Abstract
Climatic factors can influence the establishment and growth of wood species, but little is known about the effect of these factors on monodominant communities in wetlands. Therefore, we asked how climatic factors, such as ENSO (El Niño-Southern Oscillation), precipitation and flooding, influence growth and establishment of the dominant species Erythrina fusca in the Pantanal. We determined the age of sampled individuals, the age of the population and evaluated the effects of climate on tree growth. We obtained samples for dendrochronological analyses using destructive (seven individuals) and non-destructive methods. We cross-dated and built a chronology, correlating results with climatic factors. We sampled 0.6 ha of the population and separated individuals into diameter classes to determine age based on diameter/age ratio obtained through dendrochronological analyses. We obtained a chronology with individuals up to 34 years old, while in the population sample, the oldest individual was 54 years old. The factors that influenced growth during the study period were precipitation (positive correlation) and El Niño (negative correlation). E. fusca individuals seem to grow more during the period of highest precipitation, and El Niño events reduce precipitation in the Pantanal, resulting in a decrease in the growth of E. fusca individuals. We detected a decrease of young individuals in the last nine years, which seems to be related to the decrease in minimum flood levels. This indicates a future decline in the number of individuals. These results allow us to propose measures to protect these monodominant formations, which mainly involve avoiding further anthropic activities, that could reduce flooding levels.
Subject terms: Climate-change ecology, Population dynamics, Wetlands ecology, Plant ecology, Climate-change ecology
Introduction
In tropical areas, some tree species can occur as monodominant stands in which more than half the total number of arboreal individuals belong to only one species1,2. Many features and events can influence monodominance, the most common being flood, fire, succession, fungal conditions, and edaphic factors3–6. These factors act as selective pressures that exclude less tolerant species, allowing more tolerant species to dominate the environment3,4.
Knowledge about the factors that affect the dominance of a species can be gained through an understanding of its dynamics and structure. The study of tree-rings is one way to interpret both the dynamics of vegetation and estimate the growth rates of arboreal species7. The data provide clues to further understand how climate change can affect vegetation in the future, thereby arming researchers with the knowledge needed to formulate strategies to conserve these formations8. In tropical regions, the monodominance of a species is often associated with seasonal flooding3,9. Some studies show that flood pulse and precipitation directly influence the growth and establishment of species10–12. When we associate climatic variables with wood increment patterns in some species, we can begin to understand how climate and natural events affect tree growth13,14 and, perhaps, the establishment and growth of monodominant stands. One such natural event is El Niño (El Niño Southern Oscillation - ENSO), which, in the Pantanal, causes a significant reduction in precipitation, thus affecting the growth of different species, such as Vochysia divergens Pohl15,16.
Environmental factors can also influence the establishment and development of individuals of different tree species, e.g. the spatial arrangement and the interactions between their parent plants and the past environmental characteristics, all affect the spatial structure and distribution of populations17. Therefore, the age structure of a current population reflects the conditions of recruitment and the mortality rates to which the populations were subjected17. In this context, the Pantanal is a favorable environment to study the occurrence and factors that influence species dominance, in particular because many monodominant species are found in its floodplains, such as Tabebuia aurea (Silva Manso) Benth. ex S. Morre (popularly known as “paratudal”), Byrsonima cydoniifolia A. Juss. (“canjiqueiral”), and Copernicia alba Morong ex Morong and Britton (“carandazal”), or Erythrina fusca Lour. (“abobral”), among others18.
The monodominant stands of E. fusca which occur in the Pantanal in the subregion of Cáceres, are an important resource in this subregion. E. fusca is a pioneer species, heliophyte and tolerant to flooding19,20. Its flowers are fleshy, showy, and frequently visited by birds and pollinators. The dry legume fruit produces numerous seeds, which, during dehiscence, fall into the river and are normally ingested by fish21,22. Furthermore, the roots present a particular arrangement that provides shelter for a variety of species, especially reptiles and mammals. Therefore, studies that report on the establishment and growth of a monodominant species in relation to environmental features are important for maintenance and conservation of these stands.
Usually, growth is negatively affected by inundation; therefore, the growth of woody species occurs principally during the dry season, when flooding and precipitation levels are lower, and stagnates during the wet season, when the flooding and precipitation levels are higher12,23,24. Our hypothesis is that the establishment and growth of individuals of E. fusca in the monodominant stands in the Pantanal subregion of Cáceres is negatively influenced by higher flooding and higher precipitation levels and positively influenced by the occurrence of El Niño since this event can cause significant precipitation reduction. Therefore, in this study, we aimed to build a chronology of E. fusca growth from monodominant stands in the Pantanal sub-region of Cáceres, verify how the structure of the monodominant population is related to variations in inundation and climate, and evaluate effects of climate (local and global) and inundation on its growth. We intended to obtain data to support the conservation of these monodominant stands, considering their ecological importance in the study region.
Methods
Study area
The Pantanal is a vast floodplain crossed by the Paraguay River and its tributaries, extending throughout Brazil, Paraguay, and Bolivia, with an area of approximately 140,000 km2 25. This large wetland has a predictable and monomodal flood pulse, with low amplitude and long duration26, and the structure and dynamics of the Pantanal are dependent on the fluctuating levels of flooding10,27.
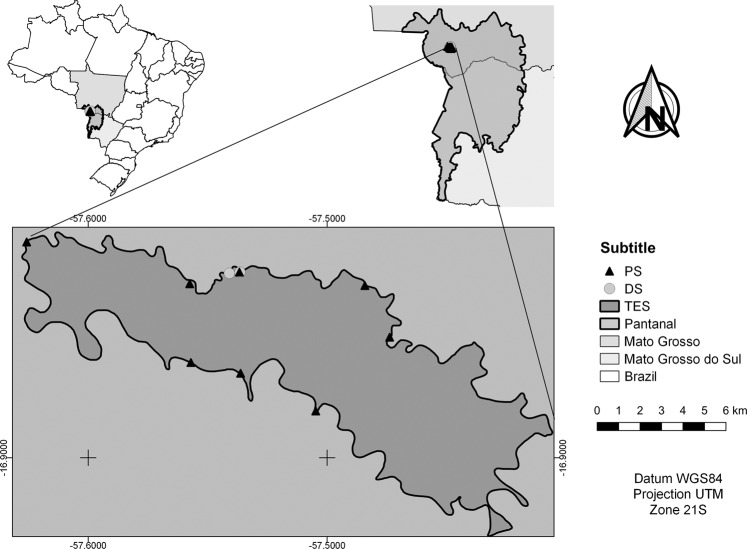
We conducted the study at Taiamã Ecological Station (TES) (Fig. 1) and the nearby areas of Sararé Island and the Jubran Private Reserve in the Pantanal subregion of Cáceres where it is possible to find floodplains covered by grasses and other herbaceous plants, floating meadows (batumes), pioneer forests, and large monodominant stands of E. fusca, locally known as abobral28. We created the map using the software QGis Versão 3.429, all the shapefiles used are open access. The shapefiles of Brazilian states was provided by the Instituto Brasileiro de Geografia e Estatística30, Pantanal provided by Ministério do Meio Ambiente31 and TES provided by Instituto Chico Mendes de Conservação da Biodiversidade32.
Figure 1.
Monodominant stands of E. fusca sampled from population structure analysis (PS) and areas of destructive sample collection (DS) at Taiamã Ecological Station (TES) in the northern Pantanal subregion of Cáceres, Mato Grosso State, Brazil.
