Abstract
Nitric oxide (NO) shows great role in tumor biology. Recent years, more and more researches utilized NO donor in tumor targeting drug delivery and treatment. In this review, we summarized the NO donors by their endogenous and exogenous stimuli. Then the application of NO donors, which was the main aim of the review, was discussed in detailed according to their functions, including inducing tumor cell apoptosis, reversing tumor multidrug resistance, inhibiting tumor metastasis and improving drug delivery.
Keywords: Nitric oxide donor, Multidrug resistance, Tumor metastasis, Enhanced permeability and retention effect
Graphcial abstract
In the review, authors summarized the nitric oxide donors, and paid main attention on the functions of NO donors in drug delivery.
1. Introduction
Cancer is a most serious threat for human being. The number of cancer patients in China accounts as high as 21.07% of the national population in 2014 [1]. Chemotherapy, radiotherapy, surgery and combinational therapy are the most common strategies to manage the cancer. Unfortunately, the cancer treatment outcome is far from satisfactory. The mortality rate is still as high as 167.89/100 000 at 2014, and it is stable in past several years [1], [2], [3]. The development of nanotechnology provided novel formulations that can partially resolve the problems faced in clinical application of chemotherapy, including reducing the requirement of toxic surfactants, prolonging blood circulation time and improving tumor targeting capacity [4], [5], [6], [7], [8], [9]. However, most of these nanoparticles are limited by their poor clinical translation potential, while the clinical available nanomedicines are benefited from their low toxicity and high tolerance dose rather than good tumor targeting capacity [10], [11]. Therefore, many researchers have dedicated their effort to developing new strategies to improve the antitumor effect.
Gaseous transmitters show wide activities in biology. Until now, three gaseous transmitters are widely evaluated, i.e. nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) [12]. Among which, NO is a most attractive gaseous transmitter that have been evaluated over two decades, and a highly complex role of NO was discovered in tumor biology [13]. At high dose (µM–mM), it can directly induce cell apoptosis through several pathways, including generating oxidative and nitrosative stress, inhibiting DNA synthesis, harming DNA and inhibiting DNA repair, suppressing cellular respiration, impairing cellular function and enhancing inflammatory reactions [14], [15]. Furthermore, it can yield various activities, such as reversing tumor multidrug resistance (MDR), inhibiting tumor metastasis, dilating tumor vessels and improving drug delivery [16]. Due to the wide and high activity of NO, researchers have developed many kinds of NO delivery donors that can specifically release NO in target site under various stimuli to reduce systemic toxicity [12], [17], [18], [19], [20], [21].
In this review, we focus on the application of NO donors in tumor diagnosis and treatment, and divide the sections by the functions of NO. Additionally, the NO donors were simply summarized.
2. NO donors
Small molecule NO donors include S-nitrosothiols (SNOs), metal-NO-complexes, sydonomines, diazeniumdiolates (NONOates), and NO-drug hybrids [22]. Although the encapsulation of NO donors into nanoparticles could achieve long blood circulation time and sustained NO release [23], [24], the premature release is still a serious problem due to the side effects [17]. Therefore, stimuli sensitive NO release achieved great attention in recent years. Generally, the NO donors can be divided into endogenous stimuli sensitive donors and exogenous stimuli sensitive donors (Table 1).
Table 1.
Endogenous and exogenous stimuli sensitive NO donors.
| stimulus | NO donors | |
|---|---|---|
| Endogenous | pH | Diethylamino NONOate [24], S-nitrosoglutathione (GSNO) loaded calcium carbonate nanoparticle [33], glyceryl trinitrate (GTN) [34], [O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl] diazen-1-ium-1,2-diolate] (JSK) [35], |
| GSH | GSNO[36], TNO3[29] | |
| H2O2 | L-arginine (L-Arg) [27] | |
| glucose | L-Arg [28] | |
| Exogenous | light | [(PaPy3)Fe(NO)]2+ and other metal nitrosyls [37], trans-[Ru(NO)Cl(cyclam)](PF6)2 (cyclam = 1,4,8,11-tetraazacyclotetradecane) [38], [Ru(NO)(Hedta)] (Hedta = ethylenediaminetetraacetic acid) [38], [Ru(tpyCOOH)(Lyso-NINO)(NO)] (PF6)3[39], Fe3O4@PDA@PAMAM@NONOate [40], S-nitrosothiols (SNO) [31] |
| ultrasound | L-Arg [32] |
The endogenous stimuli include pH, GSH, H2O2 and glucose. The acidic cancer microenvironment can directly hydrolyze NONOate to produce NO [25], making it a widely used acidic sensitive NO donor. L-arginine can generate NO by both NO synthase and reaction with H2O2 [26], [27]. Furthermore, the glucose oxidase (GOx) can convert glucose into gluconic acid and H2O2, so the L-Arg was also be used for glucose sensitive NO donor [28]. Nitrate polymers are widely used as GSH sensitive NO donor because the reduction of nitrate could generate NO. Zhang et al. developed a nitrate functionalized D-α-tocopheryl polyethylene glycol succinate (TNO3) [29]. The TNO3 micelles showed sustained NO release in the presence of GSH while rare release was observed without GSH.
The common used external stimuli are light and ultrasound. Our group developed a 2-(Nitrooxy)acetic acid modified hyaluronic acid (HA) as NO donor. Upon 808 nm irradiation, the NO release was significantly elevated, and the intracellular release of NO increased 1.39-fold [30]. Guo et al. utilized with SNO as light sensitive NO donor and loaded it into nanoparticles [31]. In dark, there was rare NO release, but about 10 µM of NO can be released upon 5 min 808 nm irradiation at an intensity of 1 W/cm2. Ultrasound could facilitate the reaction between L-Arg and H2O2, making L-Arg into an ultrasound sensitive NO donor. Zhang et al. encapsulated the L-Arg into hollow mesoporous silica nanoparticles (HMSNs) for tumor specific delivery. Upon application of ultrasound, the NO concentration released from nanoparticles increased from 1.2 µM to 4.4 µM [32].
