Abstract
Patients with hematological malignancies are severely immunocompromised and are at high risk of invasive fungal infection (IFI), particularly those undergoing remission-induction chemotherapy for acute myeloid leukemia (AML). IFIs are a major cause of morbidity and mortality in such patients. We planned to study the incidence of IFI in patients with AML undergoing intensive chemotherapy and receiving antifungal prophylaxis. We retrospectively reviewed consecutive 46 patients with non-M3 AML, who received induction chemotherapy and systemic antifungal prophylaxis. None of the patients had IFI at the time of initiation of the chemotherapy. Patients were monitored for the occurrence of IFI using high-resolution computerized tomography of the chest or para-nasal sinus and test for galactomannan antigen on serum or broncho-alveolar lavage and were followed up for 90 days. Of the 46 patients on intensive chemotherapies, 41, 4 and 1 patients were started on posaconazole, amphotericin B and voriconazole prophylaxis, respectively. The occurrence of possible and probable IFI was observed in 16 and 4 patients respectively, in which 19 patients were on posaconazole and 1 patient was on amphotericin-B prophylaxis. Overall mortality in the study population was 11 (23.9%). Four out of 20 patients died with IFI but none of the death was attributable to IFI. IFI still remains a significant cause of morbidity and mortality in patients with AML despite universal use of antifungal prophylaxis. With effective pharmacotherapy, the mortality due to IFI is preventable. Appropriate antifungal prophylaxis strategy still needs to be developed through larger and prospective studies.
Keywords: Acute myeloid leukemia, Antifungal prophylaxis, Induction chemotherapy, Invasive fungal infection, Posaconazole
Introduction
Acute myeloid leukemia (AML) is a hematological disorder characterized by immature myeloid cell proliferation and bone marrow failure [1, 2]. The incidence of AML increases with age and approximately 80% of AML patients are adults while 20% are in the pediatric age group [3]. Patients with hematological malignancies are severely immunocompromised and are at high risk of invasive fungal infections (IFIs), particularly those undergoing remission-induction chemotherapy for AML [4–6]. IFIs are a major cause of morbidity and mortality in patients with AML undergoing chemotherapy [7, 8]. The incidence of IFIs, according to the revised European Organization for Research and Treatment of Cancer (EORTC) criteria, has been reported to range from 12 to 34% in patients with AML [9]. The reported mortality from candidiasis or aspergillosis ranges from 40 to 50% and mortality from fusariosis or zygomycosis is around 70% [10]. It is observed that the response to the antifungal treatment is generally poor and the cost of treatment of IFIs is very high. Therefore, antifungal prophylaxis (AFP) has become the standard of care in such high-risk patients. On the other hand, factors like epidemiologic features of the center, duration of neutropenia or response to the treatment for AML can influence the incidence of IFIs along with the type of AFP [11].
Limited data is available in the Indian population on the incidence and mortality related to IFIs in patients undergoing intensive chemotherapy and AFP together [12, 13]. A retrospective analysis of AML patients receiving induction as well as salvage chemotherapy along with the AFP documented that none of the patients were given any antibacterial and antiviral prophylaxis. The majority of the patients received amphotericin-B as AFP. Further, there was heterogeneity in the chemotherapy regimens used [12]. A prospective study done on AML patients undergoing induction chemotherapy and receiving voriconazole as AFP has mentioned that voriconazole reduced the incidence of IFI and IFI related mortality [13]. Therefore, the current study has been planned to assess the incidence of IFIs in patients with AML on chemotherapy and AFP in the Indian population.
Materials and Methods
Patients and Data Collection
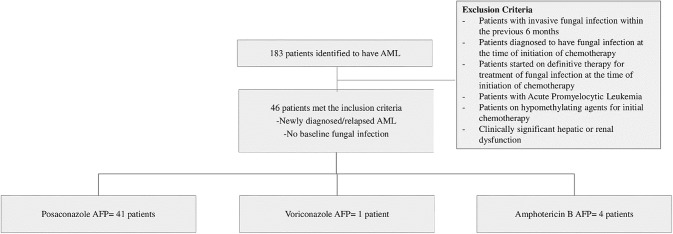
This present study was retrospective, conducted in a tertiary care center providing comprehensive cancer care in North India. The sample consisted of 46 non-M3 AML patients who received remission-induction chemotherapy from 1st August 2014 to 31st December 2016. All newly diagnosed and relapsed cases with no evidence of IFI at baseline and receiving intensive chemotherapies were included in this study (Fig. 1). All the relevant data were extracted from the charts and electronic records from the time of hospital registration until 90 days after remission-induction chemotherapy.
