Abstract
Immune platelet destruction is a significant cause for platelet refractoriness. The platelet crossmatch—a solid phase red cell adherence assay utilizes donor platelets and patient serum to assess compatibility and appears to be a feasible option in resource constrained settings. This study was done to evaluate the frequency of platelet crossmatch positivity among Paediatric Oncohaematology patients and also to assess whether a positive crossmatch is predictive of unsuccessful platelet transfusions in this group of patients. Paediatric Oncohaematology patients who received platelet transfusions between March 2013 and September 2013 were included in the study. The pre-transfusion patient sample and a segment from the transfused donor unit were used for performing the platelet crossmatch. A blood sample was collected one hour after the transfusion to assess post-transfusion platelet count. Corrected count increment (CCI) was calculated using the standard formula. CCI ≤ 7500/µL/m2/1011 was considered evidence of an unsuccessful transfusion. Seventy-three platelet crossmatches were performed for 69 patients, of which 30 patient samples (41%) showed crossmatch positivity. 25 (89.2%) of 28 unsuccessful transfusions showed crossmatch positivity, and 40 (88.9%) of 45 successful transfusions showed negative crossmatches (p = 0.03). Crossmatch positivity among transfusion dependent Paediatric Oncohaematology patients was as high as 42%, when ABO matched platelet units were allocated without further testing. Our results indicate that this test may be a reliable tool to select compatible platelet units and an effective intervention in the management of patients at risk of immune platelet refractoriness.
Keywords: Corrected count increment, Human leukocyte antigens, Platelet cross match, Platelet refractoriness, Paediatric Oncohaematology, Solid phase red cell adherent assay
Introduction
Platelet refractoriness, defined as a lack of response in terms of post-transfusion platelet increments after two or more consecutive transfusions of an adequate dose of allogeneic platelets, is a frequently encountered challenge in clinical practice [1]. Causes are multifactorial, with 80% being attributed to non-immunological causes, and 20% to immunological causes [2]. The latter is often attributed to the presence of antibodies to human leukocyte antigens (HLA) and/or human platelet antigens (HPA). The problem is greater in patients who have been multiply transfused, with 30–70% of them becoming refractory to random donor platelet transfusions [3].
Current standard of care includes testing for these antibodies, defining their specificities, and choosing HLA/HPA selected single donor apheresis platelets from a pool of voluntary donors who are HLA and HPA typed [4]. However, implementation of these algorithms remains a challenge in resource constrained settings because of the high cost involved in testing for antibodies, and maintaining a pool of HLA/HPA typed voluntary donors. Against this background, the platelet crossmatch—a solid phase red cell adherence assay (SPRCA) which helps assess if donor platelets are compatible with the patient appears to offer an alternate, cost effective and feasible option.
This study was done to evaluate the frequency of platelet crossmatch positivity among Paediatric Oncohaematology patients in a setting where platelets are allotted only on the basis of ABO matched blood group with no further compatibility testing, and also to assess whether a positive crossmatch is predictive of unsuccessful platelet transfusions in this group of patients.
Materials and Methods
This prospective study was performed in our centre after obtaining Institutional Research Board and Ethics Committee approval.
Children with thrombocytopenia, admitted in the Paediatric Oncology unit, during the period from March to September 2013, and who required platelet transfusions were included in the study, after obtaining informed consent from parents. Patients requiring repeat platelet transfusions were included again, provided the repeat transfusion was after a period of 2 weeks.
Patients with evidence of non-immunological causes of platelet refractoriness were excluded. Patients on drugs which could cause anti-platelet and anti-leukocyte antibodies were also excluded.
All patients received random, non-leukoreduced, ABO matched platelets, which were prepared by the platelet rich plasma method and were not more than 72 h old. Samples were collected from bag tubing segments prior to issuing platelet units from the blood bank, and platelet count was estimated on a Sysmex K haematology analyser. Standard transfusion guidelines were used to determine transfusion requirement [5].
