative review, we first describe the rationale of the individual components of SDD and then review the evidence base for patient-centered outcomes, where we distinguish ICUs with low prevalence of antibiotic resistance from ICUs with moderate–high prevalence of resistance. We end with future directions for research.
The concept
The concept of SDD originates from two fundamental observations made in the 1960s and 70s. In the United States, Waldemar Johanson described that the pharyngeal flora of patients changed within a few days after hospital admission; from predominantly Gram-positive to predominantly Gram-negative bacteria [4]. Subsequent studies revealed that these Gram-negative bacteria were the main causes of hospital-acquired infections (at that time), especially of nosocomial pneumonias. Around the same time, a Dutch clinical microbiologist, Dick van der Waaij, described the interaction between gut flora and infections in neutropenic gnotobiotic mice [5]. He demonstrated that conservation of the anaerobic intestinal flora prevented such mice from bacterial overgrowth with Gram-negative bacteria and subsequent infections. He called this phenomenon “colonization resistance”. In those days, these observations were based on conventional microbiological culture techniques. Yet, this concept—a healthy gut flora that prevents infection—has now been repeatedly confirmed in studies using deep sequencing technologies.
The second step was the translation of these findings to cancer patients with long-term neutropenia that frequently suffered from Gram-negative infections. Infectious disease specialists started to treat leukemia patients prophylactically with antibiotics active against Gram-negative bacteria, while preserving the protective anaerobic flora by avoiding antibiotics with anti-anaerobic activity, such as penicillins [6].
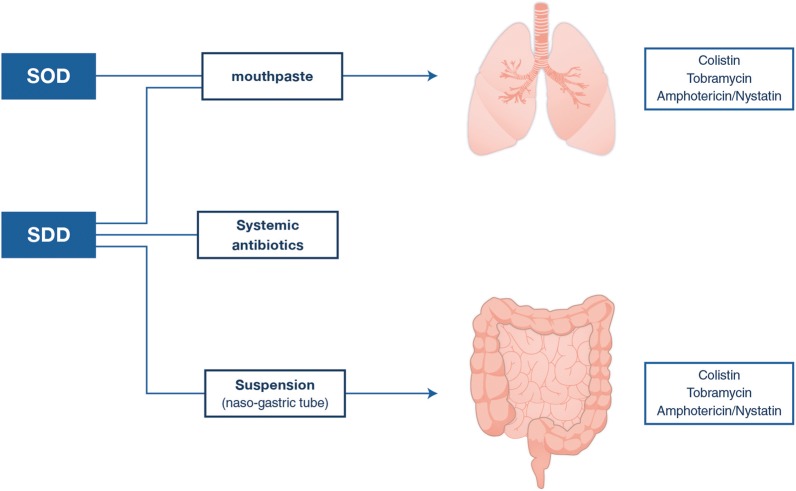
In the early 1980s, Stoutenbeek and van Saene, then working in Groningen, the Netherlands, introduced this concept in ICU patients [1]. The targeted pathogens for prevention were the Enterobacterales (e.g., Escherichia coli and Klebsiella pneumoniae), Pseudomonas aeruginosa, and Staphylococcus aureus. The intestinal flora was considered the source of these potential pathogenic micro-organisms for colonization of the upper respiratory tract during hospitalization. Therefore, topical antibiotics were chosen that were not absorbed (to avoid systemic distribution), that were active against the listed pathogens and that would not disturb the anaerobic flora. As such, the combination of an aminoglycoside (tobramycin) and colistin was selected, and these antibiotics were administered in an oropharyngeal paste applied in the buccal cavity and as a solution inserted through the nasogastric tube (both four times daily) (Fig. 1). Initial small studies, mainly in trauma patients, led to two modifications of the regimen. Amphotericin B was added to prevent overgrowth with yeasts. Furthermore, a prophylactic 4-day course of a cephalosporin—cefotaxime—(not active against anaerobes) was added to treat any incubating infection caused by the commensal flora of the respiratory tract at the time of hospital admission. The choice of cefotaxime was motivated by the assumption that trauma patients would have—at the time of trauma and immediate hospital admission—a normal respiratory tract flora susceptible to a third-generation cephalosporin. In later SDD studies, enrolled patient populations gradually changed to also include surgical and medical patients, with extensive medical histories, including prior antibiotic use and carriage with bacteria non-susceptible to cefotaxime.