Fig. 1.
Flow-chart showing the breakdown of the patients included in the study. AML acute myeloid leukemia, Ampho B amphotericin B, AFP antifungal prophylaxis
Treatment and Response Criteria of AML
Standard induction chemotherapy for non-M3 AML in this study was daunorubicin + cytarabine in 3 + 7 regime (daunorubicin 60 mg/m2 per day for 3 days, and cytarabine 100 mg/m2/day for 7 days). Other chemotherapy regimens included high dose cytarabine and mitoxantrone (HAM regimen) (Cytarabine @ 3 g/m2 twice a day on alternate days for 3 days, and mitoxantrone @8–10 mg/m2 per day for 3 days) and high dose cytarabine (HiDAC regimen) (cytarabine 1.5–3 g/m2/twice a day every alternate day for 3 days).
The outcome was measured from the first day of induction chemotherapy till the date of death or day 90 of follow-up. Patients who failed to achieve remission after 1st induction chemotherapy were censored for further occurrence of IFI, though were followed for the outcome. Induction mortality was defined as all-cause-mortality within 42 days following induction chemotherapy or till next cycle of chemotherapy. Mortality attributed to IFI was defined as death with continuing symptoms and/or signs of IFI.
During the observation period, patients received an oral suspension of 200 mg posaconazole three times a day, amphotericin-B 25 mg once every alternate day, liposomal amphotericin-B 50 mg once every alternate day or voriconazole 200 mg twice a day. Posaconazole was administered with food while voriconazole was administered irrespective of food intake. Voriconazole was administered in a dose of 200 mg twice daily. AFP was initiated 1 day before the induction chemotherapy and continued until recovery from neutropenia period or the start of another systemic antifungal agent. Data of GM antigen tests for Aspergillus and additional examinations such as brain or sinus CT, or bronchoalveolar lavage (BAL) were recorded in case record forms. A serum and BAL GM values of ≥ 0.5 and ≥ 1.0 respectively, were considered positive and suggestive of Aspergillus infection. The empirical strategy was to start antifungal treatment in cases of persistent fever despite the use of broad-spectrum antibiotics for more than 4 days without radiological evidence of IFI. AFP failure was defined as a diagnosis of possible or probable IFI as per EORTC criteria while receiving any of three AFPs. Patients who were treated empirically were not considered to be AFP failure.
Statistical Analysis
Descriptive statistics have been used to analyze the study results. Values are expressed as median (range; continuous variables) or as a percentage of the group from which they were derived (categorical variables).
Data Handling and Record Keeping
The data was recorded manually in both the hard and the soft copy and was archived for the inspection purposes. Confidentiality of the recorded data was maintained, and the data is protected by the secure password and only authorized person have access to data.
Ethics Information
The study was approved by the Scientific Committee (Res/SCM/20/2017/21) of the institute and has been performed in accordance with the ethical standards of Declaration of Helsinki. The policy that informed consent can be waived for this study was also approved by the Research Committee because the data were analyzed retrospectively from the hospital database.
