Abstract
Occurrence of skin fungal infections is increasing nowadays and their presence is more prominent in patients suffering from immunocompromised diseases like AIDS. Skin fungal infections are a major cause of visits by patients to dermatology clinics. Although, a large number of antifungal agents are available for treatment of skin fungal infections, but, their toxic profile and physicochemical characteristics reduce therapeutic outcome. When these antifungal agents are delivered topically using conventional formulations like creams and gels, they may cause various side effects like redness, burning, and swelling at the site of application. Therefore, various vesicular formulations (phospholipid based or non phospholipid based) have been explored by pharmaceutical scientists to treat skin fungal infections topically. Vesicular formulation explored for the purpose are liposomes, ethosomes, transfersomes, transethosomes, niosomes, spanlastics, oleic acid vesicles, and nanoparticles. These formulations show various advantages like bioavailability enhancement of bioactives, high skin permeation power, no side effects at application site, dosing frequency reduction, and sustained drug release. Therefore, in the present article, we have discussed about the utility of various vesicular nanocarrier systems to treat skin fungal infections.
Keywords: Conventional, Nanoparticle, Spanlastics, Transfersomes, Vesicular
Graphical abstract
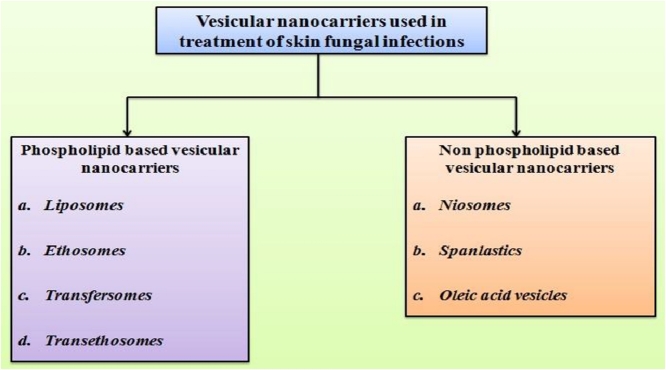
Vesicular nanocarriers used for treatment of skin fungal infection may be phospholipid based (liposomes, ethosomes, transfersomes, transethosomes) or non phospholipid based (niosomes, spanlastics, oleic acid vesicles).
1. Introduction
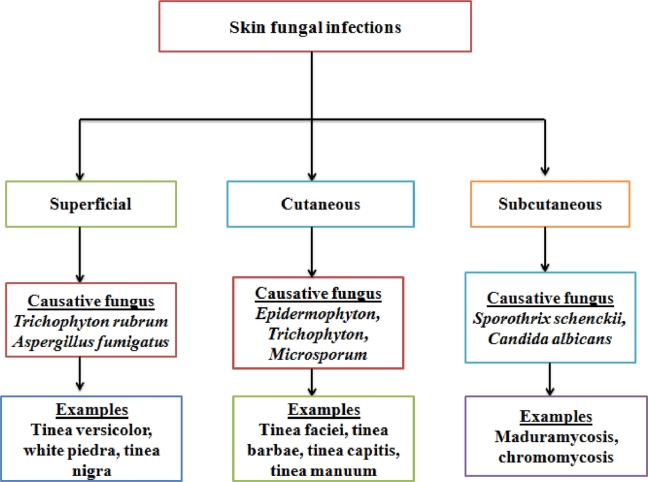
Fungi are parasitic microorganisms which can affect the skin and mucous membrane along with generation of systemic infections of various internal organs [1]. Fungal infections of skin or mucous membrane, in majority, promote visits of victims to dermatologists [2]. It has been reported that 20%–25% of human population show presence of skin fungal infections [3]. Incidences of occurrence of skin fungal infection are very high in immunocompromised patients [4]. Skin fungal infections are categorized into superficial, cutaneous, and subcutaneous depending upon the level of tissue invasion [5]. When attack of invading fungi is limited to outermost skin layers only then generated infection is called superficial fungal infection. Tinea versicolor, white piedra, and tinea nigra are examples of superficial fungal infections. Superficial fungal infection leads to increase in the skin pH along with mild scaling, redness, and inflammation at the invading site. The barrier nature of skin becomes poor in such a state [6]. Invasion of parasitic fungus into deeper epidermal skin layer develop cutaneous fungal infection. This infection is also known as dermatomycoses and it may have involvement of skin appendages like nails and hairs [7]. Dermatomycoses can also instigate cellular immune response developing pathological variations in patients [8]. Various fungi generating dermatomycoses come under three genera, namely Epidermophyton, Trichophyton, and Microsporum. Tinea faciei, tinea barbae, tinea capitis, and tinea manuum are the examples of cutaneous fungal infections [9]. Furthermore, extension of fungal infection to dermal or subcutaneous region results subcutaneous fungal infection. It is caused by fungi namely Sporothrix schenckii and Candida albicans [10]. This fungal infection is characterized by either ulcerated or infiltrated nodular lesions in the infected areas [11]. Maduramycosis and chromomycosis are other examples of subcutaneous fungal infections [12]. Fig. 1 gives a brief overview of skin fungal infections.
Fig. 1.
Classification of skin fungal infections depending upon the depth of penetration of parasitic fungus into the skin.
2. Conventional strategies for treatment of skin fungal infections
Conventionally, skin fungal infections are treated with creams, gels and lotions containing free antifungal agents [13]. Topically delivered antifungal agents show local action, therefore, they exhibit less toxic effects compared to oral antifungal agents [14]. Topical formulations used for treatment of skin fungal infections may be fungicidal or fungistatic depending upon therapeutic nature of incorporated antifungal drugs [15]. Chances of drug-drug interactions are negligible in the case of topical formulations which are more common in orally administered antifungal drug molecules [16]. However, topical antifungal formulations like creams, gels, and lotions may show redness of skin, erythema, stinging, and burning sensation as side effects [17].
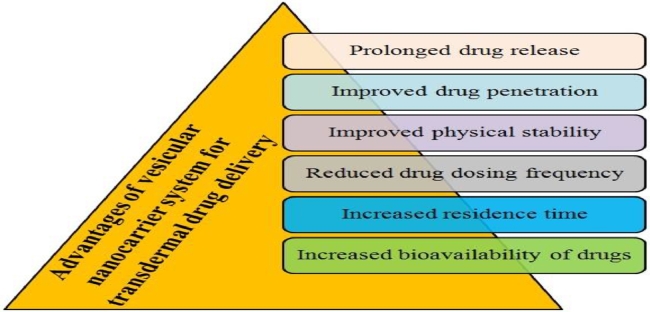
Poor skin penetration of hydrophilic antifungal drugs and high dosing frequency of conventional antifungal formulations reduce their effectiveness against skin fungal pathogens [18]. For the topical delivery, an antifungal drug must have some specified properties and lipophilic nature is most important amongst them [19]. Lipophilic drugs show excellent skin penetration upto underlying skin layer, however, their release rates should be controlled to obtain sufficient local concentrations and prolonged pharmacological effects [20]. Molecular weight of antifungal drug affects its topical delivery and it becomes more prominent for the delivery of antifungal drugs like amphotericin B whose molecular weight exceeds 500 daltons (Da) [21]. Therefore, several nanocarrier systems have been investigated by pharmaceutical scientists to fulfil these criteria and considerations for topical delivery of antifungal drugs [22]. Nanocarriers can make their way easily to hair follicles and they may show accumulation between corneocytes, merging with lipidic layer, and high intermingling with lipids present in the skin [23]. Advantages of vesicular nanocarriers over conventional delivery systems for transdermal delivery are explained in Fig. 2. Nanocarriers also have the capability to sustain the drug release, which reduce the side effects and dosing frequency of antifungal drugs [24].
Fig. 2.
Advantages of vesicular nanocarriers over conventional delivery systems for transdermal delivery.
So, in the present review our major aim was to explore the utility of various vesicular nanocarrier systems for effective treatment of skin fungal infections.
