Abstract
Introduction
Targeting inflammatory cascades is considered a promising way to prevent knee osteoarthritis (OA) progression. In terms of down-regulating the expression of inducible nitric oxide synthase (iNOS), interleukin (IL)-6, and matrix metalloproteinases (MMPs), pre-treatment with the flavonoid baicalein reportedly protects articular chondrocytes against the cytotoxicity of IL-1β. However, the benefits of post-treatment baicalein on osteoarthritic chondrocytes are not fully elucidated.
Methods
In this study, primary human chondrocytes were stimulated with IL-1β prior to baicalein application to evaluate the therapeutic effect of post-treatment.
Results
Post-treatment baicalein alleviated cell death and partially restored mitochondrial viability, while the senescence-associated secretory phenotype was not improved in IL-1β-stimulated chondrocytes. Post-treatment baicalein down-regulated the expressions of IL-1β, tumor necrosis factor-alpha, MMP-3, MMP-9, and MMP-13 mRNA as well as the protein production in stimulated cells. Even so, the levels of these factors were relative higher than those in un-treated chondrocytes. Moreover, iNOS, IL-6, IL-8, and COL1A1 expressions were consistently high, and IL-10 protein synthesis steadily increased in IL-1β-treated chondrocytes under baicalein treated status. Moreover, Western blot analyses showed that post-treatment baicalein suppressed nuclear factor kappa-light-chain-enhancer of activated B cells and p50 production while downstream cyclooxygenase-2 was still highly expressed.
Conclusion
Baicalein post-treatment to osteoarthritic chondrocytes had a minor benefit to the homeostasis of cartilaginous extracellular matrix.
Keywords: Osteoarthritis, Pro-inflammatory cytokine, Baicalein, Interleukin-10, Matrix metalloproteinase
Highlights
-
•
Pre-treatment with baicalein reportedly protects articular chondrocytes against IL-1β stimulation.
-
•
Post-treatment baicalein did not restore the expressions of inflammatory cytokines mRNA in stimulated chondrocytes.
-
•
IL-10 protein synthesis continuously increased and COX-2 was steadily highly expressed in IL-1β-treated chondrocytes.
-
•
A minor benefit of baicalein post-treatment to OA chondrocytes was showed in the present study.
1. Introduction
Since pro-inflammatory mediators participate in knee osteoarthritis (OA) initiation, targeting inflammatory cascades is considered a promising way to prevent OA progression [1]. Otherwise, it is also known that these inflammatory cytokines deteriorate the cellular antioxidant capacity to induce the senescence-associated secretory phenotype in chondrocytes and subsequently accelerate the degradation of cartilaginous extracellular matrix (ECM) in the knee joint [2]. Accordingly, interventions to improve the anti-inflammatory and antioxidant capacities of cells are proposed to treat early stage OA [3].
Several studies have reported that pre-treatment of chondrocytes with bioactive compounds can protect cells from pro-inflammatory cytokines. For example, the flavonoid baicalein has been proposed to treat OA for its anti-inflammatory, antioxidant, anti-tumor, anti-bacterial, anti-apoptotic, and anxiolytic effects, [4]. Human chondrocytes pre-treated with baicalein inhibited the interleukin-1 beta (IL-1β)-induced expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, ADAMTS-5, cathepsins, and inducible nitric oxide synthase (iNOS) mRNA; it also inhibited nitric oxide (NO) production [5]. Similarly, baicalein down-regulated the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 in IL-1β-stimulated human articular chondrocytes in vitro [6]. Another study found that baicalein treatment prior to IL-1β/tumor necrosis factor-alpha (TNF-α) stimulation attenuated apoptosis and reduced MMP-3 and MMP-13 secretions in human chondrocytes by inhibiting NO production and the downstream caspase signaling pathway [7]. It was shown that pre-treatment with baicalein could activate the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway to suppress the IL-6 expression in human osteoarthritic chondrocytes [8]. Other protective effects of baicalein on oxidative stress-induced DNA damage and apoptosis have also been demonstrated [9].
Even though these findings provide solid evidence of the therapeutic potential of baicalein to treat knee OA, the experimental condition such as pre-treatment of baicalein prior to inflammatory cytokine-stimulation may not actually represent the clinical reality. Patients who diagnosed with Kellgren–Lawrence grade II even III knee OA frequently suffer from pain and mobility impairment and have been exposure to inflammatory situation for a long time. Therefore, the therapeutic effects of post-treatment baicalein on osteoarthritic chondrocytes should be considered and examined.