The climate is seasonal Aw in the Köppen classification33 with a dry season from May to September and a rainy season from October to April15. In the Pantanal subregion of Cáceres, high flooding levels almost coincide with the rainy seasons (Fig. 2). The average annual temperatures are around 26 °C, ranging from a minimum average of 20 °C to the maximum of 31 °C, and the average annual precipitation is 1227 mm. We calculated these values for the years between 1980 and 2014 from data obtained from the Instituto Nacional de Meteorologia (Brazilian National Institute of Meteorology)34 and the Marinha Brasileira (Brazilian Navy)35.
Figure 2.
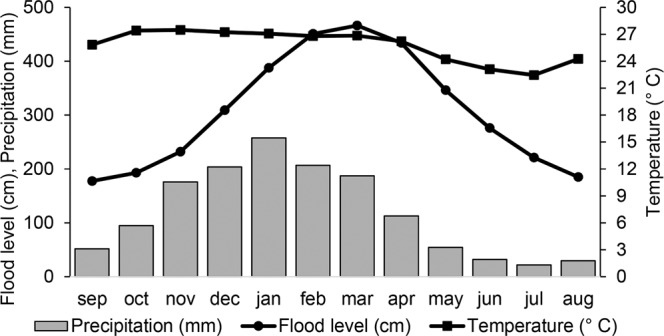
Average flood levels of the Paraguay River, annual precipitation, and temperature between 1980 and 2014 in the northern Pantanal subregion of Cáceres, Mato Grosso, Brazil.
In the subregion, the predominant soil is Eutrophic Gleysol36, which remains periodically saturated by water stagnation or the occurrence of lateral flow in the soil. The striking feature of this soil type is its strong gleization owing to a reduced environment wherein the water saturation throughout the year, combined with oxygen demand for biological activities, leads to low dissolved oxygen concentration in soil37.
Considering that the Pantanal is in Southern Hemisphere, it is important to remember that, for some species the growth of tree rings begins at the beginning of the rainy season15,38,39, which corresponds to October, that is, the growing year of the plant does not follow the calendar. Thus, in results (Figures) the months designated with (−1) indicate the growth season in the previous year and (+1) indicate the change of the calendar year during the growing season in the Southern Hemisphere, similar methodology is used by other authors in recent papers (e.g. Fortes et al.15; Locosselli et al.40; Locosselli et al.41).
Species description
E. fusca (Fabaceae) is a tree species (20–30 meters high) with a globose treetop and short and branched trunk, prickly when young. The leaves are alternate, composite, trifoliolate, with leathery leaflets, glabrous. The inflorescence is in the form of terminal racemes, with showy yellow-orange flowers19. This is a pioneer, heliophyte, deciduous species, tolerant to flooding19,20, which occurs naturally in riparian forests and blooms from May to September, with intense fructification starting in November19,21. During fieldwork, we observed that adult individuals of E. fusca showed a pattern of prop roots which seems to help improve root oxygen levels and root fixation on the soil during flooding, we also observed that the higher the flood level, the more prop roots could be found in individuals.
Monodominant stands of E. fusca occur on the plains of the northern Paraguay River, in the Cáceres subregion, and along the banks of the Aquidauana River18. In the dry season, this species occupies an area of approximately 18 km2, which represents 16% of the station area28. These monodominant stands are low-density vegetation with predominance of arboreal individuals of E. fusca (approximately 77% of the community), along with another 10 tree/shrub species (Unpublished data). The herbaceous stratum is very homogeneous, dominated by grasses, and the soil is covered by a histosol layer with leaf litter, mostly composed of E. fusca leaves.
We collected and prepared a fertile sample of E. fusca according to herbarium techniques42,43 and deposited the voucher in the CGMS Herbarium under registration CGMS 40967.
Collection and sample preparation
Considering that the area was a Brazilian Federal Conservation Unit (Taiamã Ecological Station) with a limited permission to cut trees (for this project we were allowed to collect only seven individuals), in December 2013, we selected two sample areas (Fig. 1) where we marked seven E. fusca trees of different diameters. In each tree, we used the Mariaux Windows method44, which starts by using a mechanical incision to remove part of the bark from the tree to expose the vascular cambium. After two years, in December 2015, we collected these trees using the destructive method. These destructive samples were included in the total sampling, but they were also used for knowledge of wood anatomy, growth ring pattern determination, and presence/absence of false or missing rings.
After previous analysis of the discs (destructive samples) and verification that there were no missing or false rings that could lead to misinterpretation of data, we decided to complement sampling with the use of non-destructive method. The non-destructive method has been used by several authors (e.g. Schöngart et al.45; Fonseca-Junior et al.46; Locosselli et al.41; Andrade et al.47; Neves et al.48) in recent research in tropical trees with good results of ring analysis. In December 2016, using the non-destructive method, we extracted cores from the stems of 29 trees (one or two cores per stem, but we used the one that allowed the best view of the rings) using an increment borer (Pressler borer) 5 mm in diameter. These non-destructive samples were collected over the E. fusca monodominance areas at Taiamã Ecological Station, close to where we collect the destructive samples, according to the availability of collect individuals of different diameters. In the field, we measured the diameter at breast height (DBH) with a diametric tape and the height of all trees using a hypsometer.
We kept samples at room temperature until completely dried to avoid cracking. Then we polished the samples with sandpaper of different grains (80, 100, 180, 220, 320, 400, 600, and 1200) so that the growth rings and anatomical characteristics of the wood could be visualized24.
Anatomical procedures
The growth rings were delimitated with anatomical analyses. We analyzed the anatomical samples at the Laboratório de Anatomia Vegetal of Universidade Federal de Mato Grosso do Sul (UFMS). For anatomical description and determination of tree-rings, we used 1 × 1 × 2 cm wood samples of three E. fusca individuals. We boiled the wood samples in glycerin solution (50%) for 15 minutes to soften the wood. Afterwards, we prepared sections (longitudinal and cross planes) of about 25 µm thickness using a sledge microtome (Leica SM2000R). The sections were bleached with sodium hypochlorite, washed in distilled water and acetic acid (1%), and stained with alcian blue and safranin, both in the concentration of 1% in water, proportion of 9:149. Macerations were prepared using wood segments dissociated in hydrogen peroxide and glacial acetic acid (1:1) and heated in an oven at 60 °C for 12 h. Isolated cells were stained with 1% Safranin or 12:25% basic fuchsin, in 50% ethanol and mounted in 50% glycerin solution. To detect lignified secondary wall, we performed a histochemical test with acidic phloroglucin (phoroglucinol, HCl and ethyl alcohol) in which the lignified cell wall becomes violet-red50. We analyzed and photographed the sample under a microscope. The descriptions of wood constituents followed the guidelines of the IAWA Committee51.
After defining the anatomical and growth ring characteristics, we measured the width of the tree-rings. To do this, we used a tree-ring measurement (LintabTM 6) with precision of 0.01 mm, together with TSAP-WINTM Scientific software (Time Series Analysis and Presentation), which is specific for time sequence analysis. We analyzed the samples at the Laboratório de Ecologia Vegetal of Universidade Federal de Mato Grosso do Sul (UFMS). The tree-rings were delimited and measured according to the classification of Coster52,53, as adapted by Worbes24.