3. Novel vesicular nanocarrier systems in the treatment of skin fungal infections
3.1. Phospholipid-based vesicular nanocarriers
3.1.1. Liposomes
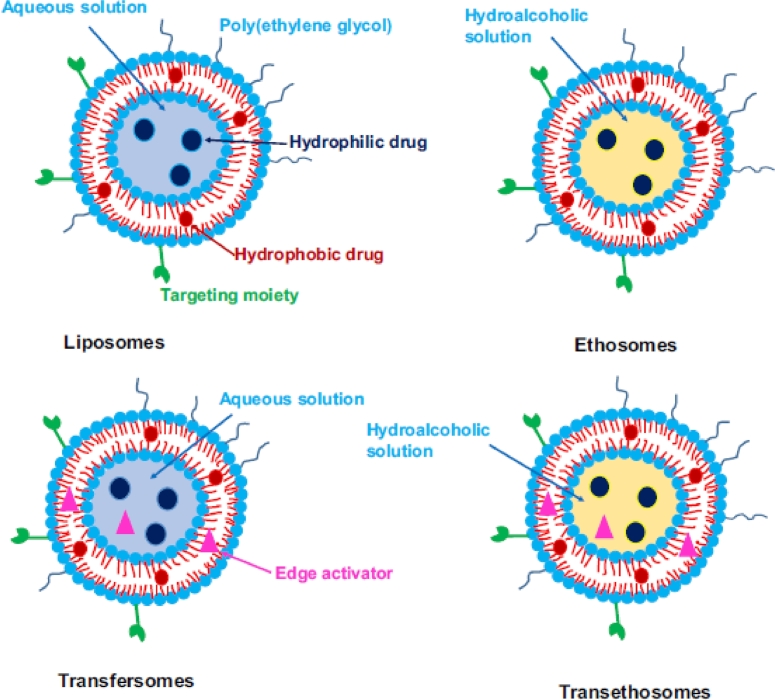
Liposomes represent firstly used phospholipid based nanocarrier systems for drug delivery, which were described during 1980s [25]. Structurally, liposomes are bilayered vesicular systems having an aqueous core along with one or several concentric phospholipid membranes [24]. Fig. 3 describes various phospholipid based nanocarrier systems used for topical delivery of antifungal drugs [26]. Due to their unique structural characteristics, liposomes have the capability to deliver both hydrophilic and lipophilic bioactive molecules [27].
Fig. 3.
Different phospholipid-based vesicles used in drug delivery (Reproduced with permission from reference [26]) Copy right 2017, Elsevier.
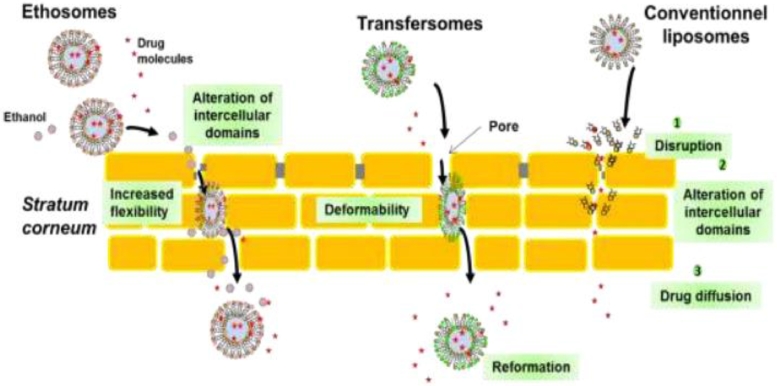
Other advantages of liposomes are high drug loading, toxicity reduction, improved stability and bioavailability along with higher biocompatibility [28]. Cholesterol is also added into the liposomal system to enhance rigidity of bilayer, improve vesicles stability, and to sustain the release of encapsulated material [29]. The topical drug delivery route is quite effective as it generates high drug concentration in local area reducing dosing frequency along with elimination of side effects of drugs. Topical delivery also reduces cost of therapy and improve patient compliance because of ease of application and removal of formulation [30]. Liposomes show effective penetration upto stratum corneum, which is a site of invasion of parasitic fungus [22]. Mechanism of transdermal delivery through various vesicular nanocarrier systems is explained in Fig. 4 [31]. Sudhakar et al. evaluated terbinafine HCl loaded liposomes dispersed in gum karaya gel for ex-vivo drug retention in rat skin. Developed liposomes showed approximately 70% entrapment of terbinafine HCl and prolonged retention of drug in rat skin compared to plain gum karaya gel containing free terbinafine HCl upto 24 h [32].
Fig. 4.
Schematic representation of the main permeation mechanisms of lipid-based vesicles (Reproduced with permission from reference [31]) Copy right 2018, Elsevier.
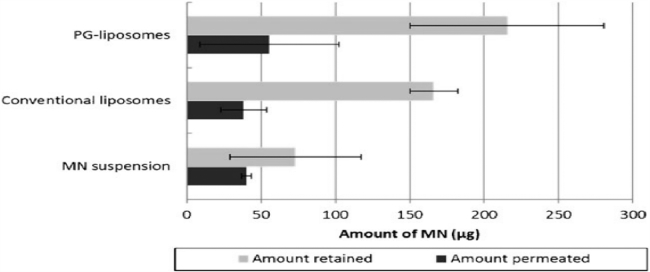
Furthermore, a comparative assessment between propylene glycol liposomes and conventional liposomes loaded with miconazole nitrate was carried out by Elmoslemany et al. for transdermal delivery. Propylene glycol liposomes loaded with miconazole nitrate showed minimum inhibitory concentration (MIC) value of 1.46 µg/ml against Candida albicans which was low compared to the MIC value of conventional liposomes (2.93 µg/ml). Propylene glycol (PG) liposomes also showed high skin retention and skin permeation of miconazole nitrate compared to conventional liposomes and miconazole nitrate (MN) suspension in human skin (Fig. 5) [33].
Fig. 5.
MN retained in and permeated through skin after 24 h, determined at 32 °C, using human skin in Franz diffusion cells under non-occlusive conditions (Reproduced with permission from reference [33]) Copy right 2012, Springer Nature.
Agarwal and Katare performed an evaluation of liposomes prepared from two different phospholipids namely phosphatidyl choline saturated 97.3% content (PCS) and phosphatidylcholine unsaturated 98.0% content (PCU) for topical delivery of miconazole nitrate. Liposomes developed from both phospholipids showed high stability and good colloidal characteristics. However, PCS based liposomes loaded with miconazole nitrate showed higher skin retention of miconazole nitrate compared PCU based liposomes in-vitro in mouse skin [34]. Table 1 gives an overview of liposomes as effective nanocarriers for treatment of skin fungal infections.
Table 1.
Role of liposomes in effective elimination of skin fungal infections.
| Composition of liposomes | Drug | Entrapment/size /zeta potential | Animal model/Route of Administration | Key findings | Ref. |
|---|---|---|---|---|---|
| 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine, Oligolysines (Lys-5 and Lys-7) | Fluconazole | 67.28% ± 15.86%/ 61.15 ± 4.25 nm/ + 3 mV | Not available (NA) | Oligolysines incorporation in a liposomal formulation produced structural variations in fluconazole loaded liposomes along with their size reduction, promoting their in-vitro skin retention effect | [35] |
| Soya lecithin, Cholesterol | Keto conazole | 74.05%/ 5.64 ± 0.014 µm/ NA | NA | Liposomal gel showed extended the drug release upto 24 h along with higher in-vitro skin deposition compared to the marketed gel of the same drug | [36] |
| Soya phosphatidyl choline, Cholesterol | Keto conazole | 54.41% ± 0.19%/ 0.86 µm/ NA | NA | Developed liposomes showed 34.96% ± 0.86% drug release after 12 h and were found stable at the 25 °C temperature for two months indicating their effectiveness in antifungal treatment | [37] |
3.1.2. Ethosomes
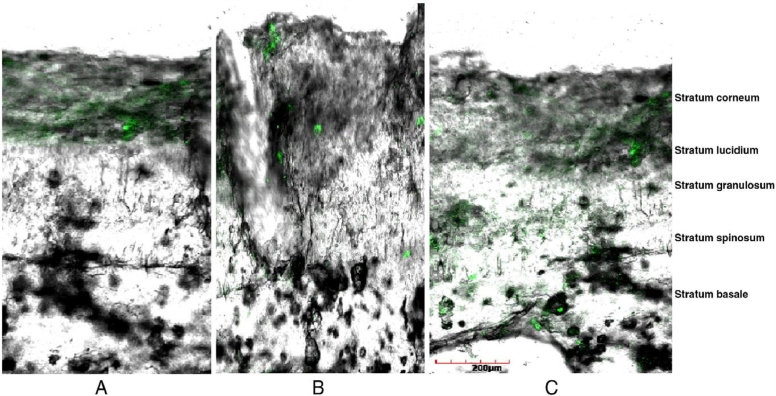
Ethosomes are nanocarrier systems which are structurally soft and having high ethanol content, phospholipids, and water in them [38]. Ethosomes may contain 2%–5% content of phospholipids and 20%–40% concentration of ethanol [39]. Skin penetration capacity of ethosomes is higher compared to liposomes due to capability of ethanol to fluidize various intercellular lipids present in the stratum corneum of skin [40]. It has been reported that as the amount of ethanol increases size of ethosomes decreases by keeping concentration of phospholipid constant [41]. Presence of ethanol in ethosome also provides a negative charge to its surface enhancing its colloidal stability [42]. However, ethosomes show high leakage of hydrophilic/ionized drugs compared to liposomes due to disruption of close packing of phospholipid bilayer by the presence of high ethanol amount [43]. Bhalaria et al. investigated fluconazole loaded ethosomes for treatment of cutaneous candidiasis in eight patients for a period of one month. Ethosomal gel containing fluconazole showed 50%–70% reduction in skin lesions in patients, which was very high compared to liposomes (30%–60%) and commercial fluconazole cream (25%–30%) [44]. Later on, econazole nitrate loaded ethosomes were compared with liposomes loaded with the same for the treatment of deep fungal infection by Verma and Pathak in gel form. Ethosomal gel showed 2 fold higher diffusion of the drug in the albino rat skin compared to liposomal gel after 12 h of application. Results of CLSM (confocal laser scanning microscopy) studies revealed accumulation of econazole nitrate loaded ethosomes in the stratum basale layer of animal skin (Fig. 6) [45]. Maheshwari et al. carried out a comparative assessment between ethosomes and ultradeformable liposomes loaded with clotrimazole for the treatment of cutaneous candidiasis. Drug loaded ethosomes showed higher in vitro antifungal activity against Candida albicans by showing 34.6 mm zone of inhibition compared to ultradeformable liposomes which showed 29.6 mm inhibition zone. Results of Fourier-transform infrared spectroscopy revealed higher in vitro skin penetration of ethosomes compared to ultradeformable liposomes [46].
