Highlights
-
•
New therapies are needed for advanced or recurrent high-grade ovarian cancer.
-
•
Genetic and molecular tests may help in directing therapeutic options according to sensitivity profiles.
-
•
Trametinib showed promising response in our patient affected by heavily pretreated high-grade ovarian cancer.
Keywords: Ovarian neoplasm, Ovarian epithelial cancer, Genetic testing, Precision medicine, Target therapy, Trametinib
Abstract
Traditional treatment failure in recurrent ovarian cancer remains a challenge for clinicians. Tumor genetic testing is a promising tool which has been proved able to identify sensitivity profiles in patients affected by cancers. This may be helpful in choosing targeted systemic treatments, aiming to overcome histology boundaries and to avoid unnecessary toxicity. We describe the case of a patient affected by recurrent high-grade serous ovarian cancer responsive to MEK-inhibitors, who had undergone multiple lines of therapy. To our knowledge, this is the first reported case of recurrent high-grade ovarian cancer showing remarkable clinical, radiologic and biochemical response to trametinib. This report suggests that trametinib could be effective in high-grade serous ovarian cancer, although most of promising scientific data on this molecule have focused on low-grade ovarian cancer. Molecular profiling has gradually become part of care for patients affected by recurrent ovarian cancer, however further randomized studies are needed to prove its efficacy in everyday clinical practice.
1. Introduction
Ovarian cancer is responsible for more than 14.000 deaths every year among women and the overall 5-year survival rate is 48%. The cornerstone of treatment is surgery and platinum-based chemotherapy. Survival rates decrease steadily in patients with platinum-resistant or recurrent disease for which therapeutic options appear limited and not very effective. Therefore, new treatment approaches need to be implemented in everyday practice. Precision medicine is an emerging field which aims to treat and prevent diseases, taking genetic and environmental factors into account. In February 2018, the most recent update of the National Comprehensive Cancer Network added the recommendation for tumor molecular testing prior to initiation of therapy for persistent/recurrent disease in ovarian cancer. While most molecular alterations do not have an FDA‐approved therapy, a demand for improved or alternative therapies is constantly rising. Foundation One CDX (Roche) is a broad-spectrum test that matches alterations in tumors’ genetic profiles to available targeted therapy agents. Our case report describes a heavily pretreated woman affected by recurrent high grade serous ovarian cancer (HGSOC) presenting a KRAS mutation. Somatic RAS mutations are highly prevalent in many human cancers that respond poorly to standard treatments, including carcinomas of the lung, pancreas, and colon, melanoma, myeloid leukemia (Diaz-Flores and Shannon, 2007) and also low-grade ovarian cancer (LGSOC). LGSOCs account for 5–10% of all serous ovarian tumors and shows alterations in the mitogen-activated protein kinase (MAPK) pathway. As part of the MAPK pathway, MEK1 and MEK2 kinases have crucial roles in tumorigenesis, cell proliferation and apoptosis inhibition and, therefore, MEK1/2 inhibition represents an attractive therapeutic strategy. The use of trametinib, which is a MEK inhibitor, in LGSOC is not only described in sporadic cases (Champer et al., 2019, Mert, 2017), but recently has been compared to standard of care in a randomized clinical trial (oncologypro, 2019). The study demonstrated that trametinib significantly improved clinical outcomes in women with recurrent LGSOC.
2. Case report
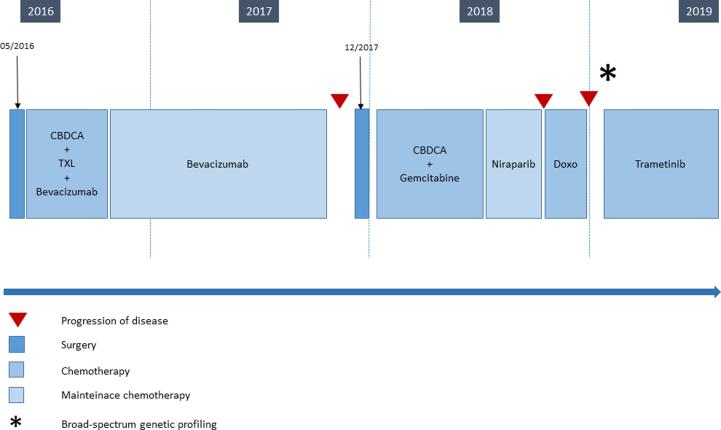
The timeline of the reported case is summarized in Figure 1.
Fig. 1.
Timeline of the case reported from the diagnosis till now. Abbreviations: CBDCA: Carboplatin, TXL: Paclitaxel, Doxo: Doxorubicin.
