Abstract
The goal of this narrative review was to summarize immunogenicity data of biosimilars or biosimilar candidates for rheumatic diseases, plaque psoriasis, or inflammatory bowel disease (IBD), available in peer-reviewed publications or regulatory documents. PubMed records and regulatory documents were searched for immunogenicity data of TNFα or CD20 inhibitor biosimilars or biosimilar candidates. Data collected included the proportion of patients positive for anti-drug antibodies (ADAbs), proportion with neutralizing antibodies (nAbs) among ADAb-positive patients, ADAb/nAb assay characteristics, cross-reactivity, and the effects of ADAbs on pharmacokinetics, pharmacodynamics, efficacy, and safety. We identified eight biosimilars or biosimilar candidates for adalimumab (BI 695501, SB5, ABP 501, GP2017, PF-06410293, MSB-11022, FKB-327, ZRC-3197) four for etanercept (SB4, GP2015, CHS-0214, LBEC0101), and three each for infliximab (SB2, CT-P13, GP1111) and rituximab (CT-P10, GP2013, PF-05280586) with immunogenicity data. Randomized, head-to-head trials with reference products varied in design and methodology of ADAb/nAb detection. The lowest proportions of ADAb-positive (0–13%) and nAb-positive patients (0–3%) were observed in the trials of etanercept and its biosimilars, and the highest with adalimumab, infliximab, and their biosimilars (ADAbs: ≤ 64%; nAbs: ≤ 100%). The most common method of ADAb detection was electrochemiluminescence, and ADAb positivity was associated with nominally inferior efficacy and safety. Overall, there were no significant immunogenicity differences between biosimilars and reference products. However, there are many discrepancies in assessing and reporting clinical immunogenicity. In conclusion, immunogenicity data of biosimilars or biosimilar candidates for TNFα or CD20 inhibitors were collected in trials that varied in design and procedures for ADAb/nAb detection. In general, immunogenicity parameters of biosimilars are similar to those of their reference products.
Electronic supplementary material
The online version of this article (10.1007/s40259-019-00394-x) contains supplementary material, which is available to authorized users.
Key Points
| Immunogenicity of biosimilars currently approved for rheumatic diseases, plaque psoriasis, or inflammatory bowel diseases is similar to that of their reference products. |
| The lowest proportions of anti-drug antibodies were reported in trials of etanercept and its biosimilars, and the highest in the trials of adalimumab, infliximab, and their biosimilars. |
| There are many discrepancies in assessing and reporting clinical immunogenicity. |
Introduction
Over the past few decades, the introduction of therapeutic proteins, also known as ‘biologics’, has resulted in significantly improved and, in some cases, transformative clinical outcomes in patients with rheumatic diseases [1–3], psoriasis [4], and inflammatory bowel disease (IBD) [5]. However, use of these highly effective biologic disease-modifying agents (bDMARDs) has been limited by high costs [6, 7]. With the expiration of patent protection for many of the original biologics, we have witnessed the development of less expensive competitor products of sufficient similarity, called ‘biosimilars’. To attain regulatory approval, biosimilars are required to be ‘highly similar’ to their reference products in terms of molecular structure, pharmacokinetics, pharmacodynamics, clinical efficacy, and safety [8–10]. Registration procedures for biosimilar products, as established by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO), differ from those used for the registration of reference products, and follow a more streamlined process based on the “totality of evidence” [8]. It is generally hoped that biosimilar entry into the market place will greatly improve patient access to these biologics.
Use of a biologic agent can trigger an immune response that may result in reduced efficacy, treatment failure, or adverse effects [11]. Detailed immunogenicity evaluations are required for approval of biosimilars [8–10], and the types of assays and sensitivity of detection are described in updated regulatory guidance documents [12, 13]. For example, the FDA recommends a sensitivity of 100 ng/mL for screening and confirmatory assays for anti-drug antibodies (ADAbs), together with acid dissociation pre-treatment or other approaches to disrupt circulating ADAb-drug complexes, which are expected to improve assay drug tolerance [13]. The assay method should specifically detect the ADAbs and not the biologic agents themselves (which are often antibodies), non-specific endogenous antibodies, or antibody reagents used in the assay. For patient populations with a high incidence or prevalence of rheumatoid factor (RF), the sponsor should demonstrate that RF does not interfere with the detection method [13].
However, regardless of the methodology by which they were obtained, immunogenicity data can be challenging to interpret [14]. For example, current assays are more sensitive and assay requirements more stringent than those used initially for the reference products [15], which complicates historical comparisons. In addition, the ability to detect ADAbs can vary greatly between various assay types [16]. Further, interpretation of the clinical impact of ADAbs is more readily understood at the group level than in individuals, where considerable variability in immune responses to therapy may be observed. Finally, the effect of immunogenicity on pharmacokinetics is less frequently reported than the incidence/titer of ADAbs and kinetics of their appearance [17], which also contributes to the lack of standardization when reporting immunogenicity data [14, 18].
In light of the increasingly complex and expanding literature on the topic, we decided to summarize the immunogenicity data for biosimilars and biosimilar candidates for treatment of rheumatic diseases, plaque psoriasis, and IBD, with the focus on agents licensed by the EMA and FDA. Those regulatory agencies were selected because of their established, rigorous procedures for evaluation and approval of therapeutic proteins, and because of readily available documentation for each approved agent.
Literature Search and Data Collection
In this review, we summarized available immunogenicity data on biosimilars approved or investigated for rheumatic diseases, plaque psoriasis, or IBD by the EMA or FDA. PubMed records, clinical summaries, or assessment reports submitted to the EMA or FDA were searched for immunogenicity data from randomized, controlled trials (RCTs), open-label extensions, or post-approval observational studies of biosimilars or biosimilar candidates in patients with rheumatic diseases, psoriasis, or IBD, as well as early studies in healthy volunteers. (The exceptions are biosimilar candidates for rituximab: their B-cell-depleting effect at the therapeutic doses would preclude studies in healthy volunteers.) In addition, abstracts from the key annual American and European conferences in rheumatology (American College of Rheumatology and the European League Against Rheumatism), dermatology (American Academy of Dermatology and European Academy of Dermatology and Venereology), and gastroenterology (American College of Gastroenterology and the United European Gastroenterology Week) were searched for agents that are currently in phase III development but whose immunogenicity data have not been published in peer-reviewed publications. Initial searches conducted using the terms ‘immunogenicity AND biosimilar AND (rheum* OR psoriasis OR bowel OR IBD OR colitis OR Crohn*) AND (adalimumab OR etanercept OR infliximab OR rituximab)’ were supplemented by additional ones (e.g., ‘anti-drug antibody’, ‘switch*’, ‘assay’) and by review of similar publications, as provided by search engines. Studies involving pediatric patients were excluded. The databases were last searched in April 2019.
