Abstract
Fuchs endothelial corneal dystrophy (FECD) is amongst one of the most common indications for endothelial keratoplasty worldwide. Despite being originally described among Caucasians, it is now known to be prevalent among a large number of populations, including Asians. While the FECD phenotype is classically described as that of central guttate and pigment deposits associated with corneal endothelial dysfunction, there are subtle yet important differences in how FECD and its phenocopies may present in Caucasians vs Asians. Such differences are paralled by genotypic variations and disease management preferences which appear to be geographically and ethnically delineated. This article provides a succinct review of such differences, with a focus on diagnostic and management issues which may be encountered by ophthalmologists practicing in the different geographic regions, when evaluating a patient with FECD.
Subject terms: Corneal diseases, Eye manifestations, Disease genetics, Transplantation
摘要
在全球范围内, Fuchs角膜内皮营养不良 (FECD) 是角膜内皮移植术最常见的适应症之一。尽管FECD最早报道于高加索人, 但目前已在包括亚洲人在内的人群中广泛流行。 FECD的典型临床表现是与角膜内皮细胞营养不良相关的中心斑点和色素沉着, 然而FECD和其拟表型在高加索人种和亚洲人种中的表现可能有细微但重要的差别。这些差别与基因多态性及与疾病管理的首选方式随区域和种族的不同相关。本篇文章简短地回顾了这些差别, 重点强调当眼科医生在不同地区面对FECD患者时, 诊断与管理方面的问题。
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a common corneal endothelial dystrophy, characterized clinically by centrally distributed Descemet membrane (DM) guttae and corneal endothelial dysfunction (Fig. 1) [1, 2]. It is a genetically heterogeneous disease attributable to a spectrum of genotypes such as the CTG trinucleotide repeat expansion in chromosome 18, single nucleotide polymorphisms within the TCF4 gene, and mutations in the SLC4A11 gene [3]. While endothelial keratoplasty (EK) is the current gold standard for management of the condition in its advanced stages, significant progress has been made in the development of novel therapeutic modalities such as cell-free descemet membrane transplantation [4, 5] and cell-injection therapy [6]. Discovery of the relatively high prevalence of the CTG trinucleotide repeat expansion sequence in FECD, in tandem with recent advances in gene editing techniques such as the CRISPR-Cas9 endonuclease platform [7, 8], implies that gene therapy may possibly benefit FECD patients as well [9–11]. This review focusses on the comparative differences in FECD phenotypes and genotypes between Asian and Caucasian populations, highlights differences in current management algorithms, and explores the potential for application of novel therapeutic modalities across these populations.
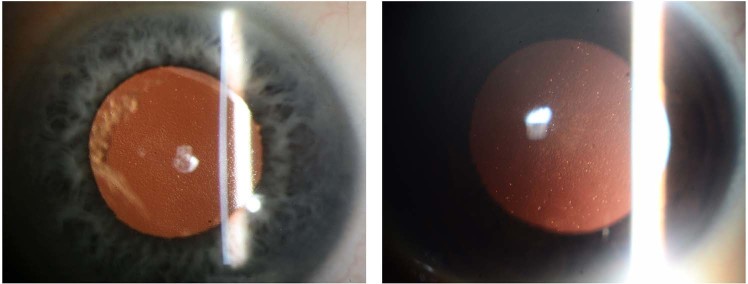
Fig. 1.
Retroilluminated anterior segment image of Fuchs endothelial corneal dystrophy. Left—Caucasian patient; Right—Asian patient
Prevalence
Among Caucasians, corneal guttae may be found in 11% of females and 7% of male participants in the Icelandic Reykjavik Eye Study [12], and the overall prevalence of FECD has been estimated to be ~21.6% from a small population residing on Tangier Island [13], in the United States of America. In Asia, a Japanese study [14] detected corneal guttae at a frequency of only 5.8% and 2.4% in female and male participants, respectively. While a slightly higher prevalence of the disease was found in a Singaporean-Chinese population (8.5% and 4.4% for females and males, respectively) [15], it is apparent that FECD is more frequent in Caucasians compared with Asians. In addition, regardless of geographical distribution, females appear to be at greater risk of harboring the disease (Table 1). While this leads to the hypothesis that the pathogenesis of FECD may possibly be subject to the influence of sex hormones, it is counter-intuitive to previous work which has established the beneficial effects of estrogen against cellular senescence [16–19].
Table 1.
Prevalence of Fuchs endothelial dystrophy in Western vs Eastern populations
| Origin | Year | Author | N (eyes) | Age (years) | Prevalence (%) | Ratio F:M | ||
|---|---|---|---|---|---|---|---|---|
| Overall | Females | Males | ||||||
| America | ||||||||
| USA | 1933 | Goar et al. [180] | 800 | >21 | 6.62 | 9.07 | 3.62 | 2.5:1 |
| 1967 | Lorenzetti et al. [181] | 1348 | >40 | 3.9 | 4 | 3.8 | 1.05:1 | |
| Europe | ||||||||
| Iceland (Reykjavik) | 2006 | Zoega et al. [12] | 1548 | >55 | 9.2 | 11 | 7 | 1.6:1 |
| Asia | ||||||||
| Chinese Singaporean | 2002 | Kitagawa et al. [15] | 920 | >50 | 6.7 | 8.5 | 4.4 | 2:1 |
| Japan | 1996 | Nagaki et al. [182] | 211 | 56–76 | 3.3 | 3.3 | – | – |
| Japan (Ishikawa Prefecture) | 2002 | Kitagawa et al. [15] | 598 | >50 | 3.7 | 5.5 | 1.5 | 3.7:1 |
| Japan (Southwestern Island) | 2011 | Higa et al. [14] | 7524 | >40 | 4.1 | 5.8 | 2.4 | 2.4:1 |
Geographical and ethnic differences in FECD prevalence may, in part, be related to genetic variations (read under section ‘Genetics’). There is also strong evidence implicating oxidative stress in the pathogenesis of FECD [20–22], which suggests that epigenetic influences such as geographical variations in UV exposure [23] may possibly affect FECD prevalence. Equatorial Asian countries, such as Singapore, are exposed to much higher levels of solar UV radiation compared with Caucasian populations, such as the Nordic countries located along higher latitudes [24], and may thus be expected to be at greater risk of UV-induced corneal endothelial cell oxidative stress/damage. However, Nordic populations are exposed to unique ecological and behavioral risk factors which may increase the risk of ocular UV damage, including: (a) increased levels of snow-reflected solar UV radiation [25], (b) prolonged, full-day UV exposure as a result of the polar day (also known as ‘midnight sun’) phenomenon during summer months [26], (c) low solar elevation angle during winter months which contributes to persistent and excessive glare [27], (d) progressive ozone layer depletion leading to increased penetrance of solar UV rays [28, 29], and (e) popularity of indoor sunbed usage due to compensate for the lack of exposure to natural sunlight [30–34].
Phenotype and phenocopies
FECD is characterized by the hallmark features of DM guttae and pigment deposition, endothelial cell pleomorphism and polymegathism, and corneal edema as a result of corneal endothelial dysfunction [1, 2]. The presence of such features allows the clinician to make an unequivocal diagnosis of FECD. However, these changes may be subtle in the early stages of FECD, during which time it may be confused with other disease phenocopies.
Posterior polymorphous corneal dystrophy
Posterior polymorphous corneal dystrophy (PPCD) is a relatively rare, autosomal dominant disease. It is clinically characterized by a corneal endothelial dystrophy which manifests as vesicles and opacities on the posterior corneal surface [35], and ectatic changes [36] resulting in abnormally steep corneal curvatures. Systemic associations include Alport syndrome and abdominal hernias [37–39]. The endothelial dystrophy in PPCD occurs secondary to an upregulation of endothelial–mesenchymal transition (EMT), which results in corneal endothelial cells exhibiting a more fibroblastic and epithelial-like phenotype [40]. In chronic and progressive disease, endothelial failure may be accompanied by features akin to iridocorneal endothelial (ICE) syndrome, such as peripheral iridocorneal adhesions, iris distortions and secondary glaucoma [40, 41]. PPCD is a genetically heterogeneous disease which may be classified into three subtypes, namely: (i) PPCD1 associated with VSX1 mutations in chromosome 20 [42, 43]; (ii) PPCD2 associated with COL8A2 mutations in chromosome 1 [44], and (iii) PPCD3 associated with ZEB1 mutations in chromosome 10, which is the most common subtype among all [38, 45]. The implication of ZEB1 mutations and its effect on upregulation of EMT in the pathogenesis of PPCD is of interest in this review, considering that the pathogenesis of FECD may involve a similar pathway. ZEB1 expression has been shown to be upregulated in FECD patients, which leads to overproduction of extracellular matrix proteins and guttae deposition [46]. TCF4 mutations, which are now known to be strongly associated with the FECD phenotype [47–49], directly affects the activity of transcription factor E2-2, which in turn regulates ZEB1 [50]. The overlapping pathogenetic pathways of these two diseases are paralleled by their relatively similar clinical presentations. In particular, the Descemet membrane (DM) vesicles and thickenings seen in PPCD may be misclassified as FECD, especially in Caucasian populations such as in the Czech Republic, which has the highest reported worldwide prevalence of PPCD (1 in 100,000 inhabitants) [51]. In contrast, PPCD is a rare disease in Asia, which represents only an insignificant minority of cases requiring keratoplasty [52].
Viral endotheliitis
While the majority of anterior uveitis cases are idiopathic, a small proportion may be attributable to viral etiologies, such as cytomegalovirus (CMV), herpes simplex virus (HSV), and rubella virus infections [53]. Regardless of the exact etiological agent, viral anterior uveitis present with a spectrum of overlapping features such as anterior segment inflammation, keratic precipitates (KPs), ocular hypertension, and various patterns of iris atrophy [54]. In particular, CMV anterior uveitis manifests as either acute and relapsing bouts of hypertensive anterior uveitis akin to Posner-Schlossman syndrome (PSS), or chronic hypertensive anterior uveitis as in the case of Fuchs heterochromic iridocyclitis (FHIC) [55]. Both presentations can be associated with a viral endotheliitis [55] which, in the presence of pigmented KPs and endothelial cell loss/dysfunction (Fig. 2), may be mistaken for asymmetrical FECD. In a patient who has previously undergone keratoplasty, CMV endotheliitis with endothelial cell loss and a low-grade anterior chamber inflammatory reaction may also be easily mistaken as acute graft rejection [56].
Fig. 2.