Our standard practice requires a platelet count assessment prior to accession of platelet units. This pre-transfusion platelet count was obtained from hospital records of the patient. For the post-transfusion platelet counts, EDTA anticoagulated blood was collected one hour after completion of the platelet transfusion. Pre- and post-transfusion platelet counts were evaluated on the Sysmex K haematology analyser. Patients’ prior transfusion history was accessed from blood bank records.
Patients’ plasma required for platelet crossmatch was obtained from pre-transfusion compatibility testing samples stored at − 30 °C. Donor platelets for crossmatching were obtained from the segments of the platelet bag, and stored on the platelet agitator in Eppendorf tubes until the crossmatch. The platelet crossmatch was performed subsequent to but within 48 h of the transfusion using the SPRCA assay (Capture P; Immucor, Norcross, GA, USA). Persons performing and interpreting crossmatches were blinded to pre- and post-transfusion platelet counts. Units tested which were reactive against patient plasma were reported as crossmatch positive and considered incompatible donor units. Units that did not show reactivity were reported as crossmatch negative and considered compatible.
Corrected count increment (CCI) was calculated by the formula shown below.
An unsuccessful platelet transfusion was defined as the occurrence of platelet count increments below 7500/µL/m2/1011 [6–8].
Solid Phase Red Cell Adherent Assay (SPRCA)
This is an anti-HPA and anti-HLA antibody detection system modified from procedures published by Rachel et al. [9] and Shibata et al. [10]. Patients’ HLA or HPA antibodies bind to donor platelet antigens which are coated on the micro-titre wells. The indicator cells used are O positive red blood cells coated with anti-immunoglobulin G (IgG). If the patient has an antibody to HPA or HLA, the anti-IgG coated indicator cells bind with platelet-bound antibodies, form a bridge, and cover the immobilized platelets forming a confluent monolayer. In the absence of platelet antibody, the indicator cells settle at the bottom forming a well-defined button indicating a negative reaction [11].
Procedure
The donor platelet count was adjusted to 30,000–300,000/µL with normal saline. 100 µL of count adjusted platelets were added to all the wells. Plates were centrifuged at 45–65 g at five minutes and the strips were washed on the Immucor CSW 100 semi-automated device to remove excess unbound plasma and to allow formation of a platelet monolayer. Within one minute of washing, 100 µL of low ionic strength saline (Capture LISS, Immucor Gamma) was added to all the wells and subsequently, 50 µL of positive control serum to the first well, negative control serum to the second well and patient plasma to remaining wells. Colour change of LISS from purple to blue confirmed presence of serum. The plate was incubated at 37 °C for 30 min, washed and immediately 50 µL of capture P indicator cells was added to all wells. The plate was centrifuged at 700–900 g for 1 min and the wells read macroscopically. A positive reaction was indicated by indicator red cells spread over the surface of the well bottom, and a negative reaction, by a tight button in the centre of the well bottom. Positive and negative controls were included in each run.
Statistical Analysis
A sample size of 80 was calculated using the formula 4 pq/d2, based on a prevalence of 40% and a targeted precision of 5%. The SSP version 16 software was used for statistical analysis. p values ≤ 0.05 were considered to be significant.
Platelet crossmatch results were correlated with CCI. A positive platelet crossmatch with CCI < 7500 was considered as true positive (TP) and a positive crossmatch with CCI ≥ 7500, as false positive (FP). Likewise, a negative crossmatch with CCI < 7500 was considered false negative (FN), and with CCI ≥ 7500, as true negative (TN). Sensitivity of the assay was then calculated as TP/TP + FN, specificity as TN/TN + FP, PPV by TP/TP + FP and NPV by TN/TN + FN.
Categorical variables were analysed with the Fisher exact test and numerical variables with Kruskal–Wallis and Wilcoxon tests. Correlation of the impact of previous history of transfusion crossmatch results and CCI was analysed using Mann–Whitney U method.
Results
Patient Characteristics
There were 73 samples from 69 patients analysed during the study period of 7 months (March–September 2013). Of these, 47 (68%) were males and 22 (32%) were females. The age of patients ranged from 3 months to 15 years. Among the 69 patients, 45 (65%) had a haematological malignancy, 18 (26%) had non-haematological malignancies and six (9%) had aplastic anaemia (Table 1).