Fig. 1.
Components of SDD and SOD. SDD selective digestive tract decontamination, SOD selective oropharyngeal decontamination
Application of SDD was accompanied by microbiological surveillance of respiratory samples and rectal swabs at the day of admission and twice weekly. Results from these cultures should serve as an early warning for selection of antibiotic resistant pathogens, but also as a measure of the efficacy of SDD. Persisting carriage with Gram-negative bacteria is—in some centers, but not everywhere—followed by more frequent application of the SDD antibiotics or addition of nebulized antibiotics such as colistin. Twice weekly surveillance, but not nebulized antibiotics, is currently part of the Dutch SDD guideline [2].
The main goal of SDD is to prevent ICU-acquired infections (and thereby improve patient outcomes). Therefore, the targeted population is comprised of patients with an expected ICU stay of at least 2 or 3 days, and receiving mechanical ventilation; the preferred moment to start SDD is immediately upon ICU admission. In most studies, SDD was continued until ICU discharge and in some until extubation [1, 7–10].
Later, as an alternative to SDD, selective oropharyngeal decontamination (SOD) was proposed, based on clinical studies suggesting a more important role of upper respiratory tract colonization in the pathogenesis of ventilator-associated pneumonia than intestinal carriage [11]. SOD consists of the oropharyngeal application of the same antibiotics without the intragastric application and without systemic prophylaxis. As at that time, many patients eligible for SDD were treated with antibiotics for clinical indications and that an increasing number of patients were colonized with bacteria resistant to second-generation cephalosporins when admitted to ICU, it was decided not to include the 4-day course of systemic antibiotics in SOD.
Considerations for study design
Since the first published studies on SDD in ICU patients, study designs have changed. Here, we briefly describe the most important considerations leading to these changes in recent studies (Supplement Table 1).
Micro-organisms are not necessarily bound to individual patients. Most ICU-acquired infections are caused by Enterobacterales (e.g., Escherichia coli and Klebsiella pneumoniae), Pseudomonas aeruginosa, and Staphylococcus aureus, and most will originate from the endogenous flora, present at the time of ICU admission. Yet, some infections may be caused by pathogens acquired through cross-transmission, and the relative importance of this may vary considerably between ICUs.
Until 2000, most studies were individually randomized trials with at most 578 patients recruited [12]. Although valid to determine the effectiveness of an intervention in many circumstances, individual randomization may reduce the generalizability of study results and is suboptimal if cross-transmission occurs. The latter implies that colonized patients pose a risk for acquisition of bacterial carriage and subsequent infection to other patients and, vice versa, decolonized patients may offer indirect protection to those not receiving SDD. Both effects will reduce the effectiveness of SDD, compared to settings where all eligible patients will receive SDD. To overcome this, investigators have adopted cluster designs, in which all eligible patients in an ICU receive SDD or not, as it would happen in real life.
Another complicating factor is the requirement of informed consent before the intervention can be started. For an intervention with an intended immediate start after ICU admission, this practically precludes enrollment of all eligible patients and will delay the start of SDD in many that do consent. This may lead to less generalizable study populations and reduce the effectiveness of SDD, as compared to settings where SDD would be started in all eligible patients upon ICU admission [13]. In the first clustered SDD study, performed in two ICUs in a single center, informed consent was still required [9]. In three more recent cluster-randomized studies, waivers for informed consent were granted in the Netherlands, Belgium, Spain, Portugal, Italy, Slovenia, and the UK. Waivers were granted, because SDD was considered part of daily ICU practice in each country. At the same time, there was true equipoise on the effects of SDD, leading to marked differences in practice between ICUs. Moreover, SDD (and SOD) were considered safe and it was acknowledged that a cluster-randomized study would not be feasible without such a waiver. The waivers allowed an immediate start of SDD after ICU admission and enrollment of 6000 patients and more [7, 8, 10].