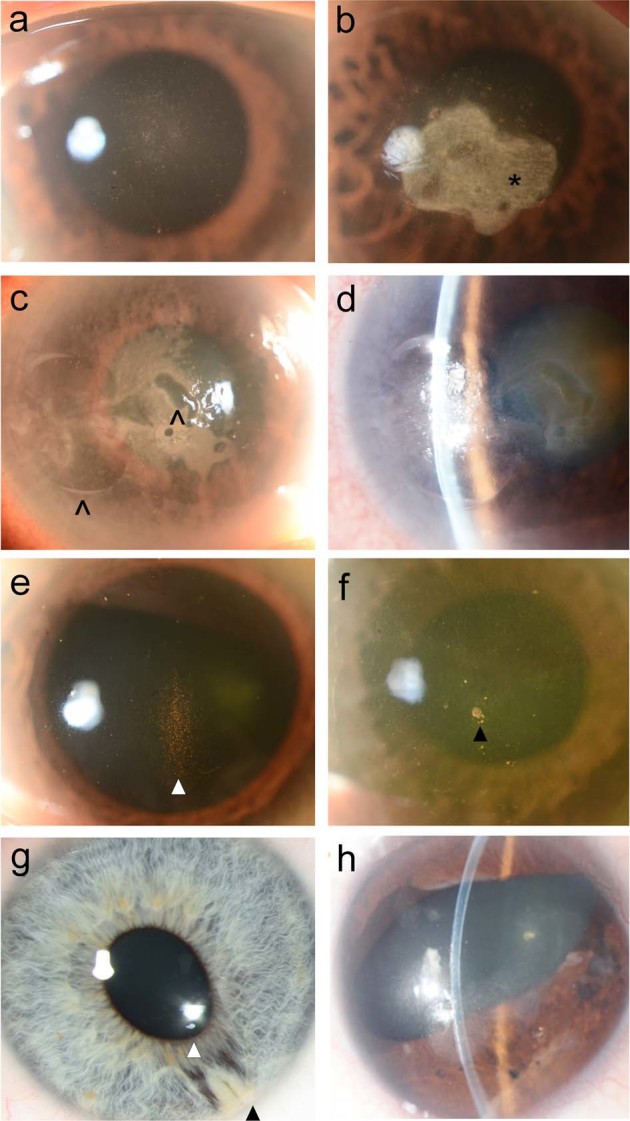
Anterior segment photographs. a Fuchs endothelial corneal dystrophy (FECD) in an Asian eye. b FECD with stromal scarring (scar indicated by *). c FECD complicated by bullous keratopathy (epithelial bullae indicated by ^). d Slit-beam demonstrating concommitant shallow anterior chamber and a brunescent cataract in the same eye. e Krukenberg’s spindle in an Asian eye with pigment dispersion syndrome (white arrowhead). f CMV endotheliitis with endothelial pigment deposits and a ‘coin-lesion’/nummular keratic precipitate (black arrowhead). g ICE syndrome in a Caucasian patient with corectopia (white arrowhead) and peripheral anterior synechiae at the 5 o’clock position (black arrowhead). h ICE syndrome in an Asian patient of the essential iris atrophy subtype
The diagnostic challenge of differentiating CMV anterior uveitis from the abovementioned non-infectious etiologies is particularly problematic in Asian patients, in whom the disease has been much more commonly reported compared with Caucasians [54, 55, 57–59]. This may be related to the higher seroprevalence of CMV in Asia [60]. CMV seropositivity rates are in the range of 70–90% within Asian countries, including Japan [61] and India [62–64], with lower rates of 50–70% in Western countries, including North America [65] and Europe [66]. Herpes simplex virus appears to be a much more important etiological agent of ocular infections in Caucasians populations, with an incidence of 20.7 per 100,000 patients per year in the United States of America [67]. While there is a paucity of published data regarding the incidence of herpetic keratitis in Asia it is, in our experience as an Asian tertiary eye-care institution, a much less common disease than would be expected in the West.
Imaging modalities such as specular microscopy and confocal microscopy may assist in the evaluation of DM guttae and hyper-reflective endothelial round bodies [68] found in FECD and CMV endotheliitis, respectively. In addition, an aqueous tap in association with either CMV antibody titer assessment (i.e., Goldmann–Witmer coefficient, GWc) or polymerase chain reaction (PCR) should be performed for all patients with anterior uveitis in whom a viral etiology cannot be confidently excluded [69]. In the presence of an active viral anterior uveitis, the treatment of choice should be that of a loading followed by chronic maintenance regime of antivirals [70]. Patients with either a combined diagnosis of FECD and CMV endotheliitis, or those with CMV endotheliitis which has progressed to end-stage endothelial decompensation, should also be adequately treated with antivirals for at least 6 months prior to consideration of keratoplasty, in order to reduce the subsequent risks of graft failure [47, 56, 71]. Specifically for CMV endotheliitis, antiviral therapeutic agents include systemic ganciclovir/valganciclovir [48] and/or topical ganiclovir ointment [49]. While there are currently no standardized guidelines for the treatment of CMV endotheliitis [72], most of such patients seen at our center are commonly treated with a combination of both systemic and topical antiviral agents, which in our opinion is a more effective approach than systemic or topical antiviral monotherapy. This practice is also in keeping with the findings of the Japan Corneal Endotheliitis Study Group published in 2014 [73]. While intravitreal ganciclovir injection has previously been demonstrated to be effective for the treatment of CMV endotheliitis in a single case report it is, to the best of our knowledge, not a commonly practised approach; similarly, despite the theoretical possibility of intracameral ganciclovir being beneficial in optimizing corneal endothelial drug bioavailability, this approach has not yet been clinically evaluated.
Pigment dispersion
In pigment dispersion syndrome (PDS), reverse pupillary block results in a concave configuration of the mid-peripheral iris and chafing of the posterior iris pigment epithelium (PPE) against the lens zonules [74]. Chronic mechanical trauma of the PPE results in characteristic mid-peripheral iris trans-illumination-defects (TIDs) in association with intraocular deposition of pigments such as in the angle of the anterior chamber (AC), lens capsule and corneal endothelium [75]. In particular, pigment deposited on the corneal endothelium may assume the characteristic configuration of a vertically oriented Krukenberg’s spindle (KS) located at the center of the cornea (Fig. 2) [76].
While iris TIDs are often readily visible in Caucasian patients with light-colored irides [75], such a change is rarely observed among Asian patients [77], in whom the iris stroma is significantly thicker and more heavily pigmented. In such cases, the centrally located KS may be mistaken for central pigment deposition analogous to that seen in FECD. The diagnosis of PDS would be missed in the absence of a careful gonioscopic examination and if the clinician fails to look out for other associated features of PDS such as glaucomatous optic disc changes.
ICE syndrome
In the ICE syndrome, corneal endothelial cells undergo EMT to assume fibroblastic characteristics. These cells migrate across the corneal endothelium, angle of the AC and onto the anterior iris surface to result in characteristic pathological changes such as a ‘beaten bronze’ appearance in Chandler syndrome, pseudopolycoria in essential iris atrophy and numerous iris nevi-like lesions in Cogan-Reese syndrome [78]. In particular, corneal endothelial changes encountered in Chandler syndrome may mimic the clinical appearance of corneal endothelial guttae associated with FECD, and it may be difficult to differentiate these disease entities especially when the other associated features of ICE syndrome such as iris atrophy and peripheral anterior synechiae (Fig. 2) are not obvious. The higher prevalence of Chandler syndrome among Caucasians [79] compared with Asians, in whom Cogan-Reese syndrome [80] and essential iris atrophy [81] were found to be more common, may lead to difficulties in differentiating Caucasians with ICE syndrome, especially those with the Chandler syndrome subtype, from those with FECD, at an early stage. Specular or confocal microscopy would be a useful adjunct in these cases, as ICE syndrome gives rise to the characteristics appearance of dark-light inversion of corneal endothelial cells [82], while FECD is not associated with such changes. While patients affected by ICE/Chandler syndrome are at risk of eventually developing bullous keratopathy just like in FECD, ICE syndrome is usually unilateral [83–86], and it has a worse visual prognosis which often involves a more complex long-term management plan, in view of its significant associations with glaucoma and the greater risk of disease recurrence even after keratoplasty [87, 88].
Drug-induced endotheliitis
For patients with chronic viral uveitis and endotheliitis, the use of ocular immunosuppressants, such as topical cyclosporine [89], topical prostaglandin analogs [90], and sustained-release intravitreal steroid implants [91], may trigger viral reactivation and recurrent endotheliitis. Amantadine—a medication used to treat Parkinson’s disease—has also been associated with a dose-dependent decline in corneal endothelial cell density, pleomorphism, polymegathism, and endothelial decompensation over time [92, 93], in a fashion similar to FECD.
Genetics
FECD is a genetically heterogeneous disease. Mutations in a large number genes including but not limited to FCD1/2/3/4 [94–97], COL8A2 [44], SLC4A11 [98], ZEB1 [99], KANK4, LAMC1, and ATP1B1 [100] have been found in association with FECD. These genetic mutations are generally associated with a relatively conserved set of phenotypic features as would be expected in typical FECD, with the exception of COL8A2 mutations which result in early onset FECD; in this group of patients, corneal endothelial dysfunction has been observed as early as in the 2nd decade of life [44, 101]. In addition, FECD has also been characterized as a trinucleotide repeat (TNR) disease [102], in which an expanded CTG repeat sequence in the TCF4 gene located on chromosome 18.1 (CTG 18.1 genotype) causes the disease phenotype by interference of mRNA splicing [103]. Among these wide spectrum of genetic changes, the CTG 18.1 genotype is the most important identified thus far, as it is associated with the FECD phenotype at a much higher frequency compared with each of the other genotypes. For example, the prevalence of ZEB1 and SLC4A11 mutations were found to be approximately 2% and 5%, respectively [99, 104]. In contrast, the CTG 18.1 was much more prevalent in FECD patients, albeit at a greater frequency among Caucasians compared with Asian populations. Within Asian populations, the CTG 18.1 genotype was found in 43.9% of Chinese patients by Xing et al. [105], 26% of Japanese patients by Nakano et al. [106], and 17.3–34% of Indian patients by Rao et al. [107] and Nanda et al. [108], respectively. In contrast, the prevalence of the CTG 18.1 allele within a North-American Caucasian population was much higher, in the range of 62.1–73.3% [102, 109–111]. A similarly higher prevalence was noted in other Caucasian populations, such as 51% of Australian patients in the study by Kuot et al. [112] and 79% of German patients in the study by Luther et al. [113].
Management
FECD is a progressive disease associated with visual symptoms ranging from transient blurring of vision upon awakening, during the early stages of the disease, to a debilitating loss of central visual acuity when the cornea endothelium eventually decompensates [1]. Management of FECD progresses in a stepwise fashion, ranging from watchful management or topical hypertonic saline ointment in its early stages, to endothelial keratoplasty (EK) or penetrating (PK) for more advanced disease [3].