Results
Over the 29-months period, a total number of 183 patients were identified having non-M3 AML who were started on chemotherapy. Forty-six patients met the inclusion criteria and others were excluded because of the presence of IFI at baseline or treatment with a hypomethylating agent or other non-intensive chemotherapies. The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of the patients
| Characteristics | n/median | Range | % |
|---|---|---|---|
| Total patients (n) | 46 | ||
| Age (in years) | |||
| Median (range) | 39 | 15–65 | |
| Gender | |||
| Male | 28 | 61 | |
| Female | 18 | 39 | |
| M/F ratio | 1.56:1 | ||
| Diagnosis (WHO classification) | |||
| AML with recurrent cytogenetic abnormality inv3, t9:11 | 1 | 2 | |
| AML with recurrent cytogenetic abnormality t6:9 | 2 | 4 | |
| AML with recurrent cytogenetic abnormality t8:21 | 4 | 9 | |
| AML-MRC | 4 | 9 | |
| AML-NOS erythroid/myeloid | 1 | 2 | |
| AML-NOS monocytic | 7 | 15 | |
| AML-NOS myelomonocytic | 8 | 17 | |
| AML-NOS with maturation | 5 | 11 | |
| AML-NOS without maturation | 12 | 26 | |
| AML-NOS with minimal differentiation | 1 | 2 | |
| Therapy-related AML | 1 | 2 | |
| Newly diagnosed | 41 | 89 | |
| Relapsed | 5 | 11 | |
| Cytogenetic risk stratification | |||
| Low | 8 | 17 | |
| Intermediate | 18 | 39 | |
| High (including relapse cases) | 18 | 39 | |
| Not available | 2 | 4 | |
| Baseline radiological parameters | |||
| Chest X-ray | 46 | ||
| Normal | 46 | 100 | |
| HRCT chest | 32/46 | 69.5 | |
| Normal | 32 | 100 | |
AML acute myeloid leukemia, MRC myelodysplasia related changes, NOS not otherwise specified, HRCT high resolution computed tomography
Chemotherapy and AFP Given in AML Patients
Among 46 patients, 40 (86.9%) patients received 3 + 7 regimen, 4 (8.6%) patients received HAM regimen and 2 (4.3%) patients received HiDAC regimen as remission-induction chemotherapy. The total duration of follow-up from the day of starting of induction chemotherapy was 90 days. Appropriate AFP was given in all the patients.
IFI Profile During Chemotherapy
During induction chemotherapy, a breakthrough of IFIs was detected in 20 (43.4%) patients. Table 2 shows the details of AFP, the methods of microbiological and radiological diagnosis of IFIs. Of the 20 patients with IFI, 16 had a possible fungal infection, while 4 had probable IFI as per EORTC–MSG criteria. Sites of fungal infection included the lungs in 19 and 1 had sinus involvement in the form of diffuse haze. Of the 19 patients with lung involvement, 17 patients had nodular opacities with or without surrounding ground-glass haze, 1 patient had consolidation, and in 1 patient the findings were non-specific. GM antigen assay test was performed in 16 patients out of 46. Samples were taken from blood (n = 15) and BAL (n = 1).
Table 2.
Invasive fungal infection (IFI) profile during induction, its treatment and outcome
| IFI parameters | N | % |
|---|---|---|
| IFI profile during induction | ||
| Possible | 16/46 | 32.6 |
| Probable | 4/46 | 8.6 |
| Proven | Zero | Zero |
| IFI site | 20/46 | 43.4 |
| Pulmonary (HRCT chest) | 19 | 41.3 |
| PNS (CT PNS) | 1 | 2.1 |
| Total no. of GM done during the induction phase | 20/46 | 43.4 |
| Positive | 4 | 20 |
| Serum | 3 | 15 |
| BAL | 1 | 5 |
| Negative | 16 | 80 |
| Day of onset of IFI | ||
| Median (range) | 13(4–24) | |
| IFI on different antifungal prophylaxis | ||
| Posaconazole (n = 41) | 19 | 46.3 |
| Amphotericin-B (n = 4) | 1 | 25 |
| Voriconazole (n = 1) | Zero | Zero |
| IFI treatment and outcome | ||
| IFI treatment (possible + probable) | 20 | 43.4 |
| Empirical antifungal treatment | 10 | 21.7 |
| Drugs used to treat fungal infection (n = 30) | ||
| Monotherapy | 22 | 73.3 |
| Voriconazole | 4 | 18.1 |
| Amphotericin-B | 14 | 63.6 |
| Caspofungin | 4 | 18.1 |
| Double antifungal therapy | 8 | 26.6 |
| Duration of antifungal treatment (in days) | ||
| Median (range) | 12.5 (6–84) | |
| Outcome of fungal infection (n = 20) | ||
| Resolved | 12 | 60 |
| Not resolved | 4 | 20 |
| Death | 4 | 20 |
IFI invasive fungal infection, HRCT high resolution computed tomography, PNS para-nasal sinus, GM galactomannan, BAL broncho-alveolar lavage
Antifungal Therapy Usage in AML Patients
The antifungal treatment was given in 30/46 (65.2%) patients. Among these, 10 (33.3%) patients received empirical treatment (antifungal treatment based on only clinical factors without having radiological or microbiological evidence of IFI) and the other 20 (66.6%) patients received pre-emptive treatment (antifungal treatment based on clinical factors along with the presence of suggestive radiological signs and/or microbiological criteria) for IFI (Table 2). After treatment, the infection was resolved in 12/20 (60%) patients and no death attributable to IFI was observed. The diagnosis of breakthrough IFIs under AFP is a challenge, so, we evaluated the role of GM in the diagnosis of breakthrough IFIs in patients with prophylaxis failure. Serum GM was positive in 3 patients and BAL GM was positive in 1 patient who received posaconazole as AFP.