2. Materials and methods
2.1. Isolation and culture of human articular chondrocytes
The retrieval and use of human tissues were reviewed and approved by the Institutional Review Board of the hospital (ECKIRB1080502). Written informed consent was provided by the eight Kellgren–Lawrence grade IV knee OA patients (five females and three males) with an average age of 72.4 years (range 58–79 years) enrolled in this study. Isolation of articular chondrocyte was performed as a previous study [10]. In brief, the articular cartilage tissues were harvested from the intact lateral compartment of the knee during total knee replacement surgery, and the samples were sectioned as thin tissue slices and minced into fragments using a scalpel. The tissues were first treated with 0.1% protease (P8811, Sigma–Aldrich, St Louis, MO, USA) for 30 min, then digested with 0.2% type II collagenase (9001-12-1; Gibco, Life Technologies, Grand Island, NY, USA) overnight. Digested tissues were collected and washed twice with phosphate-buffered saline (PBS). The isolated cells were cultured in Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F-12 (DMEM/F-12, D8900, Sigma–Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (SH30396.03, Hyclone, Logan, UT, USA), and 1% antibiotic (15140-122, Gibco, Grand Island, NY, USA) in an incubator set at 37 °C and 5% CO2. The culture medium was changed every two to three days. The cells (P2–P5) obtained from each donor were cultured and studied independently.
2.2. Evaluation of toxicity of baicalein to chondrocytes
Human chondrocytes were seeded into the 96-well culture plate (5000 cells/well) and cultured in regular DMEM/F-12 medium overnight. The cells were then washed twice with PBS and subsequent cultured in medium containing 0 (control), 5, 10, 25 and 50 μM baicalein (bai, 465119, Sigma–Aldrich, St Louis, MO, USA). After 24 h cultivation, the morphology of baicalein-treated chondrocytes was observed. The culture media were collected to measure the lactate dehydrogenase (LDH) release (G1780, CytoTox 96® NonRadioactive Cytotoxicity Assay, Promega, WI, USA). Finally, the cells were cultured in serum-free DMEM/F12 medium containing 10% water-soluble tetrazolium salt-1 reagent (11644807001, Roche, Mannheim, Germany) for 3 h to evaluate the mitochondrial activity.
2.3. IL-1β stimulation and baicalein application
Based on the results of baicalein cytotoxicity, medium containing 25 μM baicalein was selected for further study. After being cultured in DMEM/F-12 medium overnight, the chondrocytes were stimulated with 10 ng/mL IL-1β (579402, BioLegend, San Diego, CA, USA) for 24 h. The stimulated cells were washed twice and cultured in baicalein-containing media for additional 24 h. The LDH release and mitochondrial viability were determined as in the previous section. In addition, cell survival was identified using a live/dead double staining assay (R37601, LIVE/DEAD® Cell Imaging Kit, Thermo Fisher Scientific, Waltham, MA, USA). Senescent cells were also detected based on the presence of β-galactosidase (β-gal, K320-250, senescence detection kit, Biovision, Milpitas, CA, USA).
2.4. Semi-quantitative real-time polymerase chain reaction (PCR) analysis
After being exposed to IL-1β for 24 h and cultured in baicalein-containing media for an additional 24 h, the total RNA of chondrocytes was extracted (R2052 Direct-zol™ RNA MiniPrep Kit, Zymo Research, Irving, CA, USA). RNA yield was quantified using a nanodrop spectrophotometer, and the RNA was reverse transcribed into cDNA by reverse-transcription polymerase chain reaction (PCR) (T100™ Thermal cycler, Bio-Rad, Hercules, CA, USA) using a cDNA synthesis kit (RR037A, PrimeScript™ RT Reagent Kit, TaKaRa Bio Inc., Shiga, Japan). The semi-quantitative gene expression levels of cells were analyzed by using the real-time PCR (QuantStudio 3 Real-Time PCR system, Thermo Fisher Scientific, Waltham MA, USA) with the Taqman system (4444556, Applied Biosystems™ TaqMan™ Fast Advanced Master Mix, Applied Biosystems, Waltham, MA, USA) or the SYBR Green system (A25741, Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix, Applied Biosystems, Waltham, MA, USA). Pro-inflammatory cytokine expression, including expression of iNOS, IL-1β, IL-6, IL-8, IL-10, and TNF-α, was determined. The ECM-related genes, including MMP-3, MMP-9, MMP-13, tissue inhibitor of metalloproteinase (TIMP)-1, aggrecan (AGCN), collagen type I (COL1A1), and collagen type II (COL2) were also evaluated. In addition, the cell proliferation marker Ki-67 and the phenotype marker Sirt1 were analyzed. The mRNA expression of each target gene was normalized to that of the house keeping gene glyceraldehyde 3-phosphate dehydrogenase.
2.5. Quantification of anabolic and catabolic cytokines by enzyme-linked immunosorbent assay (ELISA)
The culture supernatants of treated chondrocytes were collected to quantify the pro-inflammatory cytokines. The levels of IL-1β (88–7010; eBioscience, San Diego, CA, USA), IL-6 (88–7066; eBioscience, San Diego, CA, USA), IL-8 (BMS204/3TEN, affymetrix; eBioscience, San Diego, CA, USA), IL-10 (88–7106; eBioscience, San Diego, CA, USA), TNF-α (88–7340; eBioscience, San Diego, CA, USA), MMP-3 (DY513; R&D Systems, Minneapolis, MN, USA), MMP-9 (DY911; R&D Systems, Minneapolis, MN, USA), TIMP-1 (DY970; R&D Systems, Minneapolis, MN, USA), and TIMP-2 (DY971; R&D Systems, Minneapolis, MN, USA) in supernatants were determined using a relevant enzyme-linked immunosorbent assay (ELISA) along with a spectrophotometer.