Additionally, we have to know that the stimuli may not directly trigger release of NO but through one or several intermediaries. For example, the glucose sensitive NO release was triggered by the H2O2 which was the product of glucose after oxidation [28]. Near-infrared laser-triggered NO release from Fe3O4@poly(dopamine)@ mesoporous silica nanoparticles was due to the heat produced from irradiation of polydopamine, while heat triggered NO release from SNO [31]. To be consistent with the published papers, we selected the first stimuli for dividing NO donors in the review.
3. Roles of NO in tumor targeting drug delivery and treatment
3.1. Inducing cell apoptosis
The effect of NO on cell proliferation is concentration-dependent. At low concentration (pM-nM), it can promote cancer cell proliferation and infiltration, while at high concentration (µM–mM), it can induce cell apoptosis [14], [15]. In a study performed by Weidensteiner et al. [41], the NO donor, JSK, could directly induce U87 glioma cell apoptosis by a dose-dependent manner. In vivo immunohistochemical analysis of the glioma bearing brain also demonstrated a significant antiproliferative effect of JSK, suggesting the NO donor could be used for tumor treatment. A NO releasing polymer, TNO3 could self-assemble into micelles and thus passively distribute into tumor [29]. Upon releasing of NO in the presence of GSH, the TNO3 micelles could directly induce HepG2 cell apoptosis. IC50 values were 45.86, 27.47, and 14.38 µg/ml at 24, 48, and 72 h. NO donor loaded silica nanoparticles also induced higher cellular apoptosis that small molecule donor, which may owe to the prolonged NO release time from nanoparticles [23]. Furthermore, Coating the NO donor decorated silica nanoparticles with bovine serum albumin (BSA) under a layer-by-layer scheme could improve the stability of nanoparticles and prolong the NO release. Using a fluorescence based NO assay, it showed the BSA coating delayed the release rate of NO [42]. Additionally, Lee et al. loaded NO into echogenic liposomes (NO-ELIP) for intracellular delivery of NO [43]. Compared with empty ELIP, the NO-ELIP showed significant antitumor effect. The IC50 on MDA-MB-468 cells was about 0.42 mg/ml.
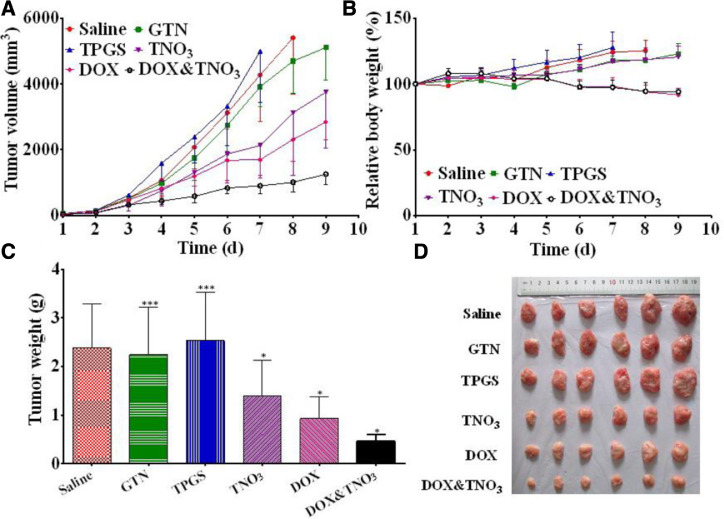
The antitumor effect of NO could be combined with chemotherapeutics to further improve the treatment outcome. The mechanism may involve in the reaction of NO with O2− produced by chemotherapeutics [44]. The reaction would produce high toxic peroxynitrite (ONOO−) [45], which can cause damage of various elements in cells, such as DNA and mitochondria membrane. The TNO3 micelles, as described above, could enhance the cytotoxicity of DOX, resulting in 6.25-fold lower IC50 at 24 h (2.65 µg/ml of DOX + TNO3 versus 16.54 µg/ml of DOX) [29]. As a result, the tumor weight after treatment with DOX&TNO3 was considerably reduced to 0.5 g, while the weight was 0.9 and 2.4 g for DOX treatment and saline group, respectively (Fig. 1). Another study modified NO donor JSK onto triblock copolymer p(Gal-b-DPA-b-Az) by click chemistry [35]. The obtained polymer could self-assemble into nanoparticles (p(GD-Az-JSK) NPs) while DOX was loaded into the nanoparticles during assembling. It can sustained release NO in the presence of GSH, and 58.86% of NO was released in 72 h from the nanoparticles. When incubated the DOX loaded p(GD-Az-JSK) NPs with HepG2 cells, the combination index (CI) of IC50 was 0.48, 0.45 and 0.33 at 24, 48, and 72 h, indicating a strong synergistic effect. Similarly, upconversion nanoparticles were utilized for loading DOX and Roussin's black salt (RBS), a photosensitive NO donor, simultaneously [46]. After accumulation in tumor, 980 nm laser irradiation (0.7 W/cm2, for 30 min) could effectively trigger the release of NO, providing synergistic antitumor effect with released DOX. Acidic and reduction sensitive NO delivery by calcium carbonate mineralized nanoparticles could first release S-nitrosoglutathione in the acidic condition of endosomes and then be reduced to NO for synergistic antitumor effect with DOX. It was showed the NO release could elevate the anti-proliferation effect of DOX by 11.7%−32.8% at various DOX concentrations. Other chemotherapeutics, such as cisplatin, also showed improved antitumor effect in combination with NO donors. The IC50 of cisplatin to BE(2)-C cells could be reduced from 7.13 µM to 1.55 µM [36].
Fig. 1.
In vivo antitumor efficiency of different treatment groups in H22 tumor-bearing mice. KM mice were injected with saline, GTN, TPGS, TNO3, DOX, and DOX&TNO3 on alternate days. Tumor volume (A); relative body weight of tumor-bearing mice (B); tumor weight of tumor-bearing mice (C); images of tumor tissues (D). Data was presented as mean ± SD (n = 6). *: P 〈 0.05. **: P < 0.01. ***: P 〉 0.05. (Reproduced with permission from [29]. Copyright 2014 American Chemical Society).