Fig. 6.
Confocal laser scanning microscopy. (A) CLSM image of control gel; (B) CLSM image of liposomal gel showing less penetration of drug; (C) CLSM image of ethosomal gel showing penetration of drug as far as the last layer (stratum basale) of epidermis (Reproduced with permission from reference [45]) Copy right 2012, Elsevier.
Furthermore, voriconazole loaded ethosomes were investigated by Faisal et al. for effective skin deposition. Developed ethosomal formulation showed six fold more ex vivo drug permeation in the rat abdominal skin compared to hydroethanolic solution of voriconazole [47]. Ethosomes having concentration of ethanol more than 30% cause excessive release of entrapped material and irritation of skin [48]. Therefore, Akhtar and Pathak developed Cavamax W7 composite ethosomes to minimize these harmful effects of high ethanol concentration in vesicles by lowering its amount in vesicles. Cavamax W7 is a permeation enhancer and it shows synergistic effect on ethanol's skin penetration power. Developed Cavamax W7 composite ethosomes showed high stability and ex vivo skin permeation, antifungal activity against Candida albicans and Aspergillus niger compared to conventional ethosomes [49].
3.1.3. Transfersomes
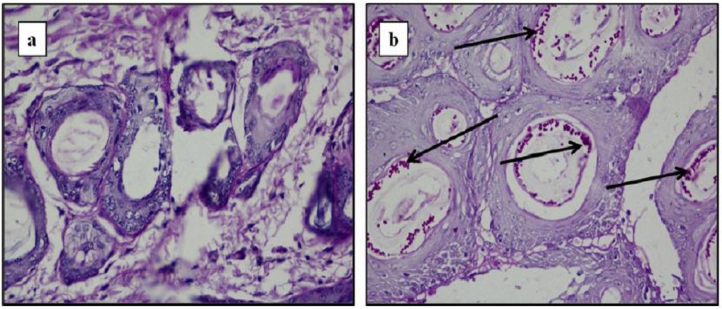
Conventional liposomes have poor penetration through the skin, which can be improved by modifying their bilayer composition [50]. Liposomes were firstly modified by Cevc and Blume by the addition of edge activators to liposomal composition and resulted modified liposomes were called ‘deformable liposomes’, ‘elastic liposomes’, or ‘transfersomes’ [51]. Various examples of edge activators used in transfersomes are sodium deoxycholate, sodium cholate, dipotassium glycyrrhizinate, Tween 80 , Tween 60, Tween 20, Span 80, Span 65, and Span 60 [52]. Transfersomes show enhanced deformability due to the weakening of their lipid bilayers because of edge activators [53]. Transfersomes have higher skin penetration compared to conventional liposomes due to their higher deformability and they can easily cross through the pores having diameter 5–10 times less compared to their own diameter [54]. Pandit et al. investigated topical antifungal efficacy of transfersomes loaded with miconazole nitrate in Sprague-Dawley rats. Developed modified liposomes showed high in vivo antifungal activity and reduced toxicity compared to conventional liposomes and free drug solution [55]. Aggarwal and Goindi carried out evaluation of transfersomes loaded with griseofulvin in guinea pigs for eradication of Microsporum canis induced dermatophytosis. Optimized transfersomal formulation showed better skin retention and permeation compared to conventional liposomes. Histopathological analysis revealed complete eradication of fungal spores from guinea pig skin within 10 d treatment by using griseofulvin loaded transfersomes (Fig. 7) [56].
Fig. 7.
Histopathology of skin of guinea pig infected with M. canis after treatment with (A) test formulation (griseofulvin loaded transfersomes) showing complete absence of fungal elements (B) placebo, arrows show presence of spored hyphae in hair follicles (n = 5) (Reproduced with permission from reference [56]. Copy right 2012, Elsevier.)
Recently, transfersomes loaded with amphotericin B were developed by Perez et al. and were evaluated for in vitro antifungal activity and human skin permeation. They reported maximum deformability in transfersomes using Tween 80 as an edge activator. In vitro sensitivity of clinical isolates of Candida albicans was very high towards transfersomes loaded with amphotericin B compared to mammal cells. Transfersomes showed forty times better accumulation in human skin compared to a marketed liposomal formulation of amphotericin B (AmBisome) [57].
3.1.4. Transethosomes
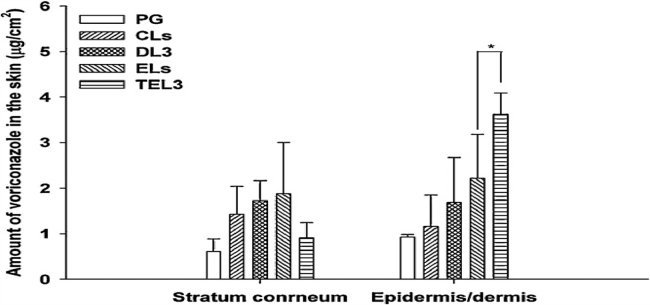
Transethosomes are highly advanced vesicular nanocarrier systems which encompass the advantages of transfersomes and ethosomes. Their composition is exactly similar to ethosomes additionally having the presence of a penetration enhancer or an edge activator [58]. Song et al. evaluated voriconazole loaded transethosomes for in vivo skin deposition of drug in mice. Transethosomes (TEL) showed increased in vivo skin deposition of voriconazole in the dermis and epidermis area compared to other nanocarriers like deformable liposomes (DL), conventional liposomes (CL), ethosomes (EL), and polyethylene glycol drug solution (PG) (Fig. 8) [59]. Table 2 gives a brief summary of research work done on phospholipid based nanocarriers other than liposomes for transdermal delivery of antifungal drugs. Overview of various advantages and disadvantages of phospholipid based nanocarrier systems is explained in Table 3.
Fig. 8.
Amount of voriconazole retained in skin at the of in-vivo skin deposition studies after applying lipid vesicles or control PG solution. Each value is the mean ± S.D. (n = 4) (*P < 0.05 vs ELs) (Reproduced with permission from reference [59]. Copy right 2012, Elsevier.)
Table 2.