2.6. Western blotting
The chondrocytes were lysed by radioimmunoprecipitation assay buffer with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) for protein extraction, and protein concentration was determined using the bicinchoninic acid (BCA) assay (23227, Pierce™ BCA protein assay kit, Thermo Fisher Scientific, Waltham MA, USA). Twenty μg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), and incubated with anti-phospho-Nrf2 (ab180844, Abcam, Cambridge, UK), anti-Nrf2 (ab62352, Abcam, Cambridge, UK), anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB, 10745-1-AP, Proteintech, Rosemont, IL, USA), anti-p50 (14220-1-AP, Proteintech, Rosemont, IL, USA), anti-cyclooxygenase-2 (COX-2, 66351-1-lg, Proteintech, Rosemont, IL, USA), and anti-β-actin (MA5-15739, Thermo Fisher Scientific, Waltham MA, USA) antibodies at 4 °C overnight. Protein expression was detected with a chemiluminescence reagent (SuperSignal West Pico PLUS Chemiluminescent Substrate, Thermo Fisher Scientific, Waltham MA, USA) and imaged using a Western blot imaging/gel documentation system (Invitrogen iBright FL1000 Imaging System, Thermo Fisher Scientific, Waltham MA, USA). The band of each targeting protein was quantified and expressed as relative densitometry.
2.7. Statistical analysis
Data obtained from each group were expressed as mean ± standard error. One way ANOVA was used to analyze the differences among groups. A difference was considered statistically significant when the value of p was < 0.05. For the asterisks on figures, * represents p < 0.05, ** represents p < 0.01, and *** represents p < 0.001.
3. Results
3.1. High dose baicalein is cytotoxic to human articular chondrocytes
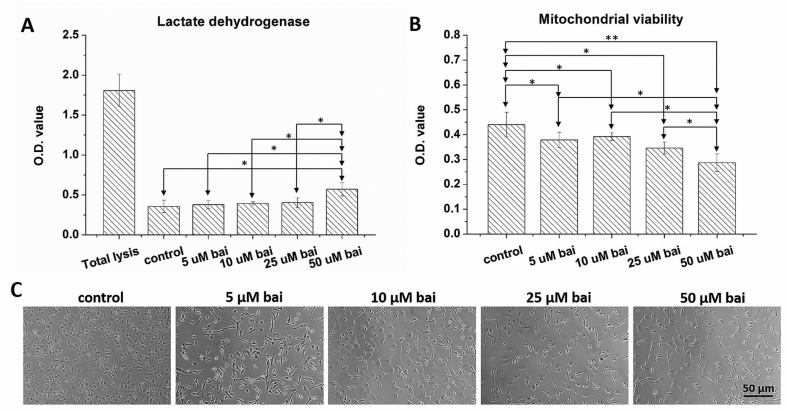
After being cultured in baicalein-containing media for 24 h, the viability and LDH release of chondrocytes were determined. Application of 50 μM baicalein to cells significantly increased LDH release (p < 0.05, Fig. 1A) and decreased mitochondrial activity (p < 0.05, Fig. 1B) when compared with those of the control group. Although no significant differences were noticed in LDH release nor mitochondrial activity among the 5, 10, and 25 μM baicalein groups, the mitochondrial activity of the above three groups was significantly lower than that of the control group (p < 0.05). Unlike the cobble-stone morphology seen in the control group, the application of baicalein to chondrocytes resulted in a slightly extended pseudopod appearance (Fig. 1C). Otherwise, no hypertrophic transformation was noticed in baicalein-treated cells.
Fig. 1.
Dose-dependent toxicity of baicalein to chondrocytes. (A) Application of 50 μM baicalein on human chondrocytes significantly increased LDH release. (B) Chondrocytes treated 50 μM baicalein also had a decrease in mitochondrial activity. (C) Chondrocytes in control group had a cobble-stone morphology, and application of baicalein on cells resulted in a slightly extending pseudopodia in appearance.