To combine with starving like therapy with gas therapy, a kind of hollow mesoporous organosilica nanoparticles (HMONs) was developed for co-delivery of glucose oxidase (GOx) and L-arginine [28]. When passively distributed into tumor, the GOx could convert glucose into gluconic acid and H2O2 [47], reducing the glucose level in tumor, providing starving like therapy. Then the H2O2 in tumor acidic condition can oxidize L-arginine into NO for enhanced gas therapy [48]. Compared with PBS group, the intratumoral blood oxygen level of HMON-GOx group quickly dropped to below 20% within 2 h. Meanwhile, the intratumoral H2O2 level was dramatically increased. In combination, the L-arginine loaded HMONN-GOx led to significant tumor cell apoptosis and necrosis, and the tumor growth was completely inhibited.
Combining NO donor with photothermal therapy may further elevate antitumor effect. N-doped graphene quantum dots (N-GQDs) is a suitable system for organelle targeted drug delivery [49]. Guo et al. functionalized N-GQDs with ruthenium nitrosyl and triphenylphosphonium (TPP) for mitochondria targeted NO delivery [50]. When the nanoparticles entered tumor cells, TPP could mediate the targeting distribution to mitochondria, Upon irradiation by 808 nm laser, the nanoparticles could convert light to heat for photothermal therapy. 10 min irradiation at a density of 1 W/cm2, the temperature of nanoparticles at 1 mg/ml could elevate about 16.7 °C. What's more, the upconversion property of N-GQDs could convert 808 nm light into short wavelength light and trigger NO release. Under constant irradiation with mild power, the NO level could reach micromolar level, which was high enough for inducing tumor cell apoptosis. After internalization into Hela cells, the TPP modified nanoparticles showed clear colocalization with mitochondria as demonstrated by confocal microscopy. The IC50 of nanoparticles was about 25 µg/ml under 808 nm laser irradiation, which was much lower than control particles lack of NO or TPP targeting ligand. In vivo, the nanoparticles almost totally inhibit the tumor growth, the tumor tissue was completely destroyed in two out of five mice.
3.2. Reversing MDR
NO could reduce the expression of P-glycoprotein (Pgp) and multidrug resistance (MDR)-associated proteins (MRPs), markers of MDR of tumor cells [51]. Therefore, the NO donor could be developed for reversing MDR. A NO donor, diethylenetriamine diazeniumdiolate (DETA NONOate), was into injectable hollow-microsphere system (HMs) for reversing of resistance to irinotecan [52]. The DETA NONOate was stable in the pH 8.0 inner water phase of the particles. After injected into the tumor, the acidic microenvironment could trigger the hydrolysis of DETA NONOate and release NO bubbles. The NO bubbles could produce permeable defects in the PLGA shell of the system and facilitate the release od irinotecan. Furthermore, the NO effectively reversed the MDR of tumor cells, increased irinotecan concentration in tumor cells and elevated the antitumor effect. In vitro, the expression of P-gp on MDR cells was reduced 45% after treatment with HMs at pH 6.6, and the cell viability was considerably reduced. In vivo, the tumor volume was significantly inhibited after treatment with HMs, which was greatly better than free drugs and free NONOate.
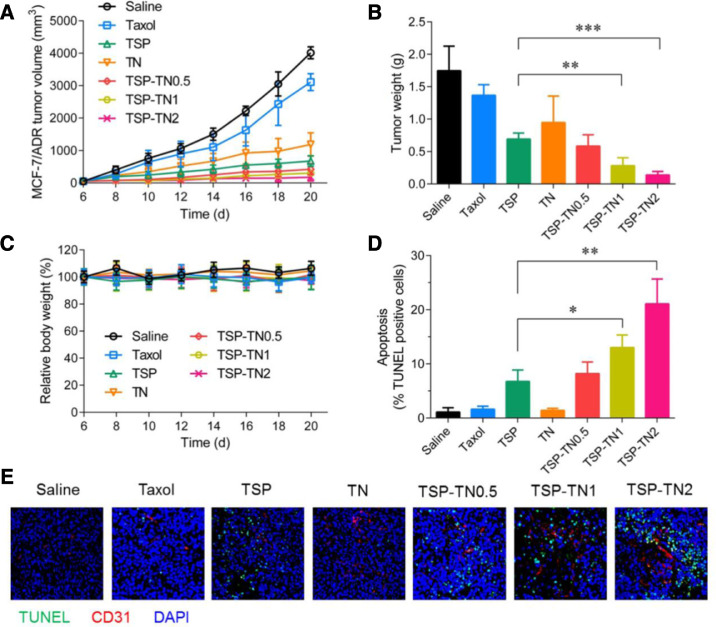
Yin et al. co-encapsulated paclitaxel (PTX) and D-α-tocopherol polyethylene 1000 glycol succinate (TPGS) derived NO donor (TPGS-NO3) into TPGS-based hybrid micelles (TSP-TN) for breast cancer targeting therapy [53]. The TSP-TN could quickly release NO in the presence of DL-Dithiothreitol (DTT), which was 2–3 folds higher than that in non-reductive condition, suggesting the NO could be specifically released in tumor cells due to the reductive condition. The TSP-TN could be effectively internalized into MCF-7/ADR cells with an intensity significantly higher than TSP (control micelles without NO donor). Furthermore, the NO released in cells could be diffused into the stroma, and enhanced microvascular permeability, blood perfusion and drug accumulation in tumor tissue, therefore, improved the antitumor effect. The tumor growth inhibition rate of TSP-TN group was 92.1%, which was much higher than the TSP group (60.8%) (Fig. 2). It was showed the treatment with TSP-TN could effectively reduce the hypoxia area from 40% of saline and TSP to 20%, which may reduce the resistance to chemotherapy.
Fig. 2.
In vivo antitumor efficacy against MDR tumors. (A) MCF-7/ADR tumor growth profile, (B) tumor weight, (C) relative body weight, and (D) induced apoptosis of mice i.v. administrated with saline, Taxol, TSP, TN, TSP-TN0.5, TSP-TN1 and TSP-TN2 at a dose of 10 mg PTX/kg. 0.5, 1 and 2 indicated the dose ratios of TN to TSP. *P < 0.05, **P < 0.01, and ***P < 0.001 vs TSP. (E) Representative immunofluorescent images of blood vessel and tumor apoptosis. Blood vessels were stained by α-CD31 antibody (red), and nuclei were stained by DAPI (blue). Apoptotic cells were stained by TUNEL (green). (Reproduced with permission from [53]. Copyright 2017 Elsevier).
