Abstract
In two independent ongoing next-generation sequencing projects for individuals with holoprosencephaly and individuals with disorders of sex development, and through international research collaboration, we identified twelve individuals with de novo loss-of-function (LoF) variants in protein phosphatase 1, regulatory subunit 12a (PPP1R12A), an important developmental gene involved in cell migration, adhesion, and morphogenesis. This gene has not been previously reported in association with human disease, and it has intolerance to LoF as illustrated by a very low observed-to-expected ratio of LoF variants in gnomAD. Of the twelve individuals, midline brain malformations were found in five, urogenital anomalies in nine, and a combination of both phenotypes in two. Other congenital anomalies identified included omphalocele, jejunal, and ileal atresia with aberrant mesenteric blood supply, and syndactyly. Six individuals had stop gain variants, five had a deletion or duplication resulting in a frameshift, and one had a canonical splice acceptor site loss. Murine and human in situ hybridization and immunostaining revealed PPP1R12A expression in the prosencephalic neural folds and protein localization in the lower urinary tract at critical periods for forebrain division and urogenital development. Based on these clinical and molecular findings, we propose the association of PPP1R12A pathogenic variants with a congenital malformations syndrome affecting the embryogenesis of the brain and genitourinary systems and including disorders of sex development.
Keywords: PPP1R12A, MYPT1, holoprosencephaly, embryogenesis, disorders of sex development, forebrain, hypospadias, facial dysmorphism, encephalocele, omphalocele
Main Text
Protein phosphatase 1, regulatory subunit 12a (PPP1R12A [MIM: 602021]) encodes a component of myosin phosphatase (MP), a key enzyme instrumental in the regulation of cell morphology and motility.1,2 PPP1R12A interacts with the protein phosphatase type 1 catalytic unit (PP1c) and M20/21 to form MP, which is a trimeric holoenzyme. MP regulates the function of non-muscle myosin II by regulating the phosphorylation state of myosin regulatory light chain.3, 4, 5 MP activates when PP1c is unphosphorylated and bound. Phosphorylation of specific consensus sites on PPP1R12A by protein kinases leads to inhibition of its activity. Pathogenic variants in PPP1R12A prevent PPP1R12A from binding to PP1c and result in a non-functional MP.6 Since the initial discovery of MP,5,7 research to define its characterization and function has been productive, but the application of these findings to human diseases has been limited. Previously published animal models illustrate an instrumental role of PPP1R12A during embryogenesis through the regulation of cell movement and adhesion. The mutated Drosophila homologue of PPP1R12A (DMYPT) demonstrates that this protein is required for cell movement during dorsal closure and morphogenesis of the eye.8,9 In C. elegans, PPP1R12A homologue MEL-11 facilitates embryonic elongation through changes in cell shape by contraction of the epidermal cell layer that encloses the embryo.10 In mice, targeted disruption of Ppp1r12a results in embryonic lethality before 7 days post conception.11 Lastly, zebrafish ppp1r12a morpholino knockdown results in gastrulation defects including complete and partial cyclopia, partial cyclopia, and microphthalmia reminiscent of the severe phenotypic changes observed in humans with holoprosencephaly (HPE).12 We report the association of loss-of-function (LoF) variants in PPP1R12A with multiple congenital anomalies, including HPE spectrum and urogenital malformations.