Indicators such as corneal pachymetry, specular or confocal microscopy findings and central visual acuity are useful adjuncts in guiding the clinician’s assessment of disease severity. In addition, the clinician would have to take into consideration the presence of other ophthalmic co-morbidities and risk factors in one assessment of a patient’s requirement and suitability to undergo surgical intervention. In Asia, the relatively higher prevalence of angle closure compared with Caucasians [114], especially in elderly females [115] who are also at greatest risk of FECD, results in a relatively common scenario wherein an elderly patient presents with the triple combination of (i) shallow anterior chamber, (ii) FECD, and (iii) cataracts (Fig. 3). Phacoemulsification surgery under such circumstances is associated with significantly greater risks of corneal endothelial decompensation. In addition, corneal endothelial reserves in such patients may have been further attenuated by earlier attacks of acute primary angle closure, chronic primary angle closure glaucoma with poorly controlled intraocular pressure or laser peripheral iridotomy (LPI) induced bullous keratopathy (BK) [116, 117]. The latter may be especially problematic among Asian patients [118] with relatively thicker irides who may require a large number of additional high-fluence argon-laser shots to achieve patency during LPI. Preoperatively, it may be worthwhile to counsel these patients on their risk of corneal endothelial decompensation following phacoemulsification, for which surgical options would include either a combined phacoemulsification + EK, or a staged phacoemulsification followed by EK as a separate procedure subsequently. In a phakic patient with concomitant narrow anterior chamber angles and FECD for whom LPI is being considered, it would be judicious for the clinician to lower the visual acuity threshold for considering cataract extraction, and to defer LPI in favor of early phacoemulsification in order to lower the risks of subsequent post-operative corneal endothelial decompensation. In addition, for patients in whom the corneal endothelium is already noted to be compromised preoperatively, it would be advantageous to consider combined phacoemulsification + EK rather than phacoemulsification alone followed by EK only upon the development of BK, as the former is associated with significantly better post-operative visual outcomes [119]. Having said that, it should be qualified that it may not be straightforward to accurately predict preoperatively, the risk of an FECD patient decompensating following phacoemulsification alone. While more severe FECD is correlated to a higher post-phacoemulsification risk of corneal decompensation, such an outcome may occur in FECD patients with milder disease as well.
Fig. 3.
Anterior segment photographs of a phakic Asian eye with the concommitant diagnoses of a Fuchs endothelial corneal dystrophy (white arrowhead indicating endothelial pigments and guttata) and b primary angle closure suspect with shallow anterior chamber, for which c laser peripheral iridotomy (black arrowhead) has been performed
Besides disease severity, the decision to undergo keratoplasty also depends on a wide spectrum of other non-medical factors such as a patient’s lifestyle and visual requirements, access to medical care and surgical expertise, and other psycho-social-economic ideas, concerns and expectations regarding corneal transplantation. In western nations such as the United States of America (USA) and Europe where FECD patients have relatively easy access to high quality medical care, there is increasingly a shift toward early surgical intervention [120]. Keratoplasty used to be offered only to patients with advanced disease who suffer from impaired central visual acuity or corneal edema. Improvements in surgical techniques over the past decade, notably developments in the field of endothelial keratoplasty (EK), have generally led to a favorable tilt in the risk-benefit ratio when considering the merits of keratoplasty for FECD [121–124]. This, coupled with increased awareness of the disease and its treatment options, has resulted in a trend wherein patients are increasingly keen to consider keratoplasty as the definitive therapeutic modality even in early stages of the disease, during which time the only visual symptoms may be those of increased higher-order-aberrations such as glare and halos, or decreased contrast sensitivity [125–127].
EK is generally perceived to be a safe and effective procedure [121–124], and is in fact the procedure of choice for treatment of FECD at most tertiary eye hospitals [128–133]. However, there are exceptions to the norm, such as a large registry study in Australia which indicated poorer survival and visual outcomes for EK compared with PK, regardless of surgeon experience [134]. While such results are in contrast to most other reports [121–124], they are derived from real-world data evaluating outcomes from multiple surgeons and institutions, as opposed to the other studies which typically described the surgical outcomes from a single or small group of surgeons [121–124]. The choice between EK or PK is less dependent on specific geographical distributions (i.e., Western vs Asian countries), than it is on a panel of multiple factors including institutional or surgeon preferences, availability of EK surgical expertise, economic feasibility considerations related to the costs incurred for the establishment of EK tissue preparation facilities, and stage of presentation (e.g., presentation at the stage of BK and stromal scarring would be a contraindication to EK).
All over the world, there are institutions in which PK is still preferred over EK for the treatment of endothelial diseases. For example, in a 2015 survey of keratoplasty trends in China, it was found that only 5% of corneal surgeons have ever received training in or performed EK, in contrast to 88% of surgeons who were adept in PK [135]. A review of keratoplasties performed in Vietnam from 2002 to 2013 showed that EK accounted for only 2% of total keratoplasty load over the decade [136]. While the average rate of EK in Iran from 2006 to 2013 was similarly low at 7.5%, there has been a significant shift toward EK in recent times, as evidenced by an increase in EK load from 0% in 2006 to 14.9% in 2013 (Iran National Registry Data) [137]. In New Zealand, a survey of corneal transplantation practices from 1999 to 2009 indicated that EK accounted for <3% of all keratoplasties [138], which mirrored the extremely low local rates of lamellar keratoplasty observed over the preceding decade [139]. While there have been reports of endothelial keratoplasty from South Korea [123, 140–143], the majority of all procedures performed are still PKs, with lamellar keratoplasties accounting only for 2.7% of all cases nationwide (collective data from 25 hospitals in South Korea) [144]. In Japan, a cross-sectional national survey of surgical management trends for bullous keratopathy in 2007 found that an overwhelming majority (97.7%) of all patients were managed with PK [145]. Nonetheless, it must once again be emphasized that such rates may differ significantly depending on the availability of surgical expertise, even within the same country—for example, the number of EKs performed at Kanazawa University Hospital in Japan has doubled from 2007 to 2016, with most cases being that of Descemet stripping automated endothelial keratoplasty (DSAEK) [146]. An audit report by the Eye Bank Association of America in 2016 found that PK was generally still the most common keratoplasty procedure performed in international centers using US donor tissue (74.4%), with EK accounting for only 14.9% of all cases [147].
In other institutions such as the University of Toronto in Canada, a contrasting trend has been observed. Over the decade from 2002 to 2012, EKs accounted for 29.5% of all keratoplasties performed, with the increasing popularity of EK accompanied by a commensurate decrease in frequency of PK by 61.8% [148]. In 2016, up to 93.1% of all FECD patients in USA who required keratoplasty were treated with EK, with only 6.9% of patients receiving penetrating keratoplasty (PK) for various reasons such as late presentation and postural difficulties [147]. This has contributed to EK being the most common keratoplasty procedure performed with preserved corneal tissue in USA (44% of total surgical load), in contrast to PK which accounted for only 38% [147]. In France, EK surpassed PK as the most commonly employed technique for the management of FECD in 2013, with EK being performed in up to 70% of FECD cases undergoing keratoplasty in 2015 [131]. The trend toward EK has been even more marked in Italy, in which EK accounted for more than 62% of all procedures performed for patients with BK [149]. EK surpassed PK as the preferred surgical technique for management of BK in Italy in 2008 [149], at about the same time as EK was noted to overtake PK as the procedure of choice for management of FECD in Scotland [150]. The popularity of EK is also rapidly rising in Germany, accounting for 57% of all keratoplasties performed in 2016 in contrast to only 1.4% in 2006 [151]. While it may appear that the preference for EK exists only in Western nations, it should be noted that such a trend has also been observed in Asian nations such as Singapore [152]. At the Singapore National Eye Centre (SNEC), EK overtook PK as the surgical technique of choice for the mangement of FECD in 2009, with PK reserved only for the infrequent patient with neglected, end-stage disease complicated by stromal scarring. In 2017, 59.9% (n = 340) of all keratoplasties performed at SNEC were EKs, in contrast to PK which accounted for only 15% of the total caseload. In Eastern India (Prova Eye Bank, Disha Eye Hospitals, Barrackpore, India), an approximately equal number of EKs and PKs were performed in 2015 [153]. A similar trend has been observed at a tertiary eye-care institution in Southern India (Ramayamma International Eye Bank, L. V. Prasad Eye Institute, Hyderabad, India)—while in 2007 only 17% of FECD patients were treated by EK in contrast to PK for the remaining 83%, this trend had reversed by 2011, with 80% of FECD patients being treated by EK instead of PK [154].
Endothelial keratoplasty—surgical techniques
DSAEK and Descemet membrane endothelial keratoplasty (DMEK) are the most common methods of performing EK. While the absence of a donor stroma in DMEK allows a more precise post-operative anatomical configuration which is associated with less refractive change [155–157] and rejection risk [158], DMEK is a more technically challenging procedure than DSAEK due to the difficulties which may be encountered during preparation, insertion and unscrolling of the delicate single-cellular endothelial layer. Both DSAEK and DMEK are widely practised, with the choice of surgical technique dependent on the availability of surgical expertise, access to donor tissue and casemix. For example, while DSAEK used to be the more popular technique accounting for most EK procedures performed at a University Eye Hospital in Germany in 2008, by 2015 the trend has reversed such that DMEK now represents the technique of choice for patients who require EK [129]. At the Singapore National Eye Centre, 58% of all keratoplasty procedures performed in 2018 were endothelial keratoplasty, out of which 50% were DSAEK and 50% were DMEK. For DMEK, the graft is prepared and folded in such a manner as to create an ‘endothelial-in’ graft configuration, followed by insertion via a pull-through technique using a donor-mat device [159]. The endo-in, donor-mat approach is preferred at our center because it facilitates graft insertion and positioning. This is a particularly important advantage for late-presenting FECD cases associated with bullous keratopathy and poor view of the anterior chamber, which are commonly encountered in our practice. While there is scant data available regarding the relative popularities of DMEK vs DSAEK in Asia, we postulate that DSAEK is likely to be more commonly practised. The relative paucity of eye-bank facilities in Asia equipped to prepare pre-stripped DMEK graft tissue likely precludes DMEK in many institutions. In addition, DSAEK is a relatively easier procedure than DMEK, especially in late-presenting patients with bullous keratopathy, which as mentioned earlier is relatively prevalent in Asia. In fact, when considering the technical difficulties associated with performing even DSAEK in such decompensated eyes, coupled with fears of inadvertent donor tissue wastage and the scarcity of cadaveric donor corneas in Asia, it is not surprising that PK remains a highly popular technique in Asia for the management of patients with FECD.
