Abstract
Diffuse large B cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma among adults, although it also affects the young and the elderly. DLBCL is treated with a chimeric monoclonal antibody against CD20, a B cell surface protein, named rituximab, in combination with a multidrug chemotherapeutic regimen. However, owing to its high cost, rituximab cannot be afforded by patients in developing or underdeveloped countries. In such cases, biosimilars of rituximab have been used instead of rituximab, with equivalent efficacy. In this single center, retrospective, observational study, we have compared patient outcomes such complete response (CR), partial response (PR), and overall response rate (ORR) in a cohort of 152 patients in an Indian hospital, who were treated either with innovator rituximab or Reditux, a biosimilar. We observed that the ORRs of both groups (88% in innnovator group and 82% in biosimilar group) were comparable. There was no statistically significant difference between the two groups in terms of CR (p = 0.353), PR (p = 0.42), ORR (p = 0.23), unfavorable responses, and stable or progressive disease (p = 0.42). The number of patients who died due to complications were few, and there was no significant difference between the two groups. The differences in the 3-year event-free survival and overall survival were not statistically significant. Biosimilar rituximab can suitably and safely replace the innovator rituximab for treatment of diffuse large B cell lymphoma.
Keywords: Rituximab, Reditux, Biosimilars, DLBCL, CD20, Overall survival
Introduction
Diffuse large B cell lymphoma (DLBCL), the most common form of non-Hodgkin lymphoma among adults [1], and accounts for 40% of all cases worldwide [2]. Although heterogeneous and aggressive, DLBCL can be cured using chemotherapy or a combination of chemotherapy and immunotherapy. Till the last two decades, DLBCL was treated with a multiagent chemotherapy regimen consisting of cyclophosphamide, vincristine, doxorubicin, and prednisone, referred to as “CHOP.” The addition of immunotherapy with rituximab to CHOP (R-CHOP) has improved overall survival (OS) and progression free survival (PFS) in young as well as elderly patients with DLBCL, as is evident from the results of the Lymphome Non Hodgkinien study 98-5 (LNH98-5) trial [3] and MabThera International Trial (MInT) [4] trials. Rituximab is a chimeric mouse-human monoclonal antibody directed at CD20 on the B cell surface [5]. Binding of rituximab to CD20 triggers cell death. Studies show 55.8% survival at 6 years among patients receiving only CHOP and 74.3% among patients receiving R-CHOP [4].
However, the cost of DLBCL therapy has increased manifold after the addition of rituximab to the standard therapeutic regimen. In fact, in developing countries such as India, this drug was out of reach for economically constrained patients till the first biosimilar of rituximab was launched in India in 2007 [6]. Since then, several biosimilars of rituximab have been introduced in the market. As the synthesis of biological molecules involves complex steps, the biosimilar may not be clinically equivalent to the reference molecule in terms of efficacy and safety, and therefore not always interchangeable even for the same indication [7]. Although cost is a cause of major concern when providing standard of care, it is imperative that efficacy and safety should not be compromised while using biosimilar molecules. Indeed previous studies have shown that the efficacies of certain biosimilars were equivalent to that of rituximab. In this study, we investigated whether the innovator rituximab molecule and its biosimilar are interchangeable, and compared the efficacy these molecules in treating patients with DLBCL in a tertiary care center in India.
Materials and Methods
This was a single center, retrospective, observational study performed in the Department of Hematology at a tertiary care centre in India. The study was approved by the institutional review board. Informed consent waiver was obtained for the purpose of the study.
Patients
Patients newly diagnosed with previously untreated DLBCL from January 2011 to December 2014, who were treated with R-CHOP chemotherapy regimen at this hospital, were included in this study. Patients who presented with relapsed disease or were initially treated outside this hospital were excluded from the study. Patients who were lost to follow-up before completion of six cycles of R-CHOP were excluded from the final analysis on outcomes but were accounted for in each arm.