Twelve individuals with de novo LoF variants in PPP1R12A were identified by multiple clinical genetic centers in the United States, Canada, and Europe and evaluated by clinical exam, brain imaging (when clinically indicated), and/or autopsy. Clinical and research laboratories identified variants by exome sequencing (see Supplemental Materials and Methods in Supplemental Information). This study was approved by the National Human Genome Research Institute Institutional Review Board (IRB), Children’s National Health System IRB, and local IRBs. Informed consent for publication was obtained from all individuals or legal guardians. The clinical manifestations in twelve individuals with de novo heterozygous LoF variants in PPP1R12A are summarized in Table 1 and described as follows (see Supplemental Note—Case Reports in Supplemental Information). The first two individuals originated from an HPE cohort of 277 individuals (135 trios and 142 singletons).13 Per protocol, Sanger sequencing of the four most common genes associated with HPE, SHH (MIM: 600725), ZIC2 (MIM: 603073), SIX3 (MIM: 603714), and TGIF1 (MIM: 602630), failed to identify any detectable pathogenic variants (see Supplemental Materials and Methods in Supplemental Information). Individual 1 had syntelencephaly or middle interhemispheric variant (MIHV) of HPE, polymicrogyria, and Chiari I malformation identified on brain MRI, as well as other medical diagnoses including intellectual disability, attention deficit hyperactivity disorder (ADHD), and seizures. Individual 2 had semilobar HPE and agenesis of the corpus callosum identified on MRI, and other medical diagnoses included myoclonus, intellectual disability, and syndactyly of the toes. Data from individuals 3 through 10 were obtained through GeneMatcher.14 In addition to agenesis of the corpus callosum and colpocephaly in the third individual and fetal acrania with exencephaly and omphalocele in the fourth individual, inclusion of urogenital anomalies and a spectrum of 46,XY disorders of sex development (DSD) was seen in individuals 5 through 10. Individuals 11 and 12 were identified through a targeted variant search in a cohort of 94 families (300 individuals) with DSD. While chromosomal sex on either CMA or karyotype was normal male on these last two individuals, their urogenital phenotypes ranged from streak gonads, rudimentary Fallopian tubes, and a urogenital sinus to ovaries which underwent gonadal degeneration, a uterus, posterior labial fusion, a clitoris, and increased labial rugation with pigmentation. Seven of 12 individuals in this study had developmental delay (Table 1: individuals 1,2,6,7,10,11, and 12); however, two of these individuals had unremarkable brain MRIs (individuals 10 and 11), and this implies that other mechanisms besides structural brain anomalies may be responsible for developmental delay. The variants in PPP1R12A from each of these individuals are noted in Table 1, and their positions and domains are annotated along the protein in Figure 1.
Table 1.
Summary of Neurologic and Urogenital Phenotypes
| - | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 15 years | 15 years | 5.5 years | 12 weeks gestation | 3 years | 6 years | 7 years | 45 years | 2 years | 2 years | 12 years | 3 years |
| Brain malformation | MIHV HPE | semilobar HPE | agenesis of the corpus callosum | acrania, anencephaly | head CT unremarkable | dysgenesis of the corpus callosum, absent septum pellucidum, Chiari malformation, cortical dysplasia/polymicrogyria, and grey matter heterotopia | leukomalacia | not evaluated | not evaluated | brain MRI unremarkable | brain MRI unremarkable | not evaluated |
| Genitourinary malformation | not evaluated | not evaluated | renal asymmetry | not described on autopsy | micropenis, chordee, scrotal hypospadias, bilateral cryptorchidism, and uterus | glandular hypospadias and chordee | hypospadias, cryptorchidism, uterus and ovaries | uterine didelphys and streak gonads | clitoral hypertrophy, UGS, posterior fusion of the labia majora | grade 2 hypospadias, cryptorchidism, removal of right inguinal hernia identified as a fallopian tube, and uterus | streak gonads with rudimentary fallopian tubes, and UGS | clitoris, posterior labial fusion, labial rugation and pigmentation, uterus, fallopian tubes and ovaries |
| Head and facial features | macrocephaly, hypertelorism | microcephaly, epicanthal folds, long philtrum | not described | hypertelorism, flattened facial profile, absent nasal bone | not described | short upslanting palpebral fissures, low-set ears, and micrognathia | long face, large protruding ears, ptosis, small pointed nose | not described | not described | not described | not described | deformed pinnae, epicanthus inversus |
| Other | Developmental delay | developmental delay, syndactyly | ADHD, kyphoscoliosis, stiff joints, decreased subcutaneous fat | omphalocele | absent | developmental delay, strabismus, astigmatism, hyperopia, and alternating esotropia | developmental delay, bilateral rod and cone dysfunction, decreased vision, and latent nystagmus | absent | absent | developmental delay | developmental delay | developmental delay, strabismus, right esotropia |
| Genotypic sex | 46,XX | 46,XX | 46,XY | 46,XX | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY | 46,XY |
| Phenotypic sex | female | female | male | female | male | male | male | female | female | male | female | female |
| Inheritance | de novo | unknown | de novo | de novo | de novo | de novo | unknown | unknown | de novo | de novo | de novo | de novo |
| Variant | c.2033_2034delCT (p.Ser678∗) | c.1415C>G (p.Ser472∗) | c.793-1G>A | c.223_224delAC (p.Thr75Cysfs∗8) | c.2739_2740delCT (p.Leu914Argfs∗14) | c.1510C>T (p.Arg504∗) | c.2573G>A (p.Trp858∗) | c.2073dupA (p.Ser692Ilefs∗2) | c.2698C>T (p.Arg900∗) | c.960dupA (p.Glu321Argfs∗6) | c.1189delA (p.Thr397Hisfs∗42) | c.681dupT (p.Lys228∗) |
Individuals 1–4 share the neurological phenotype, individuals 3 and 5–12 share diverse urogenital malformations, and individuals 3, 6, and 7 share an overlap of both. Notable discordance in genotypic and phenotypic sex is seen between the 46,XX and 46,XY individuals, but the significance requires further investigation. Transcript NM_002480.3 was used for all described variants in PPP1R12A. Abbreviations: MIHV—middle interhemispheric variant, HPE—holoprosencephaly, ADHD—attention deficit hyperactivity disorder, and UGS—urogenital sinus.