Phospholipid based vesicles other than liposomes as effective nanocarrier for treatment of skin fungal infections.
| Vesicular carrier/ Composition | Drug | Entrapment/size /zeta potential | Animal model/Route of Administration | Key findings | Ref. |
|---|---|---|---|---|---|
| Ethosomes/ Soya phosphatidyl choline, ethanol | Fluconazole | 82.68%/ 144 ± 6.8 nm/ NA | Human/ topical | Ethosomal gel containing fluconazole showed 50% - 70% reduction in skin lesions in patients, which was very high compared to liposomes (30% - 60%) and commercial fluconazole cream (25% - 30%) | [44] |
| Ethosomes/ Soya phosphatidyl choline, ethanol | Econazole nitrate | 81.1% ± 0.13%/ 202.8 ± 5.10 nm/ –75.1 ± 0.21 mV | Albino rats/ topical | CLSM (confocal laser scanning microscopy) studies revealed accumulation of econazole nitrate loaded ethosomes in the stratum basale layer of animal skin | [45] |
| Ethosomes/ Soybean phosphatidyl choline, ethanol | Clotrimazole | 68.7% ± 1.4%/ 132 ± 9.5 nm/ NA | Sprague–Dawley rats/ topical | Fourier-transform infrared spectroscopy revealed higher in vitro skin penetration of ethosomes compared to ultradeformable liposomes | [46] |
| Ethosomes/ Soybean phosphatidyl choline, ethanol | Voriconazole | 46.5% ± 2.1%/ 423.67 ± 26.64 nm/ −18.20 ± 0.30 mV | NA | Ethosomal formulation showed six fold more ex-vivo drug permeation in the rat abdominal skin compared to hydroethanolic solution of voriconazole | [47] |
| Ethosomes/ Soya lecithin, Cavamax, propylene glycol | Clotrimazole | 98.42% ± 0.15%/ 202.8 ± 4.8 nm/ 83.6 ± 0.9 mV | NA | Cavamax W7 composite ethosomes showed high stability and ex-vivo skin permeation, antifungal activity against Candida albicans and Aspergillus niger compared to conventional ethosomes | [49] |
| Transfersomes/ Soya phosphatidyl choline, sodium deoxycholate, Tween-80, Span-60, Span-80, cholesterol | Miconazole nitrate | 91.3% ± 1.20%/ 182 ± 8.53 nm/ NA | Sprague-Dawley rats/ topical | Ultraflexible liposomes loaded with miconazole nitrate showed high in-vivo antifungal activity and reduced toxicity compared to conventional liposomes and free drug solution | [55] |
| Transfersomes/ Phospholipon® 90 G, Span 85, Cholesterol | Griseofulvin | 63.44% ± 0.45%/ 284.6 nm/ − 22.0 ± 3.68 mV | Guinea pig/ topical | Histopathological analysis revealed complete eradication of fungal spores from guinea pig skin within 10 d treatment by using griseofulvin loaded transfersomes | [56] |
| Transfersomes/ Soybean Phosphatidyl choline, Sodium cholate, Tween 80, Cholesterol | Ampho tericin B | NA/ 98 ± 8 nm/ -1 ± 0.2 mV | NA | Transfersomes showed forty times better accumulation in human skin compared to a marketed liposomal formulation of amphotericin B (AmBisome) | [57] |
| Transethosomes/ Soybean Phosphatidyl choline, ethanol, sodium taurocholate, Tween 80 | Voriconazole | 96.6% ± 2.7%/ 191.9 ± 41.5 nm/ − 6.9 ± 0.6 mV | Mice (HanLim Animal, Korea)/ Topical | Transethosomes (TEL) showed increased in-vivo skin deposition of voriconazole in the dermis and epidermis area compared to other nanocarriers like deformable liposomes (DL), conventional liposomes (CL), ethosomes (EL), and polyethylene glycol drug solution (PG) | [59] |
Table 3.
Advantages and disadvantages of phospholipid based nanocarrier systems.
| Vesicular nanocarrier | Advantages | Disadvantages | References |
|---|---|---|---|
| Liposomes | Improved drug stability | Drug leakage | [22], [28], [29], [30] |
| Available in various size range | Scale-up difficulty | ||
| Reduced drug toxicity | Dose dumping | ||
| Prolonged drug release | Sterilization problems | ||
| Ethosomes | High skin permeation compared to liposomes | Chances of coalescence | [41], [42] |
| Non-toxic raw materials | Poor yield problems | ||
| Smaller size compared to liposomes | Less stability | ||
| Transfersomes | Higher penetration compared to liposomes and ethosomes | Expensive | [53], [54] |
| Systemic and topical delivery | Difficult manufacturing | ||
| Transethosomes | Higher penetration compared to ethosomes | Scale-up problems | [58] |
| Higher stability compared to ethosomes | Expensive |
3.2. Non phospholipid-based vesicular nanocarriers
3.2.1. Niosomes
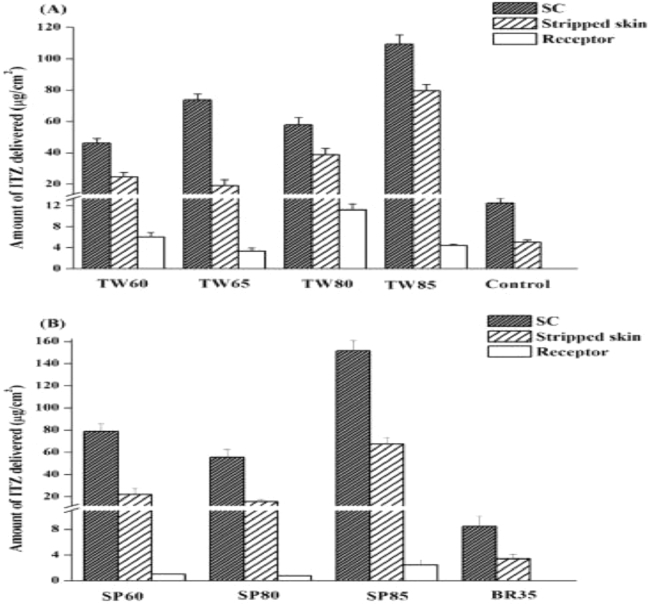
Niosomes are bilayered vesicular systems which are made up of single alkyl chain non-ionic surfactants [60]. Handjani-Vila et al. gave first description of niosomes in 1979 [61]. Structurally, they have a hydrophilic head of surfactant oriented towards the exterior and interior of bilayer while, hydrophilic tail endorsed inside the bilayer [62]. Therefore, niosomes are capable of encapsulating both hydrophilic or lipophilic drugs [63]. Cholesterol is also added in the production of niosomes to enhance the rigidity of bilayer and reduction of premature drug leakage [64]. Various characteristics like low production cost, higher chemical stability, high loading capacity, and regular conditions storage make them efficient carries than liposomes [65]. Characteristics of niosomes can be easily modified by varying their composition and preparation methods [66]. Niosomes are suitable to deliver various therapeutic agents through various routes like topical, oral, and parenteral [67]. Examples of surfactants which are used for niosome production are Tweens, Spans, polyglycerol alkyl ethers, polyoxy- ethylene alkyl ethers, Brij, and ester-linked surfactants [68]. Kassem et al. carried out a comparative assessment between niosomal gel loaded with griseofulvin, and liposomal gel loaded with same for topical treatment of tinea corporis. These formulations were investigated clinically in sixteen patients for about three week treatment period. Niosomal gel showed approximately 80% cure rate, which was very high compared to liposomal gel (50%) [69]. Later on, Alomrani et al. prepared niosomes loaded with itraconazole (ITZ) using different non ionic surfactants for transdermal delivery. A comparison was done using Span surfactants [like Span 60 (SP60), Span 80 (SP80), and Span 85 (SP85)], Tween surfactants [like Tween 60 (TW60), Tween (TW80), and Tween 85 (TW85)], and Brij 35 (BR35). Niosomes prepared from Span 85 and Tween 85 showed maximum skin penetration, however, skin permeation power of Tween 85 niosomes was higher compared to Span 85 niosomes and Brij 35 niosomes (Fig. 9) [70].
Fig. 9.
The Amount of ITZ delivered into the stratum corneum (SC), Stripped skin and the receptor fluid after 6 h of incubation with rat skin after applying different type of niosomes(Reproduced with permission from reference [70]) Copy right 2015, Elsevier.
A comparative assessment between niosomal formulation loaded with terbinafine hydrochloride and its marketed cream was carried out by Sathali and Rajalakshmi in-vitro against pathological fungus Aspergillus niger. Niosomes containing encapsulated drug (purified niosomes) and niosomes containing encapsulated drug and free drug both were dispersed into gel base and their antifungal effects were compared with marketed formulation. A zone of inhibition was found in the order - niosomal gel containing encapsulated drug and free drug both > niosomal gel containing encapsulated drug only > marketed cream formulation > gel containing pure drug [71]. A brief overview of niosomes for treatment of skin fungal infection is given in Table 4.