Wherever available, data collected included immunogenicity assay type, time of sample collection, type of antibody detected (IgG1 or IgG1 and IgG4), proportions (%) of patients positive for ADAbs, proportions (%) of patients with neutralizing antibodies (nAbs) among ADAb-positive patients, impact of immunogenicity on pharmacokinetic, pharmacodynamic, or clinical parameters, impact of ADAbs on safety, impact of switching (i.e., replacing reference product with biosimilar) on immunogenicity, and data on cross-reactivity, defined as the ability of ADAbs developed as a response to reference product to recognize the biosimilar molecule, and vice versa.
Summary of Literature Searches
Studies
Overall, we identified 52 trials of 18 biosimilars or biosimilar candidates that fit our search criteria.
There were 14 studies in healthy volunteers with immunogenicity data available in publications or regulatory documents, for five biosimilars (BI 695501 [19], SB5 [20], ABP 501 [21], FKB327 [22], and GP2017 [23]) and one biosimilar candidate (MSB11022 [24]) of adalimumab, two biosimilars (SB4 [25] and GP2015 [26]) and one biosimilar candidate (LBEC0101 [27]) of etanercept, and two biosimilars of infliximab (SB2 [28] and CT-P13 [29]). As expected (see Sect. 2), we could not identify immunogenicity data from healthy volunteer studies of rituximab biosimilars (Supplementary File, Tables 1 and 2, see electronic supplementary material [ESM]).
For rheumatic diseases, we were able to identify 25 studies with immunogenicity data for 15 biosimilars or biosimilar candidates: six with adalimumab as the reference product (biosimilars BI 695501 [30], SB5 [31], ABP 501 [32], and FKB327 [33], and biosimilar candidates PF-06410293 [34], and ZRC-3197 [35]) and three each with etanercept (biosimilar SB4 [36] and biosimilar candidates CHS-0214 [37] and LBEC0101 [38]), infliximab (biosimilars SB2 [39], CT-P13 [40–45], and infliximab-qbtx [46]), and rituximab (biosimilars CT-P10 [47–49] and GP2013 [50, 51] and a biosimilar candidate PF-05280586 [52, 53]) (Supplementary File, Tables 1 and 2, see ESM).
Immunogenicity data for patients with plaque psoriasis were available with two biosimilars of adalimumab (ABP 501 [54] and GP2017 [55]) and one biosimilar of etanercept (GP2015 [56]), with one study each (Supplementary File, Tables 1 and 2, see ESM). CT-P13 was the only biosimilar with immunogenicity data for IBD, obtained from 10 trials (Supplementary File, Tables 1 and 2, see ESM) [57–66]. Of note, the NOR-SWITCH trial included patients with IBD, rheumatic diseases, and psoriasis, but was categorized within the IBD group because those patients comprised over 50% of participants [63].
Studies in both healthy volunteers and patients varied in methodology of ADAb/nAb detection (Supplementary File, Table 1, see ESM), as well as in design and duration (Supplementary File, Table 2, see ESM). In addition, reporting of both the ADAb/nAb assay methodology and immunogenicity parameters was not standardized and the details, such as the exact numbers of patients with ADAbs or nAbs, were occasionally presented in supplementary materials or regulatory documents only.
Characteristics of Anti-drug Antibody (ADAb) and Neutralizing Antibody (nAb) Assays
Overall, publications and regulatory agency summaries were not sufficiently detailed to allow for methodical comparisons between the assays used. The most common method of detecting ADAbs was the electrochemiluminescence (ECL) assay, which was used in 86% (12/14) of trials in healthy volunteers [19–26, 28] and 55% (21/38) of trials in patients with rheumatic diseases, plaque psoriasis, or IBD (Supplementary File, Table 1, see ESM) [30–32, 34, 36, 38–42, 44–49, 52–56]. The second most commonly used detection method was the enzyme-linked immunosorbent assay (ELISA), which was employed in 7% (1/14) of trials in healthy volunteers [29] and in 21% (8/38) of patient trials [50, 51, 57, 59–61, 64, 65]. Of note, ELISA was predominantly used in open-label trials of the infliximab biosimilar CT-P13 in patients with IBD (Supplementary File, Table 1, see ESM) [57, 59–61, 64, 65]. The radioimmunoassay (RIA) was used in two (5%) patient trials [43, 66]. The remainder of the trials used either an affinity capture elution assay [healthy volunteers, 7% (1/14)] [27], an automated assay on autoDELFIA platform [patients, 5% (2/38)] [58, 62], non-specified assay methodology [patients, 11% (4/38)] [33, 35, 37], or, in the case of the NOR-SWITCH trial of the infliximab biosimilar CT-P13, assessed the presence of nAbs only, using a non-specified, in-house assay (Supplementary File, Table 1, see ESM) [33]. In general, nAbs were detected using competitive ligand-binding assays or cell-based assays, but their detection was sometimes not reported or not performed, most notably in open-label trials of CT-P13 in patients with IBD (Supplementary File, Table 1, see ESM) [58–62, 64–66].
An initial acid dissociation step, recommended by the FDA and aimed at dissociating ADAb-drug complexes [13], was mentioned in 43% (6/14) of trials in healthy volunteers [19, 24, 26, 27] and in 5% (2/38) of patient trials [36, 56]. Of note, the use of acid dissociation, although probably included in the protocols of most trials, could not be confirmed if it was not mentioned in publications or the regulatory summaries.
Regardless of the type of trial, reporting of assay sensitivity, drug trough levels, or drug tolerance was sporadic, which did not allow for meaningful comparison between studies. The only detailed descriptions of assay methodology were found in the FDA Briefing Documents for the infliximab biosimilar CT-P13 and the rituximab biosimilar CT-P10 [67, 68].