Guo et al. developed Fe3O4@poly(dopamine)@mesoporous silica nanoparticles (PTNGs) to load NO donor SNO [31]. The SNO is a heat sensitive NO donor, while polydopamine could convert NIR light to heat, enabling the PTNGs with NIR sensitive NO release. In vitro, 808 nm laser irradiation with a density of 0.3 W/cm2 obviously elevated the release of NO. After incubation with 3-amino,4-aminomethyl-2′,7′difluorescein diacetate (DAFDA) and PTNGs, the NIR irradiation of MCF-7/ADR cells led to bright fluorescence, indicating the NIR could trigger intracellular NO release and the nanoparticles could effectively deliver NO donor into MCF-7/ADR cells. After exposure the MCF-7/ADR cells to PTNGs with 5 min NIR irradiation (1 W/cm2), the expression of P-gp was effective decreased. Accordingly, the cellular uptake of DOX loaded PTNGs by MCF-7/ADR cells was enormously increased, and the IC50 was greatly reduced. Consequently, the in vivo antitumor effect was improved, the tumor growth was almost completely inhibited.
3.3. Inhibiting tumor metastasis
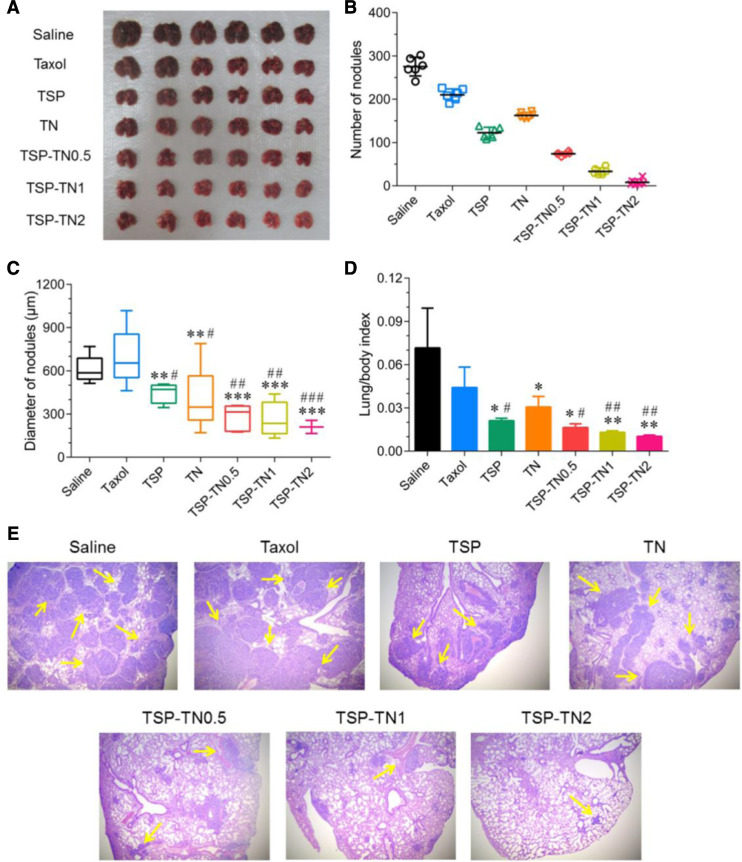
Although the NO can dilate the tumor vessels, it can reduce the hypoxia of tumor, which may contribute to the metastasis inhibition of NO donors [54]. In the study performed by Yin et al., the TSP-TN treatment reduced the hypoxia area from 40% of saline and TSP to 20%. Consequently, the surface metastasis nodules in lung were decreased from 276 of saline to 8, which was much lower than the TSP group (123) (Fig. 3). The diameter of nodules was considerably reduced after combination treatment, while similar tendency was observed in the lung/body weight ratio [53]. The results suggested the codelivery with NO could effectively inhibit tumor metastasis.
Fig. 3.
Inhibited tumor metastasis in B16F10 metastatic model. (A) Lungs were dissected for imaging. (B) Number and (C) diameter of nodules in the surface and depth of metastatic lungs, respectively. (D) Lung/body index and (E) representative H&E staining sections of lung. *P < 0.05, **P < 0.01, and ***P < 0.001 vs saline. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs Taxol. (Reproduced with permission from [53]. Copyright 2017 Elsevier).
Our group developed a kind of HA and gold nanocluster (AuNC) fabricated nanoparticles (AuNC@CBSA-PTX-ICG@HA-NO3) for tumor targeting delivery of PTX, ICG and NO [55]. After four times treatment, the tumor growth inhibition of primary breast tumor was as high as 95.3% compared with saline, which was much better than the nanoparticles lack of NO, PTX or ICG, demonstrating a synergistic effect. We further evaluated the effect on lung metastasis by bioluminescence evaluation. During the treatment, bioluminescence signal from tumor cells in lung of saline group grew quickly, and two out of three mice were dead at the end of two weeks treatment. In contrast, the signal from AuNC@CBSA-PTX-ICG@HA-NO3 treatment grew very slowly. At the end of treatment, the bioluminescence intensity of lung was lower than 10% of the control group, indicating the nanoparticles with NO could effectively inhibit tumor metastasis.
Although the promising results were obtained by the published papers, it should be taken into consideration that the leakier tumor vessels may facilitate the metastasis of tumor. Therefore, careful evaluation of the effect of NO on tumor metastasis should be performed, and sufficient amount of NO should be delivered to tumor.
