Abstract
The aim of this study was to compare the handgrip strength (HGs), assessed with a cylindrical-shape grip device, of fibromyalgia syndrome (FM) patients with healthy subjects and to demonstrate the relationship between HGs characteristics and disease severity. Consecutive female patients with FM were enrolled and compared to a group of healthy women. The correlations between HGs curve characteristics and disease severity indices were studied through the Spearman’s rho correlation coefficients (rho). The HGs threshold distinguishing the FM presence was determined using the receiver operating characteristic (ROC) curves analysis. Multivariate regression analysis was used in order to assess the contribution of covariates on the HGs. 110 patients (mean age 53.8 ± 12.4 years) and 111 healthy controls have been enrolled. Altogether all parameters related to the analysis of HGs were worse in patients with FM. The HGs cut-off distinguishing the presence of a FM was 14.2 Kg. A negative correlation was found between disease severity indices and peak force (p < 0.001). Factors significantly associated with HGs area under the curve (AUC) in multivariate analysis were the Widespread Pain Index (WPI) (p = 0.003) and the revised Fibromyalgia Impact Questionnaire (FIQR) (p = 0.016). HGs is reduced in female FM patients and is inversely related to disease severity. The force-time curves analysis may be used as a complementary tool in the FM assessment and monitoring.
Subject terms: Skeletal muscle, Fibromyalgia
Introduction
Fibromyalgia syndrome (FM) is a multi-symptom disorder, characterized mainly by chronic widespread musculoskeletal pain, chronic fatigue, sleep disturbance, symptoms of cognitive impairment, and activity limitations, the etiology of which is still to be clarified1.
The physical fitness is globally reduced over the course of FM and under this term are included multiple domains, in particular muscle strength, aerobic activity, balance and flexibility: the limitations of one or more of these aspects determine a functional limitation2,3. Adequate muscle strength is obviously necessary to perform all the usual actions of daily life4–6. In female patients with FM, a strength deficit of 20% to 36% is documented compared to healthy women7. The reduction in muscle strength in women with FM results in a deterioration in the quality of life (QoL)8.
Handgrip strength (HGs), as a substitute for overall muscle strength, is related to physical fitness9,10. HGs is traditionally measured as the maximum force that can be generated by each hand. This parameter is used to assess the degree of hand deterioration in the FM. However, peak force measurement alone does not take into account other hand strength characteristics such as force generation speed, sustainability, and grip force variability.
A more accurate description of HGs is certainly provided by the force-time (FeT) curve analysis. The FeT curve can be used to assess effort sincerity and distinguish between sincere effort and false weakness11. Through the analysis of the FeT curve it is possible to reveal peak force, mean force, total grip time, area under the curve, and variability of the curve plateau region12.
To the best of our knowledge, such kind of assessment has never been conducted in FM patients.
On the basis of these considerations, the objective of this study was to explore the FeT curve analysis of HGs, using a cylindrical-shape grip device, in patients suffering from FM compared to healty subjects.
Methods
Subjects
From July 2018 to May 2019, consecutive adult FM patients of the Rheumatological Clinic of the Università Politecnica delle Marche (Jesi, Ancona, Italy), fulfilling the 2016 American College of Rheumatology (ACR) classification criteria for FM (using the cut-off of 13 to classificate FM according the Symptom Severity Score) were enrolled in this study13,14. Only female patients were included for the purposes of this study.
All the patients underwent the rheumatologic assessment to confirm the FM diagnosis at study entry. Exclusion criteria were represented by the presence of: (a) coexisting inflammatory rheumatic diseases or connective tissue disorders, (b) orthopedic or musculoskeletal conditions that would prohibit moderate-intensity exercise (i.e. severe osteoarthritis); (c) known cardiovascular disease or uncontrolled hypertension; (d) moderate-severe chronic lung disease; (d) uncontrolled endocrine disturbances; (g) history of major depression disorder, schizophrenia or other psychosis.
Each patients underwent a comprehensive clinimetric evaluation including socio-demographic variables, disease duration, QoL items, and disease-related variables was administered to the patients.
The socio-demographic variables were: age, weight, height, marital status (single, married and divorced/separated), and level of education according to Italian school system, primary (from 6 to 10 years old); secondary (from 11 to 13 years old); high school/university (from 14 to 23 years old). FM severity was evaluated through two Italian validated patient-reported outcomes (PROs) vesions, designed for the multidimensional assessment of the disease: the revised Fibromyalgia Impact Questionnaire (FIQR) and the Fibromyalgia Assessment Status (FAS)15,16. The FIQR is a multidimensional tool widely disseminated to assess the impact of disease. It consists of 21 numerical rating scales (NRS) aimed at exploring function, overall impact, and symptoms during the last week17,18. The final score ranges from 0 to 100. The severity of the FM has been established on the basis of the FIQR score, in particular remission: FIQR ≤ 30, mild severity: 30 <FIQR ≤ 45, moderate severity: 45 <FIQR ≤ 65, and high severity: FIQR > 6519.