ADAb and nAb Incidence
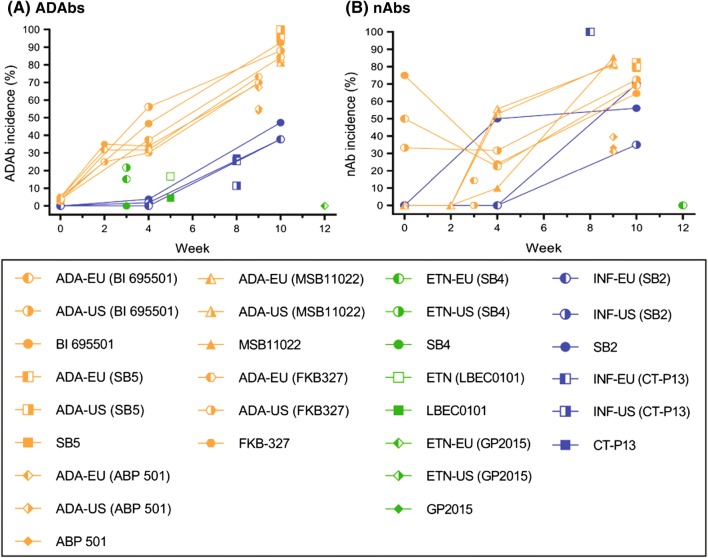
Across all trials and treatment groups, the incidence of ADAbs ranged from 0 to 100% (Supplementary File, Table 2, see ESM; Figs. 1a, 2a). A very high incidence of ADAbs (range 70–100%) was observed in phase I single-dose studies in healthy volunteers of adalimumab biosimilars BI 695501 [19], SB5 [20], ABP 501 [21], and FKB327 [22, 33] and an adalimumab biosimilar candidate MSB11022 [24] (Supplementary File, Table 2, see ESM; Fig. 1a).
Fig. 1.
Incidence of ADAbs (in treatment groups overall) and nAbs (in ADAb-positive individuals) in healthy volunteers, by study week. a, b Incidences of ADAbs and nAbs, respectively. ADAbs anti-drug antibodies, nAbs neutralizing antibodies
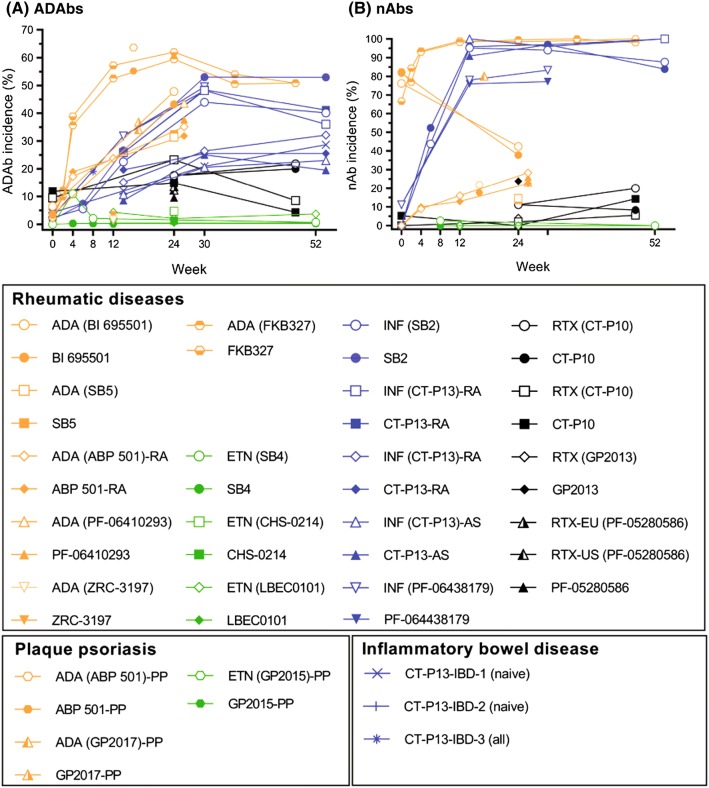
Fig. 2.
Incidence of ADAbs (in treatment groups overall) and nAbs (in ADAb-positive individuals) in patients with rheumatic diseases, plaque psoriasis, or inflammatory bowel disease, by study week* [results of switching treatments are excluded]. a, b Indicate incidences of ADAbs and nAbs, respectively. ADAbs anti-drug antibodies, nAbs neutralizing antibodies
In patients, the highest incidences of ADAbs and nAbs were observed in trials of adalimumab [23, 30–35, 54, 55, 69, 70] and infliximab [39–46, 58–66, 71–78] biosimilars (ADAbs: up to 64%; nAbs: up to 100%), and the lowest in studies of etanercept [36–38, 56, 79–81] biosimilars (ADAbs 0–13%; nAbs 0–3%) (Supplementary File, Table 2, see ESM; Fig. 2a, b).
Regardless of the trial duration or type of participants (healthy volunteers or patients), the incidence of ADAbs in individuals treated with adalimumab, infliximab, and their biosimilars appeared to exceed that of etanercept, rituximab, and their biosimilars (Figs. 1a, 2a). In addition, the incidence of ADAbs in adalimumab and infliximab trials generally increased with trial duration (reaching, in patient trials, a plateau after 12–24 weeks of treatment), a phenomenon that was not observed in trials of etanercept, rituximab, and their biosimilars (Figs. 1a, 2a). Of note, rituximab treatment regimens consisted of Course 1, which included two doses 2 weeks apart, and ADAbs assessment at week 24. In Course 2, patients in need of additional treatment received another dose, 16–24 weeks after the second one from Course 1, and ADAbs were assessed at week 48 [49, 82].
In addition, ADAb incidence in various clinical conditions (rheumatic diseases, plaque psoriasis, IBD) appears similar within biosimilars for each reference product (Fig. 2a). However, it needs to be taken into account that the trials in rheumatic diseases (n = 25) greatly outnumbered those in plaque psoriasis (n = 3) and IBD (n = 10), which, together with differences in trial design, assay type, and patient populations, makes such comparisons very limited.
Consistent with the designation of biosimilarity, the proportions of ADAb- and nAb-positive patients in individual RCTs were similar between reference products and their biosimilars (Supplementary File, Table 2, see ESM; Figs. 1, 2). Of note, in a 52-week trial of etanercept and its biosimilar SB4, the cumulative incidence of ADAbs from baseline to week 52 was significantly lower with SB4 than etanercept [1% (3/299) vs 13% (39/296), p < 0.001], the majority being transient [79]. This difference resulted mostly from samples collected at weeks 4 and 8, when 37/39 ADAbs in the etanercept group and 2/3 in the SB4 group were detected, and was not reflected in the incidence of nAbs and efficacy or safety of etanercept (Supplementary File, Table 2, see ESM). In a recent report, it was noted that there was no association between ADAbs and injection-site reactions in this trial [83].
