Abstract
Diabetes mellitus is a metabolic disorder that majorly affects the endocrine gland, and it is symbolized by hyperglycemia and glucose intolerance owing to deficient insulin secretory responses and beta cell dysfunction. This ailment affects as many as 451 million people worldwide, and it is also one of the leading causes of death. In spite of the immense advances made in the development of orthodox antidiabetic drugs, these drugs are often considered not successful for the management and treatment of T2DM due to the myriad side effects associated with them. Thus, the exploration of medicinal herbs and natural products as therapeutic sources for the treatment of T2DM is promoted because they have little or no side effects. Bioactive molecules isolated from natural sources have been proven to lower blood glucose levels via regulating one or more of the following mechanisms: improvement of beta cell function, insulin resistance, glucose (re)absorption, and glucagon-like peptide-1 homeostasis. In recent times, the mechanisms of action of different bioactive molecules with antidiabetic properties and phytochemistry are gaining a lot of attention in the area of drug discovery. This review article presents an update of the findings from clinical research into medicinal plant therapy for T2DM.
1. Introduction
Diabetes mellitus is a metabolic disorder depicted by hyperglycemia (elevated levels of blood glucose) and glucose intolerance, which brings about defects of insulin secretion or insulin's action to boost glucose uptake. This disorder causes a burden worldwide because of its high rate of morbidity, mortality, and higher health costs for management and treatment. According to the International Diabetes Federation report of 2017, 451 million adults worldwide are living with diabetes, with a predicted 693 million cases by 2045 [1]. On a global level, this disorder is prevalent more in the low-income and middle-income countries with almost 50% of the cases undiagnosed. In Africa, there is a high incidence of undiagnosed diabetes cases (69.2%) with 73.7% of all deaths due to diabetes occurring before the age of 60 [1, 2], thus showing the extent to which diabetes is destroying its workforce population. In Africa and other continents of the world, type 2 diabetes accounts for over 90-95% of diabetes cases [3]. The prevalence of diabetes is rapidly increasing in South Africa with approximately 1.8 million adults suffering from diabetes mellitus (DM), while an additional 1.5 million adults remain undiagnosed [4, 5].
The economic burden of diabetes in the Republic of South Africa per person per annum was approximately R 5000 in 2010 and R 26,743.69 in 2015 [6]. This statistic only showed the cost effect of treating diabetes without addressing the cost of loss of manpower, since 60-80% of those suffering from this ailment belong to the working class and they die before the age of 60 [6]. According to the World Bank, not more than 5% of a country's gross domestic profit (GDP) should be spent on health; however, in South Africa, 8.9% of GDP is spent on health-related matters [7].
Type 2 diabetes mellitus (T2DM) is ranked among one of the most challenging global epidemics because it affects both human health and economies. The number of people plagued with T2DM worldwide in the past 20 years has more than doubled [8].
T2DM is a chronic disease caused by the complex interactions of genetic and environmental factors (dietary and lifestyle factors) [9]. The roles of both our genetic makeup and the environment are contributing factors to insulin resistance and β-cell dysfunction [9]. In recent times, there have been arguments saying that changes in the gene makeup cannot be the main cause for the upsurge in the prevalence of T2DM but that changes in dietary and lifestyle patterns are fundamental to grasping this epidemic [10].
The management and treatment of T2DM inflict both direct and indirect costs on the subject most especially when it is linked with other comorbidities like stroke and cancer. The global community is seriously searching for a drug which is cheap and together potent against T2DM so as to cut down the number of death cases annually [11]. Furthermore, the numerous antidiabetic therapies employed by the use of conventional drugs are laborious in the sense that most of these drugs are not a single-dose program and are most of the times taken by patients for their entire life. Also, it has been reported that adverse side effects such as diarrhoea, abdominal distention, and flatulence emanate from the intake of these drugs. Thus, these limitations have prompted the exploration of management strategies in the form of medicinal plants with antidiabetic potentials which are cost effective and have fewer side effects.
At the moment, there are a number of scientific reports on the different biological activities of phytochemicals against type 2 diabetes and diabetes. However, what is lacking is a comprehensive review that gathers experimental evidence and judiciously assesses their achievement as this would provide future research direction in the area of oxidative stress-mediated diabetes related to phytochemicals in type 2 diabetes treatment. Therefore, this review investigates the link between oxidative stress and type 2 diabetes at both the cellular and molecular levels with the aim of putting forth experimental findings on the potential of phytochemicals in type 2 diabetes treatment.
2. Oxidative Stress and Diabetes
Oxidative stress describes a physiological state in which the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) attains disproportionate levels, either by excess production or reduced removal due to the overwhelming antioxidant capacity of the system [12, 13]. These highly reactive molecules are products of normal cellular metabolism, and they play crucial roles in most signalling pathways. The mitochondrion is the site where most of these highly reactive species are generated. During ATP formation in the mitochondria, electron transport and oxidative phosphorylation take place. These electrons react with oxygen (O2), thus forming superoxide anions (•O2−) which in turn reacts with molecules like Fe2+ and generates other reactive species (RS) such as the hydroxyl radical (•OH), hydrogen peroxide (H2O2), and organic peroxides [14].
Also, the production of these highly reactive molecules can be initiated in response to both extracellular and intracellular stimuli. Extracellular stimuli on plasma membrane receptors generate RS through tumor necrosis factor- (TNF-) α, hormones (insulin), and growth factors (platelet-derived growth factor (PDGF) and epithelial growth factor (EGF)). Intracellular stimuli that generate reactive species (RS) are induced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [15], nitric oxide synthase (NOS) [12, 16], and mitochondrial electron transfer [17]. In addition, RS can also be generated via some enzymatic systems such as monoamine oxidase, lipoxygenase, xanthine oxidase, and glucose oxidase [15]. All of these are the major sources of reactive species (ROS and RNS), and upon their overwhelming the body system they react by modifying and damaging cellular macromolecules such as nucleic acids, proteins, lipids, and carbohydrates to generate reversible or irreversible oxidative modifications. They also have the ability to trigger a number signalling cascades linked with decoding stress, such as the mitogen-activated protein (MAP) kinase family and c-Jun N-terminal kinase (JNK) [18]. ROS has the ability to react with motifs of certain metal ligands such as metalloproteases and the iron in oxyhemoglobin. The superoxide radical (•O2−) possesses the ability to modify and inhibit catalase, while the hydroxyl radical (•OH), a major product of a Fenton reaction, is released during prolonged exercise and disease condition such as diabetes [19, 20].
Recent findings have revealed that ROS, most especially hydroxyl and superoxide radicals, react with certain amino acids (such as cysteine, histidine, tryptophan, methionine, and tyrosine), proteins, and simple peptides, thus making them susceptible to altered function and damage, and thus modifying their structure [20–22]. The effect of ROS and RNS on fatty acids, lipoproteins, and phospholipids induces a process called lipid peroxidation, and its resultant effect is the formation of intermediates/products such as 4-hydroxynonenal, hydroperoxide lipid, and malondialdehyde. These products cause alterations and damages to the plasma membrane, and they also have the ability to diffuse to other cells within the organism, thus causing inflammation through the binding of the oxidized low-density lipoprotein receptor and also triggering apoptosis [20, 23]. According to Tsai et al. [24] and Kawamura et al. [25], elevated blood sugar levels enhance the production of ROS during lipid degradation of low-density lipoprotein (LDL).
Hydrogen peroxide at different levels in the cell can either act as a signalling molecule that enhances cellular proliferation or prompt cell death. At low/mild concentrations, H2O2 acts as a second messenger for the triggering of NF-κB and various kinases (p38 MAPK, ERK, PI3K, Akt, JAK2, and STAT), while its presence at a little higher concentration in the cell alters mitochondrial membrane integrity, thus bringing about the loss of the mitochondrial membrane potential and the release of cytochrome c and other proapoptotic proteins such as apoptosis-inducing factor (AIF) [26, 27]. Upon the liberation of cytochrome c, it triggers the activation of the intrinsic caspase-dependent apoptotic pathway [28].