Another advantage of cluster studies is the possibility to study effects of the interventions on antibiotic resistance at the ward level, as all eligible patients in an ICU receive the same intervention. For this, monthly point-prevalence surveys of antibiotic resistance were used in most of these studies [7–10].
Naturally, cluster designs may also create problems, such as baseline imbalance between groups and within clusters with time, either because of temporal changes in patient case mix or because of differences in patient enrollment (selection bias). The presence of intra-cluster variation massively affects the required sample size and the effects of changes in time, for instance in infection control practices, are difficult to measure. A cross-over design, in which SDD and its control are evaluated successively in the same setting, can be used to minimize baseline imbalance. Yet, cluster designs always require complex adjusted statistical analyses [14].
Finally, initial studies sought to quantify the effects of SDD on preventing ICU-acquired pneumonia. Yet, establishing this diagnosis is subject to ascertainment bias and, therefore, would require adequate blinding. However, as SDD modifies microbiological culture and sensitivity results, maintaining study blinding is probably unrealistic. Instead, recent studies have used patient survival and ICU-acquired bloodstream infections as primary outcome.
SDD in ICUs with low prevalence of antibiotic resistance
In 2001, a Dutch national guideline concluded that the routine use of SDD was not recommended in ICUs as the available evidence for beneficial effects was not considered to be sufficiently robust and the risks on antibiotic resistance had been insufficiently addressed. This initiated three clustered studies that eventually led—in 2018—to the recommendation to use SDD in all patients with an expected ICU stay of more than 48 h in the Netherlands [2].
In the first study, eligible patients were randomized—in a single hospital—to an ICU in which all patients received SDD or a similar control unit, in which SDD was not used [9]. Randomization to one of both units was only possible when both units had at least one bed available. It was not specified how frequently this occurred, but the 2-year enrollment period yielded two comparable patient populations of about 450 subjects. Patient outcome was better for those in the ICU with SDD, with a relative risk reduction of hospital mortality of 22% and a shortened length of stay in ICU.
As this was a single-center study, and because of the possibility of residual confounding due to differences between the two participating ICUs, there was limited adoption of the intervention at that time. Instead, a cluster-randomized cross-over study was performed in 13 ICUs, comparing SDD, but also SOD, to standard care. In this study, the potential influence of differences between individual ICUs on patient outcome was addressed using a cross-over design in which all three study interventions were applied in all participating units [7]. Yet, despite all efforts to prevent selection bias in patient enrolment, there was evidence that patients enrolled in the standard of care population were less ill than those enrolled in the SDD or SOD groups. After adjustment for baseline characteristics, both SDD and SOD were associated with a better patient outcome compared to standard care with relative reductions in mortality 28 days after ICU admission of 13% and 11%, respectively.
Finally, another similarly designed study was performed in 16 ICUs comparing 12-month periods of SDD and SOD. In this study, SDD was associated with a better patient outcome than SOD, with absolute and relative day-28 mortality reductions of 3.0% and 11.6%, respectively [8]. In a post hoc analysis of the de Smet study, SDD and SOD appeared to be cost-effective compared to standard care [15]. In a subsequent analysis based on the individual patient data from the de Smet [7] and the Oostdijk [8] study, SDD yielded significantly lower in-hospital mortality and comparable costs compared with SOD in Dutch ICUs [16].
All three studies used an SDD regimen which included systemic antibiotics and were performed in ICU settings with low prevalence of antibiotic resistance. Carriage and infection rates with feared multi-resistant pathogens, such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant enterococci (VRE), or carbapenem-resistant Gram-negative bacteria were less than 1% and proportions of infections caused by Enterobacterales resistant to the third-generation cephalosporins among all infections caused by Enterobacterales were less than 10%. An individual patient data meta-analysis of six studies, all performed in units with low levels of antibiotic resistance (from the Netherlands, Germany and France), included 17,884 ICU admissions [17]. Compared to standard care or placebo, the pooled adjusted odds ratios for hospital mortality was 0.82 (95% confidence interval (CI) 0.72–0.93) for SDD and 0.84 (95% CI 0.73–0.97) for SOD. Compared to SOD, the adjusted odds ratio for hospital mortality was 0.90 (95% CI 0.82-0.97) for SDD. The effects on hospital mortality were not modified by type of ICU admission (p values for interaction terms were 0.66 for SDD and control, 0.87 for SOD and control, and 0.47 for SDD and SOD). Similar results were found for ICU mortality.