In view of a worldwide shortage in cadaveric donor grafts, there have been efforts to maximize the therapeutic yield of cadaveric donor grafts. Descemet membrane transplantation (DMT), which involves the transplantation of acellular DM devoid of corneal endothelial cells, represents one such approach which will be discussed in detail in the in next section. In addition, Melles et al. have also successfully demonstrated the efficacy of hemi- and quarter-DMEK [160, 161], i.e., the transplantation of semicircular or quadratic endothelial graft segments instead of a standard, circular endothelial graft, which allows between 2 and 4 patients to benefit from a single cadaveric donor graft.
Timing of surgery
There have been reports of mean pre-operative best-corrected-visual-acuity being in the range of 6/12 or better, among patients undergoing DMEK in USA [120]. This contrasts with other reports wherein the majority of patients listed for keratoplasty have pre-operative visual acuities of worse than 6/24 [162, 163]. In fact, from an Asian perspective, we continue to encounter a significant proportion of patients who either present late or accept surgical intervention only in advanced stages of the disease, such as when BK (Fig. 2) leads to intractable pain and when chronic stromal edema and scarring leads to a significant loss of central visual acuity [164]. Cultural prejudices against cadaveric tissue transplantation may favor the adoption of conservative management options among elderly patients in lieu of the relatively more onerous alternative of surgical transplantation. In our experience as one of the major regional referral centers for keratoplasty in Asia, the lack of access to donor cornea tissue and paucity of surgical expertise in keratoplasty among healthcare institutions offering primary eye-care services leads to yet another unfortunate but often encountered scenario: patients suffering from moderate FECD and cataracts who have undergone phacoemulsification by a general ophthalmologist, present to our clinics only when FECD has progressed to an advanced stage or even BK, as a result of either their misconceptions or the primary physician’s failure to adequately counsel regarding the option of further surgical interventions to address their endothelial dystrophy. Regardless of the cause of late presentation, such a scenario is undesirable, as it is known that patients with advanced disease and BK tend to have poorer post-operative outcomes [119, 163]. On the contrary, early surgical intervention appears to be strongly correlated with improved visual outcomes, with up to 67% among all patients undergoing DMEK, with a mean pre-operative visual acuity of 6/12, being able to achieve visual acuity of at least 6/7.5 post-operatively [120, 165].
Future therapeutic modalities
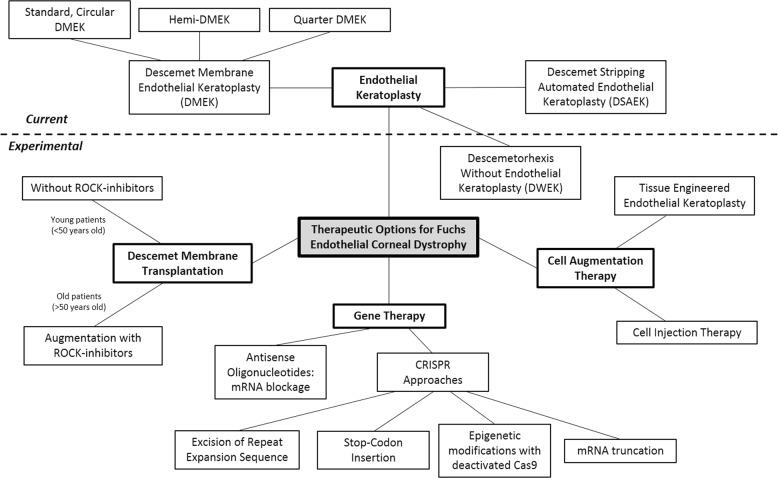
While EK is the current standard-of-care for advanced FECD, significant progress has been made over the past decade to develop novel, alternative therapeutics for FECD. These alternative therapeutics can be broadly divided as (i) Cell-free corneal transplantation, (ii) Pharmacological adjuncts, (iii) Cell-injection therapy, (iv) Tissue-engineered EK (TE-EK), and (v) Gene therapy. Current surgical management options and experimental therapeutic appproaches for FECD are summarized in Fig. 4.
Fig. 4.
Current and experimental therapeutic options for the management of Fuchs endothelial corneal dystrophy
In FECD, diseased corneal endothelial cells and DM guttae are located centrally, with relative sparing of the corneal peripheries. It has been shown in an ex vivo cadaveric human corneal organ culture study that upon removal of central corneal endothelial cells, peripheral endothelial cells may rapidly repopulate the central cornea, especially in subjects younger than 50–60 years of age [166]. In vivo rabbit studies have further demonstrated that central endothelial recovery may occur even after stripping of the central DM, provided the DM defect was replaced by an acellular DM graft [4]. In contrast, Descemet membrane stripping alone, without transplantation of a basement membrane to replace the DM defect (i.e., Descemet stripping without endothelial keratoplasty, DWEK [167]), led to highly unpredictable endothelial recovery responses in adults [3], and are likely to be successful only in young patients who retain excellent functional endothelial reserves [168]. Subsequent to these findings, a phase 1 clinical trial found that transplantation of a cell-free corneal graft, or DMT, following stripping of a central disc of diseased DM in a patient suffering from FECD, resulted in prompt recovery of the central corneal endothelium [5]. While this approach remains to be validated in more patients, it represents an improvement from the status quo of EK, as it holds the potential for greatly increasing the number of FECD patients who can benefit from low cell-count endothelial grafts which would otherwise have been discarded. When considered against the aforementioned differences in management choices between Asians and Caucasians, it appears that the latter group of patients, who are more likely to present and accept surgical intervention at an earlier stage of the disease, would be better poised to benefit from DMT, which is known to work better in younger patients [166].
Rho-associated protein kinase inhibitors (ROCK inhibitors) belong to a relatively novel class of ophthalmic pharmacological agents, with a single formulation (Ripasudil Hydrochloride 0.4% topical eyedrop) having recently been approved in Japan for the treatment of glaucoma, in 2014 [169]. In recent years, ROCK inhibitors have also been shown to be potent stimulants of corneal endothelial cell migration, at least within an in vitro cell culture environment [166, 170]. Accordingly, in 2018, a phase IIa clinical trial was initiated by Kruse et al. in Germany to evaluate the potential efficacy of ROCK inhibitors for the treatment of FECD (clinicaltrials.gov, NCT03575130). In the setting of DMT and amongst Asian patients who tend to present later with more advanced FECD, a combination strategy of DMT in association with topical ROCK inhibitors to promote corneal endothelial recovery may be possibly enhance endothelial recovery following central DM stripping [171]. Alternatively, cell-augmentation following central DM stripping, in the form of either cell-injection therapy [172] or transplantation of a TE-EK, may also be efficacious in such patients who present later. In a recent phase 1 clinical trial, cell therapy via intracameral injection of cultured corneal endothelial cells and Rho-associated protein kinase inhibitor was shown to be effective in restoring corneal transparency and re-establishing a corneal endothelial monolayer among patients suffering from BK [6].
Following the discovery of the association between the CTG 18.1 genotype and FECD, it has been proposed that FECD may be amenable to treatment via gene therapy [3]. Multiple techniques have been suggested, including the use of antisense oligonucleotides to target sense-expanded CUG repeat transcripts and inhibiting misplacing events [173]. In recent years, it has also been found that the prokaryotic CRISPR-Cas9 endonuclease platform may be reprogrammed with appropriately designed guide-RNAs to perform site-specific gene editing in eukaryotic cells [7, 8]. There is great potential in further exploration of CRISPR-Cas9’s ability to treat FECD via in vivo gene editing approaches [7, 8, 174–176] such as induction of insertion or deletion (indel) mutations via non-homologous-end-joining, truncation of the CTG repeat expansion sequence, insertion of a stop-codon at a location proximal to the promoter sequence [177], CRISPR-induced epigenetic modifications of gene expression and truncation of the deleterious RNA transcripts [178, 179]. Corneal endothelial cells are relatively accessible in vivo and thus may be easier to transfect with viral vectors, via intracameral injection, compared with cells from other deep visceral organs. In addition, any phenotypic alterations to the cornea secondary to successful knock-out of the CTG 18.1 genotype may be easily observed as corneal endothelial cell morphological changes on specular microscopy, and functionally as a reversal of corneal edema, all of which provides direct evidence of treatment efficacy. In consideration of the greater prevalence of the CTG 18.1 genotype in Caucasians [102, 109–111] compared with Asians [105], gene editing strategies targeting the CTG 18.1 genotype may be comparatively more successful in Caucasians. An additional factor to take into consideration when evaluating the practicality of gene editing for FECD pertains to the timing of disease detection and treatment. While gene editing may possibly prevent further worsening of the FECD phenotype, it is unlikely that gene editing would be successful in removing the collagenous deposits on the DM (i.e., guttata) once formed. As such, the success of a gene editing approach is premised on the ability to diagnose FECD at an earlier stage (i.e., before significant guttae develop), and on patient attitudes toward accepting treatment while asymptomatic—the discussion of which is beyond the scope of this review.