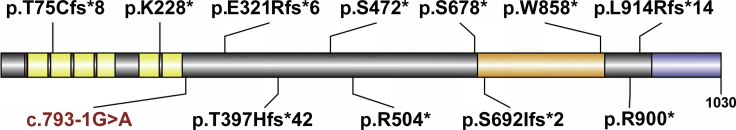
Figure 1.
PPP1R12A with Variant Annotations and Highlighted Regions
Per UniProt, domains include a Lysine-Valine-Lysine-Phenylalanine (KVKF) motif (red), multiple ankyrin repeats (yellow), rho-associated coiled-coil-containing protein kinase 1 (ROCK1) and rho-associated coiled-coil-containing protein kinase 2 (ROCK2) interaction site (orange), and a leucine zipper domain which binds a cGMP-dependent protein kinase 1. Stop gain and frameshift variants are notated in black with the splice-site variant in red at the approximate site predicted to result in a premature termination codon and nonsense-mediated decay. Diagram was created using Domain Graph version 2.0.15
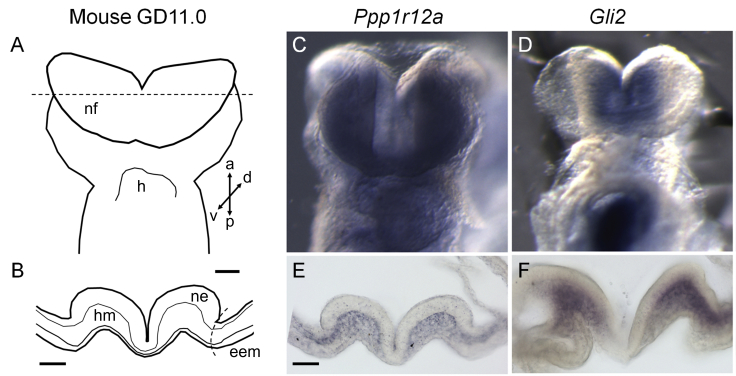
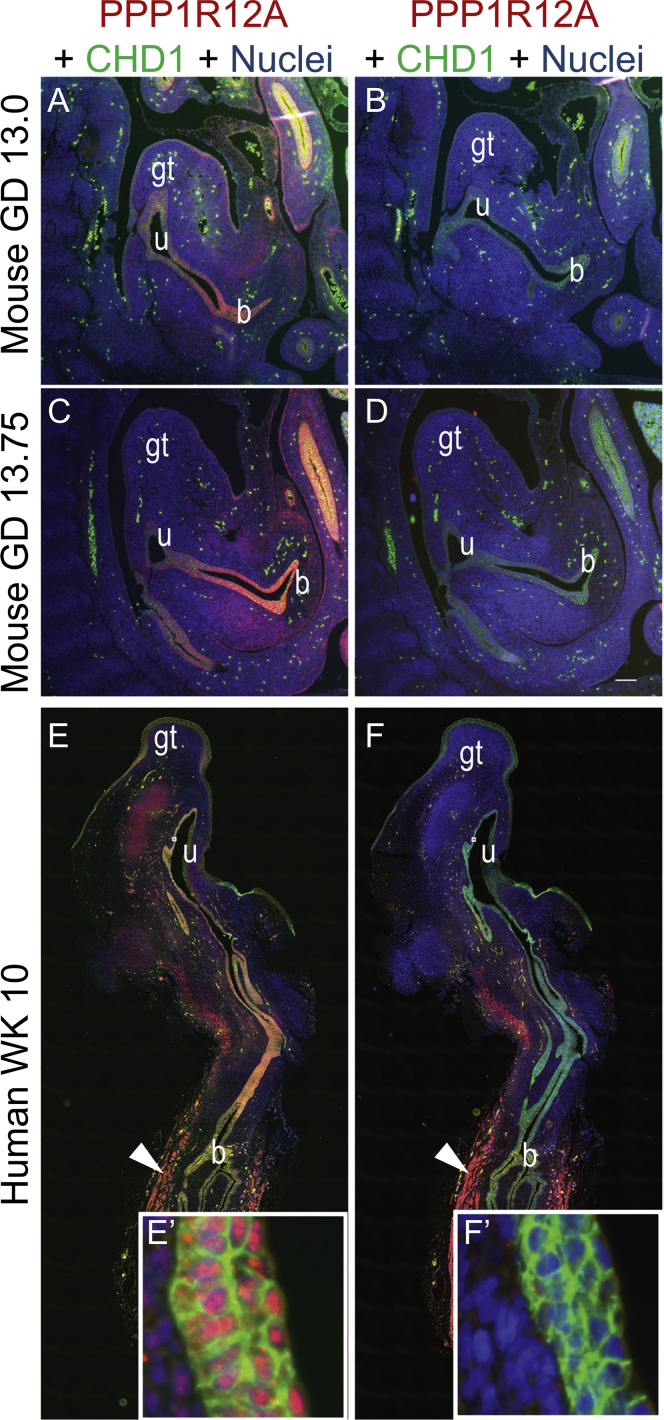
Brain in situ hybridization in mouse revealed Ppp1r12a expression in the prosencephalic neural folds of the mouse at gestational day (GD) 8.25. Staining of sections through the neural folds illustrated Ppp1r12a expression restricted to the mesenchymal compartment (Figure 2). Next, examination of Gli2 expression occurred on the same tissues as a positive control. Gli2 encodes the dominant Shh pathway transcriptional activator, and LoF variants in this gene cause HPE in both humans and mice.17, 18, 19, 20 Gli2 was expressed in the head mesenchyme adjacent to the prosencephalic neuroectoderm. These results demonstrate that Ppp1r12a is expressed in the prosencephalic neural folds during the critical period for HPE in a pattern consistent with known HPE-associated genes. Mouse urogenital immunostaining of PPP1R12A showed protein localization in the lower urinary tract, specifically in epithelium of the bladder, urethra, and genital tubercle epithelium at GDs 13 and 13.75 (Figures 3A and 3C). Immunohistochemical (IHC) staining was also conducted for PPP1R12B (MIM: 603768), which is an isoform of PPP1R12A. PPP1R12B localization was not seen in the urogenital tract at GD 13 (Figure 3B) or GD 13.75 (Figure 3D). In human embryos at week 10, IHC staining revealed PPP1R12A localization in the genital tubercle epithelium (ectoderm derived), the bladder and urethra (endoderm derived), urogenital sinus (UGS) mesenchymal cells, and bladder detrusor muscle (Figure 3E). PPP1R12B protein localization was restricted to the bladder detrusor smooth muscle (Figure 3F). These results show unique localization of PPP1R12A in the lower urinary tract during urethral development in advance of urethral plate closure.
Figure 2.
Brain: Mouse in situ Hybridization of the Prosencephalic Neural Folds
Gestational day 8.25 mouse embryos were stained via in situ hybridization in order to determine gene expression patterns. A ventral view is shown for whole mounts. Transverse sections through the prosencephalic neural folds (at the level of the dashed line in schematic) were stained in order to visualize gene expression in specific cellular compartments. Ppp1r12a localized to the head mesenchyme and is absent from extra-embryonic membranes. nf—neural folds, h—heart, ne—neuroectoderm, hm—head mesenchyme, eem—extra-embryonic membranes. Scale bar = 100 μm.
Figure 3.
Urogenital: Mouse and Human Immunostaining of the Genitourinary Tract
(A–B) Tissue sections from mouse genitourinary tracts at gestation day (GD) 13.
(C–D) Mouse genitourinary tracts at GD13.75.
(E–F) Human genitourinary tracts at week 10 were immunostained in order to detect protein localization patterns. PPP1R12A was detected in genital tubercle epithelium (ectoderm derived), bladder, and urethral epithelium (endoderm derived), and a subset or urogenital sinus mesenchymal cells (arrowhead) bladder detrusor smooth muscle. (E′) PPP1R12A localized to epithelial cell nuclei of human urethra (lower image, inset). (F′, arrowhead) PPP1R12B detected in developing human detrusor smooth muscle (B, D, F, F′) but not in developing mouse or human bladder or urogenital sinus epithelial cells. Abbreviations are B—bladder, GT—genital tubercle, U—urethra.
