Abstract
The Marburg I polymorphism (G511E) in FSAP gene was listed as one of the risk factor for idiopathic DVT among the western population. The frequency of Marburg I polymorphism in India is presently not known. Fifty DVT cases and 50 healthy controls were tested for Marburg I polymorphism using ARMS-PCR technique. The thrombophilic risk factors (Protein C, Protein S, Antithrombin III, Factor V Leiden and antiphospholipid antibodies) were also determined. Marburg I polymorphism (heterozygous) was found in 2 patients (4%) but not in control subjects. These two cases did not have any other thrombophilia markers. Among the thrombophilic markers, heterozygous FVL mutation, PS, PC, AT deficiencies and antiphospholipid antibodies were seen in 10%, 10%, 6%, 6% and 8% of the patients respectively. The controls showed only the presence of antiphospholipid antibodies in 6% of subjects. Marburg I polymorphism among Indians DVT patients was determined for the first time. Its incidence was found in 4% of cases and not in controls. Although not statically significant this may be considered as one of the contributory risk factors for the development of DVT. A larger study is required for the validation of data.
Keywords: Deep vein thrombosis, Genetic polymorphism, Marburg I, Thrombophilia
Introduction
Deep vein thrombosis-pulmonary thromboembolism (DVT-PE) is one of the commonest causes of death in hospitalized patients in western countries. In western populations, the incidence of DVT was found to be 1.24 per thousand populations per year [1]. In India, the incidence of DVT is much less when compared to western countries. According to the available literature, the common inherited risk factors for DVT are: deficiencies of Protein C (PC) (9.5%–15.9%), Protein S (PS) (6.5%–13.1%), Antithrombin III (AT) (3.6%–3.8%) and Factor V Leiden (FVL) mutation (3%–8.3%) [2, 3]. Recently, a mutation in the Factor Seven Activating Protease (FSAP) gene has been described as one of the risk factors for venous thrombosis [4–7].
FSAP is a novel serine protease and the gene encoding FSAP protein is located on chromosome 10 with 13 exons spanning 35 kb length. The function of FSAP is to maintain hemostasis by acting through both coagulation and fibrinolytic pathways hence activating Factor VII and prourokinase respectively [8]. Marburg I polymorphism (Gly511Glu) is one of the single nucleotide polymorphisms in FSAP associated with thrombosis. This polymorphism causes functional abnormality of FSAP however, quantitatively, its level remains normal. This leads to 50–80% impairment in activation of prourokinase thereby inhibiting fibrinolysis [9]. Its action towards activating Factor VII is normal which led to activation of coagulation pathway. This imbalance diverts hemostasis towards a prothrombotic state. The data related to Marburg I polymorphism in patients with DVT is very sparse in the literature and is not available for the Indian population. The aim of present study to investigate the DVT patients for Marburg I polymorphism and also to detect the correlation between other variables causing thrombosis if any.
Patients and Methods
This was a prospective case–control study carried out in the coagulation laboratory of Hematology Department over a period of 2 years (2014–16). The study was approved by the ethics committee of the Institute (Reference No. NK/1631/MD/9818). Fifty patients with deep vein thrombosis between the age of 18 and 60 years were recruited in this study after obtaining consent. The exclusion criteria included patients on oral anticoagulation and the onset of the symptoms within 4–6 weeks. Patients were classified into idiopathic and secondary cases. The patients who had no associated risk factors were included in idiopathic cases. Fifty non-DVT age-matched volunteers were included in this study after informed consent.
The Marburg I Polymorphism was detected using Amplification–Refractory–Mutation System Polymerase Chain Reaction (ARMS-PCR) technique using the following primer pairs: FSAP 1601G/wild-type Forward primer: 5′-TGGGGCCTGGAGTGTGG-3′, Reverse primer: 5′-GGTGTCCATTGTTGGCC-3′, FSAP 1601A/Marburg I Forward primer: 5′-TGGGGCCTGGAGTGTGA-3′, Reverse primer: 5′-GGTGTCCATTGTTGGCC-3′. [4] The DNA fragments which harbour the mutation were generated using specific primers designed specifically for those mutations. Automated DNA sequencing was performed to confirm the presence of polymorphism using an automated sequencer (ABI 3130 Genetic Analyzer; Applied Biosystems). The FVL mutation was detected by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) using Mnl1 as a restriction enzyme. Platelet-poor plasma (PPP) was stored for maximum 1 month at − 20 °C. The functional activity of PC, PS and AT and lupus anticoagulant (LA) were measured on automated coagulation analyzer (STA-R Evolution®, Diagnostica Stago, Asnieres, France). The low values of the tests were confirmed by repeat testing on a separate sample after a period of 1 month. The anticardiolipin antibody (ACA) and anti-beta2 glycoprotein 1 antibody (B2GP1) were measured with ELISA kits (Orgentec Diagnostika GmBH, Mainz, Germany). Antiphospholipid antibodies (APL) were considered positive if one of the three tests (ACA, B2GP1 & LA) were positive on two occasions, at least 12 weeks apart. Fischer’s exact test was used to analyse the association of Marburg I polymorphism between the patient and non-DVT control groups and also its correlation with other thrombophilic markers. A p value < 0.05 was taken as significant.