Table 4.
Niosomes as effective nanocarrier for treatment of skin fungal infections.
| Composition of Niosomes | Drug | Entrapment/size /zeta potential | Animal model/Route of Administration | Key findings | Ref. |
|---|---|---|---|---|---|
| Span 60, Cholesterol, Ethanol | Itraconazole | 89.67 ± 1.85%/ 16.02 ± 1.35 µm/ NA | NA | Itraconazole loaded niosomes showed high in vitro skin permeation and a larger zone of inhibition against Candida albicans compared to a commercially available topical formulation of itraconazole | [72] |
| Span 40, Span 60, Tween 60, Cholesterol | Keto conazole | 69.39 ± 0.94%/ 5.94 ± 2.14 µm/ NA | NA | Niosomes having Span 60 and cholesterol in the ratio 1 : 0.2 loaded with drug showed a prolonged effect than formulation containing free ketoconazole | [73] |
| Span 60, cholesterol, stearic acid | Nystatin | 80.25%/ 189 ± 0.55 nm/ −30.55 ± 0.28 mV | Albino rabbits/ Topical | Niosomal gel showed two fold increased deposition of nystatin in porcine skin and less irritation in rabbits on topical application compared to conventional gel of nystatin | [74] |
| Span 80, cholesterol | Econazole | 98%/ 0.050 µm/ NA | NA | Niosomes containing Cholesterol and Span 80 in the ratio 1 : 4 showed maximum drug entrapment and extended the drug release upto 24 h indicating their efficacy to treat skin fungal infections | [75] |
3.2.2. Spanlastics
Spanlastics are novel vesicular carriers, which are also termed as ‘modified niosomes’ as they contain edge activator in niosomal composition. Spanlastics usually contain Spans alonwith presence of edge activators like Tweens and many others [76]. Kakkar and Kaur firstly developed spanlastics loaded with ketoconazole using Span 60 as surfactant and Tween 80 as an edge activator for ocular drug delivery [77]. Elsherif et al. developed and evaluated spanlastics loaded with terbinafine hydrochloride for treatment of nail fungal infection namely onychomycosis. Study was carried out by using Span 60 and Span 65 as surfactants while Tween 80 and sodium deoxycholate as edge activators. Spanlastics formulated with Span 65 as surfactant and sodium deoxycholate as edge activator showed maximum drug entrapment, smaller size and good colloidal properties therefore, categorized as optimized formulation. Confocal laser scanning microscopy (CLSM) revealed efficient ex vivo nail permeation of optimized spanlastic formulation [78].
3.2.3. Oleic acid vesicles
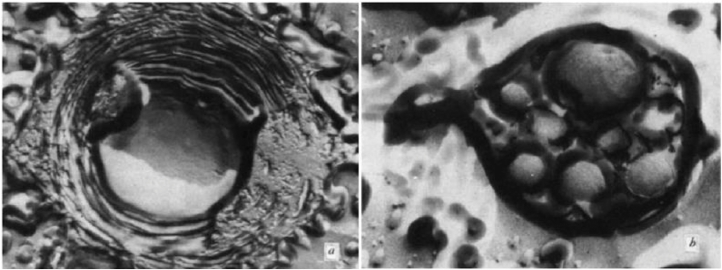
Fatty acids are amphiphilic molecules consisting of a carbon atom chain behaving as polar part while terminal carboxylic group behaving as non polar part. The presence of double bonds in structure of fatty acid governs whether they are saturated or unsaturated [79]. Gebicki and Hicks firstly reported ability of unsaturated fatty acid like oleic acid to form vesicles [80]. Fig. 10 gives structural elucidation of spherical vesicles prepared by using oleic acid.
Fig. 10.
Electron micrographs of freeze-etched oleic acid spheres ( × 41 650 ). (A) A central aqueous region surrounded by concentric membranes- the flat pitted area was cut by the microtome. (B) Smaller spheres enclosed by a common envelope (Reproduced with permission from reference [80]. Copy right 1973, Nature.)
Zakir et al. evaluated oleic acid vesicles loaded with fluconazole for efficient transdermal drug delivery. Developed vesicles showed maximum drug entrapment, acceptable size, and good colloidal properties at 7 : 3 oleic acid to drug ratio. Results of ex-vivo skin permeation and confocal microscopic studies revealed accumulation of drug loaded oleic acid vesicles in the lower epidermis area of skin after topical application indicating their effectiveness in the localized drug delivery [81]. Later on, clotrimazole loaded oleic acid were investigated by Verma et al. for cutaneous candidiasis treatment in guinea pigs. Developed oleic acid vesicles showed high skin permeation along with good skin retention in animal skin. In-vivo study revealed capability of drug loaded oleic acid vesicles to release clotrimazole upto 5 d after the time of application [82]. A brief summary of various non phospholipid based vesicular nanocarriers for skin delivery of various antifungal drugs is given in Table 5. Table 6 describes advantages and disadvantages of various non phospholipid based nanocarrier systems.
Table 5.
Role of various non-phospholipid based vesicular carriers in treatment of skin fungal infections.
| Vesicular carrier/ Composition | Drug | Entrapment/size /zeta potential | Animal model/Route of Administration | Key findings | Ref. |
|---|---|---|---|---|---|
| Spanlastics/ Span 60, Span 65, Tween 80, sodium deoxycholate | Terbinafine hydro chloride | 79.09% ± 1.46%/ 1512.5 ± 192 nm/ − 42.35 ± 0.212 mV | NA | Confocal laser scanning microscopy (CLSM) revealed efficient ex vivo nail permeation of optimized spanlastic formulation | [78] |
| Oleic acid vesicles/ Oleic acid, methanol | Fluconazole | 44.11% ± 1.13%/ 527 ± 15 nm/ NA | Guinea pigs/ Topical | Confocal microscopic studies revealed accumulation of drug loaded oleic acid vesicles in the lower epidermis area of skin after topical application indicating their effectiveness in the localized drug delivery | [81] |
| Oleic acid vesicles/ Oleic acid, methanol | Clotrimazole | 49.5% ± 1.0%/ 455 ± 22 nm/ −22.45 ± 0.25 mV | Guinea pigs/ Topical | In vivo study revealed capability of drug loaded oleic acid vesicles to release clotrimazole upto 5 d after the time of application | [82] |
Table 6.
Advantages and disadvantages of non phospholipid based nanocarrier systems.
| Vesicular nanocarrier | Advantages | Disadvantages | References |
|---|---|---|---|
| Niosomes | Cheap compared to liposomes | Poor drug loading | [65], [66] |
| High stability compared to liposomes | Special manufacturing equipments required | ||
| Reduced toxicity due presence of non ionic surfactant | |||
| Spanlastics | High skin permeation compared to niosomes | Expensive | [76], [78] |
| Oleic acid vesicles | Cheap compared to niosomes and spanlastics | Stability issues | [82] |
| High penetration power compared to niosomes | |||
3.3. Nanoparticles
Various types of nanoparticles explored for transdermal delivery of antifungal drugs are polymeric nanoparticles (NPs), solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) [83]. Polymeric nanoparticles may be present either in particulate dispersion form or solid powder form having size range 10–1000 nm. Nanoparticle matrix may have drug in entrapping, encapsulated, or dissolved form [84]. Various examples of natural polymers used for NPs preparation are gelatin, albumin, chitosan, and alginate. Synthetic polymers used for NPs preparation may be biodegradable [poly(lactide-coglycolide) (PLGA), poly(ε-caprolactone)] and non-biodegradable [poly(methyl methacrylate), polystyrene and polyacrylates] [85]. Kumar et al. evaluated clotrimazole loaded PLGA microparticles in guinea pigs for successful eradication of cutaneous candidiasis. Gel containing drug loaded PLGA microsphere showed penetration upto 50 µm in the dermis of animal skin along with better antifungal efficacy after 4 d of application [86]. Solid lipid nanoparticles (SLNs) are spherical structures having solid lipid core which may solubilize non polar drug molecules [87]. Examples of various solid lipids implemented for the preparation of SLNs are glycerol bahenate, tristearin, glycerol monostearate, stearic acid, and cetyl palmitate [88]. SLNs manufacturing involves the use of physiological lipids and less use of organic solvent which make them efficient carrier for transdermal drug delivery [89]. Bhalekar et al. performed ex-vivo skin permeation studies of miconazole nitrate loaded SLNs prepared using solid lipid Compritol 888 ATO (glycerol bahenate). Developed SLNs formulation showed better ex-vivo accumulation of drug in the skin along with better skin targeting effect compared to the marketed gel [90]. Furthermore, glycerol palmitostearate (Precirol ATO 5) based SLNs loaded with econazole nitrate were evaluated by Sanna et al. in vivo by using five healthy living subjects (females). SLNs revealed better diffusion of drug in lower epidermal skin layers after 180 min of application compared to marketed gel in living subjects [91]. Nanostructure lipid carriers (NLCs) are nanocarriers consisting of the lipid matrix embedded with special nanostructures [92]. NLCs produced using high pressure homogenization technique may be in lipid particle dispersion form having 60%–80% solid content [93]. Gupta and Vyas evaluated fluconazole loaded NLCs in vivo for effective eradication of cutaneous candidiasis in albino rats. Drug loaded NLCs showed 3.3 fold higher skin retention, better skin targeting effect, and high therapeutic efficacy compared to drug loaded SLNs [94]. Furthermore, econazole nitrate loaded thermodynamically stable NLCs were developed by Keshri and Pathak by the use of central composite design for transdermal drug delivery. Results of confocal microscopy revealed penetration of drug loaded NLCs upto stratum basale layer of animal skin. The developed formulation showed a minor change in particles size and zeta potential during 90 d stability analysis [95]. So, after reviewing literature, it can be considered that nanoparticles may be a good alternative to improve transdermal antifungal therapy.