Table 1.
Patient demographics and platelet transfusion details
| Parameter | Categories | Number of patients (%) |
|---|---|---|
| Diagnosis | Haematological malignancy | 45 (65) |
| Oncological malignancy | 18 (26) | |
| Aplastic anaemia | 6 (9) | |
| Gender | Male | 47 (68) |
| Female | 22 (32) | |
| Indications for platelet transfusions | Prophylaxis | 50 (68) |
| Mucosal bleeding | 16 (22) | |
| Prior to procedure | 7 (10) | |
| Episodes of platelet transfusion received | One | 9 (12) |
| Two | 52 (71) | |
| Three | 10 (14) | |
| Four | 2 (3) | |
| Previous history of transfusions | Yes | 59 (81) |
| No | 14 (19) |
Platelet Transfusions
A total of 151 ABO matched random donor platelet units were transfused to participants during the study period with a mean of two units per patient (range one to four platelet units) (Table 1). The mean platelet count per bag transfused was 6.4 × 1010 (range 5.8–8.8 × 1010).
Platelet Crossmatch Results
Of the 73 patient samples tested, 30 (41%) showed crossmatch positivity and 43 (59%) showed a negative crossmatch (Fig. 1). Of the 30 crossmatch positive samples, 15 (50%) had crossmatch positivity with all the transfused platelets, and 15 (50%) were crossmatch positive with a portion but not all of the transfused units (Fig. 1).
Fig. 1.
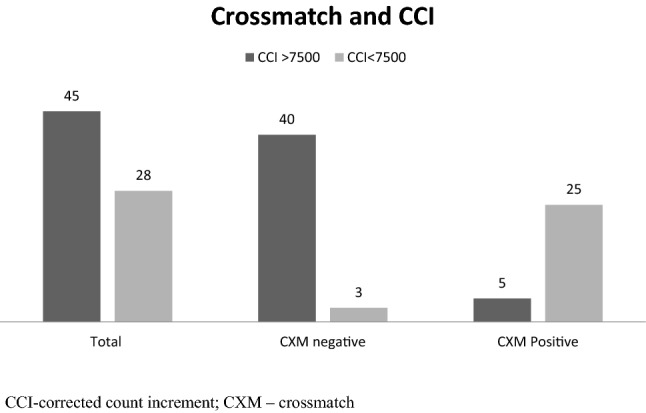
Numbers of patients with successful and unsuccessful transfusions among crossmatch positive and negative groups. CCI corrected count increment, CXM crossmatch
Correlation Between Platelet Crossmatch Results and Outcome of Platelet Transfusion (CCI)
The 1 h CCI showed that among the 73 transfusion events in 69 patients, 28 (38%) were unsuccessful transfusions demonstrated by a CCI < 7500/µL/m2/1011 and 45 (62%) were successful transfusions.
Platelet crossmatch results in the unsuccessfully transfused group showed 25 of the 28 transfusions (89%) involved incompatible platelet units, as defined by platelet crossmatch positivity. The remaining three patients in this group (11%) had unsuccessful transfusions despite negative platelet crossmatches. On the other hand, among the 45 successful transfusions, 40 (89%) received platelet crossmatch negative, compatible transfusions; while in five (11%), transfusions were successful despite platelet crossmatch positivity (Fig. 1).