Growth modeling and population structure
After we measured the width of the rings, we generated the individual radial increase rates, from which we obtained the rates of mean diameter increment (MDI) in millimeters. We built the cumulative diameter growth curves and then fitted to the DBH obtained in the field14. Through adjustments of the cumulative diameter growth curves, we obtained the relationship between age and diameter of individuals54–56. Based on the individual cumulative diameter growth curves, we calculated the mean diameter growth curve, which was fitted to a sigmoidal regression model, to obtain the relationship between age and diameter55,56, according to:
where DBH is the diameter at breast height (cm) and β0, β1 e β2 are equation parameters para for the model obtained by the non-linear regression fit.
The relationship between DBH and the height of trees sampled (H) was adapted to a non-linear regression, according to Schöngart55,56. The regression models were produced using the Xact software (SciLab).
We sampled monodominant stands of E. fusca (MSEF) in eight areas (Fig. 1), distributed throughout the TES (over 115 km2), and in each area, we established three 50 × 5 m plots with a minimum distance of 20 m between them, totaling 0.6 ha. We allocated the plots in order to best show different elevations and respective flooding durations of the Paraguay River levees. Trees with circumference at breast height (CBH) of ≥15 cm were included in the sampling. Bifurcated stems were only included if at least one had a circumference ≥15 cm. We did not find individuals below 15 cm of CBH. We transformed the data of CBH into DBH (diameter at breast height) before performing analyses. We also measured the height of trees with a hypsometer.
Using population sampling data, we constructed a new correlation of diameter with height of the individuals, adapted to a nonlinear regression55. In addition, using the values of DBH and the age of each sample, we calculated the age of the individuals included in the population sample.
We also distributed the individuals in classes of diameter with intervals of classes defined by Spiegel’s formula57 constituted by A/K, where A represents the breath of data (diameter), and K represents the algorithm of Sturges: K = 1 + 3.3 log N, where N is the number of individuals sampled. Considering the relationship between diameter/age of individuals sampled for dendrochronological analyses, we calculated the age of all the individuals sampled from the population.
Since the number of individuals in the largest diameter classes tends to experience a natural decrease, we were unable to perform a direct analysis between the environmental factors and the number of individuals in each diameter class. Therefore, we used the distribution of individuals in the first five diameter/age classes (classes with more than five individuals, totaling the last 35 years), to build a series that contains maximum and minimum values of flooding levels and total precipitation data for the years falling into each class. To verify differences in the levels of these factors between age groups, we performed analysis of variance, followed by the Tukey 5% test using R software and the agricolae package58.
We determined the duration of flooding of each plot. First, we measured from the ground the height of the water mark left by the last inundation on tree trunks inside the plots. Then, we obtained an average of the water marks per plot and compared these values with the highest level recorded at the hydrometric gauge of Cáceres during that year of sampling. Considering that the overall topography is very flat and that no tributaries flow between the gauge and the sampled area, we considered variation in water level to be the same. Using these data, we calculated the topographic position of each plot in relation to zero by the Cáceres gauge. Thus, we used ten years of data to calculate an average period of flooding for each plot.
With the calculations above mentioned we observed that the monodominant stands of E. fusca in the Pantanal subregion of Cáceres were subjected to different periods of flooding, ranging from 42 to 117 days per year. Therefore, to observe the differences in the distribution of individuals in diameter and age class subjected to different flood periods, we divided the plots into two parts (i.e., half the total length of the inundation gradient) based on the results obtained by Damasceno-Junior et al.59 and Arruda et al.60. Therefore, half of the plots were in the first category (flooding between 42–78 days per year) with 62 individuals, while the second category (flooding between 79–117 days per year) presented 91 individuals. To compare the number of individuals within diameter classes between flood periods (42–78 and 79–117), we used a chi-square test with R software61.
Environmental variables
We used the weather data corresponding to the period from 1981 to 2014. We obtained the precipitation data from KNMI Climate Explorer62. We obtained the data on daily flood level of the Paraguay River in the study subregion from the Marinha do Brasil (Brazilian Navy)35. The records of ENSO events (El Niño 1 + 2, El Niño 3, El Niño 3.4 and El Niño 4), Pacific Decadal Oscillation (PDO), and Southern Oscillation Index (SOI) were obtained from the database available on the National Oceanic Atmospheric Administration (NOAA)63.
Chronology and correlations with climatic variables
We used standard dendrochronological techniques to cross-date the time series of different individuals and combine these time series with a main chronology64. The cross-dating process comprises overlapping individual indexed curves, matches the variations in rings width between trees and checking relationships between dating to infer where rings are missing, false or incorrectly observed13,23,45,65,66. We visually and statistically checked the increment curves pairwise with cross-dating to build an average chronology based on the growth ring series of all individuals65. We used the TSAP-WINTM software to describe the similarity between individual curves. Percentage of parallel run was used to indicate the year-to-year agreement in the oscillation of two curves within the overlapping interval67. The samples without the pith were carefully analyzed, paired (visually and statistically) and cross-dating with the other curve samples according to the similarity between the individual curves, the comparison of diameters and according to the pith distance, thus, it was possible to estimate the average number of missing rings, similar methodology is used by other authors (e.g. Brienen et al.68). Cross-dating was visually and statistically validated by the coefficient of coincidence Gleichlaeufigkeit (GLK) and the T value65,69. Both concepts are used to verify the quality of agreement between temporal series, and GLK is specially developed for growth ring cross-dating65. After this process, the best individual time series were selected for the construction of the average time series (chronology).
The curves standardization removes the effects of ring-width reduction associated with environmental fluctuations, changes in age and position within the stem, and site productivity conditions66. To remove long-term growth trends related to increasing tree age and size, or even from changes in the surrounding forest, causing effects on competitive structure66, a five-year moving average was used to convert crude tree-ring curves into growth indices. This procedure results in normally distributed data, which is an elementary condition for correlation with climate variables70.
To verify if the species is sensitive to environmental variations we calculated the mean sensitivity, which expresses the relative year-to-year variation in ring width66. We tested the significance of the relationships between climatic factors and chronology of E. fusca using correlation matrices, and we built the graphics using R software61.
Results
Anatomical description and determination of growth rings
The wood of E. fusca is whitish and poorly lignified. The pores are distinct to the naked eye and form semi-porous rings, with a row of larger pores at the beginning of the growth layer. These pores are sparsely distributed and small-sized toward the end of the layer. The pores are in dendritic pattern with solitary (most) and multiple clusters. The axial parenchyma is visible to the naked eye and is confluent and in bands.
The growth layers are distinct (Fig. 3), and the beginning is marked by the distribution of the semi-porous rings and the presence of parenchyma and few fibers (Fig. 4). The rest of the layer shows a decrease in pore size and number, and the end of the layer is marked by few, or no, pores and a band of parenchyma with flattened cells (Fig. 4). When looking at the samples with Mariaux window marks, we noticed that new rings started soon after we made the scars. This means that the beginning of the growth of a new layer occurred between the months of December and January. From anatomical analysis, we observed no false, or missing, rings, allowing us to confidently perform measurements on non-destructively obtained samples.
Figure 3.

Macroscopic detail of E. fusca stem disk with Mariaux window scar collected in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. The arrows indicate the delimitations of distinct growth layers, with a band of parenchymatic flat cells, above which we see the rest of the layer showing a decrease in pore size and number. The end of the layer is marked by few, or no pores, and a band of parenchyma with flattened cells.
Figure 4.