Results
Among 50 patients, Marburg I polymorphism was detected in 2 patients (4%) and both showed heterozygous mutation (Fig. 1). For confirmation of the Marburg I polymorphism, one of the patient sample which showed the mutation was subjected to gene sequencing (Fig. 2). There was a G to A transition seen in the automated DNA sequencing which confirmed the presence of Marburg I polymorphism. None of the hundred alleles tested in the 50 control subjects showed the polymorphism. Fischer’s Exact test was applied and it was found that the difference between patients and control subjects were not statistically significant (p value − 0.495). These two patients did not have any other thrombophilia markers. The test results for FVL, PC, PS, AT and APL which were checked for correlation with Marburg I polymorphism positive patients and were found to be statistically insignificant.
Fig. 1.
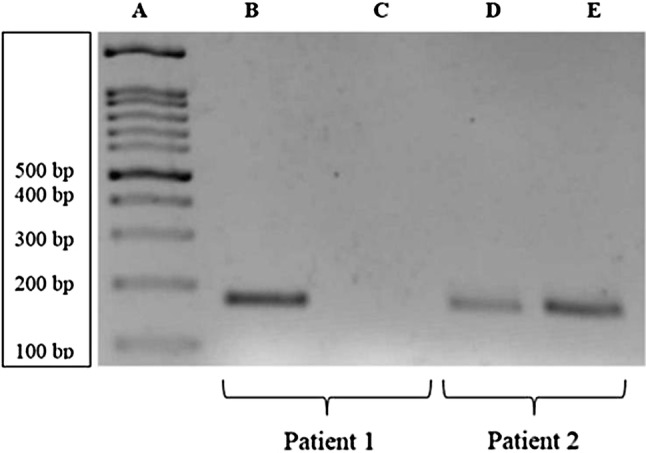
PCR amplification showing Marburg I polymorphism (Lane A—DNA Ladder; Lane B, D—Amplified product with normal pair of primers; Lane C, E—Amplified product with mutant pair of primers; Patient 1—Normal and Patient 2—Heterozygous for Marburg I polymorphism)
Fig. 2.
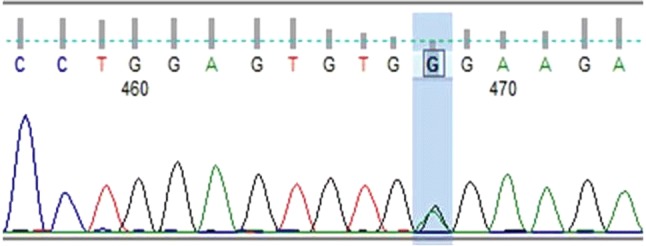
Sequence read showing Heterozygous mutation (1691G > A) in exon 13
Among the inherited thrombophilic risk factors, there were 5 (10%) cases of PS deficiency and 3 cases (6%) each for PC and AT deficiency. Five patients were heterozygous for FVL mutation which was one of the commonest thrombophilic risk factors among patients. Antiphospholipid antibodies, were present in 4 (8%) patients and 3(6%) control subjects.
In addition, the patients had many predisposing circumstantial risk factors and some of the patients had coexistent of inherited risk factors with circumstantial factors. The thrombophilic risk factors (including predisposing factors) and their frequency in percentage of patients and controls are given in Table 1. Many of the patients have more than one risk factors. These patients were grouped on the basis of number of risk factors (Table 2) and out of the 50 patients, 16 (32%) did not have any identifiable risk factors (idiopathic DVT) excluding Marburg I polymorphism. Hence the incidence of Marburg I polymorphism among those labelled as idiopathic DVT was 12.5% (2/16).
Table 1.