In summary, we present 12 individuals with LoF variants in PPP1R12A and multiple congenital anomalies. The two most common affected organ systems are the brain and the genitourinary tract. Five of the 12 individuals (∼40%) had midline brain anomalies found via MRI, and two individuals had HPE (individuals 1 and 2); the most severe brain finding was anencephaly (individual 4). Nine affected individuals (75%) had genitourinary malformations including three 46,XY individuals with female external genitalia. Only two individuals had both brain and genitourinary anomalies (individuals 6 and 7).
Among these individuals, there is a broad spectrum of manifestations, and a clear genotype-phenotype correlation was not seen associating with specific variants in PPP1R12A. The variants occurred across multiple exons (1, 5, 6, 9, 10, 11, 15, 18, and 21) as well as in intron 5. Two variants occurred in the ankyrin repeat domains, and two occurred in the rho-associated coiled-coil kinase binding domains without an observable pattern between the phenotypes. Premature termination codon (PTC) with subsequent nonsense-mediated decay (NMD) was predicted for the stop gain and frameshift variants because these followed the previously described criteria for this mechanism.20,21 In silico modeling of the splice-site variant predicted canonical splice acceptor site destruction in intron 5 with secondary PTC and NMD. Tissue-specific mRNA expression patterns were not available for individuals in this study, and review of a prior expression study on human fetal samples did not specifically include brain or urogenital tissue.22 As such, the value of comparing expression of PPP1R12A in adult tissues to expression during fetal development is limited because the impact leading to the observed phenotypes may be most influenced by these initial alterations. Review of DECIPHER revealed copy-number variants (CNV) of various sizes involving PPP1R12A. Neurologic and urogenital phenotypes were noted, but direct comparison of these CNVs remains limited due to the limited ability to quantify the haploinsufficiency from each of the other deleted genes. 12q21 deletion syndrome, which only has six reported cases, encompasses this gene and shares cryptorchidism, pylectasis/hydronephrosis, developmental delay, and various neurologic malformations including hypoplasia of the corpus callosum.23 Last, while none of these variants were present in gnomAD, of the 59.8 expected LoF variants, only three have been observed, and two of those have been flagged for further review on quality or annotation. This produces an observed over expected ratio of 0.05 and is within the range associated with genes intolerant to LoF. The remaining individual in gnomAD would need an evaluation because both the milder neurologic and urogenital phenotypic spectrum may not be apparent without further clinical examination.
The pathogenesis of brain malformations secondary to haploinsufficiency of PPP1R12A is incompletely understood. Experiments have shown that elimination of either MP subunit (PPP1R12A or PP1c) results in lost expression of the remaining subunit and is thought to contribute to decreased activity of the MP holoenzyme.24 We show here that Ppp1r12a is expressed in the neural folds of the embryonic mouse brain at the critical time for forebrain development (Figure 2). Forebrain division occurs in early embryogenesis. A complex signaling pattern, including sonic hedgehog, emanates from the prechordal plate (PrCP) beneath the telencephalon and directs median forebrain expansion and division shortly after gastrulation.25 Brain malformations including HPE, anencephaly, and agenesis of the corpus callosum were found in these individuals. HPE occurs in approximately one in 10,000 liveborns and one in 250 conceptuses. The clinical spectrum of HPE ranges from the most severe form with cyclopia and one cerebral ventricle (alobar HPE) to almost complete cerebral hemisphere division (lobar HPE). The etiology of HPE is heterogenous, and both genetic and environmental causes have been identified. However, most individuals with unremarkable karyotypes remain undiagnosed.26,27 Individuals 1 and 2 had HPE which precisely matched the cyclopia phenotype in zebrafish; the most severe finding was fetal acrania with exencephaly seen in individual 4. The pathways associated with HPE are perturbed in human and animal models of exencephaly; these pathways include Tgif (mouse model), Shh, and Gli2,28 and 13q deletions which include ZIC2 (humans). Additionally, in a series of 150 embryos with HPE, 14 were noted to have exencephaly and/or myeloschisis.29 Gathering more individuals with fetal acrania may provide another area to investigate for variants in PPP1R12A.