3.4. Improving drug delivery
NO can dilate tumor vessels, open the endothelial cell junction gaps, and elevate blood flow, which may contribute to the enhanced EPR effect [56], [57]. In 1998, Maeda et al found that the administration of NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) could greatly inhibit EPR effect of S-180 solid tumor [57], which demonstrated at the first time that NO could influence the EPR effect. Then Maeda's group used a NO donor, nitroglycerin ointment in combination with macromolecular drug administration [58]. Compared with the control, application of nitroglycerin delivered two to three times more drugs to solid tumor, suggesting the NO donor could improve the EPR effect. To provide tumor specific blood perfusion, Weidensteiner et al. utilized glutathione-S-transferases activated JS-K [O(2)-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] for U87 glioma specific vascular permeability enhancement [41]. The treatment with JS-K clearly increased dynamic contrast enhanced MRI read-out initial area, indicating the significant increase in blood-tumor barrier permeability of glioma. When combination NO donor TNO3 with DOX, the HepG2 cellular uptake was 3.42-fold higher than that of free DOX [29]. In vivo, the DOX concentration in tumor after administration of DOX&TNO3 was 4.2 and 3.2 µg/g at 1 h and 4 h, respectively, which was significantly higher than that of free DOX group (3.1 and 2.3 µg/g, respectively).
The EPR effect enhancement of NO donor could be also applicated in improving delivery of nanoparticles, liposomes and micelles. S-nitrosated HSA-Dimer (SNO-HSA-Dimer) is a NO donor that shows longer blood circulation time than the monomeric form of HSA, and it can passively accumulate in tumor by EPR effect due to its large size (30 nm) [59]. To demonstrate the EPR enhancing capacity, the SNO-HSA-Dimer (1.3 µmol NO/kg) was combined injected with N-(2-hydroxypropyl)methacrylamide (HPMA)-zinc protoporphyrin (ZnPP) and Doxil [60]. From in vivo imaging, the combination group showed much higher fluorescence in tumor (about 3.8-fold higher vs HPMA-ZnPP group) but similar concentration in blood. Similarly, the doxorubicin distribution of Doxil group was only 10 ng/mg protein in tumor, while the tumor distribution of coadministration group was as high as 33 ng/mg protein. Using Evans blue as a maker of vascular permeability, the SNO-HSA-Dimer greatly enhanced the tumor/blood ratio of Evans blue. These results demonstrated the co-administration with SNO-HSA-Dimer could improve the distribution of nanoparticles in tumor by elevating the EPR effect of tumor rather than modifying pharmacokinetics of nanoparticles. As a result, the tumor growth was considerably suppressed by coadministration compared to HPMA-ZnPP and Doxil group. What's more, the number of lung metastasis was greatly decreased from about 30 to 5, demonstrating the enhanced EPR effect could improve antitumor effect of nanoparticles.
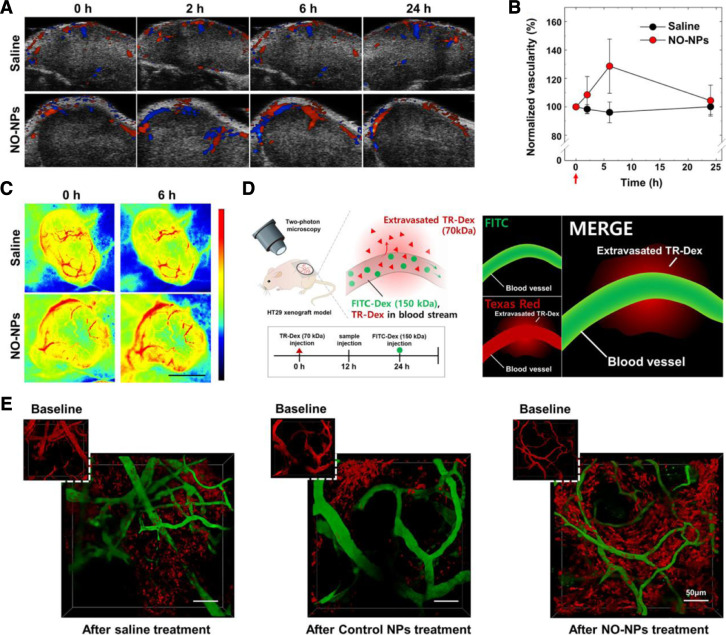
Nanoparticles based NO donor could passively target tumor and specifically release NO into tumor with longer blood circulation time and lower systemic toxicity than small molecule NO donors. Therefore, several studies developed NO delivered nanoparticles for improving drug delivery. Poly(ethylene glycol)-nitrated dextran (PEG-NO-Dex) could self-assembled into stable nanoparticles (NO-NPs) in aqueous solution and release NO in the presence of GSH [61], which was overexpressed in tumor. After intravenous injected NO-NPs to HT29 tumor bearing-mice, the blood flow in the tumor at the first 6 h was rapidly increased to 30% of the base level, while saline group showed no significant increase (Fig. 4). Through intravital imaging window, it clearly showed that the administration of NO-NPs induced more Texas Red-labeled dextran extravasation than control groups, implying the higher vascular permeability. What's more, the dilated tumor blood vessels could be observed in several regions after administration of NO-NPs as imaged using fluorescein-labeled dextran. As a result, the NO-NPs showed 1.75-fold higher accumulation in tumor compared to control group. When loading with DOX, the DOX concentration in tumor of DOX loaded NO-NPs group was 14.5 µg/g tissue at 12 h, while the control group was only 9.33 µg/g tissue. After two weeks treatment, the tumor volume was successfully suppressed to 149.9 mm3 by DOX loaded NO-NPs, which was much lower than saline and DOX control NP group (544.5 and 393.8 mm3 respectively). Similarly, N-(2-hydroxypropyl) methacrylamide (HPMA)-based polymer NO donor was also used to form nanoparticles to specifically deliver NO to tumor with capacity of improving tumor accumulation of polymer bound DOX, which showed enhanced antitumor effect attributing to the elevated intratumor DOX concentration [34], [62].
Fig. 4.
In vivo vasodilation effect after systemic administration of NO-NPs. (A) Representative power Doppler images depicting tumor vascularity. The colors indicate the flow directions toward (red) or away from (blue) the transducer. (B) Sonographic measurement of changes in vascularity upon treatment with saline and NO-NPs. The red arrow indicates the time point of intravenous administration. (C) Representative laser speckle images of changes in tumor vascularity. (D) Experimental illustrations for dual-color imaging of tumor vasculature using two-photon microscopy. (E) Representative two-photon microscopic images of tumor vasculature, labeled using TR-Dex (70 kDa) (red) or FITC-Dex (150 kDa) (green). Scale bar: 50 µm. (Reproduced with permission from [61]. Copyright 2018 American Chemical Society).