Effect of ADAbs on Pharmacokinetics, Pharmacodynamics, Clinical Efficacy, and Safety
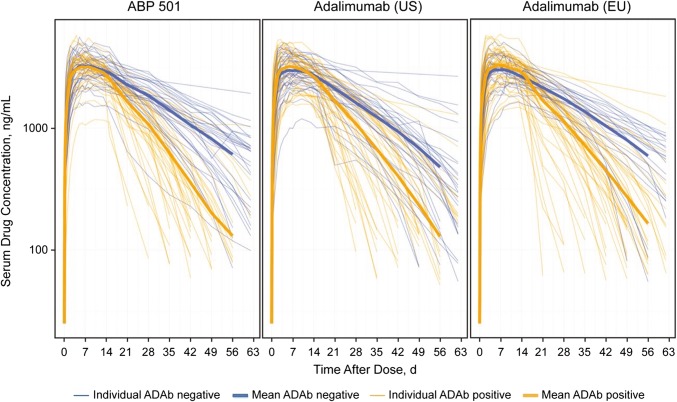
A majority of studies reported pharmacokinetics, pharmacodynamics, efficacy, or safety parameters by ADAb status (Supplementary File, Table 2, see ESM), but the information was often presented in supplementary materials or regulatory documents only. Typically, ADAb-positive individuals had lower drug concentrations and higher clearance rates compared with ADAb-negative individuals, with effects comparable between reference products and biosimilars (Supplementary File, Table 2, see ESM). A typical effect of ADAbs on pharmacokinetic parameters is illustrated in Fig. 3, using the example of the adalimumab biosimilar ABP 501 [21].
Fig. 3.
The effect of ADAbs on serum concentration of adalimumab and its biosimilar ABP 501 after a single dose in healthy volunteers. Reproduced from Kaur P et al, Ann Rheum Dis. 2017;76:526–533 [21], with permission, under the license CC BY-NC 4.0 (https://creativecommons.org/licenses/by-nc/4.0/). Lines represent individual pharmacokinetics profiles of adalimumab (US or EU) and ABP 501 in ADAb-negative (blue) and ADAb-positive patients (orange). Bold lines represent mean values. ADAbs anti-drug antibodies
Overall, there is evidence that the formation of ADAbs is associated with worsening of certain pharmacodynamic parameters such as C-reactive protein or erythrocyte sedimentation rate and diminished clinical efficacy and safety (Supplementary File, Table 2, see ESM), but the statistical significance of those differences was generally not examined in individual trials. Examination of the impact of ADAbs on pharmacokinetics, pharmacodynamics, safety, and efficacy was not reported in trials of etanercept and its biosimilars or biosimilar candidates, due to the very low incidence of ADAbs.
ADAb Cross-Reactivity
The ability of ADAbs to bind both the reference and biosimilar products has been reported in four RCTs in rheumatic diseases: one with adalimumab and its biosimilar FKB327 [33], two comparing infliximab and the biosimilar CT-P13 [40, 44], and one with rituximab and the candidate biosimilar PF-05280586 [52]. In the adalimumab-FKB327 trial (ARABESC), cross-reactivity was reported for all ADAb-positive participants [33]. In RCTs of CT-P13 in rheumatoid arthritis (RA) (PLANETRA) [44] and ankylosing spondylitis (PLANETAS) [40], 89–100% ADAbs and nAbs against infliximab were also reactive to CT-P13, indicating that the two macromolecules share similar immunodominant epitopes [84]. In addition, a single-center study in rheumatic diseases demonstrated that ADAbs against infliximab were reactive to CT-P13 in all patients assessed [85], and two studies in patients with IBD showed that ADAbs against CT-P13 were cross-reactive to infliximab [57, 86]. Both studies in IBD found an overlap in epitopes between biosimilar and reference products [57, 86]. In the RCT comparing rituximab and PF-05280586, approximately 83% of ADAb-positive samples were cross-reactive [52]. Cross-reactivity assessments available in regulatory documents only, for adalimumab’s FKB327 [22], etanercept’s GP2015 [26], and rituximab’s CT-P10 [68], suggest full cross-reactivity between ADAbs for biosimilars and reference products.
Effect of Switching on ADAb Incidence
Replacement of reference products with biosimilars in patients’ treatment regimens raises the question whether switching treatments affects immunogenicity. In the studies identified in our searches, the effect of switching was assessed with one or more cross-over steps in RCTs or their open-label extensions [30, 33, 45, 48, 54–56, 63, 69, 70, 78, 80, 81], or, subsequent to approval, in real-world observational studies [43, 58, 63–66, 73, 75, 76, 87]. Available data for the biosimilars of adalimumab (ABP 501 [54, 69], BI 695501 [30], SB5 [70], GP2017 [55], and FKB327 [33]), etanercept (SB4 [80] and GP2015 [56, 81]), infliximab (CT-P13 [43, 45, 63], SB2 [78], and unspecified [87]), and rituximab (CT-P10 [48]) indicate that switching resulted in no changes in quantitative or qualitative immunogenicity.
Discussion
Overall, the ranges of ADAb incidences in pivotal RCTs of reference products [88] are lower than those reported in recent trials comparing them to their biosimilars (Supplementary File, Table 2, see ESM), which may be a result of improvements in assay methodology (including sample handling, drug trough levels, validation techniques, sample storage, number of replicates), sensitivity (currently mandated by regulatory agencies [12, 13]), as well as patient disease status and trial design employed [89].
ECL assays used for detection of ADAbs in biosimilar RCTs are usually more sensitive, specific, and less affected by drug interference or RF presence than ELISAs and RIAs used in the trials of reference products, and should include an acid dissociation step to dissociate ADAb–drug complexes [12, 13, 90]. However, a majority of trial reports did not provide detailed descriptions of the ADAb/nAb assays employed and their specifications, including the FDA-recommended acid dissociation step [13], and it needs to be pointed out that all the trials cited here were designed and conducted before the FDA recommendation was issued, in 2019. As a result, meaningful comparisons between immunogenicity trials are challenging, if not impossible. A reliable comparison of immunogenicity effects is possible only if the same, validated test is used for both the reference product and the biosimilar within the same trial. In addition, the non-standardized reporting of immunogenicity parameters obtained in clinical trials is a recognized problem that has prompted initiatives and proposals for resolution [14, 18].
With all this in mind, comparisons of ADAb and nAb rates between different trials and different molecular entities, such as those presented in Figs. 1 and 2, are for illustrative purposes only and should be interpreted with caution, with full awareness of the aforementioned limitations. We think such comparisons are more valuable when examining the time profiles of ADAb development. For example, the observation that the incidence of ADAbs and nAbs increases with trial duration in cases of adalimumab and infliximab biosimilars (and reference products), which appears not to be the case with etanercept and rituximab (with the caveat that rituximab dosing is intermittent), should be independent of the differences in assay methodology between trials.