Treatment
The patients made an informed decision to receive either innovator rituximab or a biosimilar of rituximab, called Reditux (Dr. Reddy’s Laboratory, Hyderabad, India). The innovator molecules used were Mabthera (Roche, marketed by Roche in India), Ikgdar (Roche, marketed by Emcure Laboratories, Pune, India), and Ristova (Roche, marketed by Roche in India). Rituximab was administered at a dose of 375 mg/m2 along with the standard CHOP regimen. None of the patients received antibiotic prophylaxis.
Response to Treatment
Patients were assessed for response to chemotherapy at the end of six cycles of R-CHOP. The response assessment tools used were predominantly computed tomography (CT) or positron emission tomography (PET) scan, but in some cases included ultrasound and chest X-ray only, as per the financial suitability of the patient. The responses were categorized as complete response (CR), partial response (PR), stable disease, progressive disease or relapse as per the Lugano criteria [8].
Statistical Analysis
A comparative statistical analysis was performed between two groups who received either innovator rituximab or the biosimilar molecule. Statistical analyses were performed using SPSS 14.0 (SPSS, Chicago Ill). Patients’ demographics and clinical characteristics were expressed as proportions and compared across treatments using two-tailed Chi squared tests. Treatment efficacy was expressed as proportions of CR, PR and CR + PR, and compared across treatments using two-tailed Chi squared tests and odds ratios with 95% confidence intervals (CI). P values < 0.05 were considered statistically significant. The outcomes measured included overall response rates (ORR), event free survival (EFS) and OS. OS was calculated from the date of diagnosis to date of death due to any cause. EFS was calculated from the date of diagnosis to date of disease progression, relapse, or death. The Kaplan–Meier method was used to estimate EFS and OS, and differences were compared using the two-sided log rank test.
Results
Patient Characteristics
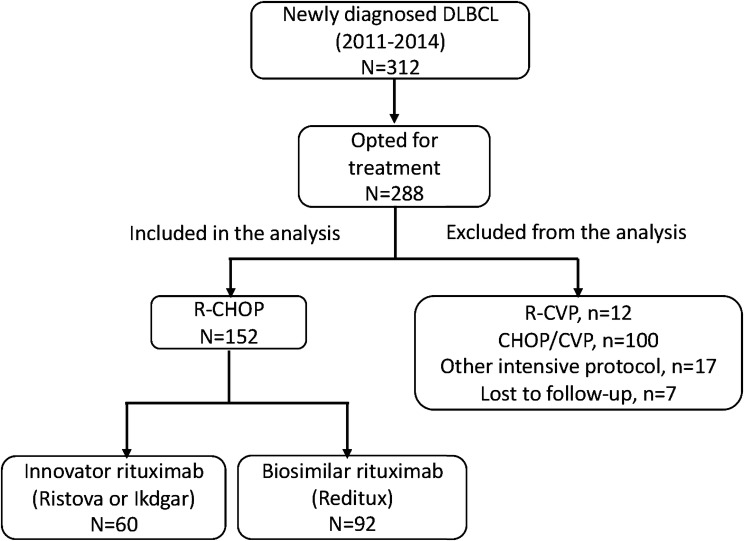
Three hundred and twelve patients were evaluated to have DLBCL between years 2011 and 2014 at this institute (Fig. 1). Among these, 288 patients opted to get treated at this hospital. One hundred and thirty-six patients were excluded from the analysis as they received regimens other than R-CHOP (due to financial constraints, 100 patients received standard CHOP/CVP regimens without rituximab, 12 patients received R-CVP at physician’s discretion due to poor performance, 15 patients received intensive protocols other than R-CHOP due to high risk disease, two patients received the modified McGrath protocol for primary CNS lymphoma, and seven patients were lost to follow-up before the completion of chemotherapy). One hundred and fifty-two patients (n = 152) with newly diagnosed diffuse large B cell lymphoma satisfied the inclusion criteria and were analyzed in this study. Out of the 152 patients, 60 patients (39.4%) received the innovator rituximab molecule and 92 patients (60.6%) received the biosimilar molecule.