While these individuals provide initial evidence supporting the importance of PPP1R12A in development, more research will be needed in order to understand the precise mechanism that PPP1R12A causes in these malformations. Current evidence does not support the possibility that PPP1R12A is part of the canonical pathways of HPE such as the hedgehog signaling pathways; however, PPP1R12A has an established role in cell migration, cell adhesion, and cytoskeletal organization.30,31 Animal models, such as zebrafish morpholino knockdown of ppp1r12a (also known as mypt), resulted in defective PrCP anterior migration,12 and removal of the PrCP resulted in cyclopia.33, 34, 35 These findings support the association between PPP1R12A LoF variants and HPE. While the zebrafish provides a commonly used model for comparison to human neurodevelopment, due to its less-understood mechanisms, it does not model human sex differentiation and gonadal development.35 As seen with the zebrafish, we draw connections between the cell migration defects and midline brain malformations.
Other genetic conditions known to have both brain and urogenital malformations include Smith-Lemli-Opitz syndrome (MIM: 270400), X-linked lissencephaly (MIM: 300215), microcephaly, facial dysmorphism, renal agenesis, and ambiguous genitalia syndrome (MIM: 618142), pontocerebellar hypoplasia type 7 (MIM: 614969), orofaciodigital syndrome IV (MIM: 258860), and other conditions with complete gonadal dysgenesis and discordance between the phenotypic and genotypic sex. Syndromic and non-syndromic causes have also been reported, but the etiology in many affected individuals remains broad due to the phenotypic overlap between individuals with partial androgen insensitivity and those with partial gonadal dysgenesis.37, 38, 39 Individuals 5–12 had a wide spectrum of genitourinary phenotypes from partial gonadal dysgenesis with micropenis, hypospadias, and ambiguous genitals with Müllerian duct remnants to complete gonadal dysgenesis in a genotypic 46,XY individual with female external genitalia. Ppp1r12a has been noted to be increased in mouse striated and smooth muscle during sexual differentiation with higher levels in males than females.39 The high number of 46,XY individuals with urogenital anomalies (nine out of 12) in this report may either reflect ascertainment bias or a probable sex-influenced mechanism, in contrast to the remaining three 46,XX individuals with severe brain anomalies (HPE and anencephaly).
Müllerian ducts are formed via several steps including specification, invagination through apical constriction, and elongation. Multiple signaling systems are involved in the process of Müllerian duct formation; these include RhoA GTPases, molecules known to modulate the processes of many epithelial tissue invaginations and morphogenesis through the indirect increase of nonmuscle myosin II activity. Given the clinical findings, we propose that alterations to this pathway could change the development of these ducts and subsequently lead to defective regression of the Müllerian ducts, in the presence of sex-determining region Y (SRY), and ultimately result in a DSD. Sexual differentiation occurs in the undifferentiated zygote through complex interactions between genetic and developmental processes. During this process, phenotypical sexual differences are evolved through the presence or absence of SRY and through impairment of the cascades of developmental events downstream. However, the developmental processes that cause DSD remain unknown, and in many instances, individuals do not receive a molecular diagnosis. Recently, the Rho-kinase pathway was found to be a major regulator of the male urogenital function and disorders.40 Notably, PPP1R12A, which is downstream of this system, is highly localized in the developing reproductive system39 and remains highly expressed in human adult uterus and vagina (GTEx). While the specific role of PPP1R12A in external and internal genitalia development has not been previously described, these individuals provide a starting point for further research regarding the role of this gene in DSD.
In summary, these 12 individuals illustrate the association of PPP1R12A with HPE spectrum phenotypes and urogenital malformations including DSD. In situ mouse hybridization studies of Ppp1r12a demonstrate expression precisely at the proper time and location for brain development implicated in HPE, and our immunostaining of PPP1R12A in the mouse embryo and human tissue reveals protein localization patterns in the developing lower urinary tract epithelium, which is responsible for bladder, urethral, and genital tubercle formation.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We thank the families for their participation in this publication. This work was supported by the National Human Genome Research Institute Intramural Research program. Work in the R.J.L. lab was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (NIH) under award numbers R01ES026819 and T32ES007015. Work in the CMV lab was supported by NIH grant U01DK110807. E.D., E.V., M.R., and P.F. are supported in part by the Disorder of Sex Development Translational Research Network (R01 HD093450). The DSD whole-genome sequencing project was funded by the Gabriella Miller Kids First Initiative (XO1HL132384, E.V.). We are grateful for the help of Linda Ramsdell, MS, CGC (Seattle Children’s Hospital) and Kimberly Kennedy, RN (Cincinnati Children’s Hospital) for individuals 11 and 12. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the NIH, and by the National Cancer Institute (NCI), the National Human Genome Research Institute (NHGRI), the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 10/10/19.