Cross-reactivity data were reported for only a small number of biosimilars or biosimilar candidates (CT-P13, FKB327, and PF-05280586 in publications and CT-P10 and GP2015 in regulatory documents), and they suggest that the ADAbs against reference products are cross-reactive with those generated against biosimilars. To date, epitope recognition by cross-reactive ADAbs has been compared only in a study by Goncalves et al. [86], which revealed, on a group level, a complete overlap in epitopes recognized by ADAbs against reference infliximab and CT-P13. In addition, there is no evidence that switching between reference products to biosimilars (in some cases in various sequences [33]) affects the incidence of ADAbs or the quality of the immunogenic response, and consequently does not impact pharmacokinetics, efficacy, or the incidence of adverse events.
Similar profiles in terms of ADAb/nAb incidence and the effect of ADAbs on pharmacokinetics, pharmacodynamics, and clinical parameters all imply that, for the most part, reference products and biosimilars induce a very similar immunological response. Interestingly, some reports exist indicating differences in the effects of reference products and biosimilars on the immune system, which may not be reflected in the development of ADAbs. For example, a small study conducted using an in vitro stimulation test of mononuclear cells collected from 55 patients with RA and 10 healthy participants suggests that T-helper-9 cells are involved in responses to infliximab but not the biosimilar CT-P13 [91].
Conclusion
Immunogenic responses to the approved biosimilars or biosimilar candidates have been shown to be similar to those of their reference products. These results indicate that the biosimilar development guidelines in the US and Europe have led to the approval of biosimilars with a highly similar within-class immunogenicity and have not resulted in immunogenic differences between biologic agents of the same class. Finally, our literature confirmed previous observations about the many discrepancies in assessing and reporting clinical immunogenicity [18]. We hope that, in the future, journals will take a more resolute stance and consider requesting some form of standardized reporting of immunogenicity data, along the lines of the Innovative Medicines Initiative ABIRISK (Anti-Biopharmaceutical Immunization Prediction and Clinical Relevance to Reduce the Risk) consortium [18] and similar to the standardized list of CONSORT (Consolidated Standards of Reporting Trials) requirements for randomized trials [92].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing assistance was provided by Vojislav Pejović, Ph.D., of Engage Scientific Solutions, and was funded by Pfizer.
Author contributions
The study was initially conceived by VS and LM; the initial concept was further refined and expanded by JG, JDI, TPH, and HEJ, LS, JG, JDI, and LM supervised data acquisition and analysis. All authors contributed to data interpretation, drafting, and critical revision of the drafts. All authors provided a final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data Sharing
This is a review of published studies; data sharing is not applicable.
Compliance with Ethical Standards
Funding
This review was sponsored by Pfizer.
Conflict of interest
Professor Isaacs and work in the Isaacs’ laboratory are supported in part by NIHR Newcastle Biomedical Research Centre, based at Newcastle Hospitals NHS Foundation Trust and Newcastle University and by the Research into Inflammatory Arthritis Centre Versus Arthritis. V. Strand has received consulting fees or honoraria from AbbVie, Amgen, Asana, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Celltrion, EMD Serono, Genentech/Roche, GSK, Janssen, Kezar, Kypha, Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Sanofi, and UCB. J. Gonçalves has received speaker fees from Amgen, Biogen, Celltrion, Libbs, Novartis, Pfizer, Samsung Bioepis, and Sandoz. J. D. Isaacs has received speaker or consulting fees from AbbVie, Biogen, BMS, Celltrion, Eli Lilly, Gilead, Janssen, Merck, Merck Serono, Pfizer, and Roche. T. P. Hickling is an employee of Pfizer and may own company stock. H. E. Jones and L. Marshall were employees of Pfizer during the development of the manuscript and may own company stock.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Heather E. Jones and Lisa Marshall were Pfizer employees at the time this review was conducted.
Change history
2/26/2020
The article Immunogenicity of Biosimilars for Rheumatic Diseases, Plaque Psoriasis, and Inflammatory Bowel Disease: A Review from Clinical Trials and Regulatory Documents, written by Vibeke Strand, Joao Gon��alves, Timothy P. Hickling, Heather E. Jones, Lisa Marshall and John D. Isaacs, was originally published Online First without Open Access.
References
- 1.Dörner T, Strand V, Castaneda-Hernandez G, Ferraccioli G, Isaacs JD, Kvien TK, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis. 2013;72(3):322–328. doi: 10.1136/annrheumdis-2012-202715. [DOI] [PubMed] [Google Scholar]
- 2.Dörner T, Strand V, Cornes P, Goncalves J, Gulacsi L, Kay J, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75(6):974–982. doi: 10.1136/annrheumdis-2016-209166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov. 2007;6(1):75–92. doi: 10.1038/nrd2196. [DOI] [PubMed] [Google Scholar]
- 4.Ellis AG, Flohr C, Drucker AM. Network meta-analyses of systemic treatments for psoriasis: a critical appraisal. Br J Dermatol. 2017;180(2):282–288. doi: 10.1111/bjd.17335. [DOI] [PubMed] [Google Scholar]
- 5.Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215–226. doi: 10.2147/JIR.S165330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 7.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- 9.Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues (EMEA/CHMP/BMWP/42832/2005 Rev1). 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed 10 Apr 2019.
- 10.Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Guidance for Industry. 2015. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed 10 Apr 2019.
- 11.Krieckaert C, Rispens T, Wolbink G. Immunogenicity of biological therapeutics: from assay to patient. Curr Opin Rheumatol. 2012;24(3):306–311. doi: 10.1097/BOR.0b013e3283521c4e. [DOI] [PubMed] [Google Scholar]
- 12.Guideline on Immunogenicity assessment of therapeutic proteins (EMEA/CHMP/BMWP/14327/2006 Rev 1). 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/06/WC500228861.pdf. Accessed 10 Apr 2019.
- 13.Immunogenicity Testing of Therapeutic Protein Products—Developing and Validating Assays for Anti-Drug Antibody Detection. Guidance for Industry. 2019. https://www.fda.gov/media/119788/download. Accessed 15 May 2019.