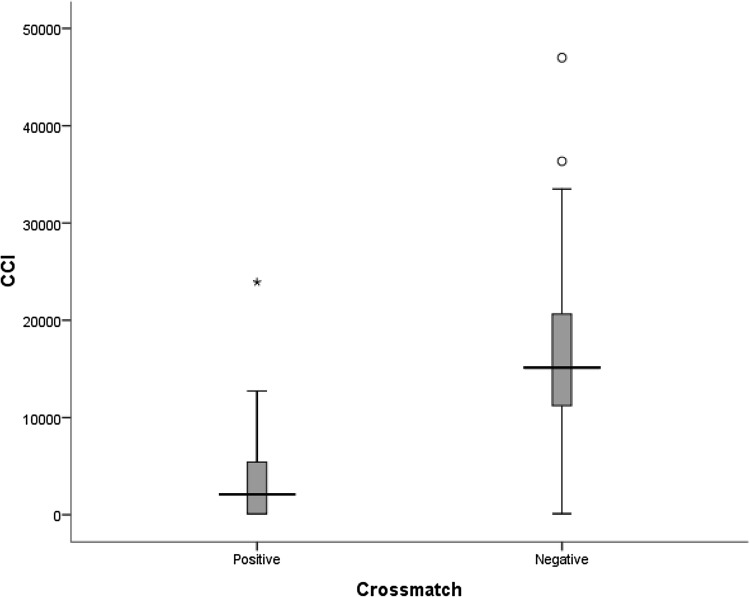
Statistical analysis using Mann–Whitney test showed a statistically significant difference in CCIs between crossmatch positive (median CCI: 2083/µL/m2/1011; range 100–7453/µL/m2/1011) and negatives groups (median CCI: 15,121/µL/m2/1011; range 100–36,343/µL/m2/1011; p = 0.000) (Fig. 2).
Fig. 2.
Box and whisker plot showing distribution of corrected count increments in crossmatch positive and negative groups
The sensitivity of the platelet crossmatch in predicting an unsuccessful platelet transfusion test was 89.2% and the specificity 88.9%. The positive predictive value of the platelet crossmatch for predicting an unsuccessful transfusion was83.3% while negative predictive value was 93.0% (Table2).
Table 2.
Two by two table showing the relation between platelet crossmatches and corrected count increments (CCI)
| Platelet crossmatch | CCI < 7500 | CCI ≥ 7500 | Total |
|---|---|---|---|
| Positive | 25 | 5 | 30 |
| Negative | 3 | 40 | 43 |
| Total | 28 | 45 | 73 |
CCI corrected count increment
Sensitivity 89.28%, specificity 88.88%, positive predictive value (PPV) 83.3% and negative predictive value (NPV) 93%
Impact of Previous Transfusion
Of the 30 patients who were crossmatch positive, 29 (97%) patients had received previous transfusions and only one patient had not received any prior transfusions, whereas among the 43 crossmatch negative patients, only 30 (69%) had received prior transfusions. It was observed that prior transfusion was significantly associated with crossmatch positivity (p = 0.003).
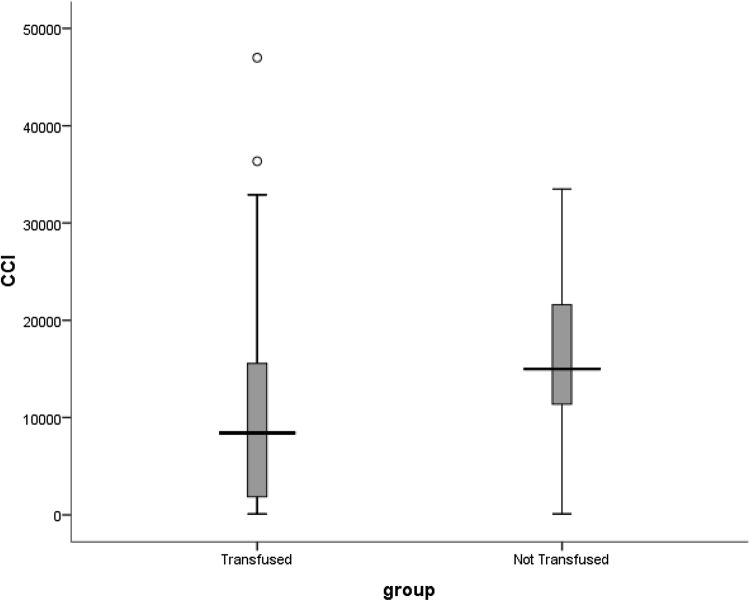
We found that prior transfusions were also significantly associated with unsuccessful transfusions (p = 0.000). The median CCI in the group who had received prior transfusions was 8423/µL/m2/1011 (range 100–36,343/µL/m2/1011) as compared to a median CCI of 14,992/µL/m2/1011 (range 100–33,496/µL/m2/1011) in the previously untransfused group (Fig. 3).