The FAS combines questions addressing fatigue, quality of sleep, and non-articular pain assessed by the Self-Assessment Pain Scale (SAPS)16. The final score ranges from 0 to 48, converted into a 0–10 scale.
For each participant was also registered the Patient Acceptable Symptoms State (PASS)20. PASS was recorded as “yes” or “no” answering to the anchor question: “Considering all the different ways your disease is affecting you, if you were to stay in this state for the next few months, do you consider that your current state is satisfactory?”21.
The present study was conducted in accordance with the 1964 Declaration of Helsinki principles and its later amendments or comparable ethical standards, and according to the rules of the Good Clinical Practice. The study design was approved by the the institutional review Board of the Hospital Clinic ethics committee (Comitato Etico Unico Regionale, prot. AV2 n° 22418) and informed written consent was provided by all participants.
Handgrip strength measurement
The HGs was assessed using a cylindrical-shape grip device made of five force sensors (FSR-402) manufactured by Interlink Electronics and connected to an Arduino Mega 2560. The device used in this study allowed the measurement of grip force in a time frame of 30 seconds, making one measurement per sensor every five seconds. Through these repeated measurements, it was possible to trace the FeT curves. A graphical representation of a typical FeT curve is provided in Fig. 1. In this study we used the peak grip force and the area under the curve force as the main outcomes, and time to reach maximum plateau of the curves as a secondary outcome. The HGs was measured in the dominant hand twice, and the mean of the two measurement values was used. The subjects were tested while sitting in a standardized position with elbow flexed at an angle of 90°.
Figure 1.
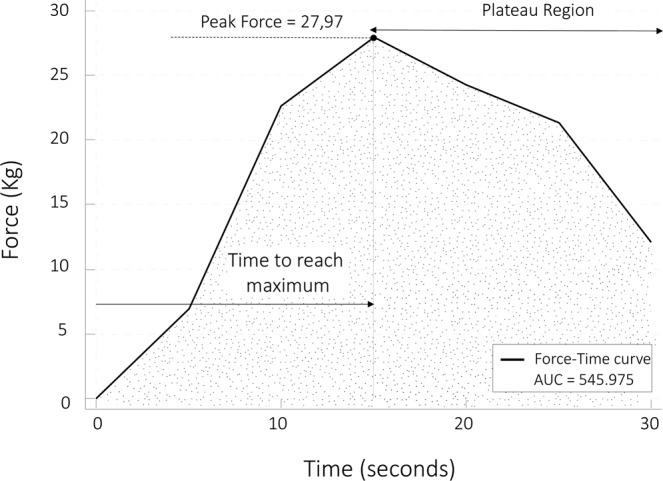
Force–time (FeT) curve showing the method of calculation of the various force attributes.
The HGs features were also determined in a control group (111 patients) consisting of voluntary healthy women, properly comparable in terms of age (55.2 ± 14.9 years) and BMI, identified among the staff of the Rheumatological Clinic and in the relatives of the patients.
Statistical analysis
The X–Y coordinates generated were transferred to a spreadsheet (Microsoft Excel 2011 for Mac, version 14.7.7) to generate digital curves, which were further processed with the MedCalc Statistical Software, version 18.0 (Ostend, Belgium), for Windows XP. Several parameters were assessed for each FeT curve. Peak force was the maximum grip strength achieved on the FeT curve, and the and variability of the time to reach maximum plateau of the curve was calculated from readings in the middle half of the force–time curve excluding the first and last quartiles, as this represents the force generation and relaxation segments. Total grip time was calculated using the X-axis of the FeT curve. The serial measurement method (first observation taken as baseline value of 0) was used in MedCalc to calculate the total area under the curve of HGs (HGs-AUC).
Median and interquartile ranges, as well as means and standard deviations (SDs) were presented where appropriate.
In order to study a possible role of obesity on the FeT curves characteristics (peak force, area under the curve, and time to reach maximum plateau), the One way Analysis of Variance (ANOVA) test with Bonferroni post-hoc analysis has been applied, distinguishing subjects by categories of BMI (BMI ≤24.9 normal, BMI ≥25 and ≤29.9 overweight, BMI ≥30 obese).
The correlations between HGs curve characteristics and questionnaires were studied through the Spearman’s rho correlation coefficients (rho). Correlations >0.90 were interpreted as very high, 0.70–0.89 as high, 0.50–0.69 as moderate, 0.26–0.49 as low, and ≤0.25 as little if any correlation22. The HGs thresholds that best discriminates between the presence or the absence of FM, as well as between severe or not severe (not severe included modetate-mild-remission states) FM were determined using the receiver operating characteristic (ROC) curve analysis, applying the PASS as external criterion.
To identify the best HGs threshold, the closest point to (0, 1) of the ROC curve was chosen.
Finally, socio-demographic variables (age, BMI, level of education) and clinimetric parameters (WPI FIQR, sleep and fatigue), were entered as possible explanatory variables in the multivariate regression model, with HGs considered as dependent variable. The level of significance was set at P less than 0.05 for all the analyses.