Mortality
Of the 46 patients in whom induction chemotherapy was initiated, 4 patients died with refractory disease during their second induction while 7 deaths were attributable to bacterial infections. Though 4 out of 11 patients had IFI but none of the deaths were IFI attributable (Table 3). At a median follow-up of 90 days, the overall survival was 70%. Since the number of patients included in the study were low, we were not able to analyze the outcome of the patients with IFIs based on treatment with different classes of antifungal agents.
Table 3.
Mortality
| Phase | N | % |
|---|---|---|
| Induction mortality (n = 46) | ||
| 1st induction (n = 46) | 5 | 10.8 |
| 2nd induction (n = 15) | 4 | 26.7 |
| Total induction mortality | 9 | 19.5 |
| Post-remission (n = 27) | ||
| Cause of death | 2 | 7.4 |
| Disease | 0 | 0 |
| Bacterial infection | 2 | 7.4 |
| Overall mortality (n = 46) | ||
| Cause of death | 11 | 23.9 |
| Induction mortality | 5 | 45.4 |
| Refractory disease | 4 | 36.3 |
| Infections (post remission) | 2 | 18.1 |
Discussion
In the present study, the incidence of IFIs in the AML patients who were given the induction chemotherapy and were on AFP was assessed. Also, an attempt was made to know the survival rate in the patients who received different AFP along with chemotherapy. A total of 46 patients with newly diagnosed and relapsed AML were recruited for the present study.
Evidence supports the beneficial effects of AFP on prevention of IFI and improvement in overall survival [14–17]. A recent meta-analysis has shown posaconazole to be the best IFI prophylaxis option and for avoiding IFI-related mortality, over voriconazole and other antifungal agents [18]. As per the literature, posaconazole is the most prescribed antifungal agent for the prophylaxis of fungal infection in patients undergoing induction chemotherapy [14, 19–22]. Our study also have most of patients receiving Posaconazole prophylaxis.
A retrospective study done on AML patients has shown that posaconazole prophylaxis is effective and well-tolerated protection against IFI. The study has shown positive effect of AFP and reported no mortality after 76 cycles of remission-induction chemotherapy followed by posaconazole prophylaxis [23]. In a prospective study of AFP enrolled 82 patients in the polyene prophylaxis group and 77 in the posaconazole prophylaxis group, reported that patients on polyene prophylaxis were more likely to experience the breakthrough of IFIs than patients on posaconazole prophylaxis [24].
A randomized multicentric study, compared the efficacy and safety of posaconazole (n = 304) with those of fluconazole or itraconazole (n = 298) as prophylaxis in patients with AML. IFIs (probable or proven) were reported in 2% and 8% of patients in the posaconazole versus other group. Significantly lesser patients had invasive aspergillosis and longer survival in the posaconazole group [10]. International guidelines recommend this approach because of the high level of evidence [25–27]. Concurrently, majority of patients in our study too received AFP with posaconazole as a standard policy.
In the present study, IFI was reported in about 43% patients despite being given the AFP while a prospective study done by Girmenia et al. [28] has reported the overall incidence of IFI during front-line chemotherapy to be 30.3% in the patients of posaconazole group. The study had shown a lower overall incidence of IFI in the posaconazole group which was higher in our study findings [28]. This high incidence of IFI in the present study can be possibly related to environmental factors (humid environment, construction work in surrounding areas) [29]. Twenty patients were given pre-emptive treatment for possible and probable IFI and 10 patients were given empirical treatment. After treatment, IFI resolved in 12 patients but 4 patients did not respond to the treatment and 4 patients died during this period. The data shows that IFI resolved in 60% of the patients after receiving systemic antifungal therapy. In the present study, the overall mortality during the study period was 24% (n = 11). Seven patients died of bacterial infections (5 during first induction while 2 patients during post-remission therapy) while 4 patients died of refractory disease. Further, the overall mortality after acquiring IFI was observed to be 20% and there was no IFI attributable mortality in our study, which were lesser when compared to the findings of a prospective study which has reported a mortality of 40% after acquiring IFI and the IFI attributable mortality of 12.3% [28].