Oxidative stress has been attributed to be one of the major determinants for the development of diabetes [29, 30]. The overwhelming of the antioxidant system by oxidants promotes the pathogenesis of diabetes and that is why we have more oxidative cells in diabetic subjects than in healthy subjects, i.e., a higher level of ROS production [31, 32]. Also, several reports have shown that there is a close association between oxidative stress and DM due to increased oxidative damage to vital macromolecules. According to reports of Grimsrud et al. [33] and Muellenbach et al. [34], there is an increased level of protein carbonylation and nitrosylation in insulin-sensitive tissues and in the type 2 diabetes mellitus (T2DM) state. Also, research findings have shown a strong association between increased oxidative stress and protein unfolding which causes the loss of protein function in a number of animal models [35, 36]. In diabetic patients, oxidative stress causes the alteration of two major mechanisms which are insulin resistance and insulin secretion. Oxidative stress causes the adipocytokine dysregulation and inhibition of insulin signals, thus bringing about insulin resistance. There are also increased levels of malondialdehyde (MDA), protein carbonyls, protein oxidation products, 4-hydroxy-2-nonenal, glycation end products, isoprostanes, carbohydrate modifications, and 8-hydroxy-2′-deoxyguanosine (8-OH-dG), which are biomarkers of oxidative stress in diabetic subjects [37–39]. Furthermore, the upsurge production of ROS in T2DM subjects has been shown to trigger harmful pathways such as glucosamine pathways, advanced glycation end products (AGEs), and PKCβ1/2 kinase [40].
In addition, high levels of leptin, free fatty acids (FFA), and nonesterified FFAs promote excessive production of ROS in T2DM subjects. These unnecessary FFAs go into the tricarboxylic acid cycle to produce acetyl-CoA and loads of NADH, which causes the overproduction of mitochondrial superoxide.
3. The Signalling Pathways Involved in Glucose Metabolism Disorder in Diabetes
Elevated blood sugar levels have been implicated in the induction of oxidative stress via a number of mechanisms, viz., autoxidation of glucose, AGE formation, polyol pathway, and PKCβ1/2 kinase [41]. Elevated free fatty acids, leptin, and other circulating factors in T2DM patients may also contribute to causing ROS overproduction [42].
In recent years, clinical and epidemiological studies in diabetes research have confirmed that hyperglycemia and lipid metabolism abnormalities have grave influence in the onset of both micro- and macrovascular diseases. To this end, four key hypotheses have been put up through clinical trials to see specific inhibitors of hyperglycaemia causing T2DM (Figure 1). These four key hypotheses are activation of protein kinase C (PKC) isoforms, increased advanced glycation end product (AGE) formation, and increased hexosamine biosynthetic pathway flux and increased poly(ADP-ribose) pathway flux (PARP).
Figure 1.
Multiple signalling pathways underlying hyperglycemic cellular damage in type 2 diabetes mellitus.
3.1. Activation of Protein Kinase C (PKC) and Diacylglycerol Formation
The protein kinase C (PKC) family consists of not less than eleven isoforms of serine-threonine kinases, which contribute to the regulation of endothelial cell permeability, stimulating cell proliferation and vascular growth [43]. According to the reports of Aiello et al. [44] and Geraldes and King [45], PKCβ has been described to be a potential target for the improvement of diabetic complication. It was revealed that its activation is enhanced by increased glucose levels in diabetic animals and vascular cells [44, 45]. In recent times, high glucose levels induce the activation of PKC and the increase in the levels of diacylglycerol (DAG) in a number of tissues (retina, aorta, heart, and renal glomeruli) are involved in diabetic vascular complications using diabetic animal models and patients [46–48]. Also, a large amount of clinical and animal experimental models implicated elevated glucose levels to be the direct activator of the polyol pathway, and it is also linked with the excessive generation of reactive oxygen species (ROS) by the activity of mitochondria, PKC, and NADPH oxidase [49, 50]. Furthermore, prolonged activation of PKC has been linked to influencing the activation of a number of growth factors, i.e., platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), and vascular endothelial growth factor (VEGF) in both cultured mesangial cells and glomeruli of diabetic rats [51, 52].
3.2. Increased Intracellular Formation of Advanced Glycation End Products
According to Degenhardt et al. [53], intracellular hyperglycaemia is a fundamental event in the formation of both intracellular and extracellular AGEs. Advanced glycation end products (AGEs) refer to a group of heterogeneous compounds that can arise from the intracellular autooxidation of glucose to glyoxal, the breakdown of the Amadori product (glucose-derived 1-amino-1-deoxyfructose lysine adducts) to 3-deoxyglucosone, and also the nonenzymatic removal of phosphate from glyceraldehyde phosphate and dihydroxyacetone phosphate to yield methylglyoxal [43, 53]. These reactive products (3-deoxyglucosone, glyoxal, and methylglyoxal) react with free amines of proteins and lipids and speed up development and accumulation of AGEs in the body [54, 55]. According to Piperi et al. [56], excessive production of AGEs inflicts greater injury to pancreatic beta cells than through hyperglycemia. In addition, hyperglycaemia has a direct effect on proteins of the electron transport chain by way of promoting the generation of ROS which in turn induces the fierce formation of AGEs [57, 58]. It is well known that the accumulation of AGEs is associated with the development of insulin resistance and also in the pathogenesis of diabetic complications [55, 59, 60].
According to Qiu et al. [61], extracellular AGEs aid the binding and activation of signal transduction receptor RAGE (receptor of AGE). Furthermore, the intracellular production of the AGE precursor causes damage to cells via three mechanisms: (i) modification of intracellular protein by AGEs, thereby causing the loss of function of cells; (ii) activation of the RAGE signalling axis, which results in cell apoptosis, proliferation, migration, and dysfunction; and (iii) plasma protein modification, which causes the binding of AGE precursors to AGE receptors (i.e., RAGE and AGE-R1, 2, and 3) on cells such as vascular smooth muscles and macrophages. The binding of AGE precursors to their respective receptors has been linked with a number of signalling pathway such as p21ras/ERK1/2MAPK, JAK/STAT, NADPH oxidase/ROS, and nuclear factor kappa B (NF-κB) activation, therefore resulting in complications such as diabetes, cancer, aging, and neurological diseases [62].
3.3. Increased Polyol Pathway Flux
The reduction of a wide variety of carbonyl compounds to their respective alcohols is stimulated by a family of aldose reductase enzymes [43, 63, 64]. The poly(ADP-ribose) pathway (PARP) involves the breakdown of tissues and cells; it also consists of key enzymes such as aldose reductase (AR) and sorbitol dehydrogenase (SDH) [64–66]. During this metabolic process, glucose is reduced to its preferred corresponding alcohol-sorbitol by the action of AR instead of being phosphorylated as 6-glucose phosphate [67, 68]. These reactions make use of nicotinic acid adenine dinucleotide phosphate (NADPH). The enzyme aldo-keto reductase (AR) determines the overall rate of the polyol pathway, and it also has a low affinity (Km > 100 mM) for glucose while in nondiabetic subjects, wherein the glucose concentration is normal. During the metabolism of glucose by the polyol pathway, a very minute percentage of total glucose is used [67, 69]. In a hyperglycemic state, AR activation is achieved by increased intracellular glucose. As a result of this reaction, resilient polar sorbitol is produced which struggles to seep into the cell membranes, thus bringing about osmotic cell swelling, impairment of cellular structure and function, a decrease of ATPase activity, and ultimately setting in motion cell metabolism and functional damage [43]. The oxidation of sorbitol to fructose by the action of sorbitol dehydrogenase causes PKC activation by way of an increased NADH/NAD+ ratio [70]. It is noteworthy that ROS is not generated in a direct way in this mechanism but it is associated with redox imbalance that brings about the onset of oxidative stress [71–73]. Recent findings have implicated PARP to be strongly associated with a myriad pathogenesis of diabetic complications, e.g., AGEs, PKC, and oxidative stress. In addition, it has been revealed to stimulate cardiac damage via its activation of NF-κB (nuclear factor κB) and also inducing the overexpression of vasoconstrictor endothelin-1 (ET-1) [49, 74, 75]. Furthermore, attention has shifted to PARP as one of the intense subjects in the aetiology of diabetic complications [73, 74].