Results
Demographic characteristics and descriptive statistics
One hundred and ten consecutive FM female patients were included. The mean age was 53.8 years (SD = 12.4, range: 18–80), with a mean time since the onset of chronic widespread pain of 5.6 years (SD = 4.3, range: 1–22). The mean body mass index (BMI) was 26.5 ± 2.2 kg/m2. Fewer than 25% of the patients were highly educated. The overall mean for FIQR was 52.6 (SD = 22.9), and for FAS was 6.1 (SD = 2.3). Matched female healthy subjects (111 controls) were comparable in terms of age (55.2 ± 14.9 years) and the mean BMI (25.9 ± 3.4). There was no significant difference in the distribution of age, gender marital status, and educational level between the two groups. The curve characteristis (peak force, time to reach maximum plateau, and area under the curve) in FM patients and healthy controls are described in Table 1.
Table 1.
The force–time curves characteristics (peak force, time to reach maximum plateau, and area under the curve) in fibromyalgia patients and healthy controls.
| HGs characteristics | FM (110 patients) | Healthy controls (111 subjects) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | 25–75 P | Mean | Median | SD | 25–75 P | |
| Peak Force (Kg/30 sec) | 14.78 | 13.91 | 4.74 | 11.03–18.72 | 19.90 | 19.36 | 5.39 | 16.41–23.55 |
| Time to reach maximum plateau (sec) | 15.50 | 15.00 | 6.36 | 10.00–20.00 | 11.82 | 10.00 | 5.67 | 7.50–15.00 |
| Area under the curve | 363.79 | 342.76 | 115.47 | 267.66–464.96 | 501.84 | 496.55 | 137.86 | 410.12–585.26 |
Abbreviations: HGs = handgrip strength, FM = fibromyalgia, SD = standard deviation, P = percentile.
Figure 2 depicts the median values of the peak force detected during the measurement interval in FM patients and healthy controls. HGs-AUC and peak force were lower in FM patients compared with healthy women (median values 342.7 vs 496.5 and 13.9 Kg vs 19.9 Kg, both p < 0.001), and in women with severe FM compared with those with not severe FM (Kruskal-Wallis test, p < 0.0001) (Tables 2 and 3). The time to reach the maximum plateau of the curves was significantly higher in patients with FM than healthy women (15.50 sec vs 11.82 sec, p < 0.001) (Table 1).
Figure 2.
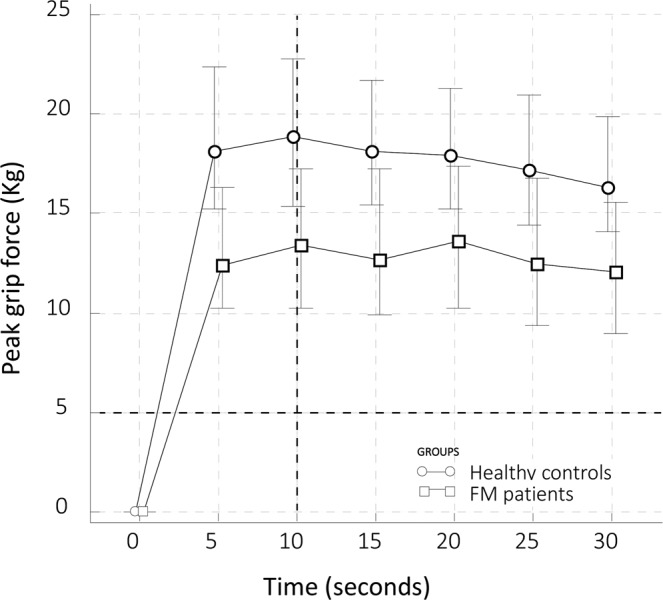
Median values (and 25–75 percentiles) of the handgrip strength peak force detected every five second during the 30 second of assessment in fibromyalgia syndrome patients and healthy controls.
Table 2.
Comparison of HGs peak force levels and HGs-AUCs for different disease severity states measured by FIQR.
| FIQR vs HGs peak force levels | n | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|
| High severity | 39 | 6.36 | 9.52 | 11.51 | 13.68 | 20.57 |
| Mild severity | 11 | 9.54 | 14.61 | 18.52 | 21.39 | 25.84 |
| Moderate severity | 33 | 5.39 | 10.27 | 13.08 | 15.21 | 21.42 |
| Remission | 27 | 10.72 | 17.18 | 18.80 | 22.05 | 23.98 |
| Test statistic | 37.88 | |||||
| Corrected for ties Ht | 37.88 | |||||
| Significance level | p < 0.0001 | |||||
| FIQR vs HGs-AUC | n | Minimum | 25th percentile | Median | 75th percentile | Maximum |
| High severity | 39 | 157.06 | 239.48 | 275.55 | 334.08 | 518.78 |
| Mild severity | 11 | 233.73 | 371.17 | 442.12 | 503.91 | 642.91 |
| Moderate severity | 33 | 134.58 | 248.36 | 324.96 | 384.77 | 537.55 |
| Remission | 27 | 258.70 | 422.57 | 468.11 | 525.58 | 594.30 |
| Test statistic | 37.62 | |||||
| Corrected for ties Ht | 37.62 | |||||
| Significance level | p < 0.0001 | |||||
Abbreviations: HGs = handgrip strength, AUC = area under the curve, FIQR = revised Fibromyalgia Impact Questionnaire.