S. G. is a 50-year-old woman who was diagnosed in 2016 with HGSOC. Her past medical history was unremarkable except for hypothyroidism, appendectomy and acute angle glaucoma on topical medication. She had a positive family history for colon cancer in a first degree relative. In April 2016 she presented with pelvic pain; the ultrasound scan during the gynecologic examination showed bilateral adnexal masses. Ca 125 serum level was 360 U/mL. Magnetic Resonance Imaging (MRI) confirmed the presence of adnexal tumors and additionally showed multiple nodules within the pouch of Douglas and adjacent to the anterior abdominal wall. Computed Tomography (CT) scan reported a 12 cm pelvic mass not clearly originating from the adnexa; the pelvic adipose tissue was infiltrated, and many nodules detected on the right hemi-diaphragmatic peritoneum, the Glissonian capsule and within the omentum were suspicious for peritoneal carcinomatosis. The patient underwent a diagnostic laparoscopy. Given the presence of peritoneal nodules on the right hemidiaphragm and within the pelvis, the surgeon defined a Fagotti’s score of 2 (Fagotti, 2013). The surgeons decided for a surgical debulking surgery, which was performed on the same day. She underwent a radical hysterectomy with bilateral salpingo-oophorectomy, omentectomy, recto-sigmoid and cecum resections, right diaphragmatic peritoneum resection, pelvic and para-aortic lymphadenectomy. Macroscopic residual tumor was absent. Final histology confirmed a stage IIIC HGSOC. BRCA analysis showed wild type genes. The patient received six cycles of carboplatin and paclitaxel in the adjuvant setting, with the addition of bevacizumab starting from the second cycle. Bevacizumab was then administered as a maintenance monotherapy for a total number of 22 cycles. As side effect of this treatment, the patient developed hypertension which was controlled by oral medications. The Ca 125 serum level decreased to 34.8 U/mL at the end of treatment. Unfortunately, after a platinum-free-interval of 13 months, the CT scan and the Positron Emission Tomography (PET) scan detected solid lesions suspicious for a peritoneal recurrence on the right hemidiaphragm. In December 2017, the patient underwent secondary cytoreductive surgery consisting of resection of the residual right hemi-diaphragmatic peritoneum and pelvic peritonectomy. After surgery, she received six cycles of carboplatin-gemcitabine, followed by niraparib as a maintenance therapy for a total number of three months. A surveillance CT scan showed new recurrent disease with peritoneal, nodal and adrenal gland metastasis. Treatment with doxorubicin was subsequently prescribed, however the administration was suspended after two cycles because of disease progression. Ca 125 serum levels rose to 250 U/mL and a new CT scan confirmed indeed an increase in size of a lesion infiltrating the liver and the right thoracic wall. The patient complained of thoracic and abdominal pain.
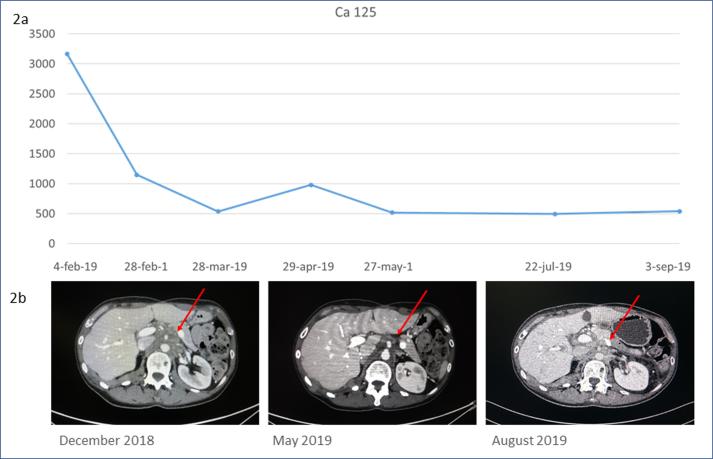
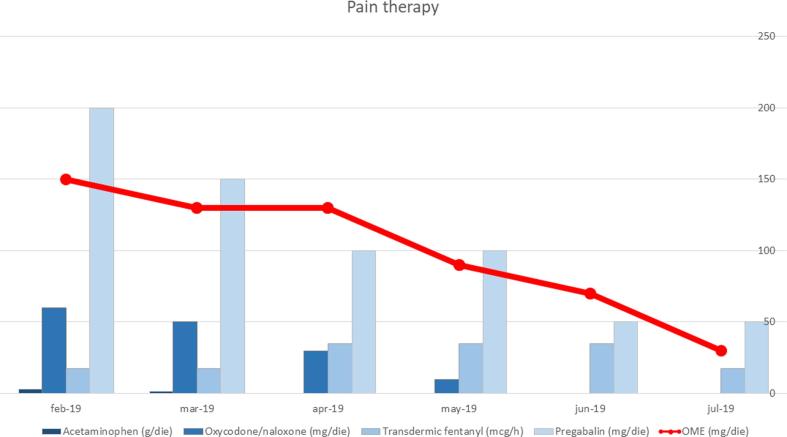
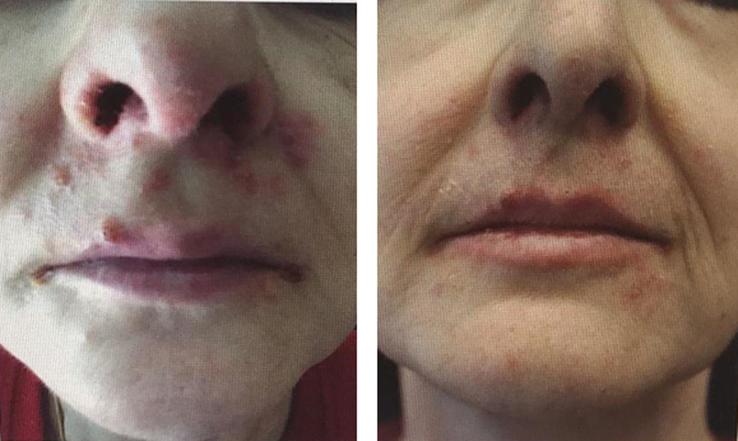