3.4. Increased Flux through the Hexosamine Biosynthetic Pathway
Abnormally high blood sugar levels and insulin resistance-induced fatty oxidation plays a key role in the onset and advancement of diabetic complications via increasing the flux of fructose-6-phosphate into the hexosamine biosynthetic pathway [76, 77]. This abnormal blood glucose level triggers the premature activation of some metabolic pathways, which in turn causes the usual expression of certain cytokines such as CTGF, ICAM-1, PAI-1, TGF-β, TNF-α, and VCAM-1, which are involved in the development of lesion [78, 79]. Upon the absorption of glucose by cells, a majority are digested and shoved via glycogen synthesis, metabolism of the pentose phosphate, and glycolysis; furthermore, approximately 1-3% of glucose also go into the hexosamine biosynthetic pathway [79, 80]. According to Qin et al. [81], the excessive shunting of intracellular glucose via the hexosamine biosynthetic pathway has been implicated in a myriad of diabetic complications. Furthermore, the hexosamine biosynthetic pathway allows fructose 6-phosphate from glycolysis to be used as substrates for reactions that require of UDP-N-acetylglucosamine such as in the case of the formation of O-linked glycoproteins and also the synthesis of proteoglycans. Another thing peculiar to this pathway is that 6-phosphate monophosphate transaminase catalyzes its first step of reaction, and it is also the rate-limiting enzyme of the pathways [82, 83]. The ability to inhibit glutamine : fructose-6-phosphate amidotransferase (rate-limiting enzyme) which converts glucose to glucosamine helps blocks hyperglycaemia-induced increases in the transcription of TGF-α, TGF-β1, and PAI-1 [76, 84–86]. Lastly, the activation of the hexosamine biosynthetic pathway via hyperglycaemia could bring about the overexpression of a number of cytokines such as TGF-α, TGF-β, VEGF, and PDGF in non-insulin-sensitive tissues and also lead to the onset of diabetic complications [62].
Some other molecular mechanisms have also been implicated in the generation of free radicals during hyperglycemia in both in vitro and in vivo models. Such mechanisms include the mitochondrial mechanism, dysfunction of cellular antioxidative defense system (ADS), glucose autoxidation, lipid peroxidation, and activation of free-radical generator enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, xanithine oxidase, cytochrome P450 (CYP450), myeloperoxidase, and uncoupled endothelial nitric oxide synthase (eNOS). These mechanisms are summarized in Figures 2 and 3.
Figure 2.
Some summarized pathways with increasing reactive oxygen species in a hyperglycemia state.
Figure 3.
Enzymatic reactions that generate ROS in diabetic state.
3.5. Mitochondrial Mechanisms
The mitochondrial respiratory chain (MRC) is constituted of five multimeric enzyme complexes (I-V). The MRC is an established site for the production of free radicals throughout hyperglycemia. Research findings have highlighted the originators of mitochondria free radical formation. These include accelerated electron disposition into the electron transport chain of the mitochondria through influx electron donation aided by complexes I, III, and IV; escape of electrons; repression of the function of the mitochondria antioxidative defense system (ADS); and alteration of mitochondrial DNA [87]. The chief role of the mitochondria in the cell is energy generation (ATP) through oxidative phosphorylation. This process involves two main stages: (a) oxidation of NADH/FADH2 which aids in the supply of electrons to METC and (b) phosphorylation of ADP to ATP [88]. In a hyperglycemic condition, the glycolytic and tricarboxylic acid pathways cause elevated levels of NADH and FADH2 [76, 88], thus promoting the accumulation of electrons in complex I and ultimately aiding in the excessive production of superoxide anion (O2−) [89–91].
The escape of electrons from the mitochondria brings about free radical production via the disruption in electron transfer induced by leakages in electrons at complexes I and III and also aids the breakdown of O2 to •O2−. When mitochondrial ADS levels are lessened, they boost free radical production. The presence of manganese-dependent superoxide dismutase (MnSOD) in the mitochondria transforms O2− to H2O2 and O2. Research findings have implicated the hyperglycemic state to be one of the reasons why there is a diminished level in mitochondria ADS expression in addition to a weakened buffering potential [87, 88, 92]. Mutation in mDNA influenced by hyperglycemia promotes the decline in the level of MnSOD, peroxiredoxins (PRX), thioredoxin (TRX), and 8-hydroxydeoxyguanosine [93].
3.6. Dysfunction of Cellular Antioxidative Defense System
Literature is replete with information that hyperglycemia gives rise to a defect in the antioxidative defense system (ADS) [94, 95]. In a diabetic state, there are a number of decreases in some enzyme activity levels. For instance, in the brain, there is a drastic decrease in the activities of SOD, CAT, and GPx. In addition, lower levels of erythrocytes, hepatic cells, lymphocytes, and vascular endothelial cells are predominant in diabetic subjects. All these diminished levels are the resultant effects of an upsurge in the activity of free radicals [87, 96–98]. Several mechanisms have been highlighted to be the likely causes of ADS-induced diabetes. The first of such mechanisms is via insulin, which is thought to be a strong catalyst in the expression of the antioxidative enzyme system [94, 99]. Its absence/shortage could trigger an aberration in ABS expression. Antioxidative enzyme glycation and inactivation is another mode by which ADS is altered in diabetes [87, 94]. According to Kakkar et al. [100], a deficiency in insulin promotes the activation of fatty acyl-coenzyme A oxidase which results in excessive generation of H2O2. Another mode by which ADS is altered in diabetes is through several possible mechanisms that may be responsible for the effect of diabetes on the ADS. Simonyan et al. in 1987 proposed another mechanism that involves the depletion in gene expression levels of CAT and SOD by a reactive species in the event of hyperglycemia [101]. This process is aided by DNA degradation and disturbance in tRNA. Sindhu et al. [97] corroborate the findings of Simonyan et al. [101] that elevated levels of H2O2 chiefly affects DNA degradation. Finally, it has been documented that the alteration in glutathione metabolism and the decline in the activity of glutathione reductase are linked with a hyperglycemic condition.
3.7. Glucose Autoxidation
According to Wolff and Dean [102] and Yaribeygi et al. [87], during hyperglycemia, autoxidation of glucose takes place, and this gives rise to the generation of harmful reactive species and ketoaldehyde compounds. The production of H2O2 and malondialdehyde is linked to glucose autoxidation-induced hyperglycemia. Also, glucose autoxidation has been suggested to be the main channel for the release of reactive species in a chronic hyperglycemic state [87, 103].
3.8. Lipid Peroxidation
There is accumulation of harmful end products (aldehydes, alkanes, carboxylic acids, ketones, and polymerization products) during fat peroxidation in the cell membrane, and this is catalyzed by an upsurge in free radicals [87]. These harmful products elicit their deleterious effects on neighboring cells [104]. In a diabetic state, there is an increased fat peroxidation, thus promoting the production of free radicals and oxidative stress [87, 105].
3.9. Activation of Free-Radical Generator Enzymes
The diabetes-induced free radicals that result from enzymatic reactions and activation of the seven most important oxidative enzymes like cyclooxygenases (COX), cytochrome P450 (CYP450), lipoxygenase (LOX), myeloperoxidase (MPO), NADPH oxidase (NOX), uncoupled endothelial nitric oxide synthase (eNOS), and xanthine oxidase (XOX) contribute as much as those from the mitochondria as shown in Figure 3 [87, 88].
3.9.1. Cyclooxygenase
Several studies revealed that prolonged low-grade inflammation is attendant with type 2 diabetes (T2D), and a well-defined connection with COX-mediated inflammation has been ascertained [106–108]. In the past, it was thought that COX existed in only two isoforms, i.e., COX-1 and 2 [109–111], but lately, there have been findings that corroborate the presence of COX-3 [112, 113]. COX-1 and 2 are both expressed in mammalian cells and play biological roles, while COX-3 is a splice variant of COX-1 [114, 115]. COX-1 is the most essential of all the isoforms as it is found in nearly all tissues, whereas COX-2 is conveyed in minute or trace quantities and most of the time it is released as a result of stimuli taken from mitogens, pathogens, oxidative stress, and inflammation [116–119].
According to Verma et al. [120], the COX-1 abundance level is enhanced at the initiation of diabetes and it has also been implicated with greater death rate in heart-related diseases. Guo et al. revealed that there is a vast amount of COX-2 in the vascular smooth muscle cells of the type 2 diabetes mouse model [121]; furthermore, in the coronary arterioles of diabetic subjects, there are elevated levels of COX-2 and antiapoptotic protein Bcl-2 [122, 123]. Also, the elevated levels of COX-2 inside podocytes make the kidney liable to diabetic glomerular injury which occurs by way of a (pro)renin-mediated mechanism [124]. The presence of COX-2 inhibitors in diabetic patients aid in shielding against the incidence of nephropathy [125–127]. In addition, nimesulide, a known COX-2 inhibitor, averts endothelial malfunction in the hind leg of diabetic rats [128].