There were several limitations to the present study, which include: (a) none of the patients underwent invasive diagnostic tests like FNAC or biopsy of infected lung/PNS tissue to prove the fungal infection. This caused skewed high rates of possible/probable IFI while no proven case of IFI could have been documented in the study; (b) The baseline serum GM testing were done in only a small number of patients, also the follow up serum GM tests were not performed routinely; (c) Patients were subjected to BAL, as and when required. BAL was not done routinely in all patients. This paucity of indirect microbiological test data led to a high number of possible IFI while lower numbers of probable IFI; (d) Heterogeneity in AFP was there; (e) Comparison among the various antifungal strategies used, was not possible, as almost 90% of the patient population was given posaconazole as the AFP; (f) Most sources of error due to confounding and bias were more common as it was a retrospective study; (g) As a high incidence of IFI was observed despite using universal AFP, the study could not support the beneficial effects of AFP on prevention of IFI; (h) Adverse effects of antifungal drugs could not be recorded due to the retrospective nature of the study and limited accessibility to the data; (i) Small cohort and a high drop-out rate is also an important limitation.
Despite these limitations, the results of our study conclude that AML patients are more prone to IFI. The prophylaxis with antifungal agents did not demonstrated significant prevention of IFI rather high incidence was reported. However, IFI attributable mortality was not there in our study. Antifungal agents for prophylaxis need to be studied further on larger cohorts to clearly define its role in AFP in Indian settings.
Abbreviations
- AML
Acute myeloid leukemia
- AFP
Antifungal prophylaxis
- IFI
Invasive fungal infection
- EORTC
European Organization for Research and Treatment of Cancer and the Mycoses Study Group
- CT
Computed tomography
- BAL
Bronchoalveolar lavage
- HAM
High dose cytarabine and mitoxantrone
- HiDAC
High dose cytarabine
- GM
Galactomannan
- HRCT
High-resolution computerized tomography
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pinki Mishra, Email: pinkimishra161190@gmail.com.
Nidhi Bharal Agarwal, Email: nidhiagarwal@jamiahamdard.ac.in, Email: nidhi.bharal@gmail.com.
References
- 1.Saultz J, Garzon R. Acute myeloid leukemia: a concise review. J Clin Med. 2016;5:33. doi: 10.3390/jcm5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khwaja A, Bjorkholm M, Gale RE, et al. Acute myeloid leukaemia. Nat Rev Dis Primer. 2016;2:16010. doi: 10.1038/nrdp.2016.10. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor A, Beniwal S, Kalwar A, et al. Metronomic therapy with oral 6-mercaptopurine in elderly acute myeloid leukemia: a prospective pilot study. South Asian J Cancer. 2016;5:70. doi: 10.4103/2278-330X.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellinghoff SC, Panse J, Alakel N, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) Ann Hematol. 2018;97:197–207. doi: 10.1007/s00277-017-3196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin G-L, Chang H-H, Lu C-Y, et al. Clinical characteristics and outcome of invasive fungal infections in pediatric acute myeloid leukemia patients in a medical center in Taiwan. J Microbiol Immunol Infect. 2018;51:251–259. doi: 10.1016/j.jmii.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Hu J, Sun Y, et al. Does high-dose cytarabine cause more fungal infection in patients with acute myeloid leukemia undergoing consolidation therapy: a multicenter, prospective, observational study in China. Medicine (Baltimore) 2016;95:e2560. doi: 10.1097/MD.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt VR, Viola GM, Ferrajoli A. Invasive fungal infections in acute leukemia. Ther Adv Hematol. 2011;2:231–247. doi: 10.1177/2040620711410098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanna M, Caocci G, Ledda A, et al. Glucose-6-phosphate dehydrogenase deficiency and risk of invasive fungal disease in patients with acute myeloid leukemia. Leuk Lymphoma. 2017;58:2558–2564. doi: 10.1080/10428194.2017.1312666. [DOI] [PubMed] [Google Scholar]