Published: December 26, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.12.004.
Accession Numbers
The accession number for the sequence reported in this paper is ClinVar: VCV000450254.
Web Resources
DECIPHER, https://decipher.sanger.ac.uk/
OMIM, https://omim.org/
UniProt, https://uniprot.org/
Supplemental Data
References
- 1.Grassie M.E., Moffat L.D., Walsh M.P., MacDonald J.A. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 2011;510:147–159. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Kiss A., Erdődi F., Lontay B. Myosin phosphatase: Unexpected functions of a long-known enzyme. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:2–15. doi: 10.1016/j.bbamcr.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Ito M., Nakano T., Erdodi F., Hartshorne D.J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 4.Shichi D., Arimura T., Ishikawa T., Kimura A. Heart-specific small subunit of myosin light chain phosphatase activates rho-associated kinase and regulates phosphorylation of myosin phosphatase target subunit 1. J. Biol. Chem. 2010;285:33680–33690. doi: 10.1074/jbc.M110.122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu H., Ito M., Miyahara M., Ichikawa K., Okubo S., Konishi T., Naka M., Tanaka T., Hirano K., Hartshorne D.J. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J. Biol. Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- 6.Huang H., Ruan H., Aw M.Y., Hussain A., Guo L., Gao C., Qian F., Leung T., Song H., Kimelman D. Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development. 2008;135:3209–3218. doi: 10.1242/dev.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi D., MacDougall L.K., Sola M.M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan C., Stronach B., Perrimon N. Roles of myosin phosphatase during Drosophila development. Development. 2003;130:671–681. doi: 10.1242/dev.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno T., Tsutsui K., Nishida Y. Drosophila myosin phosphatase and its role in dorsal closure. Development. 2002;129:1215–1223. doi: 10.1242/dev.129.5.1215. [DOI] [PubMed] [Google Scholar]
- 10.Wissmann A., Ingles J., Mains P.E. The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev. Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto R., Ito M., Suzuki N., Kongo M., Moriki N., Saito H., Tsumura H., Imanaka-Yoshida K., Kimura K., Mizoguchi A. The targeted disruption of the MYPT1 gene results in embryonic lethality. Transgenic Res. 2005;14:337–340. doi: 10.1007/s11248-005-3453-3. [DOI] [PubMed] [Google Scholar]
- 12.Weiser D.C., Row R.H., Kimelman D. Rho-regulated myosin phosphatase establishes the level of protrusive activity required for cell movements during zebrafish gastrulation. Development. 2009;136:2375–2384. doi: 10.1242/dev.034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruszka P., Berger S.I., Casa V., Dekker M.R., Gaesser J., Weiss K., Martinez A.F., Murdock D.R., Louie R.J., Prijoles E.J. Cohesin complex-associated holoprosencephaly. Brain. 2019;142:2631–2643. doi: 10.1093/brain/awz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren J., Wen L., Gao X., Jin C., Xue Y., Yao X. DOG 1.0: illustrator of protein domain structures. Cell Res. 2009;19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- 16.Chiang C., Litingtung Y., Lee E., Young K.E., Corden J.L., Westphal H., Beachy P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 17.Hong S., Hu P., Marino J., Hufnagel S.B., Hopkin R.J., Toromanović A., Richieri-Costa A., Ribeiro-Bicudo L.A., Kruszka P., Roessler E., Muenke M. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum. Mol. Genet. 2016;25:1912–1922. doi: 10.1093/hmg/ddw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon B.D., Bear K.A., Wyllie A., Keaton A.A., Dubourg C., David V., Mercier S., Odent S., Hehr U., Paulussen A. Genotypic and phenotypic analysis of 396 individuals with mutations in Sonic Hedgehog. J. Med. Genet. 2012;49:473–479. doi: 10.1136/jmedgenet-2012-101008. [DOI] [PubMed] [Google Scholar]