3.9.2. Cytochrome P450 (CYP450)
Cytochrome P450 (CYP450) is a large family of enzymes linked with drug metabolism, and they are a crucial target in drug pharmacokinetics and response. They are chiefly derived from cells of the liver but are also expressed in body tissues associated with great oxidative capability [129]. They are haemoproteins whose sole aim is to aid in the biotransformation of endogenous and exogenous compounds [129, 130]. They are mostly positioned in the sarcoplasmic reticulum and inner membrane of the mitochondria where they function in processes such as metabolism and synthesis [131]. CYP2E1 and CYP4A are the two predominant CYP450 enzymes that aid in the production of oxidants such as hydrogen peroxides, hydroxyl radicals, and anion superoxide in the body [87]. According to Bansal et al. [132], isoforms of CYP4A have the tendency for producing hydrogen peroxide and superoxide. In addition, some CYP450 isoforms (2E1, 2C6, 2C7, 3A2, 4A3, and 2A1) have been activated and implicated in the onset of hyperglycaemia via the hydroxylation of fatty acids and ketone bodies in streptozotocin-induced diabetic animals [132–134].
3.9.3. Lipoxygenase
Lipoxygenases (LOXs) are a heterogeneous family that catalyzes the oxygenation of polyunsaturated fatty acids such as arachidonic acid and linoleic acid to produce their hydroperoxy derivatives and in the process generate free radicals [135–137]. The resulting ROS produced binds to the enzymes' active site while in a diabetic state they cause collateral damage to surrounding tissues upon their escape [136]. LOX enzymes and their products, such as hydroxyeicosatetraenoic acids (HETEs) and hydroxyocatadecadienoic acids, have been linked with the development of diabetes-induced oxidative stress. Hyperglycemia promotes the upregulation LOX enzymes and boost their activities [136]. An upsurge in the activities of 12/15-LOX has been associated with the pathogenesis of diabetes and atherosclerosis [138–140]. Bleich et al. revealed that 12-LOX knockout (12-LOX KO) mice were resistant to the diabetes development [141].
3.9.4. Myeloperoxidase
Myeloperoxidase (MPO) belongs to a superfamily of mammalian heme peroxidase enzymes, which also includes eosinophil peroxidase (EPO) and lactoperoxidase (LPO) [142]. They possess antimicrobial and antiviral potentials because of their ability to produce ROS [143]. MPO is a protein predominantly expressed in neutrophils, while smaller expression has been observed in the monocytes and macrophages [144]. MPO utilizes H2O2 to make hypochlorous acid (HOCl) and tyrosyl free radicals which possess bactericidal potential, thus creating ROS [143, 144]. Upon the activation of neutrophils and monocytes, they employ ROS for the destruction of pathogens which acquire access into the cell; however, these radicals wield a great deal of cytotoxic effect in the host cells. In a diabetic state, MPO activation gives rise to an upsurge in the production of oxidants which exert cytotoxic and oxidative activity [87]; lingering hyperglycemia is commonly linked with elevated levels of an activated MPO enzyme [145]. Furthermore, the inhibitory effect of N-acetyl-lysyltyrosyl-cysteine amine on the MPO enzyme enhances the function of an endothelial cell and abates oxidative stress in diabetic mice [87].
3.9.5. NADPH Oxidase
Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidases (NOXs) have also been linked as one of the sources of ROS generation during a diabetic state [146]. The NOX family is composed of seven members (Nox1–Nox5, Duox1, and Duox2) that transfer electrons across the biological membranes to generate ROS and are myriads of organs in the body [147]. These different isoforms stimulate superoxide generation by causing a reduction in oxygen molecules via an electron donor (NADPH) [148]. Also, these isoforms are expressed in disparate patterns within the organs of the body. These enzymes possess diverse regulatory subunits crucial for their activity. For instance, Nox1 requires NOXO1, NOXA1, and Rac and Nox2 requires p47phox, p67phox, p40phox, and Rac, whereas NOX4 is constitutively active [149]. In addition to their various activities, Nox1 and Nox2 are renowned for their copious generation of superoxide anion as their immediate product, whereas NOX4 generates hydrogen peroxide enzymes without the slightest presence of a superoxide [150]. Fakhruddin et al. in 2017 affirmed that in a hyperglycemic state, NOX enzymes are activated directly or by way of impeding adenosine monophosphate- (AMP-) activated protein kinase [88]. Furthermore, in a hyperglycemic state, there is enhancement of NOX4 expression and oxidant production in the kidney [151], while Eid et al. [152] and Lee et al. [153] showed that in the same state, there is a subduing effect on AMP-activated protein kinase, thus bringing about the upregulation of NOX4 and ultimately promoting NOX activity in the glomerulus.
3.9.6. Uncoupled Endothelial Nitric Oxide Synthase (eNOS)
Uncoupled eNOS is an occurrence symbolized by an electron transfer within the eNOS molecule by way of L-arginine oxidation, which ultimately breaks down molecular oxygen into a superoxide rather than a nitric oxide (NO) [154]. Thus, it has been revealed that uncoupled eNOS plays a dual role by way of causing an upsurge in ROS production and a decline in NO bioavailability. These two processes have been linked to the development of diabetes [155]. Xia et al. in 2017 revealed that vital physiological processes in the body (cellular proliferation, cellular signalling, platelet aggregation, and vascular tone) are dependent on NO [156]. The mechanism by which uncoupling eNOS is initiated can be grouped into four pathways, viz., accumulation of methylarginines, depletion of L-arginine, eNOS S-glutathionylation, and oxidation of tetrahydrobiopterin (BH4) [157–159]. Nitric oxide binds to BH4 as a cofactor. In a diabetic condition where BH4 is absent, eNOS is transformed to its monomeric form (uncoupled eNOS). In this state, eNOS enzyme basically produces O2− instead of NO [160]. Peroxonitrite (ONOO−) is another powerful oxidant derived from the reaction between NO and O2−. The depleted bioaccessibility of BH4 in the body has been connected with diabetes development [161], and it has been suggested that it is a potential cause for endothelial dysfunction and oxidative stress in diabetes subjects [161]. Uncoupled eNOS is a major source of oxidative damage in diabetes kidneys that was reversed by BH4 treatment [162].
3.9.7. Xanthine Oxidase
Xanthine oxidase (XO) is a metalloflavoenzyme that catalyzes the oxidation of hypoxanthine, thus causing the production of xanthine and some oxidants (e.g., superoxide and peroxynitrite) [163, 164]. XO also generates oxidants, which are key players in the T2DM development process [165–168]. In a diabetic state, there is an upsurge in XO production and the treatment with an inhibitor (allopurinol) aids in the reduction of XO activity, generation of superoxide anion, and ultimately, alleviation of oxidative stress [165]. There is an exceptionally immense upsurge in the activity of XO in a diabetic state, thus promoting oxidative damage as well as inflammatory response [169].
4. Transcriptional Factors and Proteins Implicated in Oxidative Stress-Mediated Diabetes
T2DM is depicted by its myriad of stimuli, decisive factors whereby proinflammatory mediators play a vital role in the onset of insulin resistance and pathogenesis of T2DM through the involvement of oxidative stress and activation of several transcriptional mediator pathways [170].
Oxidative stress has been shown to increase the production of cytokine by a number of signalling pathways. A substantial amount of research findings has revealed that oxygen derivatives act as a second messenger which activate transcription factors such as nuclear factor kappa B (NF-κB), which in turn leads to the production of inflammatory cytokine such as tumor necrosis factor-α (TNF-α), interleukins (ILs), growth factors, and ECM proteins [171, 172].
4.1. Tumor Necrosis Factor-Alpha
The TNF superfamily contains 19 legends and 29 receptors that play a myriad of roles in the body, with all members exhibiting proinflammatory activity [173]. TNF-α is among the first proinflammatory biomarkers to be associated with the pathogenesis of insulin resistance and glucose-related abnormalities that link to T2DM [174, 175].
It plays a vital role in the development of insulin resistance by reducing the expression of glucose transporter type 4 (GLUT 4) that regulates insulin. It is situated in adipocytes and in skeletal and cardiac muscles [176, 177].