Conclusion
While FECD was first described among Caucasian patients, it is now known to affect multiple ethnic groups across various geographical borders. The spectrum of phenotypic and genotypic differences among these different populations is paralleled by variations in patient and physician attitudes toward treatment, including keratoplasty. Even when considering novel therapeutic approaches, differences in stage of presentation and prevalence of CTG repeats between Caucasian and Asian populations would most likely be important in guiding the therapeutic modality of choice.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 2.Elhalis H, Azizi B, Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocul Surf. 2010;8:173–84. doi: 10.1016/s1542-0124(12)70232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soh YQ, Peh GS, Mehta JS. Evolving therapies for Fuchs’ endothelial dystrophy. Regen Med. 2018;13:97–115. doi: 10.2217/rme-2017-0081. [DOI] [PubMed] [Google Scholar]
- 4.Bhogal M, Lwin CN, Seah X-Y, Peh G, Mehta JS. Allogeneic Descemet’s membrane transplantation enhances corneal endothelial monolayer formation and restores functional integrity following Descemet’s stripping. Invest Ophthalmol Vis Sci. 2017;58:4249–60. doi: 10.1167/iovs.17-22106. [DOI] [PubMed] [Google Scholar]
- 5.Soh YQ, Mehta JS. Regenerative therapy for Fuchs endothelial corneal dystrophy. Cornea. 2018;37:523–7. doi: 10.1097/ICO.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, Tanaka H, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378:995–1003. doi: 10.1056/NEJMoa1712770. [DOI] [PubMed] [Google Scholar]
- 7.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Angela Y., Jaskula-Ranga Vinod, Jun Albert S. Gene Editing as a Potential Therapeutic Solution for Fuchs Endothelial Corneal Dystrophy. JAMA Ophthalmology. 2018;136(9):969. doi: 10.1001/jamaophthalmol.2018.2324. [DOI] [PubMed] [Google Scholar]
- 10.Williams KA, Irani YD. Gene therapy and gene editing for the corneal dystrophies. Asia-Pac J Ophthalmol Phila Pa. 2016;5:312–6. doi: 10.1097/APO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 11.Christie KA, Courtney DG, DeDionisio LA, Shern CC, De Majumdar S, Mairs LC, et al. Towards personalised allele-specific CRISPR gene editing to treat autosomal dominant disorders. Sci Rep. 2017;7:16174. doi: 10.1038/s41598-017-16279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoega GM, Fujisawa A, Sasaki H, Kubota A, Sasaki K, Kitagawa K, et al. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006;113:565–9. doi: 10.1016/j.ophtha.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Eghrari AO, McGlumphy EJ, Iliff BW, Wang J, Emmert D, Riazuddin SA, et al. Prevalence and severity of Fuchs corneal dystrophy in Tangier Island. Am J Ophthalmol. 2012;153:1067–72. doi: 10.1016/j.ajo.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higa A, Sakai H, Sawaguchi S, Iwase A, Tomidokoro A, Amano S, et al. Prevalence of and risk factors for cornea guttata in a population-based study in a southwestern island of Japan: the Kumejima study. Arch Ophthalmol. 2011;129:332–6. doi: 10.1001/archophthalmol.2010.372. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa K, Kojima M, Sasaki H, Shui Y-B, Chew SJ, Cheng H-M, et al. Prevalence of primary cornea guttata and morphology of corneal endothelium in aging Japanese and Singaporean subjects. Ophthalmic Res. 2002;34:135–8. doi: 10.1159/000063656. [DOI] [PubMed] [Google Scholar]
- 16.Breu A, Sprinzing B, Merkl K, Bechmann V, Kujat R, Jenei-Lanzl Z, et al. Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res Publ Orthop Res Soc. 2011;29:1563–71. doi: 10.1002/jor.21424. [DOI] [PubMed] [Google Scholar]
- 17.Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005;23:1699–706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- 18.Imanishi T, Kobayashi K, Hano T, Nishio I. Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens Res. 2005;28:763–72. doi: 10.1291/hypres.28.763. [DOI] [PubMed] [Google Scholar]
- 19.Imanishi T, Tsujioka H, Akasaka T. Endothelial progenitor cell senescence—is there a role for estrogen? Ther. Adv. Cardiovasc Dis. 2010;4:55–69. doi: 10.1177/1753944709353173. [DOI] [PubMed] [Google Scholar]
- 20.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–89. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katikireddy KR, White TL, Miyajima T, Vasanth S, Raoof D, Chen Y, et al. NQO1 downregulation potentiates menadione-induced endothelial-mesenchymal transition during rosette formation in Fuchs endothelial corneal dystrophy. Free Radic Biol Med. 2018;116:19–30. doi: 10.1016/j.freeradbiomed.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthaei M, Zhu AY, Kallay L, Eberhart CG, Cursiefen C, Jun AS. Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy. Exp Eye Res. 2014;129:13–17. doi: 10.1016/j.exer.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young AR. Acute effects of UVR on human eyes and skin. Prog Biophys Mol Biol. 2006;92:80–85. doi: 10.1016/j.pbiomolbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Henriksen K, Stamnes K, Volden G, Falk ES. Ultraviolet radiation at high latitudes and the risk of skin cancer. Photodermatol. 1989;6:110–7. [PubMed] [Google Scholar]
- 25.Willmann G. Ultraviolet keratitis: from the pathophysiological basis to prevention and clinical management. High Alt Med Biol. 2015;16:277–82. doi: 10.1089/ham.2015.0109. [DOI] [PubMed] [Google Scholar]
- 26.Kroesch P. Summer eye safety: too often, a glaring omission. Occup Health Saf (Waco Tex). 2015;84:14. [PubMed] [Google Scholar]
- 27.Boulos EN, Jack D, Surowiec R, Bomback JL, Subramanian S, Simmons CJ, et al. Fundamental issues in automotive veiling glare. Warrendale, PA: SAE Technical Paper; 1997. https://www.sae.org/publications/technical-papers/content/970227/. Accessed 7 Aug 2018.
- 28.Bornman JF, Barnes PW, Robinson SA, Ballaré CL, Flint SD, Caldwell MM. Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems. Photochem Photobiol Sci. 2015;14:88–107. doi: 10.1039/c4pp90034k. [DOI] [PubMed] [Google Scholar]
- 29.Bais AF, McKenzie RL, Bernhard G, Aucamp PJ, Ilyas M, Madronich S, et al. Ozone depletion andclimate change: impacts on UV radiation. Photochem Photobiol Sci. 2015;14:19–52. doi: 10.1039/c4pp90032d. [DOI] [PubMed] [Google Scholar]
- 30.Doré J-F, Chignol M-C. UV driven tanning salons: danger on main street. Adv Exp Med Biol. 2017;996:335–46. doi: 10.1007/978-3-319-56017-5_28. [DOI] [PubMed] [Google Scholar]
- 31.Savoye I, Cervenka I, Mahamat-Saleh Y, Boutron-Ruault M-C, Kvaskoff M. Factors associated with sunbed use in women: the E3N-SunExp Study. Am J Health Behav. 2018;42:85–98. doi: 10.5993/AJHB.42.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Arnold M, Kvaskoff M, Thuret A, Guénel P, Bray F, Soerjomataram I. Cutaneous melanoma in France in 2015 attributable to solar ultraviolet radiation and the use of sunbeds. J Eur Acad Dermatol Venereol Jeadv. 2018;32:1681–6. doi: 10.1111/jdv.15022. [DOI] [PubMed] [Google Scholar]
- 33.Moan JE, Baturaite Z, Grigalavicius M, Juzeniene A. Sunbed use and cutaneous melanoma in Norway. Scand J Public Health. 2013;41:812–7. doi: 10.1177/1403494813496601. [DOI] [PubMed] [Google Scholar]
- 34.Køster B, Thorgaard C, Philip A, Clemmensen H. Sunbed use and campaign initiatives in the Danish population, 2007–9: a cross-sectional study. J Eur Acad Dermatol Venereol Jeadv. 2011;25:1351–5. doi: 10.1111/j.1468-3083.2010.03960.x. [DOI] [PubMed] [Google Scholar]
- 35.Strachan IM, Maclean H. Posterior polymorphous dystrophy of the cornea. Br J Ophthalmol. 1968;52:270–2. doi: 10.1136/bjo.52.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldave AJ, Ann LB, Frausto RF, Nguyen CK, Yu F, Raber IM. Classification of posterior polymorphous corneal dystrophy as a corneal ectatic disorder following confirmation of associated significant corneal steepening. JAMA Ophthalmol. 2013;131:1583–90. doi: 10.1001/jamaophthalmol.2013.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldave AJ, Yellore VS, Yu F, Bourla N, Sonmez B, Salem AK, et al. Posterior polymorphous corneal dystrophy is associated with TCF8 gene mutations and abdominal hernia. Am J Med Genet A. 2007;143A:2549–56. doi: 10.1002/ajmg.a.31978. [DOI] [PubMed] [Google Scholar]
- 38.Krafchak CM, Pawar H, Moroi SE, Sugar A, Lichter PR, Mackey DA, et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005;77:694–708. doi: 10.1086/497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teekhasaenee C, Nimmanit S, Wutthiphan S, Vareesangthip K, Laohapand T, Malasitr P, et al. Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology. 1991;98:1207–15. doi: 10.1016/s0161-6420(91)32152-3. [DOI] [PubMed] [Google Scholar]
- 40.Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–75. [PMC free article] [PubMed] [Google Scholar]
- 41.Cibis GW, Krachmer JA, Phelps CD, Weingeist TA. The clinical spectrum of posterior polymorphous dystrophy. Arch Ophthalmol. 1977;95:1529–37. doi: 10.1001/archopht.1977.04450090051002. [DOI] [PubMed] [Google Scholar]
- 42.Héon E, Greenberg A, Kopp KK, Rootman D, Vincent AL, Billingsley G, et al. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002;11:1029–36. doi: 10.1093/hmg/11.9.1029. [DOI] [PubMed] [Google Scholar]
- 43.Valleix S, Nedelec B, Rigaudiere F, Dighiero P, Pouliquen Y, Renard G, et al. H244R VSX1 is associated with selective cone ON bipolar cell dysfunction and macular degeneration in a PPCD family. Invest Ophthalmol Vis Sci. 2006;47:48–54. doi: 10.1167/iovs.05-0479. [DOI] [PubMed] [Google Scholar]
- 44.Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–23. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 45.Vincent AL, Niederer RL, Richards A, Karolyi B, Patel DV, McGhee CNJ. Phenotypic characterisation and ZEB1 mutational analysis in posterior polymorphous corneal dystrophy in a New Zealand population. Mol Vis. 2009;15:2544–53. [PMC free article] [PubMed] [Google Scholar]
- 46.Okumura N, Minamiyama R, Ho LT, Kay EP, Kawasaki S, Tourtas T, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab Investig. 2015;95:1291–304. doi: 10.1038/labinvest.2015.111. [DOI] [PubMed] [Google Scholar]
- 47.Ang M, Sng CCA, Chee S-P, Tan DTH, Mehta JS. Outcomes of corneal transplantation for irreversible corneal decompensation secondary to corneal endotheliitis in Asian eyes. Am J Ophthalmol. 2013;156:260–6. doi: 10.1016/j.ajo.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Anshu A, Chee S-P, Mehta JS, Tan DTH. Cytomegalovirus endotheliitis in Descemet’s stripping endothelial keratoplasty. Ophthalmology. 2009;116:624–30. doi: 10.1016/j.ophtha.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Waduthantri S, Zhou L, Chee S-P. Intra-cameral level of ganciclovir gel, 0.15% following topical application for cytomegalovirus anterior segment infection: a pilot study. PloS One. 2018;13:e0191850. doi: 10.1371/journal.pone.0191850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sánchez-Tilló E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. β-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA. 2011;108:19204–9. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liskova P, Gwilliam R, Filipec M, Jirsova K, Reinstein Merjava S, Deloukas P, et al. High prevalence of posterior polymorphous corneal dystrophy in the Czech Republic; linkage disequilibrium mapping and dating an ancestral mutation. PLoS One. 2012;7:e45495. doi: 10.1371/journal.pone.0045495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandrowala H, Bansal A, Vemuganti GK, Rao GN. Frequency, distribution, and outcome of keratoplasty for corneal dystrophies at a tertiary eye care center in South India. Cornea. 2004;23:541–6. doi: 10.1097/01.ico.0000126324.58884.b9. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez A, Calonge M, Pedroza-Seres M, Akova YA, Messmer EM, D’Amico DJ, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114:593–9. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 54.Touhami S, Qu L, Angii M, Bojanova M, Touitou V, Lehoang P, et al. Cytomegalovirus anterior uveitis: clinical characteristics and long-term outcomes in a French series. Am J Ophthalmol. 2018;194:134–42. doi: 10.1016/j.ajo.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Chan NS-W, Chee S-P, Caspers L, Bodaghi B. Clinical features of CMV-associated anterior uveitis. Ocul Immunol Inflamm. 2018;26:107–15. doi: 10.1080/09273948.2017.1394471. [DOI] [PubMed] [Google Scholar]