Table 3.
Comparison of HGs peak force levels and HGs-AUCs for different disease severity states measured by FAS.
| FAS vs HGs peak force levels | n | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|
| High severity | 52 | 5.39 | 9.51 | 11.97 | 13.95 | 21.53 |
| Mild severity | 24 | 8.21 | 13.62 | 16.85 | 18.96 | 22.14 |
| Moderate severity | 18 | 6.36 | 12.17 | 14.04 | 18.34 | 21.52 |
| Remission | 16 | 11.28 | 17.98 | 20.65 | 23.61 | 25.84 |
| Test statistic | 33.36 | |||||
| Corrected for ties Ht | 33.36 | |||||
| Significance level | p < 0.0001 | |||||
| FAS vs HGs-AUC | n | Minimum | 25th percentile | Median | 75th percentile | Maximum |
| High severity | 52 | 134.58 | 234.56 | 285.26 | 342.76 | 546.12 |
| Mild severity | 24 | 210.42 | 350.31 | 426.91 | 472.55 | 511.22 |
| Moderate severity | 18 | 157.06 | 291.95 | 351.80 | 464.88 | 509.65 |
| Remission | 16 | 284.90 | 441.25 | 511.34 | 570.12 | 642.91 |
| Test statistic | 34.54 | |||||
| Corrected for ties Ht | 34.54 | |||||
| Significance level | p < 0.0001 | |||||
Abbreviations: HGs=handgrip strength, AUC = area under the curve, FAS = Fibromyalgia Assessment Status.
ROC curve analysis revealed that the HGs peak force threshold that best discriminated between the presence or the absence of FM was 14.2 Kg (AUC 0.801; 95% confidence interval [CI] 0.723–0.844, p < 0.001), whereas the HGs peak force threshold that best discriminate the PASS was 16.3 kg (AUC 0.834; 95% CI. 0.751–0.898; p < 0.001).
As regards a potential role of obesity in FeT curves characteristics, investigated by distinguishing patients in three categories of BMI (Table 4), the ANOVA test did not allow to document significant differences of FeT curve characteristics between the different categories (Table 5).
Table 4.
Comparison of force-time curves characteristics (peak force, area under the curve, and time to reach maximum plateau) in relation to BMI categories (BMI ≤24.9 normal, BMI ≥25 and ≤29.9 overweight, BMI ≥30 obese).
| BMI (Kg/m2) | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||
| HGs Peak Force (Kg/30 sec) | Normal | 23 | 15.33 | 5.76 | 1.22 | 12.77 | 17.890 | 5.40 | 23.98 |
| Overweight | 77 | 14.72 | 4.41 | 0.50 | 13.72 | 15.728 | 6.56 | 25.84 | |
| Obese | 10 | 14.20 | 5.37 | 1.70 | 10.36 | 18.051 | 6.36 | 21.10 | |
| Total | 110 | 14.78 | 4.74 | 0.45 | 13.89 | 15.684 | 5.40 | 25.84 | |
| HGs-AUC | Normal | 23 | 378.87 | 1.41 | 30.16 | 316.14 | 441.601 | 134.58 | 594.30 |
| Overweight | 77 | 360.87 | 1.06 | 12.12 | 336.73 | 385.010 | 163.36 | 642.91 | |
| Obese | 10 | 357.36 | 1.35 | 42.87 | 260.37 | 454.349 | 157.06 | 530.90 | |
| Total | 110 | 363.79 | 1.15 | 11.00 | 341.96 | 385.612 | 134.58 | 642.91 | |
| Time to reach the maximum plateau (sec) | Normal | 23 | 14.32 | 5.83 | 1.24 | 11.73 | 16.90 | 5 | 25 |
| Overweight | 77 | 16.23 | 7.35 | 0.83 | 14.57 | 17.90 | 5 | 30 | |
| Obese | 10 | 18.50 | 7.09 | 2.24 | 13.43 | 23.57 | 5 | 30 | |
| Total | 110 | 16.09 | 7.05 | 0.67 | 14.76 | 17.42 | 5 | 30 | |
Abbreviations: HGs = handgrip strength; AUC = area under the curve; BMI = body mass index.
Table 5.
One way Analysis of Variance (ANOVA) with Bonferroni post-hoc analysis of the role of BMI categories (BMI ≤24.9 normal, BMI ≥25 and ≤29.9 overweight, BMI ≥30 obese) in force-time curves characteristics (peak force, area under the curve, and time to reach maximum plateau).