The patient underwent a genetic testing on the tumoral tissue sampled during her secondary surgery (Foundation One CDX-Roche). The molecular profiling of her tumor showed the presence of KRAS and NF1 (neurofibromin 1) mutations. Both mutations have been proved to be responsive to MEK inhibitors such as binimetinb, cobimetinib, or trametinib. Trametinib acts downstream of KRAS to suppress signaling through the MAPK cascade and has been approved in combination with dabrafenib for treatment of advanced or metastatic anaplastic thyroid cancer, non-small cell lung cancer and melanoma. Because of the continuous rise of Ca 125 serum levels (3165 U/mL), after extensive counselling, the patient gave consent to commence oral treatment with trametinib 2 mg/day in February 2019. During the subsequent course of treatment, Ca 125 serum levels constantly decreased, and then remained stable. The CT scan evaluation after 3 months of treatment, confirmed partial response consisting in a reduction of all intraperitoneal and retroperitoneal nodules; the 6-months’ CT scan showed a substantially stable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) (Figures 2a–b). As trametinib has been associated with several adverse effects such as dermatological, cardiovascular and ocular diseases (Daud and Tsai, 2017), specialist doctors were consulted regularly aiming to achieve a comprehensive periodic checkup. The patient experienced an improvement of pain during treatment, which allowed us to adapt her pain-management medications accordingly (Figure 3). She reported a grade 2 cutaneous toxicity which subsided applying topical moisturizing glycerin-based cream and betamethasone valerate twice a day; she was also advised to avoid friction at the fingertips, toes and heels and to opt for protective footwear. Complete remission of skin lesions was achieved after one month; no cutaneous lesions recurrence was observed despite the continuation of treatment with trametinib (Supplementary figure 1). No other adverse effects were recorded. Unfortunately, after 8 months of treatment, the patient was admitted to our hospital for abdominal pain. CT scan showed ascites with initial signs of bowel obstruction, Ca 125 was 1007 U/ml in October 2019.
Fig. 2.
Treatment response (a) Ca 125 serum level trend after the last recurrence showed biochemical treatment response. (b) Comparison of paraaortic nodal metastasis before starting the treatment with Trametinib (left), after 3 months (middle) and after 6 months (right). Red arrows indicate the target lesion. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Pain medication dosage, which achieve a compensation of the symptoms has been reduced during the treatment period. Every agent used (Acetaminophen, Oxycodone/Naloxone, Fentanyl, and Pregabalin) was administered at a lower dose as a clinical sign of positive response to Trametinib. After conversion to Oral Morphine Equivalent (OME) the reduction trend was confirmed.
Treatment with trametinib was discontinued and she was supported by total parenteral nutrition and a nasogastric tube was placed. After few days she recovered and subsequently was started on a traditional chemotherapy scheme with weekly taxol.
3. Discussion
In our heavily pretreated patient with a KRAS-mutated HGSOC, the treatment with trametinib showed an impressive response which lasted 8 months (VS about 3 months of the standard of care).