4. Intellectual property rights (IPR) related to use of various vesicular nanocarriers for treatment of skin fungal infections
Vesicular nanocarriers show high therapeutic potential in the treatment of skin fungal infections. These novel carriers can be considered as an effective alternative to currently available marketed products to treat skin fungal infections. Therefore, pharmaceutical scientists have filed various patents regarding the use of various vesicular nanocarriers for treatment of skin fungal infections. Table 7 gives an overview of various patents granted regarding the use of vesicular nanocarriers in treatment of skin fungal infections.
Table 7.
List of patents regarding the use of vesicular nanocarriers for treatment of skin fungal infections.
| Title of patent | Brief description | Inventors | Patent number | Ref. |
|---|---|---|---|---|
| Topical liposomes compositions for delivering hydrophobic drugs and methods preparing same | This invention describes a method of loading amphotericin B in liposomes and their role in the treatment of cutaneous candidiasis and cutaneous leishmaniasis | Mahmoud Reza Jaafari, Ali Khamesipour | US20150147382 A1 | [96] |
| Allylamine-containing liposomes | This patent deals with a method of preparation of terbinafine encapsulating liposomes and their utility to treat fungal infections through topical delivery | David Bodmer, Thomas Kissel, Friedrich Richter, Harry Tiemessen | US6623753 B1 | [97] |
| Topical terbinafine formulations and methods of administering same for the treatment of fungal infections | This invention describes about the utility of niosomes loaded with terbinafine for effective removal of skin fungal infections | Gregor Cvec, Ulrich Vierl | US7820720 B2 | [98] |
| Terbinafine compositions for onychomycosis treatment | This patent explains a method of preparation of nanoethosomes containing 60% (w/w) ethanol and loading of terbinafine into them for onychomycosis treatment | Elka Touitou | WO2010086723 A1 | [99] |
| Design of terbinafine hydrochloride loaded liposome included pullulan film system for ungual treatment of onychomycosis | This invention describes the development method of terbinafine hydrochloride containing liposomes and their efficacy to treat onychomycosis when delivered in the form of the pullulan film system | Kevser Ozgen Ozer, Sakine Tuncay Tanriverdi | WO2014209246 A1 | [100] |
5. Limitations and challenges in the use of vesicular nanocarriers for treatment of skin fungal infections
Vesicular nanocarrier systems are very effective to treat skin fungal infections due to their capability to modify the delivery of bioactive molecules to different skin layers and target diseased portion of the skin. Vesicular nanocarriers show high penetration of drug molecules in the skin through various mechanisms like fusion, absorption, and lipid exchange in skin layers. But, the excessive skin penetration enhancement may become a double edged sword because after heavy penetration, drug molecules may reach in blood circulation which can be harmful for localized treatment of skin fungal infections. Therefore, serious concerns regarding this must be taken by pharmaceutical scientists. Many other factors like safety profile, clinical efficacy, scale up techniques, and the fate of vesicular nanocarriers in transdermal therapeutics are still challenging for the scientists. Successful future research will help to answer these challenges and develop an ideal model of vesicular nanocarrier for effective transdermal antifungal therapeutics.
6. Conclusions
Significant increase in mortality rate has been observed in patients from last one decade due to fungal infections whether topical or systemic. There are several effective antifungal drugs available, but their therapeutic efficacy is limited due to unfavorable physicochemical characteristics and high toxicity profiles. Vesicular nanocarriers have capability to minimize these drawbacks of antifungal drugs due to their unique properties like high biocompatibility, ease of surface modification, and smaller size. Vesicular nanocarriers may be very effective to treat invasive skin fungal associated with immunosuppressive disease like AIDS as they show controlled drug release which do not activate the immune system of the patient. Beside all this, vesicular nanocarriers may improve stability and targeting effect of antifungal drugs to infected tissues along with the enhancement of their solubility and antifungal efficacy. Clinical evaluation of vesicular carriers is still a challenge because amphotericin B is the only antifungal drug which has been clinically evaluated in liposomal form and available in the market therefore, their presence in pharmaceutical market will be governed by successful clinical evaluation.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Authors are grateful to the Department of Research, Innovation & Consultancy (RIC), I. K. Gujral Punjab Technical University, Jalandhar, Punjab for providing access to reputed scientific journals via OpenAthens account to carry out this work.
References
- 1.Kim JY. Human fungal pathogens: why should we learn? J Microbiol. 2016;54(3):145–148. doi: 10.1007/s12275-016-0647-8. [DOI] [PubMed] [Google Scholar]
- 2.Hainer BL. Dermatophyte infections. Am Fam Phys. 2003;67(1):101–108. [PubMed] [Google Scholar]
- 3.Gupta AK, Ryder JE, Chow M, Cooper EA. Dermatophytosis: the management of fungal infections. Skinmed. 2005;4(5):305–310. doi: 10.1111/j.1540-9740.2005.03435.x. [DOI] [PubMed] [Google Scholar]
- 4.Gretzula J, Penneys NS. Complex viral and fungal skin lesions of patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1987;16(6):1151–1154. doi: 10.1016/s0190-9622(87)70149-2. [DOI] [PubMed] [Google Scholar]
- 5.Bseiso EA, Nasr M, Sammour O, Abd El Gawad NA. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol. 2015;81(5):457–463. doi: 10.4103/0378-6323.162328. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins DM, Smidt AC. Superficial fungal infections in children. Pediatr Clin North Am. 2014;61(2):443–455. doi: 10.1016/j.pcl.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycoses. A North Am Perspect Drugs. 1998;55(5):645–674. doi: 10.2165/00003495-199855050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe S. Dermatomycosis–classification, etiology, pathogenesis, and treatment. Nihon Rinsho. 2008;66(12):2285–2289. [PubMed] [Google Scholar]
- 9.Nenoff P, Krüger C, Ginter-Hanselmayer G, Tietz HJ. Mycology - an update. Part 1: Dermatomycoses: causative agents, epidemiology and pathogenesis. J Dtsch Dermatol Ges. 2014;12(3):188–209. doi: 10.1111/ddg.12245. [DOI] [PubMed] [Google Scholar]
- 10.Elgart GW. Subcutaneous (deep) fungal infections. Semin Cutan Med Surg. 2014;33(3):146–150. doi: 10.12788/j.sder.0112. [DOI] [PubMed] [Google Scholar]
- 11.Patel U, Chu J, Patel R, Meehan S. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17(10):19–35. [PubMed] [Google Scholar]
- 12.Arenas R, Moreno-Coutiño G, Welsh O. Classification of subcutaneous and systemic mycoses. Clin Dermatol. 2012;30(4):369–371. doi: 10.1016/j.clindermatol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Güngör S, Erdal MS, Aksu B. New formulation strategies in topical antifungal therapy. J Cosmet Dermatol Sci Appl. 2013;3:56–65. [Google Scholar]
- 14.Amichai B, Grunwald MH. Adverse drug reactions of the new oral antifungal agents – Terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998;37:410–415. doi: 10.1046/j.1365-4362.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Chow M, Daniel CR, Aly R. Treatments of tinea pedis. Dermatol Clin. 2003;21:431–462. doi: 10.1016/s0733-8635(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 16.Robert EM, Kalia YN. New developments in topical antifungal therapy. Am J Drug Deliv. 2006;4:231–247. [Google Scholar]
- 17.Goldstein A, Smith K, Ives T. Mycotic infections: effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40–52. [PubMed] [Google Scholar]