- 14.Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658–673. doi: 10.1208/s12248-014-9599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pineda C, Castaneda Hernandez G, Jacobs IA, Alvarez DF, Carini C. Assessing the immunogenicity of biopharmaceuticals. Biodrugs. 2016;30(3):195–206. doi: 10.1007/s40259-016-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorovits B, Baltrukonis DJ, Bhattacharya I, Birchler MA, Finco D, Sikkema D, et al. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin Exp Immunol. 2018;192(3):348–365. doi: 10.1111/cei.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YM, Wang J, Hon YY, Zhou L, Fang L, Ahn HY. Evaluating and reporting the immunogenicity impacts for biological products—a clinical pharmacology perspective. AAPS J. 2016;18(2):395–403. doi: 10.1208/s12248-015-9857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rup B, Pallardy M, Sikkema D, Albert T, Allez M, Broet P, et al. Standardizing terms, definitions and concepts for describing and interpreting unwanted immunogenicity of biopharmaceuticals: recommendations of the Innovative Medicines Initiative ABIRISK consortium. Clin Exp Immunol. 2015;181(3):385–400. doi: 10.1111/cei.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynne C, Altendorfer M, Sonderegger I, Gheyle L, Ellis-Pegler R, Buschke S, et al. Bioequivalence, safety and immunogenicity of BI 695501, an adalimumab biosimilar candidate, compared with the reference biologic in a randomized, double-blind, active comparator phase I clinical study (VOLTAIRE(R)-PK) in healthy subjects. Expert Opin Investig Drugs. 2016;25(12):1361–1370. doi: 10.1080/13543784.2016.1255724. [DOI] [PubMed] [Google Scholar]
- 20.Shin D, Lee Y, Kim H, Kornicke T, Fuhr R. A randomized phase I comparative pharmacokinetic study comparing SB5 with reference adalimumab in healthy volunteers. J Clin Pharm Ther. 2017;42(6):672–678. doi: 10.1111/jcpt.12583. [DOI] [PubMed] [Google Scholar]
- 21.Kaur P, Chow V, Zhang N, Moxness M, Kaliyaperumal A, Markus R. A randomised, single-blind, single-dose, three-arm, parallel-group study in healthy subjects to demonstrate pharmacokinetic equivalence of ABP 501 and adalimumab. Ann Rheum Dis. 2017;76(3):526–533. doi: 10.1136/annrheumdis-2015-208914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri A, Niewiarowski A, Arai Y, Nomura H, Baird M, Dalrymple I, et al. Pharmacokinetics, safety, tolerability and immunogenicity of FKB327, a new biosimilar medicine of adalimumab/Humira, in healthy subjects. Br J Clin Pharmacol. 2017;83(7):1405–1415. doi: 10.1111/bcp.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assessment Report: Hyrimoz (Procedure No. EMEA/H/C/004320/0000). 2018. https://www.ema.europa.eu/documents/assessment-report/hyrimoz-epar-public-assessment-report_en.pdf. Accessed 10 Apr 2019.
- 24.Hyland E, Mant T, Vlachos P, Attkins N, Ullmann M, Roy S, et al. Comparison of the pharmacokinetics, safety, and immunogenicity of MSB11022, a biosimilar of adalimumab, with Humira((R)) in healthy subjects. Br J Clin Pharmacol. 2016;82(4):983–993. doi: 10.1111/bcp.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YJ, Shin D, Kim Y, Kang J, Gauliard A, Fuhr R. A randomized phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel(R)) in healthy subjects. Br J Clin Pharmacol. 2016;82(1):64–73. doi: 10.1111/bcp.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assessment Report: Erelzi (EMA/CHMP/302222/2017). 2017. https://www.ema.europa.eu/documents/assessment-report/erelzi-epar-public-assessment-report_en.pdf. Accessed 10 Apr 2019.
- 27.Lee H, Chung H, Lee S, Lee H, Yang SM, Yoon SH, et al. LBEC0101, a proposed etanercept biosimilar: pharmacokinetics, immunogenicity, and tolerability profiles compared with a reference biologic product in healthy male subjects. Biodrugs. 2017;31(4):349–355. doi: 10.1007/s40259-017-0230-9. [DOI] [PubMed] [Google Scholar]
- 28.Shin D, Kim Y, Kim YS, Kornicke T, Fuhr R. A randomized, phase I pharmacokinetic study comparing SB2 and infliximab reference product (Remicade((R))) in healthy subjects. Biodrugs. 2015;29(6):381–388. doi: 10.1007/s40259-015-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park W, Lee SJ, Yun J, Yoo DH. Comparison of the pharmacokinetics and safety of three formulations of infliximab (CT-P13, EU-approved reference infliximab and the US-licensed reference infliximab) in healthy subjects: a randomized, double-blind, three-arm, parallel-group, single-dose, phase I study. Expert Rev Clin Immunol. 2015;11(Suppl 1):S25–S31. doi: 10.1586/1744666X.2015.1090311. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SB, Alonso-Ruiz A, Klimiuk PA, Lee EC, Peter N, Sonderegger I, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis. 2018;77(6):914–921. doi: 10.1136/annrheumdis-2017-212245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinblatt ME, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Jaworski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70(1):40–48. doi: 10.1002/art.40336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Genovese MC, Choy E, Perez-Ruiz F, Matsumoto A, Pavelka K, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76(10):1679–1687. doi: 10.1136/annrheumdis-2016-210459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assessment Report: Hulio (EMEA/H/C/004429/0000). 2018. https://www.ema.europa.eu/documents/assessment-report/hulio-epar-public-assessment-report_en.pdf. Accessed 11 Apr 2019.