Recent reports have revealed the pivotal role TNF-α plays in the induction of tissue-specific inflammation, which brings about the pathogenesis of T2DM [178–180]. According to Swaroop et al. [181], an elevated level of TNF-α in the blood is associated with the development of insulin resistance and diabetes. Hu et al. [182] reported that TNF-α activates adhesion molecules such as intracellular adhesion molecule-1 that stimulates the growth of insulin resistance.
In addition, reports have shown that in metabolic disorders such as hyperglycemia and hyperinsulinemia, which are closely related to diabetes, there is an enhanced production of TNF-α from monocytes and macrophages in an in vitro model [183, 184]. Also, there is a positive relationship between the increase in age and levels of TNF-α [185].
In the pathogenesis of T2DM, increased production of TNF-α in adipose tissues is also related to the obesity-associated insulin resistance that leads to the development of T2DM [186]. Phytochemicals like anthocyanidins, which possess potent antioxidants, have been proven to inhibit TNF-α activity and its related prodiabetic effects [187, 188].
There is a cross-talk between the IKK/NF-κB signalling pathway and its implicated linkage to metabolism, inflammation, and insulin action [189–191]. Almost all metabolic stress signals that are induced either intracellularly or extracellularly bring about insulin resistance or pancreatic β-cell dysfunction by converging on the NF-κB-activating kinase IKKb. Furthermore, the IKK/NF-κB pathway influences glucose metabolism via its activity on the central metabolism networks in pancreatic islets. This brings about elevated damages on the islet and also causes a malfunction in β-cell response to metabolic stress and proinflammatory signals in insulin-resistant subjects which are the hallmark of glucose intolerance and full-blown type 2 diabetes [190, 192, 193].
4.2. Transforming Growth Factor-Beta
TGF-β belongs to a superfamily of three isoforms. The most prevalent of this isoform is TGF-β1; it is produced in its latent form where it is intertwined with protein and concealed in the extracellular matrix. TGF-β1 is made active when its complexed form is cleaved by a proteolytic enzyme [194]. A number of research findings have pointed to a high level of TGF-β1 expression in advance glycation end products, high blood glucose level, and other outcomes of oxidative stress [195–197]. TGF-β1 has been implicated as a major stimulator of tissue fibrosis, and a prolonged dosage of TGF-β1 aids in restoring normal functioning of the kidney in type 1 and 2 diabetes experimental models [198, 199].
It is noteworthy that TGF-β2 has not been well studied in comparison with TGF-β1, but it has been associated with diabetes-related problems most especially in diabetic conditions relating to the kidney [200, 201]. In recent times, isoforms of TGF have been studied closely for its downstream effects on certain microRNA (miRNAs) species [202]. It has also been reported that extreme glucose levels may possibly upsurge transcription of TGF-β genes which in turn promotes the elevation levels of TGF-β and its downstream signalling [203–205]. Although the mode by which TGF-β activation causes heart problems in diabetic subjects is vague, its activation in such subjects could result from the modulation of the expression of certain changes in miRNAs. These miRNAs are noncoding ribonucleic acid molecules tasked with the responsibility of controlling the expression of genes [206]. It has been reported that miRNAs modify the focal points associated with the TGF-β pathway which in turn alters the signalling process of the pathway [207]. An example of such modification of miRNAs has been implicated in its ability to control ERK-MAPK activity in a diabetic state [208].
4.3. Plasminogen Activator Inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) is a serine protease inhibitor that functions as the principal inhibitor of tissue-type plasminogen activator and urokinase-type plasminogen activator, the activators of plasminogen and hence fibrinolysis. PAI-1 is dramatically upregulated in obesity, a complex condition associated with increased risk for myocardial infarction, accelerated atherosclerosis, hypertension, glucose intolerance, insulin resistance, hyperinsulinemia, and type 2 diabetes [209, 210]. Moreover, we recently demonstrated that PAI-1 is involved in streptozotocin-induced type 1 diabetic bone loss in female mice [211].
4.4. Soluble Adhesion Molecules
Diabetes and its macrovascular diabetic complications are multifactorial diseases, which could be brought about by genetic and environmental factors [212]. In most locations of diabetic macrovascular complications and hyperglycemia, there is a tendency to stimulate the initiation of inflammation in the endothelium by way of dysregulation of NOS, NF-κB activation, the formation of advanced glycation end products (AGEs), and oxidative stress. Upon the activation/initiation of the endothelium in diabetic subjects, there is increased expression of soluble adhesion molecules such as E-cadherin, E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) [213]. These aforementioned molecules enable conscription of leukocytes and also bring about their permeation into tissues at locations of macrovascular diabetic complications [214, 215]. It has been observed in both type 1 and 2 murine models that the erasure of ICAM-1 in diabetic nephropathy fends off the advancement of renal diseases [216, 217]. In addition, the impasse of ICAM-1 aids in averting blood-retinal barrier collapse and endothelial cell mutilation [218, 219].
According to Leinonen et al. [220], upon the activation of endothelial cells, some soluble adhesion molecules such as VCAM-1 and ICAM-1 are liberated which are biomarkers of the inflammatory reaction. Also, P-selectin and sICAM-1 levels are notably greater in diabetic neuropathy subjects and it has also been implicated in the weakened pace of nervous conduction [221, 222].
4.5. Interleukins
Type 2 diabetes mellitus (T2DM) arises out of impaired insulin secretion and insulin resistance. This metabolic disorder is connected with inflammatory responses which are typified by the modification of cytokine production such as interleukins (ILs). Interleukins have been implicated in the pathophysiology of T2DM and insulin resistance by way of their respective signalling pathways [171]. On a large scale, cytokines could either be pro- or anti-inflammatory in their activity. IL-1 has been revealed to be a key proinflammatory cytokine which is mostly liberated from immune cells, and it is concealed in certain secretory cells such as adipocytes, monocytes, macrophages, and a number of cells located around diabetic macrovascular complications [171]. IL-1 has two isoforms, IL-1α and β, with a slight difference in their biological functions. IL-1 in collaboration with other cytokines stimulate inflammation [171]. In addition, Spranger et al. [223] revealed that subjects possessing a combination of discernible IL-1β and uplifted IL-6 levels are three times more prone to exhibit T2D in comparison with subjects having trace IL-1β and dwindling IL-6 levels. Genomic analysis has pointed to certain IL-1 genes to closely link with glucose breakdown, non-insulin-dependent diabetes, and a myriad of cardiovascular diseases aftermaths [224–228].
5. Potentials of Phytochemicals in Type 2 Diabetes Mellitus Therapy
A number of naturally occurring chemical materials/substances known as phytochemicals (phenols, terpenoids, nitrogen-containing alkaloids, and sulphur-containing compounds) found in plants have been implicated to possess antidiabetic effects [229]. Phenolic compounds have been implicated in altering inflammatory activity (CRP, IL-6, IL-1β, and TNF-α), transpirational factor enzymes (NF-κB, PPARγ), and genes pertinent for the occurrence of T2DM [230].
Researchers have explored different parts of plants for their antioxidant and antidiabetic properties [231–233]. Some antioxidants present in the human body such as glutathione and thioredoxin mop up ROS via the donation of reducing equivalents in the form of a hydrogen atom or electron to the free radicals, thus making them less harmful in the body system. Certain plant-derived compounds have been ascribed with the following attributes with relation to T2DM therapy: activate the ERK1/2 and AMPK pathways [234–236]; downregulate gene expression associated with COX-2, thus promoting the increased liberation of proinflammatory mediators [237, 238]; increase glucose tolerance and insulin sensitivity [239, 240]; lessen influx of inflammatory cells [241]; decrease levels of proinflammatory cytokines IL-1β, IL-6, and TNF-α in the serum [242]; restrain the activation of NF-κB pathways [243]; and repress the expression of macrophage chemostatic protein (MCP-1) and ICAM [241]. Figure 4 exemplifies the possible function of phytochemical or secondary metabolites with antioxidant potential in the oxidative stress-induced T2DM pathway. ROS/RNS influenced oxidative stress results in diabetes through the following:
Insulin resistance
Dysfunction of beta and endothelial cells due to prolonged exposure to high glucose, elevated free fatty acid level, or the combination of both
Decreased insulin secretion and dysfunction of mitochondrial energy product
Figure 4.