3.2. IL-1β stimulation and baicalein application
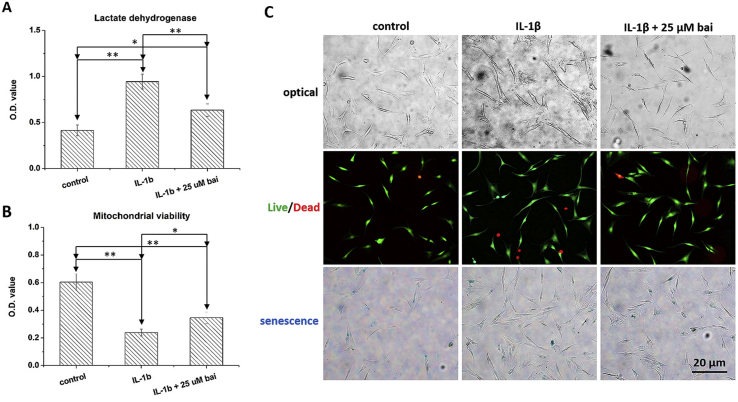
Exposure to IL-1β for 24 h significantly increased the LDH level (p < 0.01, Fig. 2A) and impaired mitochondrial activity (p < 0.01, Fig. 2B) relative to those of the control group. Application of 25 μM baicalein to IL-1β-stimulated chondrocytes decreased LDH release (p < 0.05) and restored mitochondrial activity (p < 0.05) when compared with those of the IL-1β group. However, IL-1β-stimulated chondrocytes treated with 25 μM baicalein still had a significantly higher LDH level (p < 0.05) and lower mitochondrial activity (p < 0.05) than those of the control group.
Fig. 2.
The effects of baicalein to IL-1β-stimulated chondrocytes. (A) Application of 25 μM baicalein decreased LDH release to stimulated cells. (B) The mitochondrial activity of chondrocytes was partially restored. (C) IL-1β exposure transformed chondrocytes to an irregular morphology, resulted in an increase in the number of dead cells, and caused cell senescence. Baicalein application decreased the number of dead cells, but the hypertrophic morphology and the cell senescence were not changed.
IL-1β stimulation transformed chondrocytes into an irregular morphology and resulted in an increase in the number of dead cells (red fluorescence) as well as senescent cells (β-gal staining, blue color). Although baicalein application decreased the number of dead cells, the hypertrophic morphology and cell senescence were not ameliorated (Fig. 2C).
3.3. Baicalein did not correct mRNA expression of pro-inflammatory cytokines
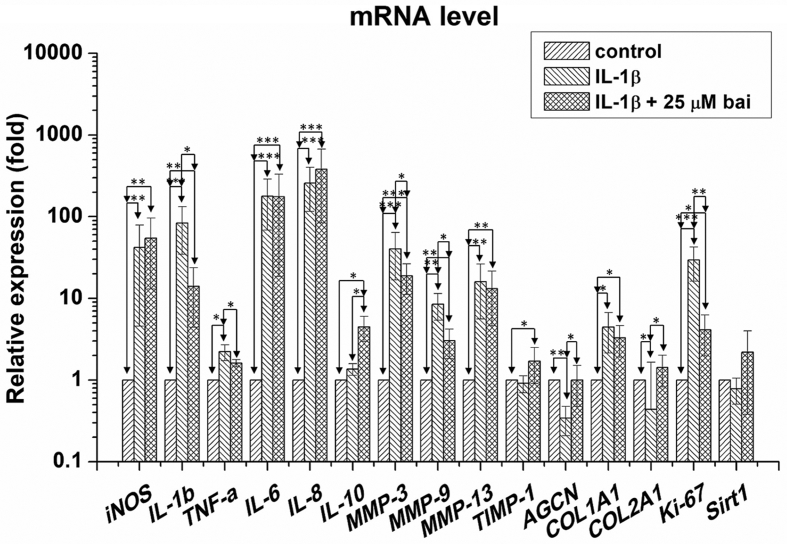
IL-1β exposure significantly up-regulated the mRNA expression of iNOS (p < 0.01), IL-1β (p < 0.001), TNF-α (p < 0.05), IL-6 (p < 0.001), IL-8 (p < 0.001), MMP-3 (p < 0.001), MMP-9 (p < 0.01), MMP-13 (p < 0.01), COL1A1 (p < 0.05) and Ki-67 (p < 0.001); and down-regulated the expression of AGCN (p < 0.01) and COL2A1 (p < 0.05) in treated chondrocytes, relative to those of the control group (Fig. 3). However, IL-1β stimulation caused no substantial changes in IL-10, TIMP-1 and Sirt1 expression in chondrocytes. Baicalein application significantly down-regulated the mRNA expression of IL-1β (p < 0.01), TNF-α (p < 0.05), MMP-3 (p < 0.05), MMP-9 (p < 0.01), and Ki-67 (p < 0.01), and up-regulated IL-10 (p < 0.05), AGCN (p < 0.05), and COL2A1 (p < 0.05) when compared with IL-1β-stimulated cells. However, baicalein did not change the expression of levels of iNOS, IL-6, IL-8, MMP-13, COL1A1, TIMP-1 and Sirt1 in treated chondrocytes.
Fig. 3.
The mRNA expressions of baicalein-treated chondrocytes. IL-1β exposure significantly up-regulated the mRNA expressions of iNOS, IL-1β, TNF-α, IL-6, IL-8, MMP-3, MMP-9, MMP-13, Ki-67, COL1A1 and down-regulated AGCN, COL2A1 to chondrocytes relative to those of control group. Baicalein application significantly down-regulated the mRNA expression of IL-1β, TNF-α, MMP-3, MMP-9, Ki-67 and up-regulated IL-10, AGCN, COL2A1 when compared with those of IL-1β-stimulated cells. However, baicalein did not change iNOS, IL-6, IL-8, TIMP-1, COL1A1 and Sirt1 levels in treated chondrocytes.