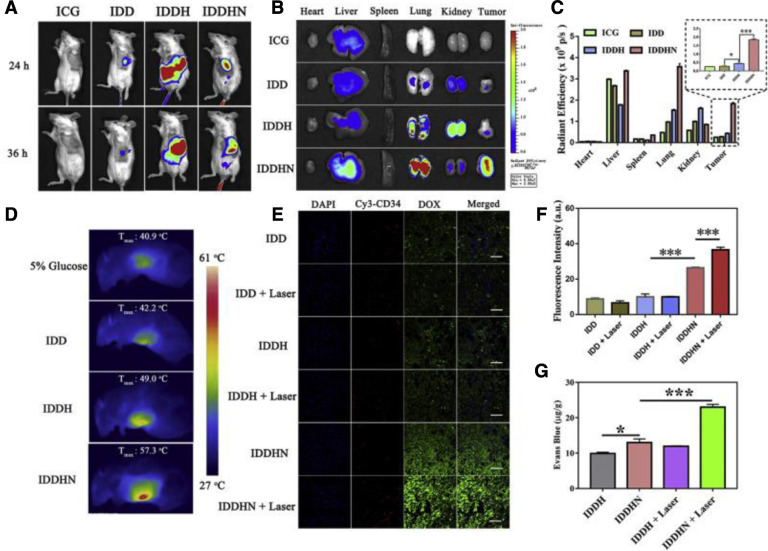
Our group constructed several size-reducible nanoparticles that has relative large initial size (about 200 nm) and can decrease to lower than 50 nm in the response of tumor overexpressed enzymes for high tumor retention and penetration [63], [64], [65]. However, the large initial size of these nanoparticles still restricted the extravasation and penetration into tumors [66], [67]. Therefore, we developed a NO donor decorated dendritic poly-L-lysine (DGL) and HA fabricated nanoparticles (IDDHN) for codelivery of DOX and indocyanine green (ICG) [30]. When the IDDHN distributed in tumor and retained near the tumor vessels, the hyaluronidase would degrade HA and release the DOX conjugated DGL with smaller size. Simultaneously, NO would be released due to reduction condition and the laser irradiation can further promote the release because of the photothermal conversion capacity of ICG. The released NO could facilitate the intratumor penetration of DGL, resulting in homogenous intratumor distribution with high concentration. In vitro, the size of IDDHN could effectively reduce from 264 nm to lower than 50 nm after incubation with hyaluronidase for 4 h. Consequently, the penetration in tumor spheroids was apparently increased. In vivo, the IDDHN showed higher tumor accumulation, while laser irradiation further elevated the concentration in tumor (Fig. 5). Using Evans blue as a marker, we could observe that the tumor permeability was considerably increased after treatment with IDDHN and laser. Additionally, coadministration the IDDHN with iRGD, a tumor-homing penetration peptide, could further improve the tumor targeting delivery which was mediated by neuropilin-1 [68].
Fig. 5.
(A) In vivo fluorescence imaging of IDDHN distribution in 4T1 breast cancer-bearing mice (Ex. 780 nm; Em. 845 nm). (B) Ex vivo imaging of tumors and other tissues at 36 h post treatment. (C) ROI analysis of ICG fluorescence signals from the tumors and normal tissues (means ± SD, n = 3, *P < 0.05, ***P < 0.001). (D) Infrared thermal imaging of laser irradiation-induced temperature elevation in tumor region of 4T1 breast cancer-bearing mice post-injection of 5% glucose, IDD, IDDH or IDDHN. (E) Laser-enhanced NO release of IDDHN triggered deep tumor penetration of IDDHN, DAPI channel (blue), Cy3-tagged CD34 channel (red) and DOX channel (green), scale bars represent 50 mm. (F) Semi-quantitative intensity of DOX fluorescence signals from the tumors (means ± SD, n = 3, ***P < 0.001). (G) Extravasation of Evans blue induced by NO donor and laser irradiation of IDDHN in 4T1 tumor bearing mice (means ± SD, n = 3, *P < 0.05, ***P < 0.001). (Reproduced with permission from [30]. Copyright 2018 Elsevier).
4. Conclusion and perspective
To date, many studies are published utilizing NO donor to improve the tumor diagnosis and treatment. As an active gaseous transmitter, NO improved tumor treatment by inducing tumor cell apoptosis, reversing MDR, inhibiting tumor metastasis and elevating drug delivery. Due to the multi-function of NO donor, there is a trend to combine NO donors with various nanomedicines. Although it is good news, the potential side effect, especially premature release of NO should be paid with particular attention. Furthermore, the effect of NO is concentration-dependent, and low concentration of NO could promote tumor growth while high concentration could inhibit. Therefore, it is important to evaluate the concentration of NO delivered into tumors. Unfortunately, although most studies claimed that they could delivery high level of NO (µM even mM) to the tumor, they only detected the intracellular NO concentration by in vitro experiments, no in vivo evaluation was reported. Additionally, the on-demand release capacity of current available NO donors was not specific enough. Donors with more sensitive to stimuli should be developed.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
Acknowledgment
The work was supported by National Natural Science Foundation of China (31571016, 81872806).