KRAS mutations are detected in approximately 40% of patients with LGSOC and <1% of patients with HGSOC (mycancergenome). Since KRAS mutation is a unique occurrence for HGSOC, some may believe that this could be one of the rarest cases of evolution to a HGSOC from a LGSOC which kept the same genetic background (Garg et al., 2012). This hypothesis may also account for the observed chemo-resistance to multiple traditional therapies. Although this is a very intriguing reasoning, it needs further studies to be validated.
In the past decade, researchers showed an increasing interest in studying the association between NF1 and ovarian cancer (Integrated genomic analyses of ovarian carcinoma, 2011). Since neurofibromin is a negative regulator of RAS-signaling, in cell lines harboring NF1 defects an increased MAPK activation has been shown (Sangha et al., 2008), supporting the hypothesis that NF1 mutation may contribute to enhance RAS-related cellular proliferation.
Trametinib is an FDA-approved allosteric molecule inhibiting MEK1 and MEK2 in several cancer types and, either as a monotherapy or in combination with other drugs, has been proved to be effective in treating advanced or metastatic anaplastic thyroid cancer, non-small cell lung cancer and some types of melanoma.
Various case reports documented successful treatment of LGSOC either with trametinib alone (Champer et al., 2019), or in association with metformin given a newly discovered synergic effect between these molecules in patients affected by RAS mutations (Mert, 2017). Recent data showed promising results also in comparison to standard of care in a randomized trial (oncologypro, 2019).
To the best of our knowledge, the case presented is the first reported of HGSOC responsive to a MEK-inhibitor after searching for “high grade ovarian cancer” or “ovarian cancer” AND “MEK inhibitor” on PubMed. It provides the first evidence supporting the hypothesis of the efficacy of MEK-inhibitors in this subset of aggressive ovarian cancer. Additionally, our case showed that tumor genetic sequencing could become an essential tool to direct therapeutic choices for our patients according to their sensitivity to targeted therapies. Furthermore, knowing the tumor sensitivity profile clinicians could be able to develop a patient-tailored therapy. However, further trials investigating the benefits of such management, the optimal timing of testing and incorporation in everyday practice are strongly needed.
Declaration of Competing Interest
All authors declare they have nothing to disclose.
Acknowledgments
Acknowledgments
We thank the pain therapists (Dr. M. R. and B. A. Z.) and the Dermatologist (Dr. P. S.) for their cooperation in the clinical care of the patient and for their suggestions for the present paper. We thank also all the personnel of our hospital for the great job they do every day. And lastly, but most importantly, we thank our patient (and all of them) for giving us the consent to share her story to improve the clinical practice and the cure for ovarian cancer.
Author contribution section
Study conception: SC, VG, GS.
Collection and assembly of data: SC, VG.
Manuscript writing: SC and all authors.
Manuscript editing and Final approval: All authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2020.100547.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Diaz-Flores E., Shannon K. Targeting oncogenic Ras. Genes Dev. 2007;21(16):1989–1992. doi: 10.1101/gad.1587907. [DOI] [PubMed] [Google Scholar]
- Champer M., Miller D., Kuo D.Y. Response to trametinib in recurrent low-grade serous ovarian cancer with NRAS mutation: A case report. Gynecol. Oncol. Rep. 2019;28:26–28. doi: 10.1016/j.gore.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert I. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol. Oncol. 2017;146(2):319–326. doi: 10.1016/j.ygyno.2017.05.019. [DOI] [PubMed] [Google Scholar]
- https://oncologypro.esmo.org/Meeting-Resources/ESMO-2019-Congress/A-Randomized-Phase-II-III-Study-to-Assess-the-Efficacy-of-Trametinib-in-Patients-with-Recurrent-or-Progressive-Low-Grade-Serous-Ovarian-or-Peritoneal-Cancer, 2019.
- Fagotti A. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am. J. Obstet. Gynecol. 2013;209(5):462.e1–462.e11. doi: 10.1016/j.ajog.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Daud A., Tsai K. Management of treatment-related adverse events with agents targeting the MAPK pathway in patients with metastatic melanoma. Oncologist. 2017;22(7):823–833. doi: 10.1634/theoncologist.2016-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.mycancergenome.org/content/disease/ovarian-cancer/kras.
- Garg K., Park K.J., Soslow R.A. Low-grade serous neoplasms of the ovary with transformation to high-grade carcinomas: a report of 3 cases. Int. J. Gynecol. Pathol. 2012;31(5):423–428. doi: 10.1097/PGP.0b013e31824ae6f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated genomic analyses of ovarian carcinoma, Nature 2011, 474(7353), 609–615. [DOI] [PMC free article] [PubMed]
- Sangha, N., et al., 2008. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia 10(12), 1362–1372, following 1372. [DOI] [PMC free article] [PubMed]