The thrombophilic risk factors (inherited and circumstantial) in study population
| Risk factors | Patients (%) | Controls | |
|---|---|---|---|
| Surgery | 10 (20%) | 1 (2%) | |
| Smoking | 10 (20%) | 1 (2%) | |
| Trauma | 3 (6%) | 1 (2%) | |
| Recurrence | 4 (8%) | Nil | |
| Pregnancy related | 7 (14%) | Nil | |
| OCP use in female cohort | 3 (13%) | Nil | |
| Recurrent fetal loss in female cohort | 2 (8.7%) | Nil | |
| Protein S deficiency | 5 (10%) | Nil | |
| Protein C deficiency | 3 (6%) | Nil | |
| Antithrombin deficiency | 3 (6%) | Nil | |
| Antiphospholipid antibodies | 4 (8%) | 3(6%) | |
| Factor V Leiden | Homozygous | Nil | Nil |
| Heterozygous | 5 (10%) | Nil | |
| Marburg I polymorphism | Homozygous | Nil | Nil |
| Heterozygous | 2 (4%) | Nil | |
Table 2.
The DVT patients with the presence of known inherited and circumferential risk factors (The Marburg I polymorphism was not included)
| Number of risk factors | Number of patients—n (%) |
|---|---|
| 0 | 16 (32%) |
| 1 | 17 (34%) |
| 2 | 12 (24%) |
| 3 | 2 (4%) |
| 4 | 2 (4%) |
| 5 | 1 (2%) |
Discussion
Marburg I polymorphism has been reported as an independent risk factor for venous thromboembolism among the western population. Hoppe et al. showed that there was a significant difference in prevalence of Marburg I polymorphism, between the patients with a history of venous thromboembolism (8.0%) or idiopathic venous thromboembolism (11.7%) and normal healthy controls (2.3%) [4]. Subsequently, Ahmad-Nejad et al. had detected Marburg I polymorphism in 7.63% of the patients with venous thrombosis and 5.14% of the control population [5]. In contrast to these studies, van Minkelen et al. reported that Marburg I polymorphism is not associated with DVT [6]. This study analysed 471 individuals each in patient and control groups of which 27 patients (5.73%) and 30 control subjects (6.36%) showed Marburg I polymorphism. This study differs from the above studies could be attributed to the variation in the frequency of Marburg I polymorphism in the control groups. Gulesserian et al. analysed the relevance of this polymorphism with respect to recurrent venous thromboembolism [7]. In this study, the incidence of Marburg I variant is reported as 4.8%. Over a period of 3 years, the probability of recurrence in patients harbouring the mutation is 20% when compared to the 12% of the patients without the mutation. The relative recurrence risk in patients with this variant is 1.3 before and 1.5 after adjusting the potential confounding factors. Hence it was concluded that the presence of Marburg I is only a mild risk factor for recurrent thromboembolism.
In the present study, which included 50 patients and 50 non-DVT controls, Marburg I polymorphism was detected in 2 patients (4%) while the incidence of polymorphism in idiopathic DVT is 12.5% (2/16). None of the non-DVT control subjects was found to harbour this polymorphism. The difference between both the groups was not statistically significant which could be attributed to small population size. There was no significant association of Marburg I polymorphism with other known thrombophilic risk factors. Even though the Marburg I polymorphism may not be considered as a strong independent risk factor among Indian population it can be speculated that like the other risk factors, the presence of the polymorphism may confer added risk, though this study group does not give enough data to support this.
In western literature, the incidence of FVL mutation (both heterozygous and homozygous) accounted for about 20–25% whereas in cases of familial thrombophilia it was noted in 50% of the patients [10]. As per the epidemiology, FVL mutation was seen in 18% of Caucasians with venous thrombosis. However, the incidence was less among African–Americans and other non-Caucasians [11]. Studies done in the Indian population with venous thrombosis reported the incidence of FVL mutation ranging from 3 to 12% of the patients [3, 12, 13]. In the present study, FVL mutation was seen in 5 patients (10%) and all were heterozygous. The overall incidence of deficiencies of anticoagulant factors PC, PS and AT among patients in several studies from varies between 3 and 21% [3, 14]. In the current study, three patients with PC deficiency (6%) were identified. Five patients had a PS deficiency (10%), and three patients were deficient for AT (6%). The incidence of antiphospholipid antibodies among Indian patients with venous thrombosis ranges from 5.5 to 10% [3, 13–15] The present study also had similar incidence (4 cases; 8%). The presence of antiphospholipid antibodies among 6% (3 controls) of controls is uncommon. Among these 2 were female and one was male. None of these females have any history of recurrent pregnancy losses. The male subject also did not have any symptoms. These control subjects need follow-up for development of any morbidity.
To conclude, Marburg I polymorphism was previously reported as an independent risk factor for venous thromboembolism. But statistically, the relationship is not proven in our study. Since there are no published Indian (Asian) data regarding the role of Marburg I polymorphism in DVT patients, our study could be considered as the first among them. The difference from other studies could be explained by various factors like population group analysed and the sample size. Larger prospective studies would be able to provide more concrete answers.