References
- 1.Chen W., Sun K., Zheng R. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Zhang S. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Zuo T., Zeng H., Zhang S., He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28(1):1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q., Zhou Z., Qiu N., Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29(14) doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 7.Park K. Controlled drug delivery systems: past forward and future back. J Control Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K., Yang P., Zhang J., Wang L., Wang H. Recent advances of transformable nanoparticles for theranostics. Chin Chem Lett. 2017;28(9SI):1808–1816. [Google Scholar]
- 9.Yu L., Chen Y., Chen H. H2O2-responsive theranostic nanomedicine. Chin Chem Lett. 2017;28(9SI):1841–1850. [Google Scholar]
- 10.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Fan W., Yung B.C., Chen X. Stimuli-responsive NO release for on-demand gas-sensitized synergistic cancer therapy. Angew Chem Int Ed Engl. 2018;57(28):8383–8394. doi: 10.1002/anie.201800594. [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S., Bronte V., Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev. 2007;27(3):317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S., Ahmad N., Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58(9):1785–1788. [PubMed] [Google Scholar]
- 15.Carpenter A.W., Schoenfisch M.H. Nitric oxide release: Part II. Therapeutic applications. Chem Soc Rev. 2012;41(10):3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J., Yung B.C., Kim W.J., Chen X. Combination of nitric oxide and drug delivery systems: tools for overcoming drug resistance in chemotherapy. J Control Release. 2017;263:223–230. doi: 10.1016/j.jconrel.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn J.F., Whittaker M.R., Davis T.P. Delivering nitric oxide with nanoparticles. J Control Release. 2015;205:190–205. doi: 10.1016/j.jconrel.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Wang M.R., Chiu S.J., Chou H.C., Hu T.M. An efficient S-NO-polysilsesquioxane nano-platform for the co-delivery of nitric oxide and an anticancer drug. Chem Commun (Camb) 2015;51(86):15649–15652. doi: 10.1039/c5cc06087g. [DOI] [PubMed] [Google Scholar]
- 19.Luo M., Boudier A., Pallotta A., Maincent P., Vincourt J.B., Leroy P. Albumin as a carrier for NO delivery: Preparation, physicochemical characterization, and interaction with gold nanoparticles. Drug Dev Ind Pharm. 2016;42(12):1928–1937. doi: 10.1080/03639045.2016.1182546. [DOI] [PubMed] [Google Scholar]
- 20.Kim J., Saravanakumar G., Choi H.W., Park D., Kim W.J. A platform for nitric oxide delivery. J Mater Chem B Mater Biol Med. 2014;2(4):341–356. doi: 10.1039/c3tb21259a. [DOI] [PubMed] [Google Scholar]
- 21.Deng Q., Xiang H.J., Tang W.W. Ruthenium nitrosyl grafted carbon dots as a fluorescence-trackable nanoplatform for visible light-controlled nitric oxide release and targeted intracellular delivery. J Inorg Biochem. 2016;165:152–158. doi: 10.1016/j.jinorgbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Seabra A.B., de Lima R., Calderon M. Nitric oxide releasing nanomaterials for cancer treatment: current status and perspectives. Curr Top Med Chem. 2015;15(4):298–308. doi: 10.2174/1568026615666150108122918. [DOI] [PubMed] [Google Scholar]
- 23.Stevens E.V., Carpenter A.W., Shin J.H., Liu J., Der C.J., Schoenfisch M.H. Nitric oxide-releasing silica nanoparticle inhibition of ovarian cancer cell growth. Mol Pharm. 2010;7(3):775–785. doi: 10.1021/mp9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrales P., Han G., Roche C., Nacharaju P., Friedman A.J., Friedman J.M. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49(4):530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai L.A., Wang Y.C., Yang C.S. Heat-activated sustaining nitric oxide release from zwitterionic diazeniumdiolate loaded in thermo-sensitive liposomes. Nitric Oxide. 2010;23(1):60–64. doi: 10.1016/j.niox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Kudo S., Nagasaki Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J Control Release. 2015;217:256–262. doi: 10.1016/j.jconrel.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Yang F., Chen P., He W. Bubble microreactors triggered by an alternating magnetic field as diagnostic and therapeutic delivery devices. Small. 2010;6(12):1300–1305. doi: 10.1002/smll.201000173. [DOI] [PubMed] [Google Scholar]
- 28.Fan W., Lu N., Huang P. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed Engl. 2017;56(5):1229–1233. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 29.Song Q., Tan S., Zhuang X. Nitric oxide releasing d-alpha-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol Pharm. 2014;11(11):4118–4129. doi: 10.1021/mp5003009. [DOI] [PubMed] [Google Scholar]
- 30.Hu C., Cun X., Ruan S. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Guo R., Tian Y., Wang Y., Yang W. Near-infrared laser-triggered nitric oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv Funct Mater. 2017;27 [Google Scholar]
- 32.Zhang K., Xu H., Jia X. Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. ACS Nano. 2016;10(12):10816–10828. doi: 10.1021/acsnano.6b04921. [DOI] [PubMed] [Google Scholar]
- 33.Lee H.J., Kim D.E., Park D.J. pH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf B Biointerfaces. 2016;146:1–8. doi: 10.1016/j.colsurfb.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 34.Studenovsky M., Sivak L., Sedlacek O. Polymer nitric oxide donors potentiate the treatment of experimental solid tumours by increasing drug accumulation in the tumour tissue. J Control Release. 2018;269:214–224. doi: 10.1016/j.jconrel.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Song H., Ji S. NO prodrug-conjugated, self-assembled, pH-responsive and galactose receptor targeted nanoparticles for co-delivery of nitric oxide and doxorubicin. Nanoscale. 2018;10(9):4179–4188. doi: 10.1039/c7nr08176f. [DOI] [PubMed] [Google Scholar]
- 36.Duong H.T., Kamarudin Z.M., Erlich R.B. Intracellular nitric oxide delivery from stable NO-polymeric nanoparticle carriers. Chem Commun (Camb) 2013;49(39):4190–4192. doi: 10.1039/c2cc37181b. [DOI] [PubMed] [Google Scholar]
- 37.Heilman B., Mascharak P.K. Light-triggered nitric oxide delivery to malignant sites and infection. Philos Trans A Math Phys Eng Sci. 2013;371(1995) doi: 10.1098/rsta.2012.0368. [DOI] [PubMed] [Google Scholar]