3.4. Influence of baicalein on anabolic and catabolic cytokines
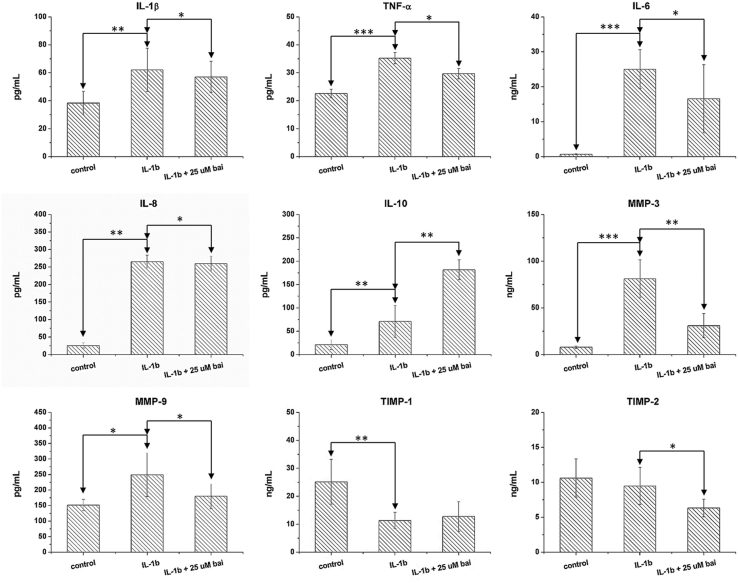
In chondrocytes, external IL-1β stimulation increased cellular production of IL-1β (p < 0.01), TNF-α (p < 0.001), IL-6 (p < 0.001), IL-8 (p < 0.001), IL-10 (p < 0.01), MMP-3 (p < 0.001), and MMP-9 (p < 0.01); it decreased TIMP-1 (p < 0.01), but TIMP-2 production was not changed. Baicalein application to IL-1β-stimulated chondrocytes decreased TNF-α (p < 0.01), MMP-3 (p < 0.01), MMP-9 (p < 0.05), and TIMP-2 (p < 0.05) protein levels and increased IL-10 level (p < 0.01). However, the synthesis of IL-1β, IL-6, IL-8, and TIMP-1 were not changed.
3.5. Baicalein modulates NF-κB and p50 syntheses
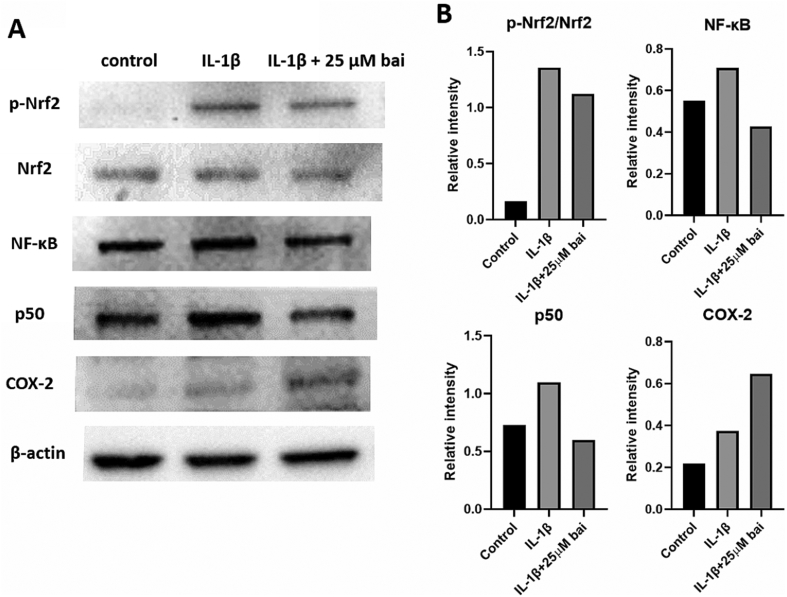
Relative to the control group, the p-Nrf2/Nrf2 ratio increased dramatically in IL-1β-exposed chondrocytes, and the syntheses of NF-κB, p50, and COX-2 were also increased (Fig. 5). Baicalein application did not change the p-Nrf2/Nrf2 ratio obviously. Otherwise, in IL-1β-stimulated cells, baicalein further increased COX-2 synthesis while NF-κB and p50 synthesis were decreased.
Fig. 5.
Western blot of baicalein-treated chondrocytes. (A) p-Nrf2/Nrf2 ratio increased dramatically in IL-1β-exposed chondrocytes, and the syntheses of NF-κB, p50, and COX-2 were also increased relative to those of control group. (B) In spite of the p-Nrf2/Nrf2 ratio was not changed obviously in IL-1β stimulated cells, baicalein application decreased NF-κB and p50 productions. However, baicalein further increased COX-2 synthesis.