Fig. 3.
Box and whisker plot showing distribution of corrected count increments in previously transfused and non-transfused groups
Discussion
Refractoriness to platelet transfusions pose a large problem in multi-transfused patients. Current standard of care, which includes defining antibody specificities, and the use of HLA/HPA matched single donor platelets from a pool of voluntary HLA/HPA typed donors, remains a challenge in developing countries. A pool size of up to 3000 donors is required to meet transfusion demands at a good HLA-match grade level [12]. This is an unfeasible option for many blood centres in a country like India considering low rates of voluntary donation and limited resources.
The platelet crossmatch test appears to offer an interim feasible and cost effective solution. This study was performed to evaluate the prevalence of platelet crossmatch positivity among Paediatric Oncohaematology patients by using the solid phase red cell adherence (SPRCA) assay and to assess if crossmatch negativity predicts a successful platelet transfusion, and crossmatch positivity, an unsuccessful one.
Prevalence of platelet crossmatch positivity observed in our study was 41%. This was consistent with available literature that describes prevalence of allo-immunisation as ranging from 15 to 50% in patients receiving non-leukoreduced blood components [13–18].
Our study aimed at assessing the clinical utility of this test. Our data showed a significant correlation of crossmatch positivity with unsuccessful transfusion. The crossmatch was able to predict a successful transfusion with a sensitivity of 89.2% and a specificity of 88.8%.
The positive predictive value was 83.3% and the negative predictive value, 93%, suggesting that the test would be a valuable instrument in selection of platelet units that are likely to produce acceptable increment (Table 2). These results support the findings of previous studies that also describe a significant association between crossmatch positivity and CCI [19, 20].
At the time of the study, irradiated, non-leukoreduced random donor platelets were the mainstay of platelet transfusion therapy for most patients at our centre. The ‘Trial to Reduce Alloimmunization to Platelets Study Group’ (TRAP) reported an incidence of lympho-cytotoxic antibodies of 45% in patients receiving non-leukoreduced blood as compared to 17–21% in patients receiving leukoreduced or leukocyte inactivated components [21]. Random donor platelets further increase the risk for alloimmunization due to the fact that there is greater donor exposure with transfusion of multiple units. The significant role that transfusion played in the development of immune refractoriness among our study group was evident in that among the 30 patients who were crossmatch positive, 29 (97%) patients had received previous transfusions (1–55 units) and only one patient had not received any prior transfusions, whereas among the 43 crossmatch negative patients, only 30 (69%) had received prior transfusions.
Of the 59 patients who had a history of prior transfusion, 24 (40%) had an unsuccessful transfusion in the absence of other known causes for refractoriness, as opposed to one out of 14 with no history of prior transfusion. Prior blood or blood component transfusion in our study showed a significant association with crossmatch positivity (p = 0.003) as well as unsuccessful transfusion (p = 0.000). These findings reiterate the fact that transfusions are a significant source of allo-sensitisation as has been described in other studies [15, 22]. It also suggests that the platelet crossmatch will particularly benefit patients who have been previously transfused.
While some authors report that HLA allo-immunisation represents the main cause of platelet transfusion refractoriness, a study from our centre identified antibodies to human platelet antigens in as many as 41.25% of platelet refractory patients [23]. In this context, platelet crossmatching by the solid-phase red cell adherence method holds the advantage of detecting antibodies directed against platelet-specific antigens as well as HLA antigens. The great diversity of platelet antigens and their variable expression has been cited as a factor in limiting the usefulness of most commercial test kits for antibody detection [24].
In our study, in five instances patients had successful transfusions in spite of having a positive crossmatch. All had received prior transfusions, so were potentially at risk of being allo-immunised. In two, the patient had received at least one compatible unit which could have contributed to the successful transfusion. Setting aside false positivity due to technical factors, other potential explanations include too low a concentration of alloantibodies to trigger physiological destruction, or differences in IgG subclasses, which may have contributed to low in vivo destruction of platelets. IgG2 is reported to be less effective than IgG1 and IgG3 for inducing phagocytosis and complement mediated cell lysis [25]. On the other hand, it has been suggested that IgG2 causes platelet dysfunction and causes significant bleeding even with normal platelet count [26].