Fig. 1.
Patient inclusion diagram
The baseline characteristics of all patients are shown in Table 1. In the biosimilar group, a significantly higher number of patients had elevated serum lactate dehydrogenase (LDH) levels (88% vs. 62% in the innovator group) (P = 0.001), which is a predictor of central nervous system relapse in DLBCL. The revised International Prognostic Index (R-IPI) was significantly lower in patients receiving the innovator molecule (R-IPI 0/5 was 24% in the innovator group compared to 5% in the biosimilar group (P = 0.001). There was no statistically significant difference in number of extra-nodal sites, stage at presentation, or bulk disease between the two groups.
Table 1.
Characteristics of 152 eligible patients with newly diagnosed DLBCL in the innovator and biosimilar rituximab groups
| Characteristic | Innovator rituximab | Biosimilar rituximab | P value |
|---|---|---|---|
| Total (N) | 60 | 92 | |
| Gender [n (%)] | |||
| Male | 46 (76) | 65 (70) | 0.414 |
| Female | 14 (24) | 27 (30) | |
| Median age at diagnosis [y (range)] | 52 (15–78) | 50 (20–76) | 0.443 |
| Elderly (> 60 years) [n (%)] | 17 (28) | 21 (23) | 0.443 |
| LDH(IU/L) > ULNa [n (%)] | 37 (62) | 81 (88) | 0.001 |
| Extra-nodal sites ≥ 2 [n (%)] | 2 (1) | 2 (2) | 0.225 |
| Bulk disease [n (%)] | 8 (13) | 20 (22) | 0.191 |
| Stage III/IV [n (%)] | 30 (50) | 49 (54) | 0.323 |
| R-IPIb [n (%)] | |||
| 0 | 14 (24) | 4 (5) | 0.001 |
| 1–2 | 24 (40) | 54 (58) | |
| 3–5 | 22 (36) | 34 (37) | |
aULN Upper limit of normal
bR-IPI Revised International Prognostic Index
Response Assessment Tool
Out of 60 patients who received the innovator rituximab, the response to six cycles of R-CHOP was evaluated with CT scan in 87% patients, with PET-CT scan in 9% cases, and with X-ray/ultrasound in 4% cases. Out of the 92 patients who received the biosimilar molecule, the response was evaluated with CT scan in 81% patients, with PET-CT scan in 10%, and with X-ray/ultrasound in 8% cases.
Outcomes
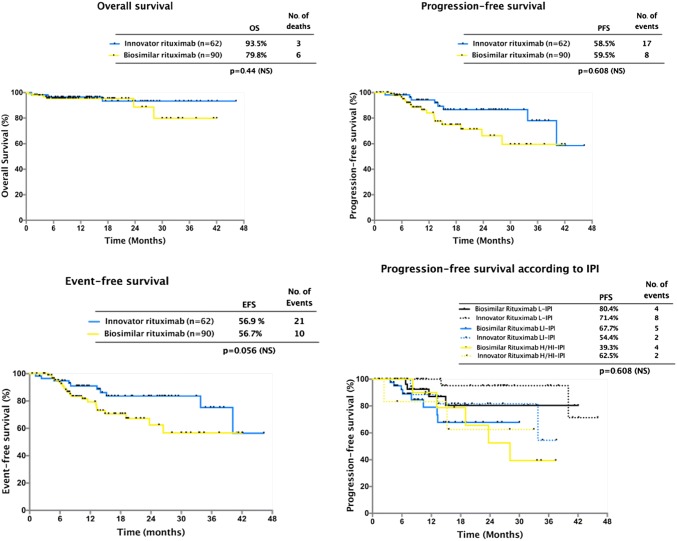
The response rates of the whole group treated with R-CHOP were as follows: CR was observed in 69.07% (105/152) and PR in 15.7% (24/152) cases, with ORR of 84.8%. Stable or progressive disease was observed in 11.1% (17/152) patients. Table 2 shows the outcomes in the two groups. In the innovator rituximab group, CR rate was 76% (46/60) and PR rate 11.6% (7/60). Five out of 60 (8.3%) patients showed unfavorable responses (four showed progressive disease and one showed stable disease). Two patients died in this group due to febrile neutropenia. In the biosimilar group, the CR rate was 64.1% (59/92), whereas PR was observed in 18.4% (17/92) patients. Twelve out of 90 (13.4%) showed unfavorable responses. (nine patients showed progressive disease and three had stable disease). Four patients died in this group, all due to complications related to febrile neutropenia. There was no statistically significant difference between the two groups in terms of CR (P = 0.353), PR (P = 0.42), ORR (P = 0.23), unfavorable responses, and stable or progressive disease (P = 0.42). The number of patients who died due to complications was few, and there was no significant difference between the two groups. One patient in the innovator rituximab group and four patients in the biosimilar group