4. Discussion
Both proinflammatory cytokines and oxidative stress participate in OA initiation and development, and thus, targeting inflammatory and antioxidant cascades have been proposed as an useful way to prevent the progression of knee OA. Although the therapeutic effects of pre-treatment of osteoarthritic chondrocytes with baicalein have been shown, the effects of baicalein post-treatment has not been fully elucidated.
The possible cytotoxicity of baicalein in human chondrocytes was studied first. Previous studies reported that 50–100 μM baicalein had no adverse effect on cell viability nor did it cause cell death in human chondrocytes [[5], [6], [7]]. However, Li et al. found that high dose baicalein (100 μM) did not impair viability to primary rat chondrocytes [11]. Our experiments reveal that human knee OA chondrocytes display more sensitive potential to the cytotoxicity of baicalein than health chondrocytes. Likewise, another study showed that 50 μM baicalein had no cytotoxic effects on rat nucleus pulposus cells [12]. However, Zandi et al. noticed that baicalein (50, 100, and 200 μM) dramatically impaired the viability of kidney epithelial Vero cells [13]. Li et al. also found that baicalein (50 and 100 μM) decreased the viability HeLa cells [14]. Similarly, 150 μM baicalein showed deleterious effects on HEI-193 human schwannoma cells [15]. These studies indicate that the cytotoxic dose of baicalein may depend on the cell type. In addition, our results showed that 50 μM baicalein slightly increased LDH release and decreased mitochondrial viability (Fig. 1). It is known that the severity of OA influences the responses of chondrocytes to external stimulation [16]. Taken these researches and the result in this study, human OA chondrocytes may have different potential sensitivity to the toxicity of baicalein.
Human chondrocyte exposure to IL-1β resulted in hypertrophic transformation, cell death, impaired cell viability, and cell senescence [17]. We also noticed that IL-1β stimulation resulted in up-regulations in COL1A1, MMP-13, and down-regulations in COL2A1 (Fig. 3), which were evidences for hypertrophic differentiation of chondrocytes [18,19]. We also noticed that baicalein treatment after inflammation stimulation alleviated cell death and partially recovered mitochondrial viability, while the senescence-associated secretory phenotype was not affected in chondrocytes (Fig. 2). In fact, baicalein has been proposed as an antioxidant and shown to stimulate free radical formation [20]. Since excessive oxidative stress is known to cause cell senescence and apoptosis in chondrocytes, the dose-dependent toxicity of baicalein in chondrocytes should be further studied. Furthermore, chondrocyte hypertrophic transformation is known to play a role in early and late stage OA [21].
IL-1β stimulation caused a serious inflammatory reaction in human chondrocytes (Fig. 3, Fig. 4). Otherwise, the cartilage-specific ECM synthesis was also disturbed through down-regulations in AGCN, COL2A1 and up-regulation in COL1A1 and MMPs mRNA expressions. Even though baicalein post-treatment decreased the mRNA expressions and protein productions of IL-1β, TNF-α, MMP-3, and MMP-9, the levels were consistently higher than those of the control group. Several studies found that pre-treat with baicalein in chondrocytes down-regulated the IL-1β-induced MMP-1, MMP-3, MMP-9 and MMP-13 mRNA expressions dramatically in a dose-dependent manner [[5], [6], [7]], and baicalein application also decreased the of MMP-3, MMP-9, and COX-2 protein productions [11]. Otherwise, we found that baicalein post-treatment decreased MMP-3 and MMP-9 levels slightly. Furthermore, the mRNA expression of MMP-13 was not changed in IL-1β-stimulated chondrocytes. In addition, other studies reported that baicalein pre-treatment (25 and 50 μM) down-regulated iNOS mRNA expression in IL-1β-treated human chondrocytes and nucleus pulposus cells efficiently [5,12]. However, this study showed that baicalein post-treatment did not decrease the iNOS expression in chondrocytes. In addition to MMPs, Jin et al. reported that baicalein pre-treatment (25 and 50 μM) also down-regulated IL-6 mRNA expression in IL-1β-stimulated nucleus pulposus cells [12]. However, our findings showed that baicalein post-treatment did not affect either IL-6 expression or IL-6 production. A high level of IL-6 in synovial fluid was found to be associated with the increasement of MMP-1 and MMP-3 levels in OA patients [22]. We also noticed that IL-8, another highly secreted inflammatory cytokine in the synovial fluid of OA patients, was up-regulated in IL-1β-stimulated chondrocytes, even after treatment with baicalein. IL-8 is also known to induce chondrocyte hypertrophic differentiation [23]. Furthermore, IL-6 and IL-8 are known to synergistically stimulate cartilage ECM degradation, and thus the concern of highly expressed IL-6 and IL-8 cannot be ignored [24].
Fig. 4.