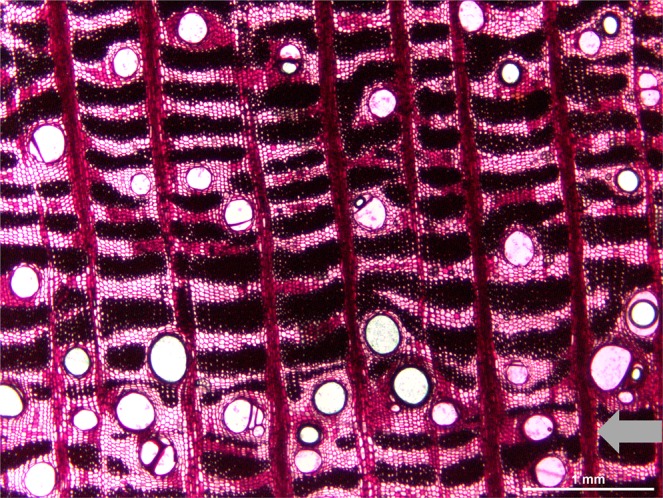
Microscopic detail of E. fusca wood collected in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. The gray arrow indicates the region where the growth layer begins, with a higher concentration of parenchyma and large pores.
Growth modelling and population structure
The ages of sampled individuals of E. fusca ranged from 6 to 34 years. The relationship between age and DBH of the trees was highly significant (r2 = 0.84, P < 0.05, Fig. 5). The maximum diameter increment rate of E. fusca was observed at 7 years (1.6 cm year−1); after this period, the rate decreased linearly.
Figure 5.
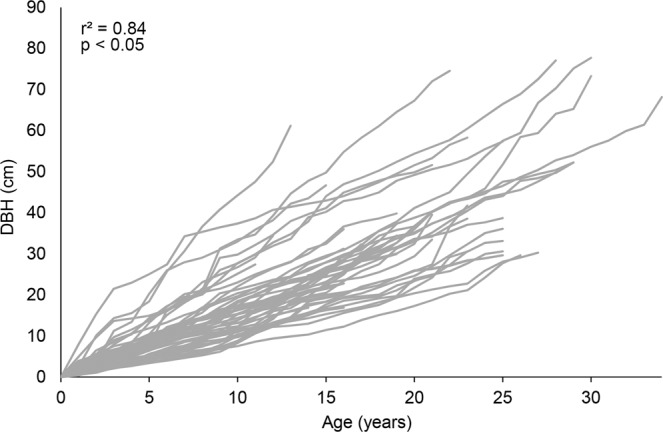
Relationship between diameter growth at breast height (DBH) and age of individuals of E. fusca in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. Lines represent the individual growth in diameter of each sample.
Diameter was highly correlated (r2 = 0.93, P < 0.05) with height of individuals. The same was observed for individuals in the population structure samples (r2 = 0.74, P < 0.05, Fig. 6), as described by a nonlinear regression model.
Figure 6.
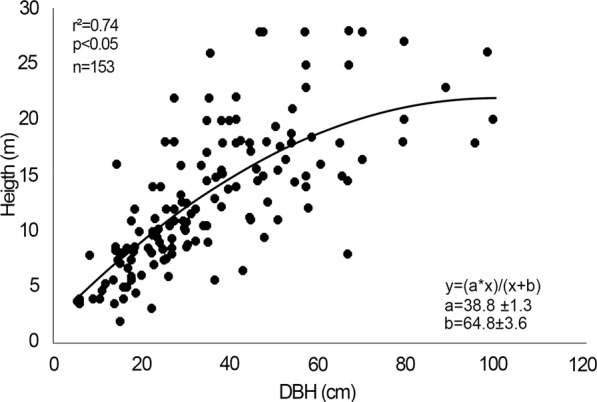
Relationship between diameter at breast height (DBH) and height of 153 individuals of E. fusca in the Pantanal subregion of Cáceres, Mato Grosso, Brazil, adjusted with a nonlinear regression analysis.
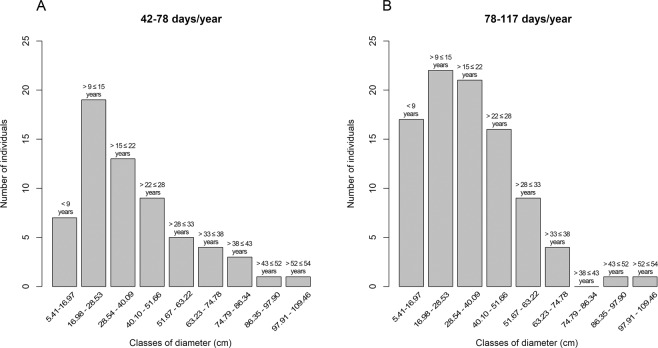
Most individuals had established between the middle and the end of the 1990s (Fig. 7a,b). For the older classes, we observed a pronounced decrease in the number of individuals with a maximum age of 54 years (Fig. 7a,b). We observed a lower number of individuals in the lower diameter classes (less than 9 years old). In this group, we also observed that areas which remained inundated a mean of 42–78 days per year (Fig. 7a) showed an even lower number of individuals (χ2 = 4.17, P = P < 0.05) in the first diameter class compared to areas subjected to 79–117 days of flooding (Fig. 7b). In these last 9 years, we also observed a decrease in the annual minimum flooding levels compared to the previous 26 years (P < 0.05).
Figure 7.
Number of E. fusca individuals sampled in the Pantanal subregion of Cáceres, Mato Grosso, Brazil, distributed into diameter classes (DBH) and age among classes, a) individuals occurring in areas where the flood period varies between 42–78 days/years, b) individuals occurring in areas where the flood period varies between 78–117 days/years.
Chronology and correlations with climatic variables
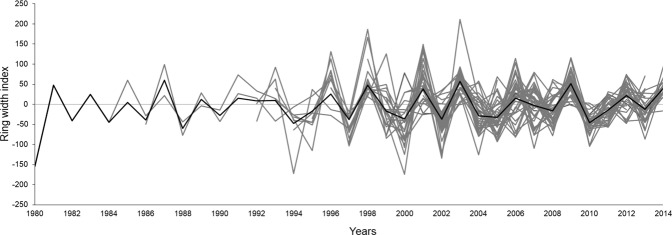
The construction of an average chronology was possible for the period from 1981 to 2014 (Fig. 8), using the 36 individuals (seven destructive samples and 29 non-destructive samples). We verified a mean sensitivity of 0.477. We observed a positive correlation between the width of the rings obtained in our mean chronology and data from precipitation (December previous year) (r = −0.49, P < 0.05; Fig. 9) and a negative correlation between the width of the rings and data from the regions with El Niño: El Niño 1 + 2 (r = −0.38, P < 0.05; Fig. 10) and El Niño 3 (r = −0.34, P < 0.05; Fig. 11).
Figure 8.
Indexed chronology of annual rings of E. fusca trees sampled (39 individuals) in an area of monodominance in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. The gray curves represent the individual indexed curves, and the black curve represents the average chronology.
Figure 9.