- 34.Fleischmann RM, Alten R, Pileckyte M, Lobello K, Hua SY, Cronenberger C, et al. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira(R)) in the treatment of active rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):178. doi: 10.1186/s13075-018-1676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jani RH, Gupta R, Bhatia G, Rathi G, Ashok Kumar P, Sharma R, et al. A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis. 2016;19(11):1157–1168. doi: 10.1111/1756-185X.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emery P, Vencovsky J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–57. doi: 10.1136/annrheumdis-2015-207588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Dell J, Takeuchi T, Tanaka Y, Louw I, Tiabut T, Kai M, et al. OP0226 randomized, double-blind study comparing Chs-0214 with etanercept in patients with active rheumatoid arthritis (RA) despite methotrexate (MTX) therapy. Ann Rheum Dis. 2016;75(Suppl 2):143.1. [Google Scholar]
- 38.Matsuno H, Tomomitsu M, Hagino A, Shin S, Lee J, Song YW. Phase III, multicentre, double-blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann Rheum Dis. 2018;77(4):488–494. doi: 10.1136/annrheumdis-2017-212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choe JY, Prodanovic N, Niebrzydowski J, Staykov I, Dokoupilova E, Baranauskaite A, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):58–64. doi: 10.1136/annrheumdis-2015-207764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–1612. doi: 10.1136/annrheumdis-2012-203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park W, Yoo DH, Miranda P, Brzosko M, Wiland P, Gutierrez-Urena S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76(2):346–354. doi: 10.1136/annrheumdis-2015-208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi T, Yamanaka H, Tanaka Y, Sakurai T, Saito K, Ohtsubo H, et al. Evaluation of the pharmacokinetic equivalence and 54-week efficacy and safety of CT-P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2015;25(6):817–824. doi: 10.3109/14397595.2015.1022297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tweehuysen L, van den Bemt BJF, van Ingen IL, de Jong AJL, van der Laan WH, van den Hoogen FHJ, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. 2018;70(1):60–68. doi: 10.1002/art.40324. [DOI] [PubMed] [Google Scholar]
- 44.Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–1620. doi: 10.1136/annrheumdis-2012-203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, Lanzon A, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76(2):355–363. doi: 10.1136/annrheumdis-2015-208786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen SB, Alten R, Kameda H, Hala T, Radominski SC, Rehman MI, et al. A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy. Arthritis Res Ther. 2018;20(1):155. doi: 10.1186/s13075-018-1646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park W, Bozic-Majstorovic L, Milakovic D, Berrocal Kasay A, El-Khouri EC, Irazoque-Palazuelos F, et al. Comparison of biosimilar CT-P10 and innovator rituximab in patients with rheumatoid arthritis: a randomized controlled Phase 3 trial. MAbs. 2018;10(6):934–943. doi: 10.1080/19420862.2018.1487912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park W, Suh CH, Shim SC, Molina FFC, Jeka S, Medina-Rodriguez FG, et al. Efficacy and safety of switching from innovator rituximab to biosimilar CT-P10 compared with continued treatment with CT-P10: results of a 56-week open-label study in patients with rheumatoid arthritis. Biodrugs. 2017;31(4):369–377. doi: 10.1007/s40259-017-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo DH, Suh CH, Shim SC, Jeka S, Cons-Molina FF, Hrycaj P, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–570. doi: 10.1136/annrheumdis-2016-209540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smolen JS, Cohen SB, Tony HP, Scheinberg M, Kivitz A, Balanescu A, et al. A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2017;76(9):1598–1602. doi: 10.1136/annrheumdis-2017-211281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tony HP, Kruger K, Cohen SB, Schulze-Koops H, Kivitz AJ, Jeka S, et al. Brief report: safety and immunogenicity of rituximab biosimilar GP 2013 after switch from reference rituximab in patients with active rheumatoid arthritis. Arthritis Care Res (Hoboken). 2019;71(1):88–94. doi: 10.1002/acr.23771. [DOI] [PubMed] [Google Scholar]
- 52.Cohen S, Emery P, Greenwald M, Yin D, Becker JC, Melia LA, et al. A phase I pharmacokinetics trial comparing PF-05280586 (a potential biosimilar) and rituximab in patients with active rheumatoid arthritis. Br J Clin Pharmacol. 2016;82(1):129–138. doi: 10.1111/bcp.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen SB, Burgos-Vargas R, Emery P, Jin B, Cronenberger C, Vazquez-Abad MD. Extension study of PF-05280586, a potential rituximab biosimilar, versus rituximab in subjects with active rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70(11):1598–1606. doi: 10.1002/acr.23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–1102. doi: 10.1016/j.jaad.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Blauvelt A, Lacour JP, Fowler JF, Jr, Weinberg JM, Gospodinov D, Schuck E, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–631. doi: 10.1111/bjd.16890. [DOI] [PubMed] [Google Scholar]
- 56.Griffiths CEM, Thaci D, Gerdes S, Arenberger P, Pulka G, Kingo K, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938. doi: 10.1111/bjd.15152. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Horin S, Yavzori M, Benhar I, Fudim E, Picard O, Ungar B, et al. Cross-immunogenicity: antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut. 2016;65(7):1132–1138. doi: 10.1136/gutjnl-2015-309290. [DOI] [PubMed] [Google Scholar]
- 58.Buer LC, Moum BA, Cvancarova M, Warren DJ, Medhus AW, Hoivik ML. Switching from Remicade(R) to Remsima(R) is well tolerated and feasible: a prospective open-label study. J Crohns Colitis. 2017;11(3):297–304. doi: 10.1093/ecco-jcc/jjw166. [DOI] [PubMed] [Google Scholar]
- 59.Farkas K, Rutka M, Balint A, Nagy F, Bor R, Milassin A, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis—experiences from a single center. Expert Opin Biol Ther. 2015;15(9):1257–1262. doi: 10.1517/14712598.2015.1064893. [DOI] [PubMed] [Google Scholar]
- 60.Farkas K, Rutka M, Golovics PA, Vegh Z, Lovasz BD, Nyari T, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016;10(11):1273–1278. doi: 10.1093/ecco-jcc/jjw085. [DOI] [PubMed] [Google Scholar]
- 61.Gecse KB, Lovasz BD, Farkas K, Banai J, Bene L, Gasztonyi B, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, Nationwide cohort. J Crohns Colitis. 2016;10(2):133–140. doi: 10.1093/ecco-jcc/jjv220. [DOI] [PubMed] [Google Scholar]
- 62.Jahnsen J, Detlie TE, Vatn S, Ricanek P. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol. 2015;9(Suppl 1):45–52. doi: 10.1586/17474124.2015.1091308. [DOI] [PubMed] [Google Scholar]
- 63.Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316. doi: 10.1016/S0140-6736(17)30068-5. [DOI] [PubMed] [Google Scholar]
- 64.Kolar M, Duricova D, Bortlik M, Hruba V, Machkova N, Mitrova K, et al. Infliximab biosimilar (Remsima) in therapy of inflammatory bowel diseases patients: experience from one tertiary inflammatory bowel diseases centre. Dig Dis. 2017;35(1–2):91–100. doi: 10.1159/000453343. [DOI] [PubMed] [Google Scholar]
- 65.Razanskaite V, Bettey M, Downey L, Wright J, Callaghan J, Rush M, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis. 2017;11(6):690–696. doi: 10.1093/ecco-jcc/jjw216. [DOI] [PubMed] [Google Scholar]
- 66.Smits LJ, Derikx LA, de Jong DJ, Boshuizen RS, van Esch AA, Drenth JP, et al. Clinical outcomes following a switch from Remicade(R) to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohns Colitis. 2016;10(11):1287–1293. doi: 10.1093/ecco-jcc/jjw087. [DOI] [PubMed] [Google Scholar]
- 67.CT-P13 (infliximab biosimilar). Briefing Document for the Arthritis Advisory Committee. 2016. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm484860.pdf. Accessed 9 Apr 2019.
- 68.CTP-10—a proposed biosimilar to Rituxan(R)—FDA Advisory Committee Meeting Briefing Document. 2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM622647.pdf. Accessed 9 Apr 2019.