Influence of baicalein on anabolic and catabolic cytokines. The cellular productions of IL-1β, TNF-α, IL-6, IL-8, IL-10, MMP-3, MMP-9 were increased while the TIMP-1 was decreased upon IL-1β stimulation. However, the TIMP-2 was not influenced in stimulated-chondrocytes. Baicalein application decreased TNF-α, MMP-3, MMP-9, TIMP-2 protein levels and further increased IL-10 in IL-1β-stimulated chondrocytes. However, the synthases of IL-1β, IL-6, IL-8 and TIMP-1 were not changed.
Another interesting finding of baicalein post-treatment was the up-regulation of IL-10 mRNA expression as well as IL-10 protein synthesis in IL-1β-stimulated chondrocytes. The role of IL-10 in OA pathophysiology is not fully understood, but IL-10 has been proven to inhibit osteoarthritic chondrocyte proliferation and MMP-3 expression [25,26]. Therefore, we hypothesize that baicalein post-treatment up-regulated IL-10 may further down-regulate MMP-3 as well as the proliferative marker Ki-67. Furthermore, up-regulation of TIMP-1 and restored AGCN and COL2A1 expressions in baicalein post-treatment chondrocyte were also found. Previously, Zhang et al. also showed that baicalein attenuated collagen type II and glycosaminoglycans loss in stimulated mouse articular cartilage explants [7]. IL-10 overexpression is known to antagonize AGCN expression, which is down-regulated by TNF-α stimulation [27]. Based on these findings, our study reveals that baicalein post-treatment may restore cartilage-specific ECM synthesis through the up-regulation in IL-10 and down-regulation of TNF-α. Moreover, other research also showed baicalein application partially restored SOX-9, a phenotype marker of chondrocyte in IL-1β-stimulated rat articular chondrocytes [11]. On the contrary, Sirt1, a gene involved in the chondrocyte hypertrophy, was still found to be disrupted in our study [28].
Similar to the study by Jeong et al., we showed that IL-1β stimulation enhanced phosphorylation of Nrf2 [15]. Whereas they showed that baicalein pre-treatment further increased p-Nrf2/Nrf2, baicalein post-treatment did not change the ratio in our study. In contrast to the report of Khan et al. that baicalein pre-treatment activated the Nrf2 signaling pathway to suppress IL-6 expression in human osteoarthritic chondrocytes, we showed that baicalein post-treatment did not further enhance Nrf2 signaling to suppress IL-6 [8]. By analysis of p65 synthesis, Li et al. showed that baicalein inhibited NF-κB pathway, which is in consist with our finding in the suppression of p50 and NF-κB [11].
A limitation of this study is the therapeutic effects of baicalein application on IL-1β-stimulated human knee OA chondrocytes were studied at one time-point only (4–24 h). In spite of our previous study found that the effective period of antioxidant was relatively short [10], Li et al. study showed that the functions of baicalein on IL-1β-stimulated rat articular chondrocytes was time-dependent [11]. Therefore, the long-term effects of baicalein on knee OA shall be further demonstrated.
5. Conclusion
We studied the benefits of baicalein post-treatment to osteoarthritic chondrocytes. Baicalein post-treatment alleviated cell death and partially recovered mitochondrial viability, while the senescence-associated secretory phenotype was not improved in IL-1β-stimulated human chondrocytes. Baicalein down-regulated IL-1β, TNF-α, MMP-3, MMP-9 and MMP-13 expressions to stimulated cells; however, levels of these factors were still higher than those of normal chondrocytes. Moreover, iNOS, IL-6, IL-8 and COLA1A1 were consistently highly expressed, and IL-10 protein production was further increased in stimulated cells even after baicalein was provided. Baicalein post-treatment suppressed NF-κB and p50 production while the downstream COX-2 was still highly expressed. The benefits of baicalein post-treatment to osteoarthritic chondrocytes was showed in this study which implying a possible treatment to OA patients especially in the homeostasis of cartilaginous ECM. Therefore, more studies in baicalein is needed.
Declaration of Competing Interest
There are no conflicts of interest.