Potential targets of antioxidants in type 2 diabetes mellitus therapy.
Antioxidants embedded in natural phytocompounds have gained greater attention, and they are now being employed therapeutically for mopping up reactive species, consequently attenuating oxidative stress-mediated diabetes. Oxidative stress in a diabetic subject causes insulin resistance, beta cell dysfunction, and insulin secretion which could be modulated by phytocompounds with strong antioxidant potential via either regulating blood sugar levels or attenuating no less than one of the following mechanisms linked with insulin resistance: beta cell function, glucose (re)absorption, and incretin-related pathways [244].
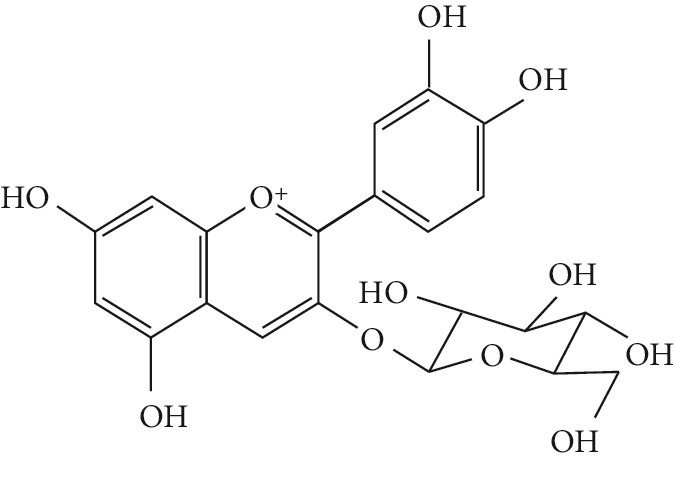
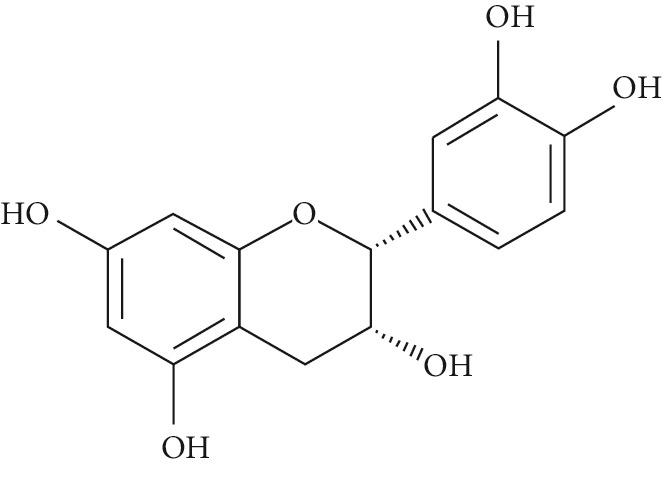
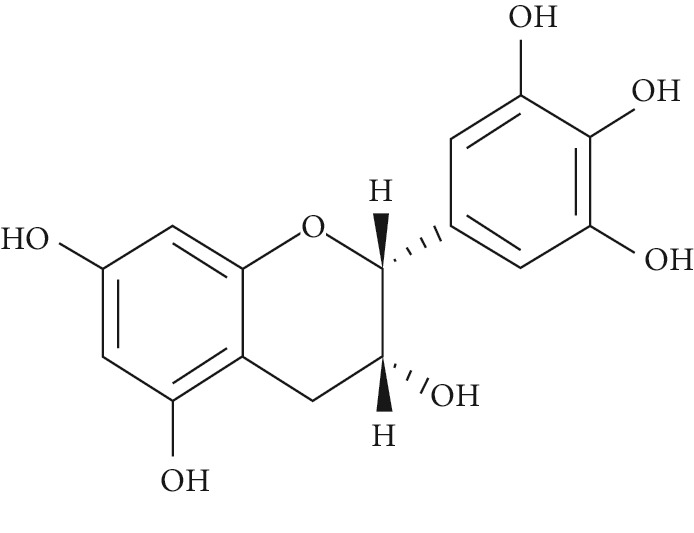
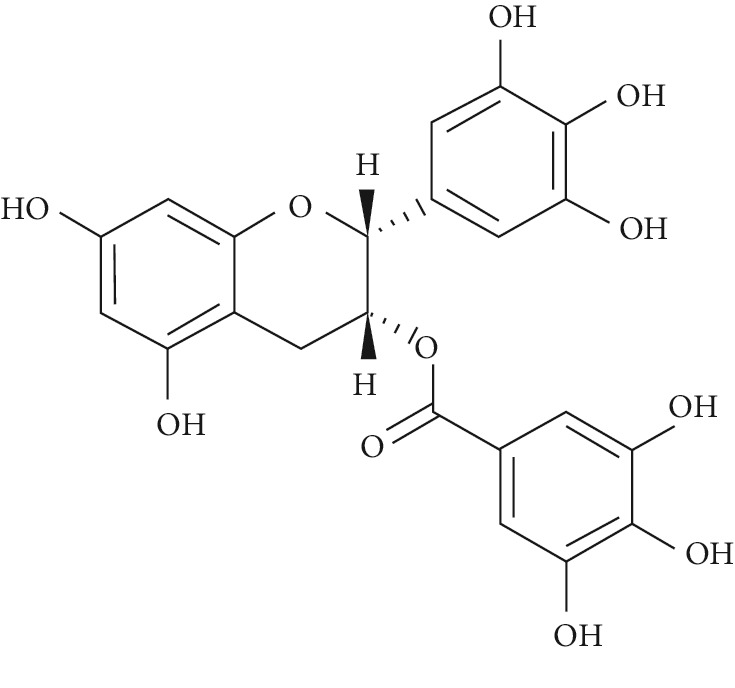
6. Antidiabetic Effects of Phytochemicals
6.1. Preclinical In Vitro/In Vivo (Animal) Studies
Several plant species having hypoglycemic activity have been available in the literature; most of these plants contain bioactive compounds such as glycosides, alkaloids, terpenoids, flavonoids, carotenoids, peptidoglycans, hypoglycans, guanidine, and amino acids, that are frequently implicated as having an antidiabetic effect.
The antidiabetic property of the hydroalcoholic extract of the Dioscorea rhizome was revealed by its ability to reduce blood sugar level in a high-fat-induced rat model [245]. Its mode of action is its ability to attenuate insulin resistance via lessening the phosphorylation of ERK and pS6K and causing an upsurge in Akt and GLUT 4 phosphorylation [245].
Another research finding examined the antidiabetes effects and mechanism of action of Astragalus membranaceus root extract on a diabetic rodent model [246, 247]. The result showed that the extract has the ability to surge insulin sensitivity via Akt activation and increase receptor response to GLUT 4 [247, 248].
The ethanolic extract of Glycyrrhiza uralensis was able to reduce blood sugar, body fats, and blood pressure in a rat model [244, 249]. Another member of this genus, G. foetida, possesses a bioactive molecule (licorice) which also helps in reducing blood sugar and body fats. The mode of action of licorice is achieved by its binding and activation of PPARγ which is pivotal in glucose and lipid metabolism, thus pointing to its antidiabetic potential [250].
Gastrodia eleta Blume is a medicinal herb in China. The aqueous extract of G. eleta has been shown to enhance insulin resistance by causing a reduction in body fat of diet-induced obese rats [251]. The presence of two potent bioactive molecules (vanillin and 4-hydroxybenzaldehyde) in this extract brings about its enhancement of insulin resistance by way of attenuating the fat accumulated in adipose tissues and causing a surge in fat oxidation [251].
Cinnamon (Cinnamomum verum and Cinnamomum zeylanicum) has a rich history of being used as a flavouring agent and medicinal plant for treating a myriad of ailments such as common cold, diarrhoea, diabetes, and rheumatism [252, 253]. Its antidiabetes activity is attributed to its ability to lower blood glucose levels by way of diminishing insulin resistance and promoting hepatic glycogenesis [252, 254]. Cinnamaldehyde, a water-soluble polyphenol compound isolated from cinnamon, acted as an antihyperglycemic and antihyperlipidemic agent in a diabetic rat experimental model [255].