- 18.Akhtar N, Verma A, Pathak K. Topical delivery of drugs for the effective treatment of fungal infections of skin. Curr Pharm Des. 2015;21(20):2892–2913. doi: 10.2174/1381612821666150428150456. [DOI] [PubMed] [Google Scholar]
- 19.Kircik LH. Advancements in topical antifungal vehicles. J Drugs Dermatol. 2016;15(2 Suppl) :s44--8. [PubMed] [Google Scholar]
- 20.Kyle AA, Dahl MV. Topical therapy for fungal infections. Am J Clin Dermatol. 2004;5(6):443–451. doi: 10.2165/00128071-200405060-00009. [DOI] [PubMed] [Google Scholar]
- 21.Firooz A, Namdar R, Nafisi S, Maibach HI. Nano-sized technologies for miconazole skin delivery. Curr Pharm Biotechnol. 2016;17(6):524–531. doi: 10.2174/1389201017666160301102459. [DOI] [PubMed] [Google Scholar]
- 22.Kumar L, Verma S, Bhardwaj A, Vaidya S, Vaidya B. Eradication of superficial fungal infections by conventional and novel approaches: a comprehensive review. Artif Cells Nanomed Biotechnol. 2014;42(1):32–46. doi: 10.3109/21691401.2013.769446. [DOI] [PubMed] [Google Scholar]
- 23.Firooz A, Nafisi S, Maibach HI. Novel drug delivery strategies for improving econazole antifungal action. Int J Pharm. 2015;495(1):599–607. doi: 10.1016/j.ijpharm.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Kaur IP, Kakkar S. Topical delivery of antifungal agents. Expert Opin Drug Deliv. 2010;7(11):1303–1327. doi: 10.1517/17425247.2010.525230. [DOI] [PubMed] [Google Scholar]
- 25.Forssen EA, Tökès ZA. Use of anionic liposomes for the reduction of chronic doxorubicin-induced cardiotoxicity. Proc Natl Acad Sci U S A. 1981;78(3):1873–1877. doi: 10.1073/pnas.78.3.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soliman GM. Nanoparticles as safe and effective delivery systems of antifungal agents: achievements and challenges. Int J Pharm. 2017;523(1):15–32. doi: 10.1016/j.ijpharm.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 27.McClements DJ. Encapsulation, protection, and release of hydrophilic active components: potential and limitations of colloidal delivery systems. Adv Colloid Interf Sci. 2015;219:27–53. doi: 10.1016/j.cis.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deniz A, Sade A, Severcan F. Celecoxib-loaded liposomes: effect of cholesterol on encapsulation and in vitro release characteristics. Biosci Rep. 2010;30(5):365–373. doi: 10.1042/BSR20090104. [DOI] [PubMed] [Google Scholar]
- 30.Akhtar N. Vesicles: a recently developed novel carrier for enhanced topical drug delivery. Curr Drug Deliv. 2014;11(1):87–97. doi: 10.2174/15672018113106660064. [DOI] [PubMed] [Google Scholar]
- 31.Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactionsand medical applications. Int J Pharm. 2018;535(1-2):1–17. doi: 10.1016/j.ijpharm.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 32.Sudhakar B, Varma JN, Murthy KV. Formulation, characterization and ex vivo studies of terbinafine HCl liposomes for cutaneous delivery. Curr Drug Deliv. 2014;11(4):521–530. doi: 10.2174/1567201810666140109113830. [DOI] [PubMed] [Google Scholar]
- 33.Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS Pharm Sci Tech. 2012;13(2):723–731. doi: 10.1208/s12249-012-9783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal R, Katare OP. Preparation and in vitro evaluation of miconazole nitrate loaded topical liposomes. Pharm Tech. 2002:1–12. [Google Scholar]
- 35.Schwarz JC, Kählig H, Matsko NB. Decrease of liposomal size and retarding effect on fluconazole skin permeation by lysine derivatives. J Pharm Sci. 2011;100(7):2911–2919. doi: 10.1002/jps.22513. [DOI] [PubMed] [Google Scholar]
- 36.Paul D, Babu VS. Formulation and evaluation of liposomal gel containing antifungal activity – ketoconazole. Ind Am J Pharm Res. 2016;6(07):6154–6170. [Google Scholar]
- 37.Patel PR, Patel HH, Baria HA. Formulation and evaluation of carbopol gel containing liposomes of ketoconazole. Int J Drug Del Tech. 2009;1:42–45. [Google Scholar]
- 38.Akhtar N, Varma A, Pathak K. Ethosomes as vesicles for effective transdermal delivery: from bench to clinical implementation. Curr Clin Pharmacol. 2016;11(3):168–190. doi: 10.2174/1574884711666160813231352. [DOI] [PubMed] [Google Scholar]
- 39.Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomedicine. 2013;8:3171–3186. doi: 10.2147/IJN.S33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blume A, Jansen M, Ghyczy M, Gareiss J. Interaction of phospholipid liposomes with lipid model mixtures for stratum corneum lipids. Int J Pharm. 1993;99:219–228. [Google Scholar]
- 41.Campani V, Biondi M, Mayol L. Nanocarriers to enhance the accumulation of vitamin K1 into the skin. Pharm Res. 2016;33(4):893–908. doi: 10.1007/s11095-015-1836-6. [DOI] [PubMed] [Google Scholar]
- 42.Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45–59. doi: 10.1517/17425247.2013.860130. [DOI] [PubMed] [Google Scholar]
- 43.Godin B, Touitou E. Ethosomes: new prospects in transdermal delivery. Crit Rev Ther Drug Carrier Syst. 2003;20(1):63–102. doi: 10.1615/critrevtherdrugcarriersyst.v20.i1.20. [DOI] [PubMed] [Google Scholar]
- 44.Bhalaria MK, Naik S, Misra AN. Ethosomes: a novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J Exp Biol. 2009;47(5):368–375. [PubMed] [Google Scholar]
- 45.Verma P, Pathak K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine. 2012;8(4):489–496. doi: 10.1016/j.nano.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Maheshwari RG, Tekade RK, Sharma PA. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi Pharm J. 2012;20(2):161–170. doi: 10.1016/j.jsps.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faisal W, Soliman GM, Hamdan AM. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J Liposome Res. 2016;2016:1–8. doi: 10.1080/08982104.2016.1239636. [DOI] [PubMed] [Google Scholar]
- 48.Lachenmeier DW. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol. 2008;3:26. doi: 10.1186/1745-6673-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhtar N, Pathak K. Cavamax W7 composite ethosomal gel of clotrimazole for improved topical delivery: development and comparison with ethosomal gel. AAPS Pharm Sci Tech. 2012;13(1):344–355. doi: 10.1208/s12249-012-9754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomed. 2013;8:3171–3186. doi: 10.2147/IJN.S33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992;1104(1):226–232. doi: 10.1016/0005-2736(92)90154-e. [DOI] [PubMed] [Google Scholar]
- 52.Benson HA. Transfersomes for transdermal drug delivery. Expert Opin Drug Deliv. 2006;3(6):727–737. doi: 10.1517/17425247.3.6.727. [DOI] [PubMed] [Google Scholar]
- 53.Benson HA. Elastic liposomes for topical and transdermal drug delivery. Curr Drug Deliv. 2009;6(3):217–226. doi: 10.2174/156720109788680813. [DOI] [PubMed] [Google Scholar]
- 54.Hussain A, Singh S, Sharma D. Elastic liposomes as novel carriers: recent advances in drug delivery. Int J Nanomed. 2017;12:5087–5108. doi: 10.2147/IJN.S138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandit J, Garg M, Jain NK. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J Liposome Res. 2014;24(2):163–169. doi: 10.3109/08982104.2013.871025. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal N, Goindi S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membranevesicles in optimized guinea pig model of Microsporum canis–dermatophytosis. Int J Pharm. 2012;437(1-2):277–287. doi: 10.1016/j.ijpharm.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Perez AP, Altube MJ, Schilrreff P. Topical amphotericin B in ultradeformable liposomes: formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf B Biointerf. 2016;139:190–198. doi: 10.1016/j.colsurfb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Kumar L, Verma S, Singh K, Prasad DN, Jain AK. Ethanol based vesicular carriers in transdermal drug delivery: nanoethosomes and transethosomes in focus. NanoWorld J. 2016;2(3):41–51. [Google Scholar]