relapsed during the follow-up period of this study (median follow-up period was 17 months for the innovator rituximab group (range 6.3–46.3 months) and 13.5 months (range: 5.6–42 months) for the biosimilar group). The 3-year EFS in the innovator rituximab group and biosimilar groups were 82.1% ± 6% versus 68.2% ± 1%, respectively. This difference was not statistically significant. (P = 0.353). The 3-year OS in the innovator and biosimilar groups were 93.5% ± 3.8% versus 79.8% ± 10.4%. These differences were not statistically significant (P = 0.425) (Fig. 2). The factors predicting survival were investigated in a univariate analysis. ECOG status > 2 at diagnosis and R-IPI 3–5 predicted significantly inferior survival. More importantly, Rituximab type- innovator or biosimilar, did not impact overall survival. (Table 3). In the multivariate analysis, both ECOG status and R-IPI both did not affect overall survival.
Table 2.
Comparison of outcomes in patients treated with innovator and biosimilar rituximab
| Response category | Innovator rituximab | Biosimilar rituximab | P value |
|---|---|---|---|
| Total (N) | 60 | 92 | |
| Overall response [n (%)] | 53 (88.3) | 76 (82.6) | 0.235 |
| Complete response [n (%)] | 46 (76) | 59 (64.1) | 0.353 |
| Partial response [n (%)] | 7 (11.6) | 17 (18.4) | 0.425 |
| Stable/progressive [n (%)] | 5 (8.3) | 12 (13) | 0.435 |
| Neutropenia-associated mortality [n (%)] | 2 (3.3) | 4 (4.3) | 0.900 |
Fig. 2.
Overall. progression-free, and event-free survival in patients treated with innovator and biosimilar rituximab
Table 3.
Univariate analysis of factors affecting overall survival in patients treated with innovator and biosimilar rituximab
| Characteristic | Univariate analysis | Multivariate analysis |
|---|---|---|
| Age [y (< 60 vs. > 60)] | HR 1.7, CI 0.3–8.6, p = 0.43 | |
| Gender (Male vs. Female) | HR 1.7, CI 0.3–8.6, p = 0.43 | |
| ECOG (0–1 vs. 2–4) | HR 10.3, CI 2.2–48.1, p = 003* | HR 1.6, CI 0.5–4.6, p = 0.3 |
| LDH (IU/L) > ULNa | HR 1.4, CI 0.3–6.4, p = 0.62 | |
| Extra-nodal sites ≥ 2 | HR 0.26, CI 0.06–1.0, p = 0.08 | |
| Bulk disease | HR 2.7, CI 0.4–16.4, p = 0.15 | |
| Stage III/IV | HR 3.0, CI 0.75–12.1, p = 015 | |
| R-IPI (0–1 vs. 2–3 vs. 4–5) | p = 0.03* | HR 1.4, CI 0.6–3.2, p = 0.3 |
aULN Upper limit of normal
*p < 0.05
Discussion
In this retrospective single center study, the efficacy of biosimilar rituximab was compared with that of innovator rituximab in patients with newly diagnosed DLBCL. The baseline characteristics of the patients with DLBCL in this study were comparable to those of patients in the prospective GELA LNH 98-5 and MInT trials [3, 4]. In addition, the baseline characteristics were comparable between the biosimilar and innovator rituximab-treated groups. The ORRs of both groups (88% in innnovator group and 82% in biosimilar group) were comparable and were similar to those observed in other trials (GELA LNH-98 and MInT) [3, 4]. The EFS and OS in the two groups were similar to those demonstrated in previous trials (in GELA LNH 98-5 and in MInT trials). The innovator group had similar outcome as compared to biosimilar rituximab in terms of 3-years EFS (82.1% vs. 68.2%; P = 0.353) and OS (93.5% vs. 79%; P = 0.425), with no statistically significant difference in EFS or OS between the two molecules.