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| HGs Peak Force (Kg/30 sec) | Between Groups | 12.22 | 3 | 4.07 | 0.177 | 0.912 |
| Within Groups | 2438.83 | 106 | 23.01 | |||
| Total | 2451.06 | 109 | ||||
| HGs-AUC | Between Groups | 7885.91 | 3 | 2628.64 | 0.193 | 0.901 |
| Within Groups | 1.44 | 106 | 13636.93 | |||
| Total | 1.45 | 109 | ||||
| Time to reach the maximum plateau (sec) | Between Groups | 144.02 | 3 | 48.01 | 0.965 | 0.412 |
| Within Groups | 5275.06 | 106 | 49.76 | |||
| Total | 5419.09 | 109 |
Abbreviations: BMI = body mass index; HGs = handgrip strength; AUC = area under the curve.
In FM patients, a negative correlation was found between FIQR, FAS, widespread pain index (WPI) scores and peak force (all at p < 0.0001) and, furthermore, a correlation was observed between WPI and the time to reach maximum plateau of the curves (p = 0.041) (Table 6). No correlation was found between HGs and BMI in FM patients.
Table 6.
Correlations between force-time curve characteristics and questionnaires studied through the Spearman’s rho correlation coefficients (rho).
| FIQR | FAS | HGs peak force levels | Time to reach maximum plateau of the curves | HGs-AUC | |
|---|---|---|---|---|---|
| WPI | 0.732 < 0.0001 | 0.823 < 0.0001 | −0.612 < 0.0001 | −0.195 0.0415 | −0.615 < 0.0001 |
| FIQR | 0.761 < 0.0001 | −0.576 < 0.0001 | −0.054 0.5768 | −0.592 < 0.0001 | |
| FAS | −0.577 < 0.0001 | −0.167 0.0813 | −0.588 < 0.0001 | ||
| HGs peak force levels | −0.151 0.0249 | 0.991 < 0.0001 | |||
| Time to reach maximum plateau of the curves | −0.135 0.0456 |
Abbreviations: HGs = handgrip strength, FIQR = revised Fibromyalgia Impact Questionnaire, FAS = Fibromyalgia Assessment Status, AUC = area under the curve, WPI = Widespread Pain Index.
Factors significantly associated with HGs-AUC in multivariate analysis were WPI (p = 0.003) and FIQR (p = 0.016) (Table 7).
Table 7.
Multivariate logistic regression analysis of the variables contributing to HGs-AUC.
| Independent variables | β coefficient | standard error | t | p | rpartial | rsemipartial |
|---|---|---|---|---|---|---|
| (Constant) | 23.9781 | |||||
| Age (years) | −0.03262 | 0.04084 | −0.799 | 0.426 | −0.08583 | 0.05902 |
| BMI (Kg/m2) | −0.03895 | 0.09174 | −0.425 | 0.672 | −0.04573 | 0.03137 |
| Level of education,yrs | −0.1127 | 0.1300 | −0.867 | 0.388 | −0.09305 | 0.06404 |
| Disease duration,yrs | −0.02357 | 0.07027 | −0.335 | 0.738 | −0.03615 | 0.02479 |
| WPI (0–19) | −0.6788 | 0.1850 | −3.670 | 0.003 | −0.3680 | 0.2712 |
| FIQR (0–100) | −0.7892 | 0.2492 | −3.167 | 0.016 | −0.3231 | 0.2340 |
| Sleep (NRS 0–10) | 0.1213 | 0.2294 | 0.529 | 0.598 | 0.05694 | 0.03907 |
| Fatigue (NRS 0–10) | 0.2174 | 0.1463 | 1.486 | 0.141 | 0.1582 | 0.1098 |
| R2 = 0.5183 | ||||||
| R2-adjusted = 0.4773 | ||||||
Abbreviations: HGs = handgrip strength, AUC = area under the curve, BMI = body mass index, WPI = Widespread Pain Index, FIQR = revised Fibromyalgia Impact Questionnaire, NRS = numerical rating scale.
Discussion
In this study we explored the characteristics of a new method of measuring HGs in FM patients based on FeT curve analysis, using a new device. From the studies available in the literature, there are several dynamometers available for measuring HGs, each with strengths and weaknesses. Among those most studied certainly can be recorded Jamar® and Takei®. The former has been used since the 50 s of the twentieth century23, and today is the one with the most abundant evidence24,25. Among the other instruments, the Takei® digital dynamometer has found a good diffusion in recent years26. Takei® is equipped with a digital display that measures the maximum force exerted by the flexor with a decimal figure and an adjustable handle that can be adapted to different amplitude measurements of the hand (from 3.5 to 7.0 cm). The main advantages of dynamometers such as Jamar® and Takei® are the reduced cost and practicality of use, while an important limit is represented by the possibility of evaluating only the isometric force and can cause stress on weak joints developing slow leaks and hysteresis. Compared to the anatomically shaped Jamar® handle, the Takei® handle has a straight profile that does not respect the natural posture of the fingers of the hand. The force evaluated by these two dynamometers is essentially unidirectional since the handle only allows a movement from the fingers to the proximal palm and thumb. This type of force measurement may not adequately represent the activities that the hand must perform to handle a cylindrical object. A work by McDowell and colleagues revealed how much more force is required to apply to a cylindrical object, dynamometers such as Jamar® and Takei® in particular incorrectly measure the force applied by the distal portion of the fingers27.