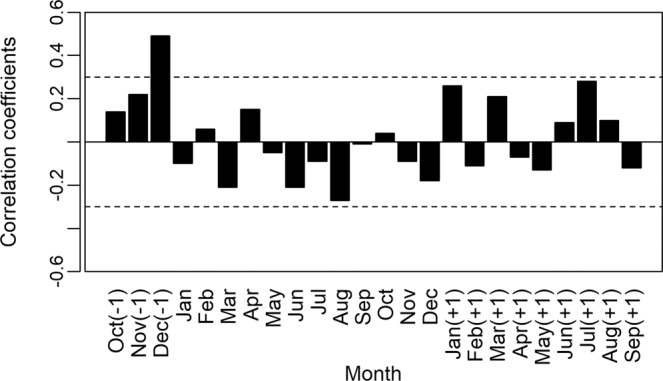
Positive correlation (December of previous year) between precipitation and E. fusca ring width index sampled from a monodominant stand in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. Months of the growth season in the previous year are indicated with (−1) and months (+1) indicate the calendar year change during the southern hemisphere growing season (as growth begins in October). Dashed line represents significance of 0.05.
Figure 10.
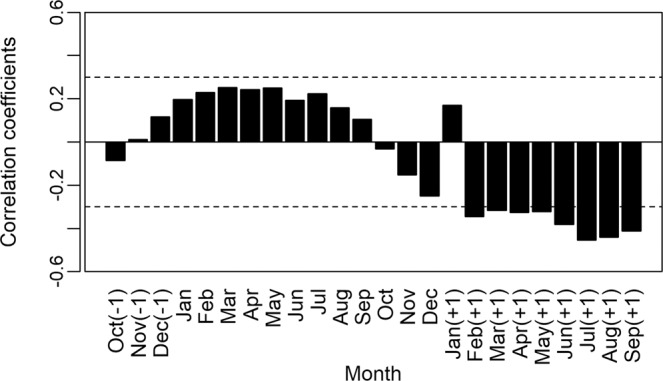
Correlations between the anomaly indices in the El Niño 1+2 region and the E. fusca ring width index sampled from a monodominant stand in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. Months of the growth season in the previous year are indicated with (−1) and months (+1) indicate the calendar year change during the southern hemisphere growing season (as growth begins in October). Dashed line represents significance of 0.05.
Figure 11.
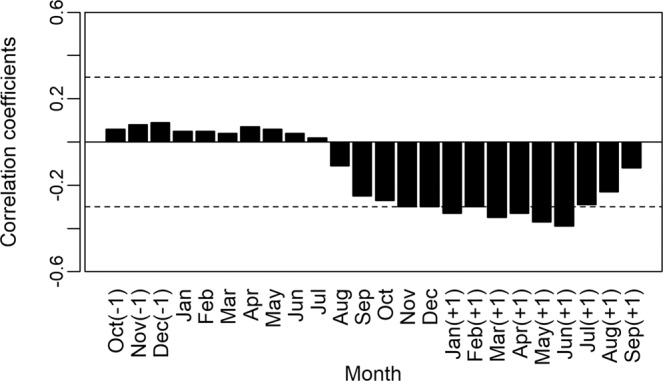
Correlations between the anomaly indices in the El Niño 3 region and the E. fusca ring width index sampled from a monodominant stand in the Pantanal subregion of Cáceres, Mato Grosso, Brazil. Months of the growth season in the previous year are indicated with (−1) and months (+1) indicate the calendar year change during the southern hemisphere growing season (as growth begins in October). Dashed line represents significance of 0.05.
We did not observe any correlation between the width of the rings and flooding data from the study subregion (r = −0.087, P > 0.05), and we did not observe any correlation with the PDO or SOI data (r = −0.17 and r = −0.10, P > 0.05, respectively), indicating that the growth of E. fusca is not affected by these factors in this subregion.
Discussion
The positive relationship between diameter and height of E. fusca in the study region allowed us to extrapolate an understanding of the whole structure from just the sampled population. We note a decrease in diameter increment of individuals from the seventh year onwards. The dendrochronological analyses showed that the sampled individuals of E. fusca (36) were young and that the oldest tree we found was 54 years. Although young, the individuals sampled were large, quite possibly the result of the rapid increase in diameter of the species, especially in the first years of life, which is an expected pattern of pioneer species71. The large and rapid increase in diameter in E. fusca has already been reported72.
When analyzing the age for the population as a whole (153 sampled individuals), irrespective of the period that individuals remain flooded (42–78 or 79–117 days of flooding), we observed that most of the stand was younger than 22 years, with few individuals over 40 and maximum determined age of 54 years. This low longevity of individuals probably results from the wood’s characteristics leading to breakage and rapid disintegration. This species is also characterized by low density (0.31–0.33 g cm−3)73 or (0.37 g cm−3)19 and low mechanical strength19,20. In the field, we observed that the trees of E. fusca break easily from events such as storms and that after such breakage, the stems disintegrate rapidly. The susceptibility of the species to rot when in contact with the soil was mentioned by Lorenzi19. In addition, we observed that the wood of the species is often attacked by wood feeder insects, which may influence its low strength and durability.
We observed a decrease in the number of individuals in first diameter class (5.41–16.97 cm) with an age of less than 9 years (Fig. 7). E. fusca is a monodominant, pioneer, light-demanding species19,20, as well as the species V. divergens, whose response pattern of growth rings to environmental factors are similar to our study15,16,74. Light-demanding species frequently have a bell-shaped distribution, with fewer individuals in larger and smaller diameter classes75, which shows mortality in the larger classes and low recruitment/establishment in the smaller classes, possibly related to the shading that larger individuals provide. In this work, we did not observe a well-defined bell-shape since the second and third classes presented a large number of individuals, but we observed a low recruitment/establishment in the first diameter class (Fig. 7). Whereas E. fusca is a light-demanding species19,20, this decrease in establishment over the past nine years could have been influenced by the low availability of light in the understory, possibly owing to shading of the larger individuals. Nevertheless, this pattern is not the same in different levels of inundation. We observed that this decrease match with the decline in flood levels over this period (last nine years). When we analyzed the climatic history of the region, we observed a decrease in the minimum flood level in the last nine years and, consequently, in the number of days these areas remained flooded. E. fusca seems to have its recruitment connected to pluriannual flood pulses, with longer periods of flooding favoring its establishment, which was apparent when we observed that areas subjected to longer flood periods (79–117 days, Fig. 7b) showed a greater number of individuals in this first diameter class when compared to areas subjected to shorter periods (between 42–78 days) (Fig. 7a). This is also supported by increased seed germination after longer periods of flooding76.
The mean sensitivity we observed is considered high (>0.40)66, which indicates high sensitivity of the species to be influenced by environmental variations. Environmental conditions greatly affect tree growth; thus, conspecific individuals can present variable growth rates, depending on the conditions to which they are exposed23,45. When we analyzed growth, the formation of new layers seemed to start between the months of December and January, during which time precipitation and flooding begin to increase in the Pantanal subregion. This is an unusual pattern since, for most species, wood growth seems to occur mainly during the dry season, and it is stagnant during the wet season12,23,24. Despite the evidence that growth starts during the period of increased flooding and precipitation, no direct statistical relationship between flooding and growth supports that idea. Although flooding influences the establishment of the species23, E. fusca growth does not seem to have a direct relationship with flooding. However, we did observe a positive correlation between precipitation in December of the previous year and E. fusca increment (Fig. 9). December has one of the higher precipitation levels in the Pantanal, which may indicate that the individual holds reserves during the period of greatest precipitation and then begins to grow in the next season. Fortes et al.15 also found, in Pantanal, a relationship between V. divergens growth and annual rainfall, but no correlation to flood patterns. In a study of two Cedrela species, in Brazilian Amazon, a correlation was observed between growth ring chronology and rainfall77.