The inclusion of Trigonella foenum-graecum leaves and seeds in diets of rats and dogs, respectively, revealed a significant diminishing effect on blood sugar [256]. The presence of compounds such as diosgenin, galactomannan, trigoneoside, and 4-hydroxyisoleucine in T. foenum-graecum promotes its antidiabetic effect [257, 258]. T. foenum-graecum brings to bear its hypoglycemic effect by way of enhancing/promoting insulin sensitivity in a clinical study [259].
Semen litchi, a common medicinal plant used by the Chinese people, also possesses antidiabetes potential. The aqueous seed extract of S. litchi causes a decrease in insulin resistance in a diabetic rat model [260]. In addition, a clinical study on the seeds has also corroborated its antidiabetes activity [261].
Gymnema sylvestre is one of the medicinal plants used in Indian folk medicine for the management and treatment of diabetes. According to Al-Romaiyan et al. [262], a novel G. sylvestre extract called OSA® showed its ability to decrease blood glucose. Its mode of action is via insulin secretion and (re)generation of beta cells in both in vivo and in vitro models. The ethanol extract of T. divaricata has been revealed to surge the insulin level in the blood and diminish blood sugar levels in STZ-induced diabetic mice [263]. The hydroalcoholic extract of Carthamus tinctorius demonstrated antidiabetic activity by way of improving insulin secretion in alloxan-treated diabetic rats [264].
Panax ginseng and P. quinquefolius are well known for their blood glucose-reducing capability in rat models [265, 266]. The ginseng mode of action of attenuating the blood glucose level is by way of diminution of beta cell function and insulin resistance [267–269]. In addition, the ethanol : water (80 : 20, v/v) extract of the ginseng root possesses a protective effect against the apoptosis of beta cells in the MIN6N8 cell line [270].
Aloeresin A, a point biomolecule derived from Aloe vera, has an antidiabetic potential due to an inhibitory action against alpha-glucosidase and glucose absorption in the intestine [271]. Apigenin is a flavonoid derived from Chamomile tea, which has been revealed to decrease the creation of proinflammatory cytokines such as IL-6, IL-1β, and TNF-α via modifying a myriad of signalling pathways in macrophages and as a result amending damage caused by a hyperglycemic state [272].
Baicalein is another flavonoid with antidiabetic potential isolated from the roots of Scutelleria baicalensis and S. lateriflora; its mode of action is via the activation of AMPK which results in lessened insulin resistance by way of phosphorylating AKT and insulin receptor substrate 1 (IRS-1), and inducing dephosphorylation of ERK, NF-κB, and JNK [273].
Berberine is a benzylisoquinoline alkaloid derived from a majority of the Mahonia genus. This biomolecule has an antidiabetic property ascribed to it since it prompts a surge of insulin resistance, diminishes blood glucose levels, and accelerates beta cell rejuvenation in T2D experimental models [274–276]. Berberine also prompts a surge in glucose uptake in L6 myocytes and C2C12 skeletal muscle cell lines by way of diminution of PTP1B activity and enhancing the phosphorylation of Akt, insulin receptor, and insulin receptor substrate [277].
Curcumin, the main bioactive compound in Curcuma longa, possesses antioxidant, antidiabetic, and other immune-boosting effects [278]. Its antidiabetic effect is attributed to its ability to enhance beta cell function and regulate insulin tolerance [279]. According to Wongeakin et al. [280], diabetic rats fed with a dose of 300 mg/kg BW of curcumin amended vascular inflammation via attenuating ROS overproduction and ICAM-1 and NOX2 expressions.
Diosmin, is a flavonoid found in oranges, lemons, and other citrus plants. Its mode of action is by attenuating ROS-induced diabetes through the impairment of NF-κB-related proinflammatory cytokines, specifically interleukins, MCP-1, and TNF-α [281].
Emodin, a potent bioactive compound found in Aloe vera, banana, and Rheum palmatum, has an antidiabetic property [229, 282]. Its mode of action is through the breakdown of IκB, a very essential part of NF-κB. In addition, the treatment of varying concentrations of emodin caused an upsurge in glucose uptake via enhancing glycogen breakdown by AMP-activated protein kinase and also aided the repression of NF-κB and ERK in C2C12 myotubes and 3T3-L1 adipocytes [282].
Epigallocatechin-3-gallate (EGCG), a catechin isolated from the leaves of Camellia sinensis has been revealed to possess antidiabetic potential in different experimental models [283–286]. Its mechanism of action is via the upsurge of insulin secretion, safeguarding the islet of Langerhans, and diminishing both insulin tolerance and generation of glucose from FFA and lipids [286, 287].
Genistein, also known as 4′,5,7-trihydroxyisoflavone, is a naturally occurring isoflavonoid derived from Glycine max and some other leguminous plants like chickpeas. 4′,5,7-Trihydroxyisoflavone has the ability to sustain islet of Langerhans mass by way of upsurging the amount of beta cells and promoting its continued existence within the pancreas [288, 289]. Its antidiabetic mechanism of action is by way of initiation of ERK1/2 and protein kinase A (PKA), thus resulting in declined insulin sensitivity [290]. The treatment of genistein to high-calorie-diet mice brought about enhanced insulin action via the initiation of AMPK [291].
Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol derived from fruits and vegetables with a very potent antioxidant activity [292]. Its antioxidant potential is due to its ability to suppress the level of IL-1β and TNF-α in diabetic neuropathy in mice [293]. Kaempferol notably reduced fast blood glucose levels of high-fat-diet mice via the initiation of the AMPK signalling pathway [294].
Morin is the main compound isolated from Maclura pomifera, M. tinctoria, and from the leaves of Psidium guajava. Abuohashish et al. revealed that morin diminished the surge of IL-1β, IL-6, and TNF-α through the SphK1 signalling pathway [295]. Another study using streptozotocin-induced diabetic rats showed that morin drastically trimmed down blood glucose, enzymes involved in glucose metabolism, and caused an upsurge of levels of insulin [296].
Myricetin is another naturally occurring flavonoid, but it is more abundant in walnut. A number of pharmacological properties (antioxidant, anti-inflammatory, and antidiabetic) have been ascribed to myricetin. It owes its antidiabetic effect to its ability to enhance insulin receptor substrate 1- (IRS-1-) related GLUT 4 and PI3-kinase transfer/movement [297]. According to Chang et al. [298], the effect of myricetin on HFD rats was through the improvement of PPARα and the suppression of sterol regulatory element-binding protein (SREBP) hepatic expression.
Naringenin is a flavanone present in citrus and grapefruits with very strong antioxidant activity. According to Sandeep and Nandini [299], streptozotocin-induced diabetic rats treated with 0.05% of naringenin had enhanced levels of IRS 1, GLUT 1, and GLUT 3. Another study on streptozotocin-induced diabetic rats administered with naringenin displayed an improvement in the signalling pathways of both PPARγ and AMPK and caused a surge in insulin sensitivity [239, 300].
Resveratrol is a stilbene abundantly found in the skin and seeds of grapes. A number of pharmacological activities such as antidiabetic, anticancer, anti-inflammatory, and immunomodulatory activities have been attributed to resveratrol [301]. The upsurge in hepatic glucose level is a crucial indicator of hyperglycemia in type 2 diabetic subjects. Resveratrol aids in the stimulation of AMPK in the liver, thus causing a decline in the production of hepatic glucose and diminishing the expression levels of certain gluconeogenic enzymes, i.e., phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) [302]. In addition, it averted apoptosis of beta cells influenced by islet amyloid polypeptide (IAPP) on culture medium [303]. Furthermore, it promotes glucose uptake in L6 myotubes by way of initiating sirtuins (SIRT1) as well as AMPK phosphorylation [304]. Clinical studies [305, 306] point toward resveratrol potential in the enhancement of glycaemic control and insulin sensitivity, and in the diminution of oxidative stress in T2DM subjects.
These selected in vitro and in vivo studies on cells and diabetic rat models directly involved or mimic cells/tissues/organs implicated in diabetes are summarized in Tables 1 and 2 thus showing the potential of phytochemicals in obtaining therapeutic agents by T2DM subjects.
Table 1.