Acknowledgements
The authors would like to thank Mr S. K. Bose and Mr. Varun Uppal for helping in laboratory-based thrombophilia testing.
Funding
The author(s) received financial support for this research from Department of Science and Technology-Union territory (DST-UT) Chandigarh, India (Letter No. S&T/Sanc/06/2014/456-461 dated 5.6.14). Dr. Archana Sundaram had received financial support for this as a thesis grant (Special Research Grant) from Medical Education and Research Cell, PGIMER Chandigarh (2014–16).
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest amongst any of the authors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost. 2000;83:657–660. doi: 10.1055/s-0037-1613887. [DOI] [PubMed] [Google Scholar]
- 2.Pai N, Shetty S, Kulkarni B, Ghosh K. Differences in etiological and clinical manifestations in upper extremity and lower limb deep venous thrombosis patients from India. Clin Appl Thromb Hemost. 2010;16:698–700. doi: 10.1177/1076029609351290. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh K, Shetty S, Madkaikar M, Pawar A, Nair S, Khare A, et al. Venous thromboembolism in young patients from western India: a study. Clin Appl Thromb Hemost. 2001;7:158–165. doi: 10.1177/107602960100700214. [DOI] [PubMed] [Google Scholar]
- 4.Hoppe B, Tolou F, Radtke H, Kiesewetter H, Dörner T, Salama A. Marburg I polymorphism of factor VII-activating protease is associated with idiopathic venous thromboembolism. Blood. 2005;105:1549–1551. doi: 10.1182/blood-2004-08-3328. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad-Nejad P, Dempfle CE, Weiss C, Bugert P, Borggrefe M, Neumaier M. The G534E-polymorphism of the gene encoding the factor VII-activating protease is a risk factor for venous thrombosis and recurrent events. Thromb Res. 2012;130:441–444. doi: 10.1016/j.thromres.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 6.van Minkelen R, de Visser MC, Vos HL, Bertina RM, Rosendaal FR. The Marburg I polymorphism of factor VII-activating protease is not associated with venous thrombosis. Blood. 2005;105:4898. doi: 10.1182/blood-2005-02-0576. [DOI] [PubMed] [Google Scholar]
- 7.Gulesserian T, Hron G, Endler G, Eichinger S, Wagner O, Kyrle PA. Marburg I polymorphism of factor VII-activating protease and risk of recurrent venous thromboembolism. Thromb Haemost. 2006;95:65–67. doi: 10.1160/TH05-09-0601. [DOI] [PubMed] [Google Scholar]
- 8.Römisch J, Feussner A, Vermöhlen S, Stöhr HA. A protease isolated from human plasma activating factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1999;10:471–479. doi: 10.1097/00001721-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Roemisch J, Feussner A, Nerlich C, Stoehr HA, Weimer T. The frequent Marburg I polymorphism impairs the pro-urokinase activating potency of the factor VII activating protease (FSAP) Blood Coagul Fibrinolysis. 2002;13:433–441. doi: 10.1097/00001721-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance) Blood. 1995;85:1504–1508. doi: 10.1182/blood.V85.6.1504.bloodjournal8561504. [DOI] [PubMed] [Google Scholar]
- 11.De Stefano V, Chiusolo P, Paciaroni K, Leone G. Epidemiology of factor V Leiden: clinical implications. Semin Thromb Hemost. 1998;24:367–379. doi: 10.1055/s-2007-996025. [DOI] [PubMed] [Google Scholar]
- 12.Garewal G, Das R, Varma S, Chawla Y, Prabhakar S. Heterogeneous distribution of factor V Leiden in patients from north India with venous thromboembolism. J Thromb Haemost. 2003;1:1329–1330. doi: 10.1046/j.1538-7836.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Chougule A, Rajpal S, Ahluwalia J, Bose SK, Masih J, Das R, et al. Coagulation factor VIII, IX and XI levels in north Indian patients with venous thromboembolism: first study from India. Blood Coagul Fibrinolysis. 2016;27:58–63. doi: 10.1097/MBC.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 14.Vora S, Ghosh K, Shetty S, Salvi V, Satoskar P. Deep venous thrombosis in the antenatal period in a large cohort of pregnancies from western India. Thromb J. 2007;5:9. doi: 10.1186/1477-9560-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahluwalia J, Sreedharanunni S, Kumar N, Masih J, Bose SK, Varma N, et al. Thrombotic Primary Antiphospholipid Syndrome: the profile of antibody positivity in patients from North India. Int J Rheum Dis. 2016;19(9):903–912. doi: 10.1111/1756-185X.12479. [DOI] [PubMed] [Google Scholar]