Taking all these considerations into account and to overcome the limitations of the currently available dynamomethers, we decided to develop a new cylindrical shape device, which would not only measure the peak force data as a result of the simple isometric muscle test, but a continuous acquisition of data each five second which lead to a more accurate information of the HGs of patients. The use of the FeT curve analysis improves the assessment of HGs, being able to evaluate magnitude and duration12. A sustained grip, as measured by HGs-AUC, is considered to reflect more closely the performance required in the execution of daily life activities compared to the peak grip force28. As far as we know, ours is the first study to use this methodology in the evaluation of HGs in FM patients.
Reduced physical performance, as measured by HGs, is not a new finding in FM5,6,8,29,30, and a research has also established physical fitness cut-offs for severity of illness31. Our results showed that HGs is reduced by about 30% in women with FM versus healthy controls. This reduction correlates with WPI and disease severity. Both HGs-AUC and peak force levels were associated with WPI, FIQR, and FAS scales, which are the instruments designated to assess pain, fatigue, activity limitations and mood.
The time to reach maximum plateau of the curves was predominantly related to the WPI. Low fitness and reduced exercise capacity have already been described in the context of FM. Compared to healthy women, muscle strength in women with FM is reduced in terms of 20–36%, while walking ability appears to be 16% lower7. Reduced endurance and easier fatigability have also been described, even for large muscle masses such as the quadriceps, but also for the abdominal and lumbar muscles32–34. Patients with FM have a lower maximum voluntary muscle strength, both isokinetic and isometric35. The causes of the altered muscle performance are to be found in the deconditioning that could result from fatigue, muscle damage, but also psychological variables4. In FM, reduced muscle strength was also related to pain36.
In healthy subjects, HGs is correlated to knee extension strength and can therefore be a surrogate for the strength of other muscle groups37. From this point of view, a relative simple measurement of the HGs can reflect a general assessment of muscle fitness in FM patients, therefore HGs can be a supportive tool to the clinical evaluation in establishing a treatment strategy. The HGs measurement can be particularly useful in the multimorbidity framework to detect patients with poor outcomes, such as the risk of hospitalization, which can benefit from close monitoring38–40.
The association between FM and reduced HGs has been demonstrated in different contexts38,41,42. Discordant data also exist, in which no associations between FM severity and reduced muscle performance, expressed in terms of isokinetic flexion-extension of the knee, has been demonstrated43.
Although somewhat controversial, the pathophysiological causes of the reduction in muscle strength should be sought in alterations of the muscle fibres themselves44, or in vasomotor dysregulation45, or in problems of energy metabolism and the regulation of growth factors46. Electrophysiological studies have also been conducted revealing the presence of functional alterations of the muscle membrane responsible for fatigue47.
Potential reasons also involve neuromuscular alterations caused by pain and decreased physical activity. Female FM patients usually dedicate more time to sedentary tasks rather than to activities requiring high muscle engagement. Pain and poor fitness cause physical inactivity which in turn reduces muscle conditioning43.
Our data did not reveal an HGs deficiency linked to the possible presence of overweight or obesity. This is consistent with what Carbonell-Baeza et al. have documented in a work in which they demonstrated an association of BMI with static balance, back scratch, and 6-minute walking test, but not with HGs34.
The present study has several limitations to mention. First of all, the cross-sectional assessment did not allow the evaluation of causal inferences (i.e., patients with higher disease severity may complain more pain and fear of movement, resulting in reduced strength). In addition and as a consequence, the cross-sectional evaluation did not allow prognostic evaluations (in terms of possible therapeutic interventions) of our new HGs measurement method. Finally, as men have not been studied, these findings are only applicable to the female population.
The device is currently not available on a large scale. However, considering the low production costs, we believe it will be widely available in the coming years.
In conclusion, confirming that HGs is reduced in patients with FM and that this correlates with the disease severity, this study proposes a new method (using a cylindrical-shape grip device) of evaluating HGs based on force-time curve analysis, trying to overcome some limitations of the dynamometers available today.