The events of El Niño showed a negative impact on the growth of E. fusca trees. In our study, the correlation of El Niño 1 + 2 data with the increment of rings occurred mainly during the months of February-September (Fig. 10), while for El Niño 3, they were from November-June (Fig. 11). This negative correlation must be associated with the decrease in precipitation caused by El Niño in the Pantanal; that is, El Niño decreases precipitation, and, thus, the growth of E. fusca is negatively affected. In a study with V. divergens the authors also found a negative correlation between the growth and El Niño 1 + 215. Analyses of time series of data obtained by Schöngart et al.13 indicated that during the last two centuries, the severity of El Niño effects has increased significantly. The events of El Niño significantly reduce precipitation in the Pantanal, which for V. divergens also resulted in a reduction in the diameter increment15,16,74.
Several studies conducted in tropical wetlands in Pantanal and Amazon show the positive/negative influence of El Niño events on tree species growth13,15,41,73 and in most cases El Niño is expected to positively affect the increment, as it increases the dry season13,41. Based on that, it should be recalled that our stated hypothesis held that the establishment and growth of individuals of E. fusca in the monodominant stands in the Pantanal subregion of Cáceres is negatively influenced by precipitation, but positively influenced by the occurrence of El Niño. Yet, this was refuted since the species E. fusca was contrary to that expected for most species. The same unusual pattern was found for V. divergens, which also occurs as monodominant in the northern Pantanal region15. This is unusual since most species are more often grow during the dry season and reduce/stagnate cambial activity during wet season due to anoxic environmental conditions12,23,24. The main difference between the studied area and the Amazon, were most of these studies were conducted, is the levels of inundation which is lower in Pantanal. Besides that, this species has an expressive number of adventiceous roots that can diminish anoxia effects during the wet season. This must be investigated further.
Therefore, based on the factors analyzed, we can conclude that precipitation and El Niño do affect the growth of the species in the study region, but that this most likely results from decreased precipitation. From the construction of a reliable chronology, we could determine that the individuals found in this monodominance of E. fusca in the Pantanal sub-region of Cáceres are quite young, with a maximum age of 54 years, but we could not make inferences about the age of the population, since it may have been established long before that, and individuals can be replaced. Furthermore, we observed a reduction in the number of young individuals, which appears to be a response to the decrease in the minimum flood level in the study region over the past few years. This indicates a future trend that this monodominant population will undergo a decline, if flood levels decrease, since a drastic reduction in the number of young individuals took place in less floodable areas. From these results, proposals aimed at protecting these formations can be supported, especially those preventing anthropic actions that lead to an even greater reduction in flood levels, such as dams for hydroelectric power and harbors and deforestation of the river headwaters. In addition, this study provides important information about the influence of El Niño on growth of arboreal species in the Pantanal, a little explored effect until now, and motivation to study this same effect with other species, including monodominant species. It also calls attention for situation of climatic changes where the levels of precipitation can diminish with changes in levels of inundation can conduct to this monodominant stand to disappear from this region.
Acknowledgements
This study was financed in part by the Fundação Universidade Federal de Mato Grosso do Sul – UFMS/MEC – Brazil. We also thank the Programa de Pós-Graduação em Ecologia e Conservação (PPGEC), and the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for financial and logistical support. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), which provided scholarships to DG, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico for the grants for GADJ. This work was supported by the Instituto Nacional de Ciência e Tecnologia em Áreas Úmidas (INAU) funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Ministério da Ciência, Tecnologia e Inovação (CNPq no. 573990/2008-5). We thank Dr. Maria Antonia Carniello for support in Cáceres, Dr. Jochen Schöngart for support with methodology, Dr. Catia Nunes da Cunha for lending necessary equipment, Marcus Urquiza for methods, Jean Carlo de Quadros, Diego Morais, Amanda Galdi Boaretto and Vitor Hugo Brito for help during the fieldwork and support with collecting and preparing the samples, and Sylvia Raíssa de Vasconcelos Lobo Melo for help with anatomical procedures. We also thank Daniel Luis Zanella Kantek, Selma Samiko, Miyazaki Onuma and Thadeu Deluque Costa Pereira (ICMBio – Cáceres) for support during the fieldwork.
Author contributions
D.G., I.H.I., M.R.M. and G.A.D.J. conceived and designed the research. D.G. collected field data. D.G., R.C.O.A. and E.P. performed the anatomical and dendrochronological analyses. D.G., E.P., R.C.O.A. and G.A.D.J. performed the data analysis and interpreted the data. D.G. wrote the main manuscript text. All authors helped to review and write the manuscript. All authors gave final approval for publication.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Connell JH, Lowman MD. Low-diversity tropical rain forests: some possible mechanisms for their existence. Am. Nat. 1989;134:88–119. doi: 10.1086/284967. [DOI] [Google Scholar]
- 2.Hart TB, Hart JA, Murphy PG. Monodominant and species-rich forests of the humid tropics: causes for their co-occurrence. Am. Nat. 1989;133:613–633. doi: 10.1086/284941. [DOI] [Google Scholar]
- 3.Hart TB. Monospecific dominance in tropical rain forests. Trends Ecol. Evol. 1990;5:6–11. doi: 10.1016/0169-5347(90)90005-X. [DOI] [PubMed] [Google Scholar]
- 4.Torti SD, Coley PD, Kursar TA. Causes and consequences of monodominance in tropical lowland forests. Am. Nat. 2001;157:141–153. doi: 10.1086/318629. [DOI] [PubMed] [Google Scholar]
- 5.Junk WJ, Piedade MTF, Nunes da Cunha C, Wittmann F, Schöngart J. Macrohabitat studies in large Brazilian floodplains to support sustainable development in the face of climate change. Ecohydrol. Hydrobiol. 2018;18(4):334–344. doi: 10.1016/j.ecohyd.2018.11.007. [DOI] [Google Scholar]
- 6.Ter Steege H, et al. Rarity of monodominance in hyperdiverse Amazonian forests. Sci. Rep. 2019;9:13822. doi: 10.1038/s41598-019-50323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worbes M, Staschel R, Roloff A, Junk WM. Tree rings analysis reveals age structure, dynamics an wood production of a natural forest stand in Cameroon. For. Ecol. Manag. 2003;173:105–123. doi: 10.1016/S0378-1127(01)00814-3. [DOI] [Google Scholar]
- 8.Brienen RJW, Lebrija-Trejos E, Zuidema PA, Martínez-Ramos M. Climate-growth analysis for a Mexican dry forest tree shows strong impact of sea surface temperatures and predicts future growth declines. Glob. Chang. Biol. 2010;16:2001–2012. doi: 10.1111/j.1365-2486.2009.02059.x. [DOI] [Google Scholar]
- 9.Campbell DG, Daly DC, Prance GT, Maciel UN. Quantitative ecological inventory of terra firme and várzea tropical forest on the Rio Xingú, Brazilian Amazon. Brittonia. 1986;38:369–393. doi: 10.2307/2807085. [DOI] [Google Scholar]
- 10.Junk, W. J., Bayley, P. B. & Sparks, R. E. In Proceedings of the International Large River Symposium (ed. Dodge D. P.) 110–127 (Canadian Journal of Fisheries and Aquatic Sciences, 1989).
- 11.Ishii, I. H. Estudos dendrocronológicos e determinação da idade de árvores das Matas Ciliares do Pantanal Sul-matogrossense, PhD thesis, Universidade Federal de São Carlos (1998).