Approval of rituximab as an immunotherapeutic in 1997 had dramatically transformed the treatment of CD20-positive lymphoproliferative disorders. Two decades later, cost-effective second-generation molecules and biosimilars with biological advantages are emerging, which necessitates critical appraisal of the accumulating evidence for selecting the appropriate anti-CD20 for therapy. Biosimilars are chemically and biologically highly similar to the original molecule. However, their clinical similarity to the original molecule must be evaluated and trials should be designed to determine whether they are equally efficacious and safe as the reference product. Owing to the lesser clinical examinations required prior to launching a biosimilar in the market, the production cost of biosimilars is considerably lower than that of the licensed innovator/reference product. The spiraling high cost of anti-cancer medication worldwide can therefore be abated partially by using suitable biosimilars. Indeed, the global biosimilars market is predicted to reach US$35 billion by 2020 [9]. A budget impact analysis in 28 European countries showed that the introduction of CT-P10 in the European Union (EU) will be associated with significant budget savings, which will enable many more patients to access rituximab treatment [10]. This will exert significant positive effects on both patients and the society by relieving national healthcare expenses, especially in developing and resource-poor countries.
Several biosimilars for rituximab exist, the efficacies of which have been clinically tested for rheumatoid arthritis [11, 12] and lymphoproliferative disorders [13–15]. A randomized, double-blind parallel-group, active-controlled study (phase III trial; NCT02162771 and NCT02260804) was performed on patients aged ≥ 18 years with Ann Arbor stage III–IV follicular lymphoma to test the pharmacokinetics and efficacy of CT-P10, a rituximab biosimilar [13, 14]. Results showed that CT-P10 exhibited non-inferior efficacy and pharmacokinetic equivalence to rituximab. The ORs of both the CT-P10 and rituximab-treated groups were similar (97% vs. 92.6%), which is in agreement with our observations with Reditux, another rituximab biosimilar [13]. Similar results were obtained in a phase III, multinational, double-blind, randomized, controlled trial of GP2013 (another rituximab biosimilar) on 858 young adults with previously untreated advanced stage follicular lymphoma (NCT01419665) [15]. Occurrence of adverse events and serious adverse events were similar between the treatment groups, and neutropenia was the most common adverse event [13, 15], which again corroborates our observations.