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (Grant number MOST 107-2314-B-385-002).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Sandy J.D., Chan D.D., Trevino R.L., Wimmer M.A., Plaas A. Human genome-wide expression analysis reorients the study of inflammatory mediators and biomechanics in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1939–1945. doi: 10.1016/j.joca.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musumeci G., Szychlinska M.A., Mobasheri A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: molecular markers of senescent chondrocytes. Histol Histopathol. 2015;30(1):1–12. doi: 10.14670/HH-30.1. [DOI] [PubMed] [Google Scholar]
- 3.Herrero-Beaumont G., Pérez-Baos S., Sánchez-Pernaute O., Roman-Blas J.A., Lamuedra A., Largo R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem Pharmacol. 2019;165:24–32. doi: 10.1016/j.bcp.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Kang K.A., Zhang R., Piao M.J., Chae S., Kim H.S., Park J.H. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol Ind Health. 2012;28(5):412–421. doi: 10.1177/0748233711413799. [DOI] [PubMed] [Google Scholar]
- 5.Chen W.P., Bao J.P., Hu P.F., Wu L.D. Baicalein inhibits inflammatory and catabolic gene expression in interleukin-1beta-induced human chondrocytes. Int J Clin Exp Med. 2016;9(2):3237–3241. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W.P., Xiong Y., Hu P.F., Bao J.P., Wu L.D. Baicalein inhibits MMPs expression via a MAPK-dependent mechanism in chondrocytes. Cell Physiol Biochem. 2015;36(1):325–333. doi: 10.1159/000374075. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Zhu Y., Chen X., Zhang Y., Zhang Y., Jia Y. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int Immunopharm. 2014;21(2):301–308. doi: 10.1016/j.intimp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Khan M.N., Ahmad I., Ansari M.Y., Haqqi T.M. Baicalein, a plant-derived small molecule, activate Nrf2/autophagy signaling axis via MEK1/2-ERK1/2-Elk1 pathway to suppress the expression of IL-6 in human osteoarthritis chondrocytes [abstract] Arthritis Rheum. 2017;69(suppl 10) [Google Scholar]
- 9.Park C., Choi E.O., Kim G.Y., Hwang H.J., Kim B.W., Yoo Y.H. Protective effect of baicalein on oxidative stress-induced DNA damage and apoptosis in RT4-D6P2T schwann cells. Int J Med Sci. 2019;16(1):8–16. doi: 10.7150/ijms.29692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai Y.F., Chen Y.R., Chen J.P., Tang Y., Yang K.C. Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process Biochem. 2019;85(2):175–184. [Google Scholar]
- 11.Li Y., Wang J., Song X., Bai H., Ma T., Zhang Z. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget. 2017;8(53):90781–90795. doi: 10.18632/oncotarget.21796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin H., Wang Q., Wu J., Han X., Qian T., Zhang Z. Baicalein inhibits the IL-1β-induced inflammatory response in nucleus pulposus and attenuates disc degeneration in vivo. Inflammation. 2019;42(3):1032–1044. doi: 10.1007/s10753-019-00965-8. [DOI] [PubMed] [Google Scholar]
- 13.Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Novel antiviral activity of baicalein against dengue virus. BMC Compl Alternative Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Ma J., Wang K.S., Mi C., Wang Z., Piao L.X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol Rep. 2016;36(5):2771–2776. doi: 10.3892/or.2016.5108. [DOI] [PubMed] [Google Scholar]
- 15.Jeong J.Y., Cha H.J., Choi E.O., Kim C.H., Kim G.Y., Yoo Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int J Med Sci. 2019;16(1):145–155. doi: 10.7150/ijms.27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y.R., Yang K.C., Lu D.H., Wu W.T., Wang C.C., Tsai M.H. The chondroprotective effect of diosmin on human articular chondrocytes under oxidative stress. Phytother Res. 2019;33(9):2378–2386. doi: 10.1002/ptr.6425. [DOI] [PubMed] [Google Scholar]
- 17.Huang T.L., Yang S.H., Chen Y.R., Liao J.Y., Tang Y., Yang K.C. The therapeutic effect of aucubin-supplemented hyaluronic acid on interleukin-1beta-stimulated human articular chondrocytes. Phytomedicine. 2019;53:1–8. doi: 10.1016/j.phymed.2018.09.233. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelo M., Yan Z., Nooreyazdan M., Pacifici M., Sarment D.S., Billings P.C. MMP-13 is induced during chondrocyte hypertrophy. J Cell Biochem. 2000;77(4):678–693. [PubMed] [Google Scholar]
- 19.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12(5):216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y.K., Chang T.C., Sheu J.R., Wen K.H., Chou D.S. Comparison of free radical formation induced by baicalein and pentamethyl-hydroxychromane in human promyelocytic leukemia cells using electron spin resonance. J Food Drug Anal. 2014;22(3):379–390. doi: 10.1016/j.jfda.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Vuolteenaho K., Koskinen-Kolasa A., Laavola M., Nieminen R., Moilanen T., Moilanen E. High synovial fluid interleukin-6 levels are associated with increased matrix metalloproteinase levels and severe radiographic changes in osteoarthritis patients. Osteoarthritis Cartilage. 2017;25(S1):S92–S93. [Google Scholar]
- 23.Merz D., Liu R., Johnson K., Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171(8):4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 24.Goldring M.B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Wang S.Z., Zheng F.H., Shen J.T., Cheng S., Yu P., You M.C. Inhibition of osteoarthritis chondrocyte proliferation by IL-10 via modulating NF-κB and related mechanisms. Int J Clin Exp Med. 2017;10(8):11688–11695. [Google Scholar]
- 26.Wang Y., Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin Med J. 2001;114(7):723–725. [PubMed] [Google Scholar]
- 27.Müller R.D., John T., Kohl B., Oberholzer A., Gust T., Hostmann A. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2008;44(3):377–385. doi: 10.1016/j.cyto.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Gabay O., Zaal K.J., Sanchez C., Dvir-Ginzberg M., Gagarina V., Song Y. Sirt1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine. 2013;80(6):613–620. doi: 10.1016/j.jbspin.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]