- 56.Shahrudin NA, Mohd Zahidin AZ, Md Noh UK, Wan Abdul Halim WH, Md Din N. CMV endotheliitis: a cause for recurrent failed corneal transplant. GMS Ophthalmol Cases. 2017;7:Doc31. doi: 10.3205/oc000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chee S-P, Bacsal K, Jap A, Se-Thoe S-Y, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–40. doi: 10.1016/j.ajo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 58.van Boxtel LAA, van der Lelij A, van der Meer J, Los LI. Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology. 2007;114:1358–62. doi: 10.1016/j.ophtha.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 59.Choi JA, Kim KS, Jung Y, Park HYL, Park CK. Cytomegalovirus as a cause of hypertensive anterior uveitis in immunocompetent patients. J Ophthalmic Inflamm Infect. 2016;6:32. doi: 10.1186/s12348-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–13. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 61.Numazaki K, Fujikawa T, Chiba S. Relationship between seropositivity of husbands and primary cytomegalovirus infection during pregnancy. J Infect Chemother. 2000;6:104–6. doi: 10.1007/pl00012146. [DOI] [PubMed] [Google Scholar]
- 62.Dar L, Pati SK, Patro ARK, Deorari AK, Rai S, Kant S, et al. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J. 2008;27:841–3. doi: 10.1097/INF.0b013e3181723d55. [DOI] [PubMed] [Google Scholar]
- 63.Sharma A, Rasul ES, Hazarika NK. A serological study of cytomegalovirus infection in pregnant and non-pregnant women at Gauhati Medical College and Hospital. J Indian Med Assoc. 2007;105:322–3. [PubMed] [Google Scholar]
- 64.Sheevani null, Jindal N, Aggarwal A. A pilot seroepidemiological study of cytomegalovirus infection in women of child bearing age. Indian J Med Microbiol. 2005;23:34–36. doi: 10.4103/0255-0857.13870. [DOI] [PubMed] [Google Scholar]
- 65.Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis Publ Infect Dis Soc Am. 2006;43:1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 66.Berry NJ, Burns DM, Wannamethee G, Grundy JE, Lui SF, Prentice HG, et al. Seroepidemiologic studies on the acquisition of antibodies to cytomegalovirus, herpes simplex virus, and human immunodeficiency virus among general hospital patients and those attending a clinic for sexually transmitted diseases. J Med Virol. 1988;24:385–93. doi: 10.1002/jmv.1890240405. [DOI] [PubMed] [Google Scholar]
- 67.Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–9. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi A, Yokogawa H, Higashide T, Nitta K, Sugiyama K. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol. 2012;153:445–53. doi: 10.1016/j.ajo.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 69.De Groot-Mijnes JDF, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, et al. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–8. doi: 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 70.Faith SC, Durrani AF, Jhanji V. Cytomegalovirus keratitis. Curr Opin Ophthalmol. 2018;29:373–7. doi: 10.1097/ICU.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 71.Hsiao Ching-Hsi, Hwang Yih-Shiou, Chuang Wen-Yu, Ma David H K, Yeh Lung-Kun, Chen Shin-Yi, Shu Jwu-Ching. Prevalence and clinical consequences of cytomegalovirus DNA in the aqueous humour and corneal transplants. British Journal of Ophthalmology. 2018;103(5):666–671. doi: 10.1136/bjophthalmol-2018-312196. [DOI] [PubMed] [Google Scholar]
- 72.Anshu A, Tan D, Chee S-P, Mehta JS, Htoon HM. Interventions for the management of CMV-associated anterior segment inflammation. Cochrane Database Syst Rev. 2017;8:CD011908. doi: 10.1002/14651858.CD011908.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koizumi N, Inatomi T, Suzuki T, Shiraishi A, Ohashi Y, Kandori M, et al. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. Br J Ophthalmol. 2015;99:54–58. doi: 10.1136/bjophthalmol-2013-304625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma—a major review. Clin Exp Ophthalmol. 2008;36:868–82. doi: 10.1111/j.1442-9071.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 75.Siddiqui Y, Ten Hulzen RD, Cameron JD, Hodge DO, Johnson DH. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135:794–9. doi: 10.1016/s0002-9394(02)02289-4. [DOI] [PubMed] [Google Scholar]
- 76.Evans W, Odom R, Wenaas E. Krukenberg’s spindle A study of 202 collected cases. Arch Ophthalmol. 2018;26:1023–56. [Google Scholar]
- 77.Qing G, Wang N, Tang X, Zhang S, Chen H. Clinical characteristics of pigment dispersion syndrome in Chinese patients. Eye Lond Engl. 2009;23:1641–6. doi: 10.1038/eye.2008.328. [DOI] [PubMed] [Google Scholar]
- 78.Walkden A, Au L. Iridocorneal endothelial syndrome: clinical perspectives. Clin Ophthalmol Auckl Nz. 2018;12:657–64. doi: 10.2147/OPTH.S143132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson MC, Shields MB. A comparison of the clinical variations of the iridocorneal endothelial syndrome. Arch Ophthalmol. 1989;107:1465–8. doi: 10.1001/archopht.1989.01070020539035. [DOI] [PubMed] [Google Scholar]
- 80.Teekhasaenee C, Ritch R. Iridocorneal endothelial syndrome in Thai patients: clinical variations. Arch Ophthalmol. 2000;118:187–92. doi: 10.1001/archopht.118.2.187. [DOI] [PubMed] [Google Scholar]
- 81.Chandran P, Rao HL, Mandal AK, Choudhari NS, Garudadri CS, Senthil S. Glaucoma associated with iridocorneal endothelial syndrome in 203 Indian subjects. PLoS One. 2017;12:e0171884. doi: 10.1371/journal.pone.0171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malhotra C, Pandav SS, Gupta A, Jain AK. Phenotypic heterogeneity of corneal endothelium in iridocorneal endothelial syndrome by in vivo confocal microscopy. Cornea. 2014;33:634–7. doi: 10.1097/ICO.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 83.Gupta V, Kumar R, Gupta R, Srinivasan G, Sihota R. Bilateral iridocorneal endothelial syndrome in a young girl with Down’s syndrome. Indian J Ophthalmol. 2009;57:61–63. doi: 10.4103/0301-4738.44493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Islam F, Azad N, Khan A. Bilateral iridocorneal endothelial (ICE) syndrome with microspherophakia. J Coll Physicians Surg --Pak Jcpsp. 2011;21:374–5. [PubMed] [Google Scholar]
- 85.Huna R, Barak A, Melamed S. Bilateral iridocorneal endothelial syndrome presented as Cogan-Reese and Chandler’s syndrome. J Glaucoma. 1996;5:60–62. [PubMed] [Google Scholar]
- 86.Zhao H, Tang X. Analysis of the misdiagnosis of bilateral iridocorneal endothelial syndrome. Zhonghua Yi Xue Za Zhi. 2012;92:1317–20. [PubMed] [Google Scholar]
- 87.Sacchetti M, Mantelli F, Marenco M, Macchi I, Ambrosio O, Rama P. Diagnosis and management of iridocorneal endothelial syndrome. BioMed Res Int. 2015;2015:763093. doi: 10.1155/2015/763093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Azari AA, Rezaei Kanavi M, Thompson MJ, Altaweel MM, Potter HD, Albert DM. Iridocorneal endothelial syndrome. JAMA Ophthalmol. 2014;132:56. doi: 10.1001/jamaophthalmol.2013.247. [DOI] [PubMed] [Google Scholar]
- 89.Siak J, Chee S-P. Cytomegalovirus anterior uveitis following topical cyclosporine A. Ocul Immunol Inflamm. 2018;26:90–93. doi: 10.1080/09273948.2017.1306083. [DOI] [PubMed] [Google Scholar]
- 90.Babu K, Murthy GJ. Cytomegalovirus anterior uveitis in immunocompetent individuals following topical prostaglandin analogues. J Ophthalmic Inflamm Infect. 2013;3:55. doi: 10.1186/1869-5760-3-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sims JL, Chee SP. Cytomegalovirus endotheliitis following fluocinolone acetonide (Retisert) implant. Eye Lond Engl. 2010;24:197–8. doi: 10.1038/eye.2009.54. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Teja S, Baig K. Bilateral corneal edema associated with amantadine. CMAJ Can Med Assoc J. 2015;187:1155–8. doi: 10.1503/cmaj.140542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang KC, Jeong JH, Kim MK, Wee WR, Lee JH, Jeon BS. The effect of amantadine on corneal endothelium in subjects with Parkinson’s disease. Ophthalmology. 2010;117:1214–9. doi: 10.1016/j.ophtha.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 94.Sundin OH, Jun AS, Broman KW, Liu SH, Sheehan SE, Vito ECL, et al. Linkage of late-onset Fuchs corneal dystrophy to a novel locus at 13pTel-13q12.13. Investig Opthalmology Vis Sci. 2006;47:140. doi: 10.1167/iovs.05-0578. [DOI] [PubMed] [Google Scholar]
- 95.Sundin OH, Broman KW, Chang HH, Vito ECL, Stark WJ, Gottsch JD. A common locus for late-onset Fuchs corneal dystrophy maps to 18q21.2-q21.32. Investig Opthalmology Vis Sci. 2006;47:3919. doi: 10.1167/iovs.05-1619. [DOI] [PubMed] [Google Scholar]
- 96.Riazuddin SA, Eghrari AO, Al-Saif A, Davey L, Meadows DN, Katsanis N, et al. Linkage of a Mild Late-onset Phenotype of Fuchs Corneal Dystrophy To A Novel Locus at 5q33.1-q35.2. Investig Opthalmology Vis Sci. 2009;50:5667. doi: 10.1167/iovs.09-3764. [DOI] [PubMed] [Google Scholar]
- 97.Riazuddin SA, Zaghloul NA, Al-Saif A, Davey L, Diplas BH, Meadows DN, et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86:45–53. doi: 10.1016/j.ajhg.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DTH, Mohamed MD, et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2) Nat Genet. 2006;38:755–7. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 99.Rao Bhavna S., Ansar Samdani, Arokiasamy Tharigopala, Sudhir Rachapalli R., Umashankar Vetrivel, Rajagopal Rama, Soumittra Nagasamy. Analysis of candidate genes ZEB1 and LOXHD1 in late-onset Fuchs’ endothelial corneal dystrophy in an Indian cohort. Ophthalmic Genetics. 2018;39(4):443–449. doi: 10.1080/13816810.2018.1474367. [DOI] [PubMed] [Google Scholar]