SDD in ICUs with moderate-to-high prevalence of antibiotic resistance
The effectiveness of SDD in improving patients’ outcome is less well studied in ICUs with moderate-to-high prevalence of antibiotic resistance (as defined by a prevalence of at least 5% of bloodstream infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae). Importantly, there were until recently no studies with a sufficiently large sample size to demonstrate effect sizes on patient outcome comparable to those reported from the studies in Dutch ICUs. Thus, based on the promising results in these studies, a clustered evaluation of SDD (but also of SOD and chlorhexidine mouthwash) was pursued in settings with moderate-to-high prevalence of antibiotic resistance. In this study, ICUs were recruited in Belgium, Spain, Portugal, Italy, Slovenia, and the UK. In the 13 participating ICUs, the proportion of bloodstream infections caused by highly resistant micro-organisms was 25.5% (and 15.1% for third-generation cephalosporin-resistant Enterobacterales), which markedly deviates from the settings in Dutch ICUs [10].
In this international study, SDD was applied during 6 months and compared to a similarly long baseline period. In addition to SDD, 6-month periods of SOD and chlorhexidine mouthwash were randomly tested in a cross-over design. The primary outcome of the study was the incidence of ICU-acquired bloodstream infection caused by multidrug-resistant Gram-negative bacteria which was 1.2% during SDD and 2.1% during baseline, yielding an adjusted hazard ratio of 0.70 (95% CI 0.43–1.14). For patient survival, adjusted hazard ratios for SDD, compared to baseline, were 0.95 (95% CI 0.81–1.11), 0.96 (95% CI 0.82–1.12), and 1.03 (95% CI 0.80–1.32) for mortality in ICU, in hospital, and at day 28 after admission, respectively.
An important modification of the SDD regime in this study was the absence of routine use of an intravenous cephalosporin during the first 4 days of ICU stay. As study sites were selected upon a moderate-to-high prevalence of antibiotic resistant bacteria, including third-generation cephalosporin-resistant Enterobacterales, such prophylaxis was considered inappropriate, as was an alternative prophylactic regimen providing a better coverage of these bacteria, such as a carbapenem. Indeed, most patients admitted to ICU will receive systemic antibiotic treatment for medical reasons, with therapy based on local epidemiology and practices. Therefore, the consequences of patients not being treated systemically during these 4 days remain unknown, as the relative contribution of systemic antibiotics to the overall effect of SDD has never been determined in a single study. In a Cochrane review, including 36 studies involving 6914 patients treated in ICUs published until March 2009, the combination of topical plus systemic antibiotics was associated with less infections and deaths, whereas regimens of only topical treatment were associated with less infections but not with less deaths [18]. Furthermore, in contrast to how SDD was used in the Dutch studies, application of antibiotics was discontinued upon extubation instead of upon discharge, but consequences of this on ICU-acquired bacteremia rates seemed limited.
Antibiotic resistance
In the setting with moderate-to-high levels of antibiotic resistance, there was, based on regular unit-wide point-prevalence surveys, no evidence of increasing levels of antimicrobial resistance, not during the use of SDD, nor during the use of SOD or chlorhexidine mouthwash [10]. This is in line with findings from a meta-analysis of 64 unique studies of SDD and SOD in ICUs, of which 47 were randomized-controlled trials and 35 included data for the detection of antimicrobial resistance [19]. When comparing patients in intervention groups (those who received SDD or SOD) to those in control groups (who received no intervention), there were no statistically significant differences in the prevalence of colonization or infection with methicillin-resistant S. aureus (odds ratio 1.46, 95% CI 0.90–2.37), vancomycin-resistant enterococci (0.63, 95% CI 0.39–1.02), aminoglycoside-resistant Gram-negatives (0.73, 95% CI 0.51–1.05), or fluoroquinolone-resistant Gram-negatives (0.52, 95% CI 0.16–1.68). In fact, prevalence of polymyxin-resistant Gram-negative bacilli (0.58, 95% CI 0.46–0.72) and third-generation cephalosporin-resistant Gram-negative bacilli (0·33, 95% CI 0.20–0.52) was lower in recipients of selective decontamination compared with those who received no intervention.