In the Indian scenario, Gota et al. (2016) investigated the pharmacokinetics and efficacy of Reditux in a small cohort of 21 patients with DLBCL on R-CHOP regimen and compared it with the profile of patients in the MabThera (rituximab) trial. They observed that the pharmacokinetic profile, B cell response, and OS of the patients on Reditux were similar to those on MabThera, indicating that Reditux can substitute MabThera in developing countries [16]. Similar observations were made by Roy et al. (2013), who compared CR, PFS, and OS for 5 years in 223 patients with DLBCL on R-CHOP therapy. Out of 223 patients, 101 received MabThera and 72 received Reditux. Toxicity, tumor response rates, PFS, and OS did not vary significantly between the two groups [17]. In a more recent study, Ganesan et al. (2017) showed no difference (P = 0.5) in the 5-year survival among those who received biosimilar (78%) versus innovator rituximab (86%) among patients treated with RCHOP (n = 119) [18]. Similarly, in a prospective, multicenter, double-blind, randomized trial of RTXM83 (a biosmilar rituximab) versus innovator rituximab (Mabthera®/Rituxan®), in combination with CHOP chemotherapy (RTXM83-CHOP vs. R-CHOP) as first-line treatment in patients with previously untreated DLBCL, the biosimilar molecule RTXM83 was non-inferior in terms of overall response rate. In 241 patients (123 in the RTXM83-CHOP arm and 118 R-CHOP arm), ORR was 84.7% versus 80.8% in RTXM83-CHOP and the R-CHOP arm, respectively with no different in safety profile [19]. Our observations with Reditux are in line with the above studies, suggesting that Reditux should be used widely in the Indian clinical setting where the health insurance coverage is at best dismal (In India, 12% urban and 13% rural population received protection coverage through any of the public funded health insurance schemes in year 2014) and more than 80% of the expenditure for treatment are met by out of pocket (OOP) primarily through household savings and on borrowings [20].
We acknowledge the limitation of a retrospective study design that is inherently subject to a selection bias, and missing data, especially on infusion-related and organ toxicities. A prospective randomized trial between comparing biosimilars to innovator molecule are required in DLBCL, similar to those which have been done in Follicular lymphoma [13–15]
In conclusion, the rituximab biosimilar Reditux can be used safely and effectively to treat patients with newly diagnosed patients with DLBCL. The prognostic parameters of patients on rituximab-CHOP and Reditux-CHOP were similar, although the latter is considerably more affordable. Rituximab biosimilars are expected to remain important treatment modalities for B cell malignancies owing to their cost-saving nature. Prospective studies on cost-effectiveness and toxicities of rituximab biosimilar are further needed to support these observations.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Human and Animal Rights
A formal consent wavier was obtained in view of the retrospective nature of the study. No identifiable human data were used for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.(1997) A clinical evaluation of the international lymphoma study group classification of non-Hodgkin’s lymphoma. Blood 89(11):3909 [PubMed]
- 2.World Cancer Report (2014) International agency for research on cancer. Accessed July 2014
- 3.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 5.Bosch X, Ramos-Casals M, Khamashta MA. Drugs targeting B-cells in autoimmune diseases. Berlin: Springer Science & Business Media; 2013. pp. 1–4. [Google Scholar]
- 6.Bloomberg (2007) Dr Reddy’s generic of Roche’s cancer drug to cost 50% less. http://www.livemint.com/Companies/K45flhIll1QCia18FMT5UK/Dr-Reddys-generic-of-Roches-cancer-drug-to-cost-50-less.html. Accessed 02 Feb 2017
- 7.Qureshi ZP, Magwood JS, Singh S, Bennett CL. Rituximab and biosimilars—equivalence and reciprocity. Biosimilars. 2013;3:19–25. doi: 10.2147/BS.S20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Lancet Haematology Biosimilars: an optimistic outlook, but vigilance is needed. Lancet Haematol. 2017;4(8):e341. doi: 10.1016/S2352-3026(17)30127-8. [DOI] [PubMed] [Google Scholar]