- 59.Song CK, Balakrishnan P, Shim CK. A novel vesicular carrier, transethosome,for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B Biointerf. 2012;92:299–304. doi: 10.1016/j.colsurfb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Hamishehkar H, Rahimpour Y, Kouhsoltani M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin Drug Deliv. 2013;10(2):261–272. doi: 10.1517/17425247.2013.746310. [DOI] [PubMed] [Google Scholar]
- 61.Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghie G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmet Sci. 1979;1(5):303–314. doi: 10.1111/j.1467-2494.1979.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 62.Choi MJ, Maibach HI. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol Physiol. 2005;18(5):209–219. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- 63.Thakkar M, Brijesh S. Opportunities and challenges for niosomes as drug delivery systems. Curr Drug Deliv. 2016;13(8):1275–1289. doi: 10.2174/1567201813666160328113522. [DOI] [PubMed] [Google Scholar]
- 64.Abdelkader H, Alani AW, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014;21(2):87–100. doi: 10.3109/10717544.2013.838077. [DOI] [PubMed] [Google Scholar]
- 65.Azeem A, Anwer MK, Talegaonkar S. Niosomes in sustained and targeted drug delivery: some recent advances. J Drug Target. 2009;17(9):671–689. doi: 10.3109/10611860903079454. [DOI] [PubMed] [Google Scholar]
- 66.Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull. 2011;34(7):945–953. doi: 10.1248/bpb.34.945. [DOI] [PubMed] [Google Scholar]
- 67.Marianecci C, Di Marzio L, Rinaldi F. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci. 2014;205:187–206. doi: 10.1016/j.cis.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 68.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oraldelivery. J Pharm Sci. 2010;99(4):2049–2060. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 69.Kassem MA, Esmat S, Bendas ER, El-Komy MH. Efficacy of topical griseofulvin in treatment of tinea corporis. Mycoses. 2006;49(3):232–235. doi: 10.1111/j.1439-0507.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 70.Alomrani AH, Al-Agamy MH, Badran MM. In vitro skin penetration and antimycotic activity of itraconazole loaded niosomes: Various non-ionic surfactants. J Drug Del Sci Tech. 2015;28:37–45. [Google Scholar]
- 71.Sathali AAH, Rajalakshmi G. Evaluation of transdermal targeted niosomal drug delivery of terbinafine hydrochloride. Int J Pharm Tech Res. 2010;2:2081–2089. [Google Scholar]
- 72.Wagh VD, Deshmukh OJ. Itraconazole niosomes drug delivery system and its antimycotic activity against candida albicans. ISRN Pharm. 2012;2012 doi: 10.5402/2012/653465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirsand S, Para M, Nagendrakumar D, Kanani K, Keerthy D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int J Pharm Investig. 2012;2(4):201–207. doi: 10.4103/2230-973X.107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desai S, Doke A, Disouza J, Athawale R. Development and evaluation of antifungal topical niosomal gel formulation. Int J Pharm Pharm Sci. 2011;3:224–231. [Google Scholar]
- 75.Kumar YP, Kumar KV, Shekar RR, Ravi M, Kishore VS. Formulation and evaluation of econazole niosomes. Sch Acad J Pharm. 2013;2(4):315–318. [Google Scholar]
- 76.Farghaly DA, Aboelwafa AA, Hamza MY, Mohamed MI. Topical delivery of fenoprofen calcium via elastic nano-vesicular spanlastics: optimization using experimental design and in vivo evaluation. AAPS Pharm Sci Tech. 2017;18(8):2898–2909. doi: 10.1208/s12249-017-0771-8. [DOI] [PubMed] [Google Scholar]
- 77.Kakkar S, Kaur IP. Spanlastics - a novel nanovesicular carrier system for ocular delivery. Int J Pharm. 2011;413(1-2):202–210. doi: 10.1016/j.ijpharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 78.Elsherif NI, Shamma RN, Abdelbary G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: In Vitro characterization and ex vivo evaluation. AAPS Pharm Sci Tech. 2017;18(2):551–562. doi: 10.1208/s12249-016-0528-9. [DOI] [PubMed] [Google Scholar]
- 79.Pohl EE, Peterson U, San J, Pohl P. Changes of intrinsic membrane potentials induced by flip-flop of long chain fatty acids. Biochemistry. 2000;39:1834–1839. doi: 10.1021/bi9919549. [DOI] [PubMed] [Google Scholar]
- 80.Gebicki JK, Hicks M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature. 1973;243:232–234. doi: 10.1038/243232a0. [DOI] [PubMed] [Google Scholar]
- 81.Zakir F, Vaidya B, Goyal AK, Malik B, Vyas SP. Development and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010;17(4):238–248. doi: 10.3109/10717541003680981. [DOI] [PubMed] [Google Scholar]
- 82.Verma S, Bhardwaj A, Vij M. Oleic acid vesicles: a new approach for topical delivery of antifungal agent. Artif Cells Nanomed Biotechnol. 2014;42(2):95–101. doi: 10.3109/21691401.2013.794351. [DOI] [PubMed] [Google Scholar]
- 83.Voltan AR, Quindós G, Alarcón KP. Fungal diseases: could nanostructured drug delivery systems be a novel paradigm for therapy? Int J Nanomed. 2016;11:3715–3730. doi: 10.2147/IJN.S93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(3):205–218. doi: 10.1002/wnan.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147–157. [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar L, Verma S, Jamwal S, Vaidya S, Vaidya B. Polymeric microparticles-based formulation for the eradication of cutaneous candidiasis: development and characterization. Pharm Dev Technol. 2014;19(3):318–325. doi: 10.3109/10837450.2013.778874. [DOI] [PubMed] [Google Scholar]
- 87.Lauterbach A, Müller-Goymann CC. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur J Pharm Biopharm. 2015;97(Pt A):152–163. doi: 10.1016/j.ejpb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 88.Kakadia PG, Conway BR. Lipid nanoparticles for dermal drug delivery. Curr Pharm Des. 2015;21(20):2823–2829. doi: 10.2174/1381612821666150428143730. [DOI] [PubMed] [Google Scholar]
- 89.Prow TW, Grice JE, Lin LL. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63(6):470–479. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 90.Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharm Sci Tech. 2009;10(1):289–296. doi: 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanna V, Gavini E, Cossu M, Rassu G, Giunchedi P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: in vitro characterization, ex-vivo and in-vivo studies. J Pharm Pharmacol. 2007;59(8):1057–1064. doi: 10.1211/jpp.59.8.0002. [DOI] [PubMed] [Google Scholar]
- 92.Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1-2):1–17. doi: 10.1016/j.ijpharm.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 93.Simoes S, Carvalheiro M, Gaspar MM. Lipid-based nanocarriers for Cutaneous Leishmaniais and Buruli Ulcer management. Curr Pharm Des. 2016;22(43):6577–6586. doi: 10.2174/1381612822666160701083812. [DOI] [PubMed] [Google Scholar]
- 94.Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165(4):454–461. doi: 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Keshri L, Pathak K. Development of thermodynamically stable nanostructured lipid carrier system using central composite design for zero order permeation of econazole nitrate through epidermis. Pharm Dev Technol. 2013;18(3):634–644. doi: 10.3109/10837450.2012.659256. [DOI] [PubMed] [Google Scholar]
- 96.Jaafari MR, Khamesipour A. Topical liposomes compositions for delivering hydrophobic drugs and methods preparing same. US20150147382[P].2013-
- 97.Bodmer D, Kissel T, Richter F, Tiemessen H. Allylamine-containing liposomes. US6623753 B1[P]. 2003.
- 98.Cvec G, Vierl U. Topical terbinafine formulations and methods of administering same for the treatment of fungal infections. Patent US7820720 B2, 2010.
- 99.Touitou E. Terbinafine compositions for onychomycosis treatment. WO2010086723 A1[P], 2009.
- 100.Ozer KO, Tanriverdi ST. Design of terbinafine hydrochloride loaded liposome included pullulan film system for ungual treatment of onychomycosis. Patent WO2014209246 A1 [p], 2013.