- 19.Heyne G.W., Everson J.L., Ansen-Wilson L.J., Melberg C.G., Fink D.M., Parins K.F., Doroodchi P., Ulschmid C.M., Lipinski R.J. Gli2 gene-environment interactions contribute to the etiological complexity of holoprosencephaly: evidence from a mouse model. Dis. Model. Mech. 2016;9:1307–1315. doi: 10.1242/dmm.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis B.P., Green R.E., Brenner S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y.F., Imam J.S., Wilkinson M.F. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- 22.Szabo L., Morey R., Palpant N.J., Wang P.L., Afari N., Jiang C., Parast M.M., Murry C.E., Laurent L.C., Salzman J. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015;16:126. doi: 10.1186/s13059-015-0690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna C.S., Saxena N., Dabir T.A., Jones J., Smith G., Morrison P.J. Phenotypic delineation of a 12q21 deletion syndrome. Clin. Dysmorphol. 2019;28:198–201. doi: 10.1097/MCD.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 24.Scotto-Lavino E., Garcia-Diaz M., Du G., Frohman M.A. Basis for the isoform-specific interaction of myosin phosphatase subunits protein phosphatase 1c beta and myosin phosphatase targeting subunit 1. J. Biol. Chem. 2010;285:6419–6424. doi: 10.1074/jbc.M109.074773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roessler E., Muenke M. The molecular genetics of holoprosencephaly. Am. J. Med. Genet. C. Semin. Med. Genet. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruszka P., Martinez A.F., Muenke M. Molecular testing in holoprosencephaly. Am. J. Med. Genet. C. Semin. Med. Genet. 2018;178:187–193. doi: 10.1002/ajmg.c.31617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruszka P., Muenke M. Syndromes associated with holoprosencephaly. Am. J. Med. Genet. C. Semin. Med. Genet. 2018;178:229–237. doi: 10.1002/ajmg.c.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kietzman H.W., Everson J.L., Sulik K.K., Lipinski R.J. The teratogenic effects of prenatal ethanol exposure are exacerbated by Sonic Hedgehog or GLI2 haploinsufficiency in the mouse. PLoS ONE. 2014;9:e89448. doi: 10.1371/journal.pone.0089448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen M.M., Jr. Holoprosencephaly: clinical, anatomic, and molecular dimensions. Birth Defects Res. A Clin. Mol. Teratol. 2006;76:658–673. doi: 10.1002/bdra.20295. [DOI] [PubMed] [Google Scholar]
- 30.Xia D., Stull J.T., Kamm K.E. Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly. Exp. Cell Res. 2005;304:506–517. doi: 10.1016/j.yexcr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Zagórska A., Deak M., Campbell D.G., Banerjee S., Hirano M., Aizawa S., Prescott A.R., Alessi D.R. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci. Signal. 2010;3:ra25. doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]
- 32.Feldman B., Gates M.A., Egan E.S., Dougan S.T., Rennebeck G., Sirotkin H.I., Schier A.F., Talbot W.S. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 33.Schier A.F. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 34.Shih J., Fraser S.E. Characterizing the zebrafish organizer: microsurgical analysis at the early-shield stage. Development. 1996;122:1313–1322. doi: 10.1242/dev.122.4.1313. [DOI] [PubMed] [Google Scholar]
- 35.Santos D., Luzio A., Coimbra A.M. Zebrafish sex differentiation and gonad development: A review on the impact of environmental factors. Aquat. Toxicol. 2017;191:141–163. doi: 10.1016/j.aquatox.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Délot E.C., Papp J.C., Sandberg D.E., Vilain E., DSD-TRN Genetics Workgroup Genetics of Disorders of Sex Development: The DSD-TRN Experience. Endocrinol. Metab. Clin. North Am. 2017;46:519–537. doi: 10.1016/j.ecl.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parivesh A., Barseghyan H., Délot E., Vilain E. Translating genomics to the clinical diagnosis of disorders/differences of sex development. Curr. Top. Dev. Biol. 2019;134:317–375. doi: 10.1016/bs.ctdb.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Délot E.C., Vilain E.J. Nonsyndromic 46,XX Testicular Disorders of Sex Development. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; Seattle: 2003 Oct 30. pp. 1993–2019. [Internet] [PubMed] [Google Scholar]
- 39.Lontay B., Bodoor K., Weitzel D.H., Loiselle D., Fortner C., Lengyel S., Zheng D., Devente J., Hickner R., Haystead T.A. Smoothelin-like 1 protein regulates myosin phosphatase-targeting subunit 1 expression during sexual development and pregnancy. J. Biol. Chem. 2010;285:29357–29366. doi: 10.1074/jbc.M110.143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gur S., Kadowitz P.J., Hellstrom W.J. RhoA/Rho-kinase as a therapeutic target for the male urogenital tract. J. Sex. Med. 2011;8:675–687. doi: 10.1111/j.1743-6109.2010.02084.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.