- 12.Schöngart J, Piedade MTF, Ludwigshausen S, Horna V, Worbes M. Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J. Trop. Ecol. 2002;18:581–597. doi: 10.1017/S0266467402002389. [DOI] [Google Scholar]
- 13.Schöngart J, et al. Teleconnection between tree growth in the Amazonian floodplains and the El Niño-Southern Oscillation effect. Glob. Chang. Biol. 2004;10:683–692. doi: 10.1111/j.1529-8817.2003.00754.x. [DOI] [Google Scholar]
- 14.Brienen RJW, Zuidema PA. Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. J. Ecol. 2006;94:481–493. doi: 10.1111/j.1365-2745.2005.01080.x. [DOI] [Google Scholar]
- 15.Fortes CF, et al. Dendrochronological records of a pioneer tree species containing ENSO signal in the Pantanal, Brazil. Braz. J. Bot. 2018;41:167–174. doi: 10.1007/s40415-017-0434-8. [DOI] [Google Scholar]
- 16.Schöngart J, Arieira J, Fortes CF, Cezarine de Arruda E, Nunes da Cunha C. Age-related and stand-wise estimates of carbon stocks and sequestration in the aboveground coarse wood biomass of wetland forests in the northern Pantanal, Brazil. Biogeosciences. 2011;8:3407–3421. doi: 10.5194/bg-8-3407-2011. [DOI] [Google Scholar]
- 17.Hutchings, M. J. In Plant ecology (ed. Crawley M. J.) 325–358 (Blackwell Oxford-Science (1997).
- 18.Pott A, Oliveira AKM, Damasceno-Junior GA, Silva JSV. Plant diversity of the Pantanal wetland. Braz. J. Biol. 2011;71:265–273. doi: 10.1590/S1519-69842011000200005. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil. (Vol. 2, Nova Odessa, São Paulo (1998).
- 20.Cordero, J. & Boshier, D. H. Erythrina. In Árboles de Centroamérica: Un Manual para Extensionistas (eds. Cordero J., Boshier, D. H.) 536–538 (OFI/CATIE (2003).
- 21.Pott, A. & Pott, V. J. Plantas do Pantanal. (Embrapa, Brasília (1994).
- 22.Parrini R, Raposo MA. Aves explorando flores de Erythrina fusca (Leguminosae, Fabaceae) durante a estação seca no Pantanal de Mato Grosso. Iheringia. 2010;100:97–101. doi: 10.1590/S0073-47212010000200001. [DOI] [Google Scholar]
- 23.Worbes M. Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the Neotropics. IAWA Bull. 1989;10:109–122. doi: 10.1163/22941932-90000479. [DOI] [Google Scholar]
- 24.Worbes M. How to measure growth dynamics in tropical trees. A review. IAWA Bull. 1995;16:337–351. doi: 10.1163/22941932-90001424. [DOI] [Google Scholar]
- 25.Adámoli, J. Fitogeografia do Pantanal. In Simpósio sobre Recursos Naturais e Socioeconômicos do Pantanal. (Embrapa CPAP/UFMS (1986)
- 26.Junk WJ, et al. Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2014;24:5–22. doi: 10.1002/aqc.2386. [DOI] [Google Scholar]
- 27.Nunes da Cunha, C. & Junk, W. J. A Classificação dos Macrohabitats do Pantanal Mato-grossense. In Classificação e Delineamento das Áreas Úmidas Brasileiras e de seus Macrohabitats (eds. Nunes da Cunha, C., Piedade, M. T. F. & Junk, W. F.). 77–122 (EdUFMT, (2014).
- 28.Frota AVB, Ikeda-Castrillon SK, Kantek DLZ, Silva CJ. Macrohabitats da Estação Ecológica de Taiamã, no contexto da Área Úmida Pantanal mato-grossense. Brasil. Bol. Mus. Paraense Emílio Goeldi – Cien. Nat. 2017;12:239–254. [Google Scholar]
- 29.QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project (2018). http://qgis.osgeo.org. Accessed August 2019.
- 30.IBGE – Instituto Brasileiro de Geografia e Estatística Índice de Organização do Território/ Malhas Territoriais/ Malhas Municipais/ Munícipio 2015/ Brasil. ftp://geoftp.ibge.gov.br/organizacao_do_territorio/malhas_territoriais/malhas_municipais/municipio_2015/Brasil/BR/. (Accessed August 2019).
- 31.MMA – Ministério do Meio Ambiente. Download de dados geográficos. http://mapas.mma.gov.br/i3geo/datadownload.htm. (Accessed August 2019).
- 32.ICMBio-MMA – Instituto Chico Mendes de Conservação da Biodiversidade e Ministério do Meio Ambiente. Estação Ecológica de Taiamã. http://www.icmbio.gov.br/esectaiama/downloads. (Accessed August 2019).
- 33.Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013;22(6):711–728. doi: 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- 34.Instituto Nacional de Meteorologia – INMET. Estações Meteorológica de Observação de Superfície Automática. http://www.inmet.gov.br/portal/index.php?r=estacoes/estacoesAutomaticas. (Accessed 15 March 2019).
- 35.Marinha do Brasil. Serviço de Sinalização Náutica do Oeste. https://www.marinha.mil.br/ssn-6/. (Accessed 15 March 2019).
- 36.Fernandes, F. A., Fernandes, A. H. B. M., Soares, M. T. S., Pellegrin, L. A. & Lima, I. B. T. Atualização do Mapa de Solos da Planície Pantaneira para o Sistema Brasileiro de Classificação de Solos (Comunicado técnico, 61). (Embrapa Pantanal, Corumbá, (2007).
- 37.Embrapa - Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos. (Embrapa, Brasília (2006).
- 38.Machado NG, et al. Growth rhythm of Vochysia divergens Pohl (Vochysiaceae) in the Northern Pantanal. Acta Sci. Biol. Sci. 2015;37:81–90. doi: 10.4025/actascibiolsci.v37i1.24330. [DOI] [Google Scholar]
- 39.Sallo FS, Sanches L, Dias VRM, Palácios RS, Nogueira JS. Stem water storage dynamics of Vochysia divergens in a seasonally flooded environment. Agric. For. Meteorol. 2017;232:566–575. doi: 10.1016/j.agrformet.2016.10.015. [DOI] [Google Scholar]
- 40.Locosselli GM, Buckeridge MS, Moreira MZ, Ceccantini G. A multi-proxy dendroecological analysis of two tropical species (Hymenaea spp., Leguminosae) growing in a vegetation mosaic. Trees. 2013;27(1):25–36. doi: 10.1007/s00468-012-0764-x. [DOI] [Google Scholar]
- 41.Locosselli GM, Schöngart J, Ceccantini G. Climate/growth relations and teleconnections for a Hymenaea courbaril (Leguminosae) population inhabiting the dry forest on karst. Trees. 2016;30(4):1127–1136. doi: 10.1007/s00468-015-1351-8. [DOI] [Google Scholar]
- 42.Mori, S. A., Mattos-Silva, L. A., Lisboa, G. & Coradin, L. Manual de Manejo do Herbário Fanerogâmico. (CEPLAC, Ilhéus (1989).
- 43.Bridson, D. M. & Forman, L. The Herbarium Handbook. (Royal Botanic Gardens, Kew (2004).