Likewise, of 43 crossmatch negative patients, three (2%) had unsuccessful transfusions. Setting aside test failure due to technical issues, this could be due to the presence of low levels of antibody which were not detected by the test, or due to undiagnosed non-immunological factors that contributed to platelet refractoriness. Despite these few discrepancies, a negative result was found to be highly reliable in predicting a successful transfusion as shown by the high negative predictive value (93%). This shows that in the absence of other causes for refractoriness, the test is valuable in choosing units that will offer an adequate increment in a patient with antibodies causing immunological refractoriness.
Other benefits of using the SPRCA platelet crossmatch as a strategy to manage platelet refractoriness includes the potential to perform a large number of platelet cross-matches in a short time, provision of a larger pool of potential donors, and practical benefits such as wide availability and relatively low cost compared to HLA matched platelets [9, 24]. One limitation is the need to perform frequent assays for patients requiring ongoing transfusion support. A more serious disadvantage as compared to using matched platelets is that crossmatched platelets while being effective in producing an increment, may cause further allo-immunisation.
Our study demonstrates the effectiveness and relevance of the SPRCA as a tool for selecting platelet units that will result in successful transfusion among frequently transfused patients. Limitations include the absence of clinical follow up, the fact that presence of antibody was not confirmed by other methods, and that breadth of sensitisation, antibody specificities or subgroups were not evaluated.
Acknowledgements
This study was supported intramurally by a Grant from the Christian Medical College, Vellore, India
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Involvement of Human Participants
All procedures performed in the study were in accordance with ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delaflor Weiss E, Mintz PD. The evaluation and management of platelet refractoriness and alloimmunization. Transfus Med Rev. 2000;14(2):180–196. doi: 10.1016/S0887-7963(00)80007-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira AA, Zulli R, Soares S. Identification of platelet refractoriness in oncohematologic patients. Clin São Paulo Braz. 2011;66(1):35–40. doi: 10.1590/S1807-59322011000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip J, Kumar S, Chatterjee T. Prevalence of alloimmunization to human platelet antigen glycoproteins and human leucocyte antigen class I in β thalassemia major patients in Western India. Indian J Hematol Blood Transfus. 2014;30(4):309–312. doi: 10.1007/s12288-013-0297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. Br J Haematol. 2015;171(3):297–305. doi: 10.1111/bjh.13597. [DOI] [PubMed] [Google Scholar]
- 5.British Committee for Standards in Haematology, Blood Transfusion Task Force Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10–23. doi: 10.1046/j.1365-2141.2003.04468.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis KB, Slichter SJ, Corash L. Corrected count increment and percent platelet recovery as measures of posttransfusion platelet response, problems and a solution. Transfusion (Paris) 1999;39(6):586–592. doi: 10.1046/j.1537-2995.1999.39060586.x. [DOI] [PubMed] [Google Scholar]
- 7.Yankee RA, Grumet FC, Rogentine GN. Platelet transfusion therapy; the selection of compatible platelet donors for refractory patients by lymphocyte HL-A typing. N Engl J Med. 1969;281(22):1208–1212. doi: 10.1056/NEJM196911272812202. [DOI] [PubMed] [Google Scholar]
- 8.Daly PA, Schiffer CA, Aisner J. Platelet transfusion therapy. One-hour posttransfusion increments are valuable in predicting the need for HLA-matched preparations. JAMA. 1980;243(5):435–8. doi: 10.1001/jama.1980.03300310023016. [DOI] [PubMed] [Google Scholar]