Author contributions
F.S. and M.D.C. gave substantial contribution to study conception and design, while S.F. gave substantial contribution to acquisition of data. All authors analyzed and interpreted data, drafted the article and revised it critically for important intellectual content, approved the final version of the article to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oudejans L, et al. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci. Rep. 2016;6:23573. doi: 10.1038/srep23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salaffi F, et al. Identifying the symptom and functional domains in patients with fibromyalgia: results of a cross-sectional Internet-based survey in Italy. J. Pain. Res. 2016;9:279–286. doi: 10.2147/JPR.S100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henriksson KG, Backman E, Henriksson C, de Laval JH. Chronic regional muscular pain in women with precise manipulation work. A study of pain characteristics, muscle function, and impact on daily activities. Scand. J. Rheumatol. 1996;25:213–223. doi: 10.3109/03009749609069990. [DOI] [PubMed] [Google Scholar]
- 4.Okumus M, Gokoglu F, Kocaoglu S, Ceceli E, Yorgancioglu ZR. Muscle performance in patients with fibromyalgia. Singap. Med. J. 2006;47:752–756. [PubMed] [Google Scholar]
- 5.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Jt. Bone Spine. 2002;69:293–299. doi: 10.1016/S1297-319X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso FS, Curtolo M, Natour J, Lombardi Júnior I. Assessment of quality of life, muscle strength and functional capacity in women with fibromyalgia. Rev. Bras. Reumatol. 2011;51:344–350. doi: 10.1590/S0482-50042011000400006. [DOI] [PubMed] [Google Scholar]
- 7.Larsson A, et al. Controlled, cross-sectional, multi-center study of physical capacity and associated factors in women with fibromyalgia. BMC Musculoskelet. Disord. 2018;19:121. doi: 10.1186/s12891-018-2047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aparicio VA, et al. Handgrip strength test as a complementary tool in the assessment of fibromyalgia severity in women. Arch. Phys. Med. Rehabil. 2011;92:83–98. doi: 10.1016/j.apmr.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AJM, Simmons RK, Kuh D, Brage S, Cooper R. NSHD scientific and data collection team. Physical activity, sedentary time and physical capability in early old age: British birth cohort study. PLoS One. 2015;10:e0126465. doi: 10.1371/journal.pone.0126465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bann D, et al. Light Intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PLoS One. 2015;10:e0116058. doi: 10.1371/journal.pone.0116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GA, Nelson RC, Sadoff SJ, Sadoff AM. Assessing sincerity of effort in maximal grip strength tests. Am. J. Phys. Med. Rehabil. 1989;68:73–80. doi: 10.1097/00002060-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dias JJ, Singh HP, Taub N, Thompson J. Grip strength characteristics using force-time curves in rheumatoid hands. J. Hand Surg. Eur. Vol. 2013;38:170–177. doi: 10.1177/1753193412439169. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe F, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 15.Salaffi F, et al. Psychometric characteristics of the Italian version of the revised Fibromyalgia Impact Questionnaire using classical test theory and Rasch analysis. Clin. Exp. Rheumatol. 2013;31:S41–S49. [PubMed] [Google Scholar]
- 16.Salaffi F, et al. Development and validation of the self-administered Fibromyalgia Assessment Status: a disease-specific composite measure for evaluating treatment effect. Arthritis Res. Ther. 2009;11:R125. doi: 10.1186/ar2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J. Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 18.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005;23:S154–S162. [PubMed] [Google Scholar]
- 19.Salaffi F, Di Carlo M, Arcà S, Galeazzi M. Categorisation of disease severity states in fibromyalgia: a first step to support decision-making in health care policy. Clin. Exp. Rheumatol. 2018;36:1074–1081. [PubMed] [Google Scholar]
- 20.Maksymowych WP, Richardson R, Mallon C, van der Heijde D, Boonen A. Evaluation and validation of the patient acceptable symptom state (PASS) in patients with ankylosing spondylitis. Arthritis Rheum. 2007;57:133–139. doi: 10.1002/art.22469. [DOI] [PubMed] [Google Scholar]
- 21.Salaffi F, Carotti M, Gutierrez M, Di Carlo M, De Angelis R. Patient Acceptable Symptom State in Self-Report Questionnaires and Composite Clinical Disease Index for Assessing Rheumatoid Arthritis Activity: Identification of Cut-Off Points for Routine Care. Biomed. Res. Int. 2015;2015:930756. doi: 10.1155/2015/930756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunnally, J. C. Psychometric Theory. 2nd ed. McGraw Hill: New York (1978).