There is also no evidence that prolonged use of SDD is associated with increases in the prevalence of antibiotic resistance in Dutch ICUs. In an analysis of trends in antibiotic resistance among Gram-negative bacteria in 38 Dutch ICUs using and not using SOD/SDD during a 4-year period, there were no statistically significant trends in antibiotic resistance among 637 blood isolates. For the 8353 respiratory isolates, resistance to cefotaxime/ceftriaxone increased in ICUs that did not use SOD/SDD (n = 13) and decreased in those that continuously used SOD/SDD (n = 17), as did resistance to ciprofloxacin. The introduction of SOD/SDD in eight ICUs was followed by statistically significant reductions in resistance rates for all antimicrobial agents [20]. In a single-center evaluation, 21 years of SDD use was also not associated with an increase of antibiotic resistance in ICU [21]. Of note, the total use of systemic antibiotics in the ICUs was 11.9% lower during the SDD period, compared to standard care, in Dutch ICUs [7], which most likely contributed to the observed ecological safety of SDD in that setting.
There is limited evidence on effects of SDD on antimicrobial resistance after ICU discharge. In one study from the Netherlands, colonization of the gut with Gram-negative bacteria was determined upon ICU discharge and on day 10 after ICU discharge, in patients that had received either SDD or SOD in ICU [22]. SDD resulted in more effective decontamination of the gut at the time of ICU discharge and led to lower acquisition rates on day 10 after ICU discharge, although colonization rates on day 10 after ICU discharge were similar in both study groups [22].
Future research directions
In contrast to the extensive clinical evaluation of SDD in ICU patients, relatively little is known about its effects on the microbial flora. In one study, changes in the gut microbiome during SDD were investigated using metagenomics in a single patient during and after ICU admission [23]. SDD was associated with an increase in the abundance of resistance genes, mostly of aminoglycoside resistance genes carried by anaerobe gut bacteria, which was confirmed in 12 other patients. In a subsequent study, the same investigators analyzed the gut microbiome in ten ICU patients that received SDD [24]. For comparison, gut microbiota were also determined twice in ten healthy subjects with a 1-year interval. The microbiota of the ICU patients differed from those of healthy subjects and was characterized by lower microbial diversity, decreased levels of Escherichia coli and of anaerobic Gram-positive, butyrate-producing bacteria of the Clostridium clusters IV and XIVa, and an increased abundance of Bacteroidetes and enterococci. Four types of resistance genes, providing resistance to aminoglycosides, macrolides, disinfectants, and tetracyclines, were significantly more abundant among ICU patients than in healthy subjects. These findings confirm the principles of SDD, eradicate Enterobacterales but not anaerobes from the gut, but more studies are needed to better determine the effects of SDD on selection and transmission of resistance genes.
The effects of SDD were disappointing in the only international multi-center study performed in settings with moderate-to-high levels of antibiotic resistance [10]. Yet, the Selective Decontamination of the Digestive Tract in Intensive Care Unit Patients (SuDDICU) trial (clinicaltrials.gov NCT02389036) is another cluster, cross-over, randomized-controlled trial of SDD in mechanically ventilated critically ill patients. It is currently recruiting a target of 12,000–15,000 patients in Canada, the UK, and Australia. Patients not already receiving an intravenous therapeutic antibiotic will be prescribed a 4-day course of intravenous cephalosporins. The study has a concurrent cohort study nested within the randomized trial for evaluating the effects of SDD on antibiotic resistance patterns. These recruiting countries are countries with the least moderate rate of antibiotic resistance, and therefore, the results will give us more information about the use of SDD in these populations. The study will also have cost-effectiveness analyses, microbiome/meta genetic analysis, and a process evaluation undertaken in Canada and Australia to understand the broader patient, health care practitioner, and ecological and health system implications of delivering SDD. The study will report in 2021.