- 9.Rachel JM, Summers TC, Sinor LT. Use of a solid phase red blood cell adherence method for pretransfusion platelet compatibility testing. Am J Clin Pathol. 1988;90(1):63–68. doi: 10.1093/ajcp/90.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Shibata Y, Juji T, Nishizawa Y. Detection of platelet antibodies by a newly developed mixed agglutination with platelets. Vox Sang. 1981;41(1):25–31. doi: 10.1159/000460609. [DOI] [PubMed] [Google Scholar]
- 11.Gelb AB, Leavitt AD. Crossmatch-compatible platelets improve corrected count increments in patients who are refractory to randomly selected platelets. Transfusion. 1997;37(6):624–630. doi: 10.1046/j.1537-2995.1997.37697335157.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolgiano DC, Larson EB, Slichter SJ. A model to determine required pool size for HLA-typed community donor apheresis programs. Transfusion. 1989;29(4):306–310. doi: 10.1046/j.1537-2995.1989.29489242795.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy MF, Waters AH. Immunological aspects of platelet transfusions. Br J Haematol. 1985;60(3):409–414. doi: 10.1111/j.1365-2141.1985.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 14.Taaning E, Simonsen AC, Hjelms E. Platelet alloimmunization after transfusion. A prospective study in 117 heart surgery patients. Vox Sang. 1997;72(4):238–41. doi: 10.1159/000462001. [DOI] [PubMed] [Google Scholar]
- 15.Bajpai M, Kaura B, Marwaha N. Platelet alloimmunization in multitransfused patients with haemato-oncological disorders. Natl Med J India. 2005;18(3):134–136. [PubMed] [Google Scholar]
- 16.Agarwal N, Chatterjee K, Sen A, et al. Prevalence of platelet reactive antibodies in patients refractory to platelet transfusions. Asian J Transfus Sci. 2014;8(2):126. doi: 10.4103/0973-6247.137453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegels JG, Bruynes EC, Engelriet CP. Serological studies in patients on platelet- and granulocyte-substitution therapy. Br J Haematol. 1982;52(1):59–68. doi: 10.1111/j.1365-2141.1982.tb03861.x. [DOI] [PubMed] [Google Scholar]
- 18.McGrath K, Wolf M, Bishop J. Transient platelet and HLA antibody formation in multitransfused patients with malignancy. Br J Haematol. 1988;68(3):345–350. doi: 10.1111/j.1365-2141.1988.tb04212.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiita AP, Nambiar A. Longitudinal management with crossmatch-compatible platelets for refractory patients, alloimmunization, response to transfusion, and clinical outcomes. Transfusion. 2012;52(10):2146–2154. doi: 10.1111/j.1537-2995.2012.03593.x. [DOI] [PubMed] [Google Scholar]
- 20.Heal JM, Blumberg N, Masel D. An evaluation of crossmatching, HLA, and ABO matching for platelet transfusions to refractory patients. Blood. 1987;70(1):23–30. doi: 10.1182/blood.V70.1.23.23. [DOI] [PubMed] [Google Scholar]
- 21.Reduce R. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 22.Kiefel V, König C, Kroll H. Platelet alloantibodies in transfused patients. Transfusion. 2001;41(6):766–770. doi: 10.1046/j.1537-2995.2001.41060766.x. [DOI] [PubMed] [Google Scholar]
- 23.Abraham AS, Chacko MP, Daniel D. Antibodies to human platelet antigens form a significant proportion of platelet antibodies detected in Indian patients with refractoriness to platelet transfusions. Transfus Med Oxf Engl. 2018;28(5):392–397. doi: 10.1111/tme.12516. [DOI] [PubMed] [Google Scholar]
- 24.Rachel JM, Sinor LT, Tawfik OW. A solid-phase red cell adherence test for platelet cross-matching. Med Lab Sci. 1985;42(2):194–195. [PubMed] [Google Scholar]
- 25.Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia, perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus. 2015;13(3):380–390. doi: 10.2450/2015.0275-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelsch R, Hutt K, Cassens U, Sibrowski W. Semi quantitiative measurement of IgG subclass and IgM of platelet specific antibodies in a glycoprotein specific platelet antigen capture assay. Br J Haematol. 2002;117(1):141–150. doi: 10.1046/j.1365-2141.2002.03375.x. [DOI] [PubMed] [Google Scholar]