Dear Editor,
A 62-year-old man, normotensive, non-diabetic, was diagnosed with CLL in October 2009, Rai stage 3, Binet stage B. He was treated initially with chlorambucil monotherapy for 12 months due to symptomatic massive splenomegaly and achieved a partial response. In 2012, he received 6 cycles of Bendamustine–Rituximab (BR) chemoimmunotherapy and achieved a partial response. After 18 months, he presented with a second relapse. Molecular studies (FISH–CLL panel) revealed both 11q and 13q deletion. Patient was initiated on Ibrutinib (Imbruvica) monotherapy. Initially, he tolerated ibrutinib well and achieved a partial response. Due to recurrent sino-nasal infections and low IgG levels (IgG, 203 mg/dl), he received intravenous immunoglobulin 0.4 g/kg monthly for 6 months. After 4 years on ibrutinib, (September 2018), he reported visual blurring, with intermittent flashes of light in both eyes for the previous 1 year. Due to these symptoms, he underwent a left eye cataract surgery; however, his symptoms persisted. On review by another ophthalmologist, he underwent optical coherence tomography (Fig. 1). His anterior segment was normal but posterior segment examination revealed vitritis with cystoid macular edema (CME) in both eyes. His visual acuity was—right eye 6/36 and left eye 6/24. He received topical steroids—prednisolone 1% eye drops QID, topical nepafenac eye drops, systemic steroids for 1 week, and his ibrutinib dose was decreased to 140 mg once a day. After some transient improvement, his symptoms worsened again. Subsequently, he underwent a single quadrant barrage laser in left eye. Ibrutinib was attributed as the cause, as there were no other concomitant drugs, or any co-morbidities, which could explain CME. We stopped ibrutinib in November 2018. Four weeks later, his visual acuity improved—right eye 6/24 and left eye 6/9. Sixteen weeks after stopping his ibrutinib, his symptoms have resolved completely and visual acuity improved to right eye 6/9 and left eye 6/6. Repeat OCT (Fig. 2) showed normal retinal examination. As per Naranjo probability score for drug induced adverse reaction (ADR), his score was 7 (probable ADR). Meanwhile, he is on venetoclax therapy and continues to be in partial remission.
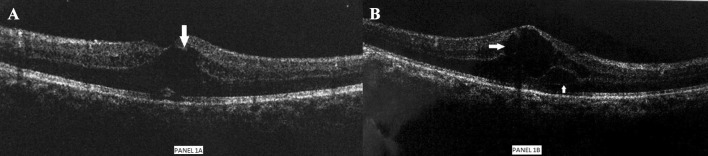
Fig. 1.
a (left) and b (right)—Optical coherence tomography (OCT) showing cystoids macular edema in left eye and right eye (marked by arrows), respectively
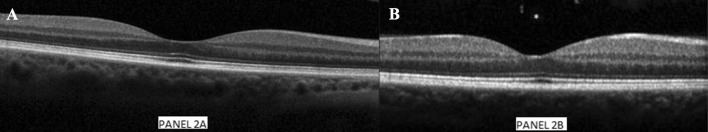
Fig. 2.
a (left) and b (right)—Optical coherence tomography (OCT) showing normal retinal layer after discontinuation of ibrutinib in left and right eye, respectively
Macular edema is a serious and important clinical sign, occurring invarious disorders. It basically, refers to accumulation of fluid in the outer plexiform and inner nuclear layer of the retina, along with swelling of Muller cells in the macular area. This abnormal fluid accumulation in the form of honeycomb-like spaces is referred to as cystoid macular edema (CME). At the cellular level, it can be either intra or extra-cellular. Common causes include ocular surgery, inherited dystrophies, ocular tumors, retinal vascular disease (commonly diabetes mellitus), vitreo-retinal adhesions, inflammatory conditions and medications. Systemic medications reported to cause CME include thiazolidinediones (pioglitazone), fingolimod, niacin, Anticancer drugs like taxanes, tamoxifen, interferon [1], and tyrosine kinase inhibitors (TKIs) (including MEK, BRAF, BCR-ABL inhibitors) [2]. Ibrutinib is an oral Bruton-Tyrosine kinase (BTK) inhibitor that is approved for patients with CLL [3]. Although the pathophysiology is unknown, it has been proposed that inhibition of MAPK pathway or increased expression of aquaporins in retinal pigment epithelium may be causative. Bernard et al. showed in patients with mantle cell lymphoma that ibrutinib can penetrate the blood brain barrier. Hence, it can also possibly reach the retina [4].
Common side effects of ibrutinib, include fatigue, rash, arthralgia, which are self-limiting and rarely prompt treatment discontinuation. Other adverse events of concern, include atrial fibrillation, diarrhea, bleeding and cytopenias [5]. Unique adverse events like palindromic rheumatoid arthritis, diffuse spongiotic dermatitis, bullous pemphigoid, recurrent paronychia, intramedullary fibrosis, and pulmonary aspergillosis [6]. Byrd et al. reported blurring of vision in 10% patients on ibrutinib and 3% incidence of cataracts in the RESONATE study. However, the cause of blurring of vision is not known. Till date, only one case has described the association of CME of ibrutinib [2]. Unlike our case, their patient’s symptoms improved with topical steroids and NSAIDs without discontinuing ibrutinib. This might be related to the prolonged duration of symptoms (1 year vs. 1 month).
With the growing popularity of ibrutinib and increasing use for B cell disorders, internists should be cognizant of this important adverse effect. Timely referral is important as long-standing CME may lead to permanent retinal structural damage.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sumeet P. Mirgh, Email: drsumeetmirgh@gmail.com
Rayaz Ahmed, Email: rayazhemat@gmail.com.
Narendra Agrawal, Email: narendra_ag1@rediffmail.com.
Sneha Bothra, Email: snehabothra2@gmail.com.
Bhaarat Mohan, Email: bhaaratfolbs@gmail.com.
Ambar Garg, Email: ambargarg04@gmail.com.
Shinto Francis Thekkudan, Email: meshinto@gmail.com.
Vishvdeep Khushoo, Email: vkhushoo@gmail.com.
Dinesh Bhurani, Email: bhurani1968@gmail.com.
References
- 1.Makri OE, Georgalas I, Georgakopoulos CD. Drug-induced macular edema. Drugs. 2013;73:789–802. doi: 10.1007/s40265-013-0055-x. [DOI] [PubMed] [Google Scholar]
- 2.Saenz-de-Viteri M, Cudrnak M. Bilateral cystoid macular edema in a patient with chronic lymphocytic leukemia treated withibrutinib. LeukLymphoma. 2019;60(3):842–844. doi: 10.1080/10428194.2018.1508673. [DOI] [PubMed] [Google Scholar]
- 3.Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16:169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous systemrelapse. Blood. 2015;126:1695–1698. doi: 10.1182/blood-2015-05-647834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh H, Khattab A, Faisal MS, Chilkulwar A, Albrethsen M, Sadashiv S, Fazal S. Case series of unique adverse events related to the use of ibrutinib in patients with B-cell malignancies—a single institution experience and a review of literature. J Oncol Pharm Pract. 2018;25:1078155218788707. doi: 10.1177/1078155218788707. [DOI] [PubMed] [Google Scholar]