Conclusion
SDD has been studied in ICU populations for almost 40 years. Its current status is that the initially perceived risks for augmented selection of antibiotic resistance have been firmly rejected. Instead, in settings with low prevalence of antibiotic resistance, SDD is consistently associated with less antibiotic resistance and with improved patient outcome. In settings with moderate-to-high prevalence of antibiotic resistance, benefits of SDD on clinically relevant patient outcomes remain to be demonstrated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984;10(4):185–192. doi: 10.1007/BF00259435. [DOI] [PubMed] [Google Scholar]
- 2.Stichting Werkgroep Antibioticabeleid (SWAB) (2018) SWAB-Richtlijn: selectieve decontaminatie bij patiënten op de intensive care (herziene versie 2018). https://www.swab.nl/swab/cms3.nsf/uploads/59AD7C3D7A4B8887C1258332002DE212/$FILE/SWAB%20richtlijn%20SDD%20revised%202018_juli_DEF.pdf. Accessed 20 Sep 2019
- 3.Francis JJ, Duncan EM, Prior ME, Maclennan G, Marshall AP, Wells EC, Todd L, Rose L, Campbell MK, Webster F, Eccles MP, Bellingan G, Seppelt IM, Grimshaw JM, Cuthbertson BH, Dsg Su. Comparison of four methods for assessing the importance of attitudinal beliefs: an international Delphi study in intensive care settings. Br J Health Psychol. 2014;19(2):274–291. doi: 10.1111/bjhp.12066. [DOI] [PubMed] [Google Scholar]
- 4.Johanson WG, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N Engl J Med. 1969;281(21):1137–1140. doi: 10.1056/nejm196911202812101. [DOI] [PubMed] [Google Scholar]
- 5.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-v. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg. 1971;69(3):405–411. doi: 10.1017/S0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiot HF, van den Broek PJ, van der Meer JW, van Furth R. Selective antimicrobial modulation of the intestinal flora of patients with acute nonlymphocytic leukemia: a double-blind, placebo-controlled study. J Infect Dis. 1983;147(4):615–623. doi: 10.1093/infdis/147.4.615. [DOI] [PubMed] [Google Scholar]
- 7.de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 8.Oostdijk EA, Kesecioglu J, Schultz MJ, Visser CE, de Jonge E, van Essen EH, Bernards AT, Purmer I, Brimicombe R, Bergmans D, van Tiel F, Bosch FH, Mascini E, van Griethuysen A, Bindels A, Jansz A, van Steveninck FA, van der Zwet WC, Fijen JW, Thijsen S, de Jong R, Oudbier J, Raben A, van der Vorm E, Koeman M, Rothbarth P, Rijkeboer A, Gruteke P, Hart-Sweet H, Peerbooms P, Winsser LJ, van Elsacker-Niele AM, Demmendaal K, Brandenburg A, de Smet AM, Bonten MJ. Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA. 2014;312(14):1429–1437. doi: 10.1001/jama.2014.7247. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet (London, England) 2003;362(9389):1011–1016. doi: 10.1016/s0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- 10.Wittekamp BH, Plantinga NL, Cooper BS, Lopez-Contreras J, Coll P, Mancebo J, Wise MP, Morgan MPG, Depuydt P, Boelens J, Dugernier T, Verbelen V, Jorens PG, Verbrugghe W, Malhotra-Kumar S, Damas P, Meex C, Leleu K, van den Abeele AM, Pimenta Gomes, de Matos AF, Fernandez Mendez S, Vergara Gomez A, Tomic V, Sifrer F, Villarreal Tello E, Ruiz Ramos J, Aragao I, Santos C, Sperning RHM, Coppadoro P, Nardi G, Brun-Buisson C, Bonten MJM. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018 doi: 10.1001/jama.2018.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164(3):382–388. doi: 10.1164/ajrccm.164.3.2005003. [DOI] [PubMed] [Google Scholar]