- 9.Gomes MZR, Mulanovich VE, Jiang Y, et al. Incidence density of invasive fungal infections during primary antifungal prophylaxis in newly diagnosed acute myeloid leukemia patients in a tertiary cancer center, 2009 to 2011. Antimicrob Agents Chemother. 2014;58:865–873. doi: 10.1128/AAC.01525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 11.Metan G, Türe Z, Pala Ç, et al. A single center experience for antifungal prophylaxis in patients with acute myelogenous leukemia. Indian J Hematol Blood Transfus. 2015;31:339–345. doi: 10.1007/s12288-014-0472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korula A, Abraham A, Abubacker FN, et al. Invasive fungal infection following chemotherapy for acute myeloid leukaemia—experience from a developing country. Mycoses. 2017;60:686–691. doi: 10.1111/myc.12646. [DOI] [PubMed] [Google Scholar]
- 13.Shah A, Ganesan P, Radhakrishnan V, et al. Voriconazole is a safe and effective anti-fungal prophylactic agent during induction therapy of acute myeloid leukemia. Indian J Med Paediatr Oncol. 2016;37:53. doi: 10.4103/0971-5851.177032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano L, Caira M. The role of primary antifungal prophylaxis in patients with haematological malignancies. Clin Microbiol Infect. 2014;20:19–26. doi: 10.1111/1469-0691.12464. [DOI] [PubMed] [Google Scholar]
- 15.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- 16.Gerber B, Köppel J, Paul M, et al. Efficacy of anti-fungal but not anti-bacterial prophylaxis in intensive primary AML therapy: a real-world, retrospective comparative single-centre study. Swiss Med Wkly. 2014 doi: 10.4414/smw.2014.13985. [DOI] [PubMed] [Google Scholar]
- 17.Yunus S, Pieper S, Kolve H, et al. Azole-based chemoprophylaxis of invasive fungal infections in paediatric patients with acute leukaemia: an internal audit. J Antimicrob Chemother. 2014;69:815–820. doi: 10.1093/jac/dkt438. [DOI] [PubMed] [Google Scholar]
- 18.Leonart LP, Tonin FS, Ferreira VL, et al. A network meta-analysis of primary prophylaxis for invasive fungal infection in haematological patients. J Clin Pharm Ther. 2017;42:530–538. doi: 10.1111/jcpt.12579. [DOI] [PubMed] [Google Scholar]
- 19.Athanasakis K, Petrakis I, Kyriopoulos J. Posaconazole vs fluconazole/itraconazole in the prophylaxis of invasive fungal infections in immunocompromised patients: a cost-effectiveness analysis in Greece. J Med Econ. 2013;16:678–684. doi: 10.3111/13696998.2013.781028. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Huang X-J, Wang J-X, et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open-label study. Int J Clin Pharmacol Ther. 2013;51:738–745. doi: 10.5414/CP201880. [DOI] [PubMed] [Google Scholar]
- 21.Kung H-C, Johnson MD, Drew RH, et al. Clinical effectiveness of posaconazole versus fluconazole as antifungal prophylaxis in hematology–oncology patients: a retrospective cohort study. Cancer Med. 2014;3:667–673. doi: 10.1002/cam4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YJ, Khoo AL, Tan G, et al. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob Agents Chemother. 2016;60:376–386. doi: 10.1128/AAC.01985-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egerer G, Geist MJP. Posaconazole prophylaxis in patients with acute myelogenous leukaemia—results from an observational study: posaconazole prophylaxis in AML patients. Mycoses. 2011;54:7–11. doi: 10.1111/j.1439-0507.2010.01979.x. [DOI] [PubMed] [Google Scholar]
- 24.Vehreschild JJ, Ruping MJGT, Wisplinghoff H, et al. Clinical effectiveness of posaconazole prophylaxis in patients with acute myelogenous leukaemia (AML): a 6 year experience of the Cologne AML cohort. J Antimicrob Chemother. 2010;65:1466–1471. doi: 10.1093/jac/dkq121. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 26.Cornely OA, Bohme A, Buchheidt D, et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the infectious diseases working party of the German society for haematology and oncology. Haematologica. 2009;94:113–122. doi: 10.3324/haematol.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 update. Bone Marrow Transpl. 2011;46:709–718. doi: 10.1038/bmt.2010.175. [DOI] [PubMed] [Google Scholar]
- 28.Girmenia C, Frustaci AM, Gentile G, et al. Posaconazole prophylaxis during front-line chemotherapy of acute myeloid leukemia: a single-center, real-life experience. Haematologica. 2012;97:560–567. doi: 10.3324/haematol.2011.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009 doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]