- 10.Gulácsi L, Brodszky V, Baji P, Rencz F, Péntek M. The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther. 2017;34(5):1128–1144. doi: 10.1007/s12325-017-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S, Emery P, Greenwald M, Yin D, Becker JC, Melia LA, Li R, Gumbiner B, Thomas D, Spencer-Green G, Meng X. A phase I pharmacokinetics trial comparing PF-05280586 (a potential biosimilar) and rituximab in patients with active rheumatoid arthritis. Br J Clin Pharmacol. 2016;82(1):129–138. doi: 10.1111/bcp.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo DH, Suh CH, Shim SC, Jeka S, Molina FFC, Hrycaj P, Wiland P, Lee EY, Medina-Rodriguez FG, Shesternya P, Radominski S, Stanislav M, Kovalenko V, Sheen DH, Myasoutova L, Lim MJ, Choe JY, Lee SJ, Lee SY, Kim SH, Park W. Efficacy, safety and pharmacokinetics of up to two courses of the rituximab biosimilar CT-P10 versus innovator rituximab in patients with rheumatoid arthritis: results up to week 72 of a phase i randomized controlled trial. BioDrugs. 2017;31(4):357–367. doi: 10.1007/s40259-017-0232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim WS, Buske C, Ogura M, Jurczak W, Sancho JM, Zhavrid E, Kim JS, Hernández-Rivas JÁ, Prokharau A, Vasilica M, Nagarkar R, Osmanov D, Kwak LW, Lee SJ, Lee SY, Bae YJ, Coiffier B. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol. 2017;4(8):e362–e373. doi: 10.1016/S2352-3026(17)30120-5. [DOI] [PubMed] [Google Scholar]
- 14.Ogura M, Sancho JM, Cho SG, Nakazawa H, Suzumiya J, Tumyan G, Kim JS, Lennard A, Mariz J, Ilyin N, Jurczak W, Lopez Martinez A, Samoilova O, Zhavrid E, Yañez Ruiz E, Trneny M, Popplewell L, Coiffier B, Buske C, Kim WS, Lee SJ, Lee SY, Bae YJ, Kwak LW. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018;5(11):e543–e553. doi: 10.1016/S2352-3026(18)30157-1. [DOI] [PubMed] [Google Scholar]
- 15.Jurczak W, Moreira I, Kanakasetty GB, Munhoz E, Echeveste MA, Giri P, Castro N, Pereira J, Akria L, Alexeev S, Osmanov E, Zhu P, Alexandrova S, Zubel A, Harlin O, Amersdorffer J. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol. 2017;4(8):e350–e361. doi: 10.1016/S2352-3026(17)30106-0. [DOI] [PubMed] [Google Scholar]
- 16.Gota V, Karanam A, Rath S, Yadav A, Tembhare P, Subramanian P, Sengar M, Nair R, Menon H. Population pharmacokinetics of Reditux™, a biosimilar rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78(2):353–359. doi: 10.1007/s00280-016-3083-x. [DOI] [PubMed] [Google Scholar]
- 17.Roy PS, John S, Karankal S, Kannan S, Pawaskar P, Gawande J, Bagal B, Khattry N, Sengar M, Menon H, Gujral S, Nair R. Comparison of the efficacy and safety of rituximab (mabthera™) and its biosimilar (reditux™) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: a retrospective analysis. Indian J Med Paediatr Oncol. 2013;34(4):292–298. doi: 10.4103/0971-5851.125248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesan P, Sagar TG, Kannan K, Radhakrishnan V, Rajaraman S, John A, Sundersingh S, Mahajan V, Ganesan TS. Long-term outcome of diffuse large B-cell lymphoma: impact of biosimilar rituximab and radiation. Indian J Cancer. 2017;54(2):430–435. doi: 10.4103/ijc.ijc_241_17. [DOI] [PubMed] [Google Scholar]
- 19.Candelaria M, Gonzalez DE, Beniwal SK, Dasappa L, Bar DO, Delamain MT, Mukhopadhyay A, Flores DH, Bhurani D, Radhakrishnan V, Salvatierra A, Kowalyszyn RD, Lipatov O, Patel M, Schusterschitz S, Volodicheva E, Perez LA. A randomized, double-blind, phase III study comparing proposed biosimilar rituximab (RTXM83) versus reference rituximab, both in combination with CHOP, in the first line treatment of patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2017;130(Suppl 1):1556. [Google Scholar]
- 20.Jayakrishnan T, Jeeja MC, Kuniyil V, Paramasivam S. Increasing out-of-pocket health care expenditure in India-due to supply or demand? Pharmacoeconomics. 2016;1:105. doi: 10.4172/pe.1000105. [DOI] [Google Scholar]