- 44.Mariaux A. Les cernes dans les bois tropicaux africains, nature et periodicité. Bois For. Trop. 1967;113:3–14. doi: 10.19182/bft2016.327.a31296. [DOI] [Google Scholar]
- 45.Schöngart J, Piedade MTF, Wittmann F, Junk WJ, Worbes M. Wood growth patterns of Macrolobium acaciifolium (Benth.) Benth. (Fabaceae) in Amazonian black-water and white-water floodplain forests. Oecologia. 2005;145:454–461. doi: 10.1007/s00442-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca-Júnior SF, Piedade MTF, Schöngart J. Wood growth of Tabebuia barbata (E. Mey.) Sandwith (Bignoniaceae) and Vatairea guianensis Aubl. (Fabaceae) in Central Amazonian black-water (igapó) and white-water (várzea) floodplain forests. Trees. 2009;23(1):127–134. doi: 10.1007/s00468-008-0261-4. [DOI] [Google Scholar]
- 47.Andrade VLC, et al. Growth rings of Brazil nut trees (Bertholletia excelsa) as a living record of historical human disturbance in Central Amazonia. PLOS ONE. 2019;14(4):e0214128. doi: 10.1371/journal.pone.0214128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neves JRD, Piedade MTF, Resende AF, Feitosa YO, Schöngart J. Impact of climatic and hydrological disturbances on blackwater floodplain forests in Central Amazonia. Biotropica. 2019;51:484–489. doi: 10.1111/btp.12667. [DOI] [Google Scholar]
- 49.Luque Rebeca, Sousa Hildeberto C. de, Kraus Jane Elizabeth. Métodos de coloração de Roeser (1972): modificado - e Kropp (1972) visando a substituição do azul de astra por azul de alcião 8GS ou 8GX. Acta Botanica Brasilica. 1996;10(2):199–212. doi: 10.1590/S0102-33061996000200001. [DOI] [Google Scholar]
- 50.Berlyn, G. & Miksche, J. Botanical Microtechnique and Cytochemistry. (The Iowa State University Press, Ames (1976).
- 51.IAWA Committee International Association of Wood Anatomists list of microscopic features of softwood identification. IAWA J. 25, 1–70 (2004)
- 52.Coster C. Zur Anatomie und Physiologie der Zuwachszonen und Jahresringbildung in den Tropen. Ann. Jard. Bot. Buitenzorg. 1927;37:49–160. [Google Scholar]
- 53.Coster C. Zur anatomie und physiologie der zuwachszonen-und jahresringbildung in den tropen II. Ann. Jard. Bot. Buitenzorg. 1928;38:1–114. [Google Scholar]
- 54.Stahle DW, Mushove PT, Cleaveland MK, Roigc F, Haynesd GA. Management implications of annual growth rings in Pterocarpus angolensis from Zimbabwe. For. Ecol. Manag. 1999;124:217–229. doi: 10.1016/S0378-1127(99)00075-4. [DOI] [Google Scholar]
- 55.Schöngart J, et al. Management criteria for Ficus insipida Willd. (Moraceae) in Amazonian white-water floodplain forests defined by tree-ring analysis. Ann. For. Sci. 2007;64:657–664. doi: 10.1051/forest:2007044. [DOI] [Google Scholar]
- 56.Schöngart JG-OL. (GOL): a new concept towards sustainable forest management in Central Amazonian Várzea floodplains. For. Ecol. Manag. 2008;256:46–58. doi: 10.1016/j.foreco.2008.03.037. [DOI] [Google Scholar]
- 57.Spiegel, M. P. Estatística. (McGraw-Hill, São Paulo (1976).
- 58.Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. Version 1.2-4. https://CRAN.R-project.org/package=agricolae. (Accessed 15 March 2019) (2016).
- 59.Damasceno-Junior GA, Semir J, Santos FAM, Leitão-Filho HF. Structure, distribution of species and inundation in a riparian forest of Rio Paraguai. Pantanal, Brazil. Flora. 2005;200:119–135. doi: 10.1016/j.flora.2004.09.002. [DOI] [Google Scholar]
- 60.Arruda WS, et al. Inundation and Fire Shape the Structure of Riparian Forests in the Pantanal, Brazil. PloS One. 2016;11:e0156825. doi: 10.1371/journal.pone.0156825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, ISBN 3-900051-07-0. http://www.R-project.org/. (Accessed 15 March 2019) (2019).
- 62.KNMI Climate Explorer. https://climexp.knmi.nl/select.cgi?id=someone%40somewhereandfield=cru_pre. (Accessed 15 March 2019).
- 63.National Oceanic Atmospheric Administration Download Climate Timeseries. http://www.esrl.noaa.gov/psd/gcos_wgsp/Timeseries/TNI. (Accessed 15 March 2019).
- 64.Stokes, M. A. & Smiley, T. L. An Introduction to Tree-ring Dating. (University of Chicago Press, Chicago (1968).
- 65.Pilcher, J. R. In Methods of dendrochronology, applications in the environmental sciences (eds. Cook, E. R. & Kairiukstis, L. A.) 40–51 (Kluwer (1990).
- 66.Fritts, H. C. Tree Rings and Climate. (Academic Press, London, (1976).
- 67.Schweingruber, F. H. Tree Rings - Basics and Applications of Dendrochronology. (Kluwer Academic Publishers, Dordrecht, (1988).
- 68.Brienen RJ, et al. The potential of tree rings for the study of forest succession in southern Mexico. Biotropica. 2009;41(2):186–195. doi: 10.1111/j.1744-7429.2008.00462.x. [DOI] [Google Scholar]
- 69.Baillie MGL, Pilcher JR. A simple cross-dating program for tree-ring research. Tree-rings Bull. 1973;33:7–14. [Google Scholar]
- 70.Cook, E. R. & Briffa, K. In Methods of Dendrochronology, Applications in the Environmental Sciences (eds. Cook, E. R. & Kairiukstis L. A.) 97–162 (Kluwer/IIASA (1990).
- 71.Swaine MD, Whitemore TC. On the definition of ecological species groups in tropical rain forests. Vegetatio. 1988;75:81–86. doi: 10.1007/BF00044629. [DOI] [Google Scholar]
- 72.Miranda EMD, Valentim JF. Desempenho de doze espécies arbóreas nativas e introduzidas com potencial de uso múltiplo no estado do Acre, Brasil. Acta Amazon. 2000;30(3):471–471. doi: 10.1590/1809-43922000303480. [DOI] [Google Scholar]
- 73.Wittmann, F. et al. Manual of Trees from Central Amazonian Várzea Floodplains. (Inpa, Manaus (2010).
- 74.Nunes da Cunha C, Junk WJ. Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Appl. Veg. Sci. 2004;7:103–110. doi: 10.1111/j.1654-109X.2004.tb00600.x. [DOI] [Google Scholar]
- 75.Sokpon N, Biaou SH. The use of diameter distributions in sustained-use management of remnant forests in Benin: case of Bassila forest reserve in North Benin. For. Ecol. Manag. 2002;161:13–25. doi: 10.1016/S0378-1127(01)00488-1. [DOI] [Google Scholar]
- 76.Gris, D. Monodominance of Erythrina fusca Lour.: influence of environmental factors, chemical ecology and dendroecology, PhD thesis, Universidade Federal de Mato Grosso do Sul (2017).
- 77.Granato-Souza D, et al. Tree rings and rainfall in the equatorial Amazon. Clim. Dyn. 2019;52(3-4):1857–1869. doi: 10.1007/s00382-018-4227-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.