- 69.Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177(6):1562–1574. doi: 10.1111/bjd.15857. [DOI] [PubMed] [Google Scholar]
- 70.Weinblatt ME, Baranauskaite A, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase iii randomized study results. Arthritis Rheumatol. 2018;70(6):832–840. doi: 10.1002/art.40444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonczi L, Gecse KB, Vegh Z, Kurti Z, Rutka M, Farkas K, et al. Long-term efficacy, safety, and immunogenicity of biosimilar infliximab after one year in a prospective nationwide cohort. Inflamm Bowel Dis. 2017;23(11):1908–1915. doi: 10.1097/MIB.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 72.Gonczi L, Vegh Z, Golovics PA, Rutka M, Gecse KB, Bor R, et al. Prediction of short- and medium-term efficacy of biosimilar infliximab therapy do trough levels and antidrug antibody levels or clinical and biochemical markers play the more important role? J Crohns Colitis. 2017;11(6):697–705. doi: 10.1093/ecco-jcc/jjw203. [DOI] [PubMed] [Google Scholar]
- 73.Høivik ML, Buer LCT, Cvancarova M, Warren DJ, Bolstad N, Moum BA, et al. Switching from originator to biosimilar infliximab—real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53(6):692–699. doi: 10.1080/00365521.2018.1463391. [DOI] [PubMed] [Google Scholar]
- 74.Park W, Yoo DH, Jaworski J, Brzezicki J, Gnylorybov A, Kadinov V, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;20(18):25. doi: 10.1186/s13075-016-0930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smits LJT, Grelack A, Derikx L, de Jong DJ, van Esch AAJ, Boshuizen RS, et al. Long-term clinical outcomes after switching from Remicade((R)) to biosimilar CT-P13 in inflammatory bowel disease. Dig Dis Sci. 2017;62(11):3117–3122. doi: 10.1007/s10620-017-4661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smits LJT, van Esch AAJ, Derikx L, Boshuizen R, de Jong DJ, Drenth JPH, et al. Drug survival and immunogenicity after switching from Remicade to biosimilar CT-P13 in inflammatory bowel disease patients: two-year follow-up of a prospective observational cohort study. Inflamm Bowel Dis. 2019;25(1):172–179. doi: 10.1093/ibd/izy227. [DOI] [PubMed] [Google Scholar]
- 77.Smolen JS, Choe JY, Prodanovic N, Niebrzydowski J, Staykov I, Dokoupilova E, et al. Comparing biosimilar SB2 with reference infliximab after 54 weeks of a double-blind trial: clinical, structural and safety results. Rheumatology (Oxford) 2017;56(10):1771–1779. doi: 10.1093/rheumatology/kex254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smolen JS, Choe JY, Prodanovic N, Niebrzydowski J, Staykov I, Dokoupilova E, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018;77(2):234–240. doi: 10.1136/annrheumdis-2017-211741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emery P, Vencovsky J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2017;56(12):2093–2101. doi: 10.1093/rheumatology/kex269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emery P, Vencovsky J, Sylwestrzak A, Leszczynski P, Porawska W, Stasiuk B, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017;76:1986–1991. doi: 10.1136/annrheumdis-2017-211591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerdes S, Thaçi D, Griffiths CEM, Arenberger P, Poetzl J, Wuerth G, et al. Multiple switches between GP2015, an etanercept biosimilar, with originator product do not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis: 30-week results from the phase 3, confirmatory EGALITY study. J Eur Acad Dermatol Venereol. 2018;32(3):420–427. doi: 10.1111/jdv.14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoo DH, Suh CH, Shim SC, Jeka S, Molina FFC, Hrycaj P, et al. Efficacy, safety and pharmacokinetics of up to two courses of the rituximab biosimilar CT-P10 versus innovator rituximab in patients with rheumatoid arthritis: results up to week 72 of a phase i randomized controlled trial. Biodrugs. 2017;31(4):357–367. doi: 10.1007/s40259-017-0232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Girolomoni G, Feldman SR, Emery P, Ghil J, Keum JW, Cheong SY, et al. Comparison of injection-site reactions between the etanercept biosimilar SB4 and the reference etanercept in patients with rheumatoid arthritis from a phase III study. Br J Dermatol. 2018;178(3):e215–e216. doi: 10.1111/bjd.16032. [DOI] [PubMed] [Google Scholar]
- 84.Reinisch W, Jahnsen J, Schreiber S, Danese S, Panes J, Balsa A, et al. Evaluation of the cross-reactivity of antidrug antibodies to CT-P13 and infliximab reference product (Remicade): an analysis using immunoassays tagged with both agents. Biodrugs. 2017;31(3):223–237. doi: 10.1007/s40259-017-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruiz-Arguello MB, Maguregui A, Ruiz Del Agua A, Pascual-Salcedo D, Martinez-Feito A, Jurado T, et al. Antibodies to infliximab in Remicade-treated rheumatic patients show identical reactivity towards biosimilars. Ann Rheum Dis. 2016;75(9):1693–1696. doi: 10.1136/annrheumdis-2015-208684. [DOI] [PubMed] [Google Scholar]
- 86.Goncalves J, Santos M, Acurcio R, Iria I, Gouveia L, Matos Brito P, et al. Antigenic response to CT-P13 and infliximab originator in inflammatory bowel disease patients shows similar epitope recognition. Aliment Pharmacol Ther. 2018;48(5):507–522. doi: 10.1111/apt.14808. [DOI] [PubMed] [Google Scholar]
- 87.Benucci M, Gobbi FL, Bandinelli F, Damiani A, Infantino M, Grossi V, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res. 2017;65(1):419–422. doi: 10.1007/s12026-016-8843-5. [DOI] [PubMed] [Google Scholar]
- 88.Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. Biodrugs. 2017;31(4):299–316. doi: 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moots RJ, Curiale C, Petersel D, Rolland C, Jones H, Mysler E. Efficacy and safety outcomes for originator tnf inhibitors and biosimilars in rheumatoid arthritis and psoriasis trials: a systematic literature review. Biodrugs. 2018;32(3):193–199. doi: 10.1007/s40259-018-0283-4. [DOI] [PubMed] [Google Scholar]
- 90.Kim JS, Kim SH, Kwon B, Hong S. Comparison of immunogenicity test methods used in clinical studies of infliximab and its biosimilar (CT-P13) Expert Rev Clin Immunol. 2015;11(Suppl 1):S33–S41. doi: 10.1586/1744666X.2015.1090312. [DOI] [PubMed] [Google Scholar]
- 91.Talotta R, Berzi A, Doria A, Batticciotto A, Ditto MC, Atzeni F, et al. The immunogenicity of branded and biosimilar infliximab in rheumatoid arthritis according to Th9-related responses. Int J Mol Sci. 2017 doi: 10.3390/ijms18102127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulz KF, Altman DG, Moher D, Group C CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;24(8):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a review of published studies; data sharing is not applicable.