- 100.Afshari NA, Igo RP, Morris NJ, Stambolian D, Sharma S, Pulagam VL, et al. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat Commun. 2017;8:14898. doi: 10.1038/ncomms14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liskova P, Prescott Q, Bhattacharya SS, Tuft SJ. British family with early‐onset Fuchs’ endothelial corneal dystrophy associated with p.L450W mutation in the COL8A2 gene. Br J Ophthalmol. 2007;91:1717–8. doi: 10.1136/bjo.2007.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wieben ED, Aleff RA, Eckloff BW, Atkinson EJ, Baheti S, Middha S, et al. Comprehensive Assessment Of Genetic Variants Within TCF4 in Fuchs’ endothelial corneal dystrophy. Investig Opthalmology Vis Sci. 2014;55:6101. doi: 10.1167/iovs.14-14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wieben ED, Aleff RA, Tang X, Butz ML, Kalari KR, Highsmith EW, et al. Trinucleotide repeat expansion in the transcription factor 4 (TCF4) gene leads to widespread mRNA splicing changes in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2017;58:343–52. doi: 10.1167/iovs.16-20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vithana EN, Morgan PE, Ramprasad V, Tan DTH, Yong VHK, Venkataraman D, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17:656–66. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- 105.Xing C, Gong X, Hussain I, Khor C-C, Tan DTH, Aung T, et al. Transethnic replication of association of CTG18.1 repeat expansion of TCF4 gene with Fuchs’ corneal dystrophy in Chinese implies common causal variant. Invest Ophthalmol Vis Sci. 2014;55:7073–8. doi: 10.1167/iovs.14-15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakano M, Okumura N, Nakagawa H, Koizumi N, Ikeda Y, Ueno M, et al. Trinucleotide repeat expansion in the TCF4 gene in Fuchs’ endothelial corneal dystrophy in Japanese. Invest Ophthalmol Vis Sci. 2015;56:4865–9. doi: 10.1167/iovs.15-17082. [DOI] [PubMed] [Google Scholar]
- 107.Rao BS, Tharigopala A, Rachapalli SR, Rajagopal R, Soumittra N. Association of polymorphisms in the intron of TCF4 gene to late-onset Fuchs endothelial corneal dystrophy: an Indian cohort study. Indian J Ophthalmol. 2017;65:931–5. doi: 10.4103/ijo.IJO_191_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nanda GG, Padhy B, Samal S, Das S, Alone DP. Genetic association of TCF4 intronic polymorphisms, CTG18.1 andrs17089887, with Fuchs’ endothelial corneal dystrophy in an Indian population. Invest Ophthalmol Vis Sci. 2014;55:7674–80. doi: 10.1167/iovs.14-15297. [DOI] [PubMed] [Google Scholar]
- 109.Soliman AZ, Xing C, Radwan SH, Gong X, Mootha VV. Correlation of severity of Fuchs endothelial corneal dystrophy with triplet repeat expansion in TCF4. JAMA Ophthalmol. 2015;133:1386–91. doi: 10.1001/jamaophthalmol.2015.3430. [DOI] [PubMed] [Google Scholar]
- 110.Vasanth S, Eghrari AO, Gapsis BC, Wang J, Haller NF, Stark WJ, et al. Expansion of CTG18.1 trinucleotide repeat in TCF4 is a potent driver of Fuchs’ corneal dystrophy. Invest Ophthalmol Vis Sci. 2015;56:4531–6. doi: 10.1167/iovs.14-16122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mootha VV, Gong X, Ku H-C, Xing C. Association and familial segregation of CTG18.1 trinucleotide repeat expansion of TCF4 gene in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2014;55:33–42. doi: 10.1167/iovs.13-12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuot A, Hewitt AW, Snibson GR, Souzeau E, Mills R, Craig JE, et al. TGC repeat expansion in the TCF4 gene increases the risk of ‘Fuchs’ endothelial corneal dystrophy in Australian cases. PLoS ONE. 2017;12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5568371/. [DOI] [PMC free article] [PubMed]
- 113.Luther M, Grünauer-Kloevekorn C, Weidle E, Passarge E, Rupprecht A, Hoffmann K, et al. TGC repeats in intron 2 of the TCF4 gene have a good predictive power regarding to Fuchs endothelial corneal dystrophy. Klin Monätter Für Augenheilkd. 2016;233:187–94. doi: 10.1055/s-0035-1546138. [DOI] [PubMed] [Google Scholar]
- 114.Qin B, Tang M, Li Y, Zhang X, Chu R, Huang D. Anterior segment dimensions in Asian and Caucasian eyes measured by optical coherence tomography. Ophthalmic Surg. Lasers Imaging. 2012;43:135–42. doi: 10.3928/15428877-20120102-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng J-W, Zong Y, Zeng Y-Y, Wei R-L. The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PLoS ONE. 2014;9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4110010/. [DOI] [PMC free article] [PubMed]
- 116.Wang PX, Koh VTC, Loon SC. Laser iridotomy and the corneal endothelium: a systemic review. Acta Ophthalmol (Copenh). 2014;92:604–16. doi: 10.1111/aos.12367. [DOI] [PubMed] [Google Scholar]
- 117.Kumar RS, Baskaran M, Friedman DS, Xu Y, Wong H-T, Lavanya R, et al. Effect of prophylactic laser iridotomy on corneal endothelial cell density over 3 years in primary angle closure suspects. Br J Ophthalmol. 2013;97:258–61. doi: 10.1136/bjophthalmol-2012-302013. [DOI] [PubMed] [Google Scholar]
- 118.Ang LPK, Higashihara H, Sotozono C, Shanmuganathan VA, Dua H, Tan DTH, et al. Argon laser iridotomy-induced bullous keratopathy a growing problem in Japan. Br J Ophthalmol. 2007;91:1613–5. doi: 10.1136/bjo.2007.120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ang Marcus, Lim Fiona, Htoon Hla M, Tan Donald, Mehta Jodhbir S. Visual acuity and contrast sensitivity following Descemet stripping automated endothelial keratoplasty. British Journal of Ophthalmology. 2015;100(3):307–311. doi: 10.1136/bjophthalmol-2015-306975. [DOI] [PubMed] [Google Scholar]
- 120.Hamzaoglu EC, Straiko MD, Mayko ZM, Sáles CS, Terry MA. The first 100 eyes of standardized descemet stripping automated endothelial keratoplasty versus standardized descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122:2193–9. doi: 10.1016/j.ophtha.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Nanavaty MA, Wang X, Shortt AJ. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst Rev. 2014;CD008420. [DOI] [PMC free article] [PubMed]
- 122.Ang M, Soh Y, Htoon HM, Mehta JS, Tan D. Five-year graft survival comparing descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2016;123:1646–52. doi: 10.1016/j.ophtha.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 123.Kim SE, Lim SA, Byun Y-S, Joo C-K. Comparison of long-term clinical outcomes between Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty in patients with bullous keratopathy. Korean J Ophthalmol. 2016;30:443–50. doi: 10.3341/kjo.2016.30.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Price MO, Gorovoy M, Price FW, Benetz BA, Menegay HJ, Lass JH. Descemet’s stripping automated endothelial keratoplasty: three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2013;120:246–51. doi: 10.1016/j.ophtha.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobashi H, Kamiya K, Shimizu K. Factors influencing visual acuity in Fuchs’ endothelial corneal dystrophy. Optom Vis Sci Publ Am Acad Optom. 2018;95:21–26. doi: 10.1097/OPX.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 126.Wacker K, McLaren JW, Amin SR, Baratz KH, Patel SV. Corneal high-order aberrations and backscatter in Fuchs’ endothelial corneal dystrophy. Ophthalmology. 2015;122:1645–52. doi: 10.1016/j.ophtha.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watanabe S, Oie Y, Fujimoto H, Soma T, Koh S, Tsujikawa M, et al. Relationship between corneal guttae and quality of vision in patients with mild Fuchs’ endothelial corneal dystrophy. Ophthalmology. 2015;122:2103–9. doi: 10.1016/j.ophtha.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 128.Ple-Plakon PA, Shtein RM. Trends in corneal transplantation: indications and techniques. Curr Opin Ophthalmol. 2014;25:300–5. doi: 10.1097/ICU.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 129.Röck T, Bartz-Schmidt KU, Röck D. Trends in corneal transplantation at the University Eye Hospital in Tübingen, Germany over the last 12 years: 2004–2015. PloS One. 2018;13:e0198793. doi: 10.1371/journal.pone.0198793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37:1198–203. doi: 10.1097/ICO.0000000000001666. [DOI] [PubMed] [Google Scholar]
- 131.Bigan G, Puyraveau M, Saleh M, Gain P, Martinache I, Delbosc B, et al. Corneal transplantation trends in France from 2004 to 2015: a 12-year review. Eur J Ophthalmol. 2018;28:535–40. doi: 10.1177/1120672118762224. [DOI] [PubMed] [Google Scholar]
- 132.Kim BZ, Meyer JJ, Brookes NH, Moffatt SL, Twohill HC, Pendergrast DG, et al. New Zealand trends in corneal transplantation over the 25 years 1991–2015. Br J Ophthalmol. 2017;101:834–8. doi: 10.1136/bjophthalmol-2016-309021. [DOI] [PubMed] [Google Scholar]
- 133.Tan JCH, Holland SP, Dubord PJ, Moloney G, McCarthy M, Yeung SN. Evolving indications for and trends in keratoplasty in British Columbia, Canada, from 2002 to 2011: a 10-year review. Cornea. 2014;33:252–6. doi: 10.1097/ICO.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 134.Coster DJ, Lowe MT, Keane MC, Williams KA. Australian corneal graft registry contributors. A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121:979–87. doi: 10.1016/j.ophtha.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 135.Hong J, Shi W, Liu Z, Pineda R, Cui X, Sun X, et al. Limitations of keratoplasty in China: a survey analysis. PLoS One. 2015;10:e0132268. doi: 10.1371/journal.pone.0132268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dong PN, Han TN, Aldave AJ, Chau HTM. Indications for and techniques of keratoplasty at Vietnam National Institute of Ophthalmology. Int J Ophthalmol. 2016;9:379–83. doi: 10.18240/ijo.2016.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rezaei Kanavi M, Javadi MA, Motevasseli T, Chamani T, Rezaei Kanavi M, Kheiri B, et al. Trends in Indications and techniques of corneal transplantation in Iran from 2006 to 2013; an 8-year review. J Ophthalmic Vis Res. 2016;11:146–52. doi: 10.4103/2008-322X.183930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crawford AZ, McKelvie J, Craig JP, McGhee CNJ, Patel DV. Corneal transplantation in Auckland, New Zealand, 1999-2009: indications, patient characteristics, ethnicity, social deprivation, and access to services. Cornea. 2017;36:546–52. doi: 10.1097/ICO.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 139.Edwards M, Clover GM, Brookes N, Pendergrast D, Chaulk J, McGhee CNJ. Indications for corneal transplantation in New Zealand: 1991–1999. Cornea. 2002;21:152–5. doi: 10.1097/00003226-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 140.Lee JS, Park YG, Yoon KC. Long-term results of Descemet’s stripping automated endothelial keratoplasty in Korea. J Korean Ophthalmol Soc. 2010;51:1431–7. [Google Scholar]
- 141.Kang DJ, Kim HK. Clinical outcomes of combined descemet-stripping endothelial keratoplasty and intraocular lens exchange. J Korean Ophthalmol Soc. 2016;57:1361–8. [Google Scholar]
- 142.Han GL, Hyun J, Lim DH, Chung ES, Chung TY. Clinical outcomes of Descemet’s membrane endothelial keratoplasty: a 1-year retrospective study. J Korean Ophthalmol Soc. 2015;56:1489–96. [Google Scholar]
- 143.Baek JW, Hwang KY, Joo C-K. Comparing clinical outcomes of Descemet’s membrane stripping automated endothelial keratoplasty between graft insertion methods. J Korean Ophthalmol Soc. 2013;54:1655–62. [Google Scholar]
- 144.Choi SH, Lee YW, Kim HM, Yang SM, Hong JU, Yoon KC, et al. Epidemiologic studies of keratoplasty in Korea. J Korean Ophthalmol Soc. 2006;47:538–47. [Google Scholar]
- 145.Shimazaki J, Amano S, Uno T, Maeda N, Yokoi N. National survey on bullous keratopathy in Japan. Cornea. 2007;26:274–8. doi: 10.1097/ICO.0b013e31802c9e19. [DOI] [PubMed] [Google Scholar]
- 146.Nishino T, Kobayashi A, Yokogawa H, Mori N, Masaki T, Sugiyama K. A 10-year review of underlying diseases for endothelial keratoplasty (DSAEK/DMEK) in a tertiary referral hospital in Japan. Clin Ophthalmol Auckl Nz. 2018;12:1359–65. doi: 10.2147/OPTH.S170263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eye Bank Association of America. Eye banking statistical report; 2016:1–99.