- 12.Verwaest C, Verhaegen J, Ferdinande P, Schetz M, Van den Berghe G, Verbist L, Lauwers P. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med. 1997;25(1):63–71. doi: 10.1097/00003246-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Wittekamp BH, Wise MP, Brun-Buisson C, Bonten MJ. Regulatory obstacles affecting ecological studies in the ICU. Lancet Infect Dis. 2014;14(10):913–915. doi: 10.1016/S1473-3099(14)70894-1. [DOI] [PubMed] [Google Scholar]
- 14.Reich NGMA. Improving efficiency in cluster-randomized study design and implementation: taking advantage of a crossover. Open Access J Clin Trials. 2014;6:11–15. [Google Scholar]
- 15.Oostdijk EA, de Wit GA, Bakker M, de Smet AM, Bonten MJ. Selective decontamination of the digestive tract and selective oropharyngeal decontamination in intensive care unit patients: a cost-effectiveness analysis. BMJ Open. 2013 doi: 10.1136/bmjopen-2012-002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hout D, Plantinga NL, Bruijning-Verhagen PC, Oostdijk EAN, de Smet A, de Wit GAd, Bonten MJM, van Werkhoven CH. Cost-effectiveness of selective digestive decontamination (SDD) versus selective oropharyngeal decontamination (SOD) in intensive care units with low levels of antimicrobial resistance: an individual patient data meta-analysis. BMJ Open. 2019;9(9):e028876. doi: 10.1136/bmjopen-2018-028876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plantinga NL, de Smet A, Oostdijk EAN, de Jonge E, Camus C, Krueger WA, Bergmans D, Reitsma JB, Bonten MJM. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clin Microbiol Infect. 2018;24(5):505–513. doi: 10.1016/j.cmi.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E, Liberati A. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Datab Syst Rev. 2009 doi: 10.1002/14651858.cd000022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(4):328–341. doi: 10.1016/s1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]
- 20.Houben AJ, Oostdijk EA, van der Voort PH, Monen JC, Bonten MJ, van der Bij AK. Selective decontamination of the oropharynx and the digestive tract, and antimicrobial resistance: a 4 year ecological study in 38 intensive care units in the Netherlands. J Antimicrob Chemother. 2014;69(3):797–804. doi: 10.1093/jac/dkt416. [DOI] [PubMed] [Google Scholar]
- 21.Buitinck S, Jansen R, Rijkenberg S, Wester JPJ, Bosman RJ, van der Meer NJM, van der Voort PHJ. The ecological effects of selective decontamination of the digestive tract (SDD) on antimicrobial resistance: a 21-year longitudinal single-centre study. Crit Care. 2019;23(1):208. doi: 10.1186/s13054-019-2480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jonge E, de Wilde RBP, Juffermans NP, Oostdijk EAN, Bernards AT, van Essen EHR, Kuijper EJ, Visser CE, Kesecioglu J, Bonten MJM. Carriage of antibiotic-resistant Gram-negative bacteria after discontinuation of selective decontamination of the digestive tract (SDD) or selective oropharyngeal decontamination (SOD) Crit Care (London, England) 2018;22(1):243. doi: 10.1186/s13054-018-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buelow E, Gonzalez TB, Versluis D, Oostdijk EA, Ogilvie LA, van Mourik MS, Oosterink E, van Passel MW, Smidt H, D’Andrea MM, de Been M, Jones BV, Willems RJ, Bonten MJ, van Schaik W. Effects of selective digestive decontamination (SDD) on the gut resistome. J Antimicrob Chemother. 2014;69(8):2215–2223. doi: 10.1093/jac/dku092. [DOI] [PubMed] [Google Scholar]
- 24.Buelow E, Bello Gonzalez TDJ, Fuentes S, de Steenhuijsen Piters WAA, Lahti L, Bayjanov JR, Majoor EAM, Braat JC, van Mourik MSM, Oostdijk EAN, Willems RJL, Bonten MJM, van Passel MWJ, Smidt H, van Schaik W. Comparative gut microbiota and resistome profiling of intensive care patients receiving selective digestive tract decontamination and healthy subjects. Microbiome. 2017;5(1):88. doi: 10.1186/s40168-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.