- 38.Gomes A.J., Espreafico E.M., Tfouni E. trans-[Ru(NO)Cl(cyclam)](PF6)2 and [Ru(NO)(Hedta)] incorporated in PLGA nanoparticles for the delivery of nitric oxide to B16-F10 cells: cytotoxicity and phototoxicity. Mol Pharm. 2013;10(10):3544–3554. doi: 10.1021/mp3005534. [DOI] [PubMed] [Google Scholar]
- 39.Xiang H.J., Deng Q., An L., Guo M., Yang S.P., Liu J.G. Tumor cell specific and lysosome-targeted delivery of nitric oxide for enhanced photodynamic therapy triggered by 808nm near-infrared light. Chem Commun (Camb) 2016;52(1):148–151. doi: 10.1039/c5cc07006f. [DOI] [PubMed] [Google Scholar]
- 40.Yu S., Li G., Liu R., Ma D., Xue W. Dendritic Fe3O4@poly(dopamine)@PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Adv Funct Mater. 2018;28(20) [Google Scholar]
- 41.Weidensteiner C., Reichardt W., Shami P.J. Effects of the nitric oxide donor JS-K on the blood-tumor barrier and on orthotopic U87 rat gliomas assessed by MRI. Nitric Oxide. 2013;30:17–25. doi: 10.1016/j.niox.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou H.C., Chiu S.J., Hu T.M. LbL Assembly of albumin on nitric oxide-releasing silica nanoparticles using suramin, a polyanion drug, as an interlayer linker. Biomacromolecules. 2015;16(8):2288–2295. doi: 10.1021/acs.biomac.5b00534. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.Y., Rim Y., McPherson D.D., Huang S.L., Kim H. A novel liposomal nanomedicine for nitric oxide delivery and breast cancer treatment. Biomed Mater Eng. 2014;24(1):61–67. doi: 10.3233/BME-130784. [DOI] [PubMed] [Google Scholar]
- 44.Bachur N.R., Gordon S.L., Gee M.V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci USA. 1979;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan L., Huang R., Li X., Liu S., Shen Y.M. Controllable release of nitric oxide and doxorubicin from engineered nanospheres for synergistic tumor therapy. Acta Biomater. 2017;57:498–510. doi: 10.1016/j.actbio.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Wang C., Ye Y., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16(4):2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 48.Yang F., Chen P., He W. Bubble microreactors triggered by an alternating magnetic field as diagnostic and therapeutic delivery devices. Small. 2010;6(12):1300–1305. doi: 10.1002/smll.201000173. [DOI] [PubMed] [Google Scholar]
- 49.Zheng M., Ruan S., Liu S. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano. 2015;9(11):11455–11461. doi: 10.1021/acsnano.5b05575. [DOI] [PubMed] [Google Scholar]
- 50.Guo M., Xiang H.J., Wang Y. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem Commun (Camb) 2017;53(22):3253–3256. doi: 10.1039/c7cc00670e. [DOI] [PubMed] [Google Scholar]
- 51.Riganti C., Miraglia E., Viarisio D. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005;65(2):516–525. [PubMed] [Google Scholar]
- 52.Chung M.F., Liu H.Y., Lin K.J., Chia W.T., Sung H.W. A pH-responsive carrier system that generates NO bubbles to trigger drug release and reverse P-glycoprotein-mediated multidrug resistance. Angew Chem Int Ed Engl. 2015;54(34):9890–9893. doi: 10.1002/anie.201504444. [DOI] [PubMed] [Google Scholar]
- 53.Yin M., Tan S., Bao Y., Zhang Z. Enhanced tumor therapy via drug co-delivery and in situ vascular-promoting strategy. J Control Release. 2017;258:108–110. doi: 10.1016/j.jconrel.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Wong P.P., Demircioglu F., Ghazaly E. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27(1):123–137. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Liu R., Xiao W., Hu C., Xie R., Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Maeda H., Nakamura H., Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Wu J., Akaike T., Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998;58(1):159–165. [PubMed] [Google Scholar]
- 58.Seki T., Fang J., Maeda H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009;100(12):2426–2430. doi: 10.1111/j.1349-7006.2009.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishima Y., Chen D., Fang J. S-Nitrosated human serum albumin dimer is not only a novel anti-tumor drug but also a potentiator for anti-tumor drugs with augmented EPR effects. Bioconjug Chem. 2012;23(2):264–271. doi: 10.1021/bc2005363. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita R., Ishima Y., Ikeda M. S-Nitrosated human serum albumin dimer as novel nano-EPR enhancer applied to macromolecular anti-tumor drugs such as micelles and liposomes. J Control Release. 2015;217:1–9. doi: 10.1016/j.jconrel.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Deepagan V.G., Ko H., Kwon S. Intracellularly activatable nanovasodilators to enhance passive cancer targeting regime. Nano Lett. 2018;18(4):2637–2644. doi: 10.1021/acs.nanolett.8b00495. [DOI] [PubMed] [Google Scholar]
- 62.Sirova M., Horkova V., Etrych T., Chytil P., Rihova B., Studenovsky M. Polymer donors of nitric oxide improve the treatment of experimental solid tumours with nanosized polymer therapeutics. J Drug Target. 2017;25(9–10):796–808. doi: 10.1080/1061186X.2017.1358724. [DOI] [PubMed] [Google Scholar]
- 63.Ruan S., Cao X., Cun X. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100–110. doi: 10.1016/j.biomaterials.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Ruan S., He Q., Gao H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale. 2015;7(21):9487–9496. doi: 10.1039/c5nr01408e. [DOI] [PubMed] [Google Scholar]
- 65.Hu G., Zhang H., Zhang L., Ruan S., He Q., Gao H. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment. Int J Pharm. 2015;496(2):1057–1068. doi: 10.1016/j.ijpharm.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 66.Cun X., Ruan S., Chen J. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater. 2016;31:186–196. doi: 10.1016/j.actbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Cun X., Chen J., Ruan S. A novel strategy through combining iRGD peptide with tumor-microenvironment-responsive and multistage nanoparticles for deep tumor penetration. ACS Appl Mater Interfaces. 2015;7(49):27458–27466. doi: 10.1021/acsami.5b09391. [DOI] [PubMed] [Google Scholar]
- 68.Hu C., Yang X., Liu R. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl Mater Interfaces. 2018;10(26):22571–22579. doi: 10.1021/acsami.8b04847. [DOI] [PubMed] [Google Scholar]