- 148.Lichtinger A, Yeung SN, Kim P, Amiran MD, Rootman DS. The era of lamellar keratoplasty, evolving surgical techniques in corneal transplantation: the University of Toronto experience. Can J Ophthalmol J Can Ophtalmol. 2012;47:287–90. doi: 10.1016/j.jcjo.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 149.Frigo AC, Fasolo A, Capuzzo C, Fornea M, Bellucci R, Busin M, et al. Corneal transplantation activity over 7 years: changing trends for indications, patient demographics and surgical techniques from the Corneal Transplant Epidemiological Study (CORTES) Transplant Proc. 2015;47:528–35. doi: 10.1016/j.transproceed.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 150.Ting DSJ, Sau CY, Srinivasan S, Ramaesh K, Mantry S, Roberts F. Changing trends in keratoplasty in the West of Scotland: a 10-year review. Br J Ophthalmol. 2012;96:405–8. doi: 10.1136/bjophthalmol-2011-300244. [DOI] [PubMed] [Google Scholar]
- 151.Flockerzi E, Maier P, Böhringer D, Reinshagen H, Kruse F, Cursiefen C, et al. Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG–section cornea and its keratoplasty registry. Am J Ophthalmol. 2018;188:91–98. doi: 10.1016/j.ajo.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 152.Tan DTH, Anshu A, Mehta JS. Paradigm shifts in corneal transplantation. Ann Acad Med Singap. 2009;38:332–8. [PubMed] [Google Scholar]
- 153.Aditya P. Evolving indications and trends in keratoplasty in Eastern India: a 5-year review. In: 75th Annual Conference of All India Ophthalmological Society (AIOS). Jaipuir, India; 2017. https://proceedings.aios.org/2017/fp1159-evolving-indications-and-trends-in-keratoplasty-in-eastern-india-a-5-year-review/.
- 154.Mohamed A, Chaurasia S, Murthy SI, Ramappa M, Vaddavalli PK, Taneja M, et al. Endothelial keratoplasty: a review of indications at a tertiary eye care centre in South India. Asia-Pac J Ophthalmol Phila Pa. 2014;3:207–10. doi: 10.1097/APO.0b013e3182a75304. [DOI] [PubMed] [Google Scholar]
- 155.Price MO, Giebel AW, Fairchild KM, Price FW. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–8. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 156.Stuart AJ, Romano V, Virgili G, Shortt AJ. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev. 2018;6:CD012097. doi: 10.1002/14651858.CD012097.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu L, Zha Y, Cai J, Zhang Y. Descemet stripping automated endothelial keratoplasty versus descemet membrane endothelial keratoplasty: a meta-analysis. Int Ophthalmol. 2018;38:897–905. doi: 10.1007/s10792-017-0533-3. [DOI] [PubMed] [Google Scholar]
- 158.Marques Raquel Esteves, Guerra Paulo Silva, Sousa David Cordeiro, Gonçalves Ana Inês, Quintas Ana Miguel, Rodrigues Walter. DMEK versus DSAEK for Fuchs’ endothelial dystrophy: A meta-analysis. European Journal of Ophthalmology. 2018;29(1):15–22. doi: 10.1177/1120672118757431. [DOI] [PubMed] [Google Scholar]
- 159.Ang M, Mehta JS, Newman SD, Han SB, Chai J, Tan D. Descemet membrane endothelial keratoplasty: preliminary results of a donor insertion pull-through technique using a donor mat device. Am J Ophthalmol. 2016;171:27–34. doi: 10.1016/j.ajo.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 160.Gerber-Hollbach N, Parker J, Baydoun L, Liarakos VS, Ham L, Dapena I, et al. Preliminary outcome of hemi-Descemet membrane endothelial keratoplasty for Fuchs endothelial dystrophy. Br J Ophthalmol. 2016;100:1564–8. doi: 10.1136/bjophthalmol-2015-307783. [DOI] [PubMed] [Google Scholar]
- 161.Baydoun L, Zygoura V, Hsien S, Birbal RS, Spinozzi D, Lie JT, et al. Clinical feasibility of using multiple grafts from a single donor for Quarter-DMEK. Acta Ophthalmol (Copenh). 2018;96:e656–e658. doi: 10.1111/aos.13720. [DOI] [PubMed] [Google Scholar]
- 162.Schlögl A, Tourtas T, Kruse FE, Weller JM. Long-term Clinical outcome after Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2016;169:218–26. doi: 10.1016/j.ajo.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Fuest M, Ang M, Htoon HM, Tan D, Mehta JS. Long-term visual outcomes comparing Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2017;182:62–71. doi: 10.1016/j.ajo.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 164.Yang M, Mehta JS, Tan DTH. Superficial keratectomy as a prelude for endothelial keratoplasty in severe bullous keratopathy with anterior stromal scarring. Cornea. 2010;29:108–9. doi: 10.1097/ICO.0b013e3181a3c516. [DOI] [PubMed] [Google Scholar]
- 165.Peraza-Nieves J, Baydoun L, Dapena I, Ilyas A, Frank LE, Luceri S, et al. Two-year clinical outcome of 500 consecutive cases undergoing Descemet membrane endothelial keratoplasty. Cornea. 2017;36:655–60. doi: 10.1097/ICO.0000000000001176. [DOI] [PubMed] [Google Scholar]
- 166.Soh YQ, Peh G, George BL, Seah XY, Primalani NK, Adnan K, et al. Predicative factors for corneal endothelial cell migration. Investig Opthalmology Vis Sci. 2016;57:338. doi: 10.1167/iovs.15-18300. [DOI] [PubMed] [Google Scholar]
- 167.Kaufman AR, Nosé RM, Pineda R. Descemetorhexis without endothelial keratoplasty (DWEK): proposal for nomenclature standardization. Cornea. 2018;37:e20–e21. doi: 10.1097/ICO.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 168.Soh YQ, Mehta JS. Selective endothelial removal for peters anomaly. Cornea. 2018;37:382–5. doi: 10.1097/ICO.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 169.Garnock-Jones KP. Ripasudil: first global approval. Drugs. 2014;74:2211–5. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 170.Peh GSL, Adnan K, George BL, Ang H-P, Seah X-Y, Tan DT, et al. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci Rep. 2015;5:9167. doi: 10.1038/srep09167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meekins LC, Rosado-Adames N, Maddala R, Zhao JJ, Rao PV, Afshari NA. Corneal endothelial cell migration and proliferation enhanced by rho kinase (ROCK) inhibitors in in vitro and in vivo models. Invest Ophthalmol Vis Sci. 2016;57:6731–8. doi: 10.1167/iovs.16-20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Okumura N, Matsumoto D, Fukui Y, Teramoto M, Imai H, Kurosawa T, et al. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PloS One. 2018;13:e0191306. doi: 10.1371/journal.pone.0191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hu J, Rong Z, Gong X, Zhou Z, Sharma VK, Xing C, et al. Oligonucleotides targeting TCF4 triplet repeat expansion inhibit RNA foci and mis-splicing in Fuchs’ dystrophy. Hum Mol Genet. 2018;27:1015–26. doi: 10.1093/hmg/ddy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–6. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–32. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551:464–71. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR–Cas13. Nature. 2017;550:280–4. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–27. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goar EL. Dystrophy of the corneal endothelium (cornea guttata), with report of a histologic examination. Trans Am Ophthalmol Soc. 1933;31:48–59. [PMC free article] [PubMed] [Google Scholar]
- 181.Lorenzetti DW, Uotila MH, Parikh N, Kaufman HE. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64:1155–8. [PubMed] [Google Scholar]
- 182.Nagaki Y, Hayasaka S, Kitagawa K, Yamamoto S. Primary cornea guttata in Japanese patients with cataract: specular microscopic observations. Jpn J Ophthalmol. 1996;40:520–5. [PubMed] [Google Scholar]