- 23.Becthol, C. O. Grip test; the use o a dynamometer with adjustable handle spacings. J Bone Joint Surg Am. 36-A, 820–824 passim (1954). [PubMed]
- 24.Massy-Westropp N, Rankin W, Ahern M, Krishnan J, Hearn TC. Measuring grip strength in normal adults: reference ranges and a comparison of electronic and hydraulic instruments. J. Hand Surg. Am. 2004;29:514–519. doi: 10.1016/j.jhsa.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Bear-Lehman J, Desrosiers J, Massy-Westropp N, Mathiowetz V. Average grip strength: a meta-analysis of data obtained with a Jamar dynamometer from individuals 75 years or more of age. J. Geriatr. Phys. Ther. 2007;30:28–30. doi: 10.1519/00139143-200704000-00006. [DOI] [PubMed] [Google Scholar]
- 26.España-Romero V, et al. Elbow position affects handgrip strength in adolescents: validity and reliability of Jamar, DynEx, and TKK dynamometers. J. Strength. Cond. Res. 2010;24:272–277. doi: 10.1519/JSC.0b013e3181b296a5. [DOI] [PubMed] [Google Scholar]
- 27.McDowell TW, Wimer BM, Welcome DE, Warren C, Dong RG. Effects of handle size and shape on measured grip strength. Int. J. Ind. Ergon. 2012;42:199–205. doi: 10.1016/j.ergon.2012.01.004. [DOI] [Google Scholar]
- 28.Pham B, et al. Validity of area-under-the-curve analysis to summarize effect in rheumatoid arthritis clinical trials. J. Rheumatol. 1999;26:712–716. [PubMed] [Google Scholar]
- 29.Aparicio VA, et al. Fitness testing in the fibromyalgia diagnosis: the al-Andalus project. Med. Sci. Sports Exerc. 2015;47:451–459. doi: 10.1249/MSS.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 30.Aparicio VA, et al. Fitness testing as a discriminative tool for the diagnosis and monitoring of fibromyalgia. Scand. J. Med. Sci. Sports. 2013;23:415–423. doi: 10.1111/j.1600-0838.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 31.Castro-Pinero J, et al. The Potential of Established Fitness Cut-off Points for Monitoring Women with Fibromyalgia: The al-Andalus Project. Int. J. Sports Med. 2017;38:359–369. doi: 10.1055/s-0043-101912. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen S, Holm B. Muscle strength and endurance compared to aerobic capacity in primary fibromyalgia syndrome. Clin. Exp. Rheumatol. 1992;10:419–420. [PubMed] [Google Scholar]
- 33.Bachasson D, et al. Neuromuscular fatigue and exercise capacity in fibromyalgia syndrome. Arthritis Care Res. 2013;65:432–440. doi: 10.1002/acr.21845. [DOI] [PubMed] [Google Scholar]
- 34.Carbonell-Baeza A, Aparicio VA, Sjostrom M, Ruiz JR, Delgado-Fernandez M. Pain and functional capacity in female fibromyalgia patients. Pain. Med. 2011;12:1667–1675. doi: 10.1111/j.1526-4637.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen S, Wildschiodtz G, Danneskiold-Samsoe B. Isokinetic and isometric strength combined with transcutaneous electrical muscle stimulation in primary fibromyalgia syndrome. J. Rheumatol. 1991;18:1390–1393. [PubMed] [Google Scholar]
- 36.Soriano-Maldonado A, et al. Association of Physical Fitness with pain in women with fibromyalgia: the al-Ándalus project. Arthritis Care Res. 2015;67:1561–1570. doi: 10.1002/acr.22610. [DOI] [PubMed] [Google Scholar]
- 37.Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46:555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahin G, Ulubas B, Calikoglu M, Erdogan C. Handgrip strength, pulmonary function tests, and pulmonary muscle strength in fibromyalgia syndrome: is there any relationship? South. Med. J. 2004;97:25–29. doi: 10.1097/01.SMJ.0000101146.95697.18. [DOI] [PubMed] [Google Scholar]
- 39.Ikegami S, et al. Physical performance reflects cognitive function, fall risk, and quality of life in community-dwelling older people. Sci. Rep. 2019;22:12242. doi: 10.1038/s41598-019-48793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur. J. Clin. Nutr. 2016;70:595–601. doi: 10.1038/ejcn.2015.207. [DOI] [PubMed] [Google Scholar]
- 41.Köklü K, Sarigül M, Özişler Z, Şirzai H, Özel S. Handgrip Strength in Fibromyalgia. Arch. Rheumatol. 2015;31:158–161. doi: 10.5606/ArchRheumatol.2016.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannerkorpi K, Svantesson U, Broberg C. Relationships between performance- based tests and patients’ ratings of activity limitations, self-efficacy, and pain in fibromyalgia. Arch. Phys. Med. Rehabil. 2006;87:259–264. doi: 10.1016/j.apmr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen M, et al. Relationships between the fibromyalgia impact questionnaire, tender point count, and muscle strength in female patients with fibromyalgia: a cohort study. Arthritis Rheum. 2009;61:732–739. doi: 10.1002/art.24512. [DOI] [PubMed] [Google Scholar]
- 44.Srikuea R, et al. Association of fibromyalgia with altered skeletal muscle characteristics which may contribute to postexertional fatigue in postmenopausal women. Arthritis Rheum. 2013;65:519–528. doi: 10.1002/art.37763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med. Hypotheses. 2007;69:517–525. doi: 10.1016/j.mehy.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 46.Bagis S, et al. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol. Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 47.Kaver-Król EG, Rasker JJ, Henriquez NR, Verheijen WG, Zwarts MJ. Muscle fiber velocity and electromyographic signs of fatigue in fibromyalgia. Muscle Nerve. 2012;46:738–745. doi: 10.1002/mus.23382. [DOI] [PubMed] [Google Scholar]