Plant extracts that elicited their antidiabetic potential using both alloxan and streptozotocin-induced diabetic rats.
| Number | Plant name | Plant part used | Extract used | Mechanism of action | Experiment model | References |
|---|---|---|---|---|---|---|
| 1 | Acacia arabica | Bark | Chloroform | Cut down the level of serum glucose and ameliorate total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels | Alloxan-induced diabetic albino rats | [307] |
| Cut down levels of serum glucose, TC, TG, LDL, and malondialdehyde (MDA) levels and also boost HDL and coenzyme Q10 | Streptozotocin-induced diabetic rats | [308] | ||||
|
| ||||||
| 2 | Acacia nilotica | Pods | Alcoholic | Aids in depleting levels of blood glucose Boosts antioxidant enzyme system (SOD and GSH), NO level, and LPO of the kidney |
Streptozotocin-induced diabetic rats | [309] |
|
| ||||||
| 3 | Achyranthes rubrofusca | Leaves | Aqueous and ethanolic | Reduction in levels of blood glucose and also aids in boosting levels of superoxide dismutase (SOD), catalase (CAT), and glutathione levels | Alloxan-induced diabetic albino rats | [310] |
|
| ||||||
| 4 | Albizzia lebbeck Benth | Stem bark | Methanol and dichloromethane | Cutting down levels of fasting blood glucose (FBG) and glycated hemoglobin and ameliorating plasma insulin. Furthermore, it caused significant diminution in levels of TC, TG, LDL, and VLDL while causing an upsurge in the level of HDL. | Streptozotocin-induced diabetic rats | [311] |
| Stem | Methanolic | Reducing levels of serum glucose, creatinine, urea, TC, TG, LDL, and VLDL and on the other hand boosting HDL level | Streptozotocin-nicotinamide-induced diabetic rats | [312] | ||
|
| ||||||
| 5 | Aloe vera | Leaves | Aqueous | Cutting down the levels of blood glucose, TG, LDL, and TC | Streptozotocin-induced diabetic mice | [313] |
| Ameliorating insulin secretion and pancreatic β-cell function, i.e., boosting pancreatic islet mass | Streptozotocin-induced diabetic rats | [314] | ||||
| Ameliorating glucose metabolism by way of cutting down the level of blood glucose | Alloxan-induced diabetic rats | [315] | ||||
|
| ||||||
| 6 | Artemisia afra | Leaves | Aqueous | Rejuvenate pancreatic beta cells Inspire insulin release and ameliorate oxidative stress in the pancreas Boost glucose utilization |
Streptozotocin-induced diabetic rats | [316] |
|
| ||||||
| 7 | Barleria prionitis | Leaves and root | Alcoholic | Cutting down levels of blood glucose and glycosylated hemoglobin Boosting levels of serum insulin and liver glycogen |
Alloxan-induced diabetic rats | [317] |
|
| ||||||
| 8 | Boerhaavia diffusa | Leaves | Aqueous | Stimulates glucose utilization and bolsters ionic balance, renal Na+-K+ ATPase activity, and renal antioxidant status (GPx, catalase, SOD, and GSH) | Streptozotocin-induced diabetic rats | [318] |
| Upsurge in levels of hepatic glucose-6-phosphatase and fructose-1,6-bisphosphatase | Alloxan-induced diabetic rats | [319] | ||||
|
| ||||||
| 9 | Bougainvillea spectabilis | Roots and barks | Aqueous and methanolic | Improve the activity of glucose-6-phosphate dehydrogenase and hepatic, skeletal muscle glycogen | Streptozotocin-induced diabetic rats | [320] |
| 3-O-methyl-chiroinositol, a bioactive compound isolated from B. spectabilis | Rejuvenate pancreatic beta cells, thus causing a rise in plasma insulin and c-peptide Employ insulin-like effects |
Streptozotocin- and alloxan-induced diabetic rats | [321]. | |||
|
| ||||||
| 10 | Byrsonima crassifolia | Fruits and seeds | Hexane and chloroform | Upsurge in levels of CAT, GSH, GSSG, and SOD Boosting activities of glucose-6-phosphatase (G6Pase), hepatic glycogen content, and plasma insulin and cutting down blood glucose level |
Streptozotocin-induced diabetic rats | [322] |
|
| ||||||
| 11 | Caesalpinia ferrea | Stem bark | Aqueous | Cutting down the level of blood glucose, TC, and TG | Streptozotocin-induced diabetic rats | [323] |
|
| ||||||
| 12 | Casearia esculenta | Root | Aqueous | Rejuvenation in levels of glucose, urea, uric acid, creatinine, and albumin; the albumin/globulin ratio; and marker enzymes AST, ALT, alkaline phosphatase (ALP), and γ-glutamyltranspeptidase (GGT) | Streptozotocin-induced diabetic rats | [324] |
| 13 | Cassia fistula | Stem bark | Alcoholic | Cutting down the level of blood glucose and also rejuvenating the levels of serum cholesterol, TG, creatinine, albumin, total proteins, and body weight | Alloxan-induced diabetic rats | [325] |
|
| ||||||
| 14 | Catharanthus roseus | Leaves and twigs | Dichloromethane-methanol | Cut down levels of blood glucose and hepatic enzyme activities such as glycogen synthase, glucose 6-phosphate dehydrogenase, succinate dehydrogenase, and malate dehydrogenase | Streptozotocin-induced diabetic rats | [326] |
|
| ||||||
| 15 | Ceriops decandra | Leaves | Ethanol | Adjusting levels of blood glucose, hemoglobin, liver glycogen, and some carbohydrate metabolic enzymes in comparison with the control group | Normal and alloxan-induced diabetic rats | [327] |
|
| ||||||
| 16 | Cinnamomum zeylanicum | Hydroalcohol | Whole plant (cinnamon polyphenols) | Diminish the expressions of inducible nitric oxide synthase (iNOS) and nuclear transcription factor-κB (NF-κB) and also rejuvenate pancreatic beta cells | Streptozotocin-induced diabetes | [255] |
|
| ||||||
| 17 | Cistus laurifolius | Leaves | Ethanol Three known flavonoids (quercetin-3-O-methyl ether, quercetin, and genkwanin) |
Cut down the level of blood glucose level and inhibit activities of α-amylase and α-glucosidase | Streptozotocin-induced diabetic rats | [328] |
|
| ||||||
| 18 | Citrullus colocynthis | Roots | Aqueous, chloroform, and ethanol | Cut down the levels of blood glucose in comparison with the control group | Normal and alloxan-induced diabetic rats | [329] |
|
| ||||||
| 19 | Combretum lanceolatum | Flowers | Ethanol | Activation of AMPK in the liver and also inhibiting hepatic glucose production | Streptozotocin-induced diabetic rats | [330] |
|
| ||||||
| 20 | Emblica officinalis | Fruits, leaves | Hydromethanol | (1) Boost high-density lipoprotein-cholesterol level and diminish low-density lipoprotein-cholesterol level (2) Upsurge in the levels of GSH, GPx, SOD, and CAT (3) Boost activities of hepatic and renal SOD and CAT (4) Cut down the level of thiobarbituric acid reactive substances (TBARS) |
Streptozotocin-induced diabetic rats | [331] |
|
| ||||||
| 21 | Gynura procumbens | Leaves | Aqueous | Enhancement of glucose uptake in the muscles | Streptozotocin-induced diabetic rats | [332] |
|
| ||||||
| 22 | Helicteres isora | Roots | Butanol and aqueous ethanol | Cut down levels of blood glucose, TC, TG, and urea levels | Alloxan-induced diabetic rats | [333] |
|
| ||||||
| 23 | Hiptage benghalensis | Leaves | Methanolic | Rejuvenation of pancreatic beta cells and improvement of insulin secretion, thus bringing about a reduction in the level of blood glucose | Alloxan-induced diabetic rats | [334] |
|
| ||||||
| 24 | Hyptis suaveolens | Leaves | 50% aqueous ethanol | Induction of glucose utilization | Streptozotocin-induced rats | [335] |
|
| ||||||
| 25 | Kigelia pinnata | Flowers | Methanol | Cuts down levels of blood glucose, serum cholesterol, and triglycerides | Streptozotocin-induced diabetic rats | [336] |
|
| ||||||
| 26 | Momordica charantia | Seeds | Methanol | Cutting down levels of serum glucose, insulin, TNF-α, and interleukin 6 (IL-6) | Streptozotocin-induced diabetic rat | [337] |
|
| ||||||
| 27 | Murraya koenigii | Leaves | Aqueous | Cuts down level of blood glucose | Alloxan-induced diabetic rats | [338] |
|
| ||||||
| 28 | Parquetina nigrescens | Leaves | Aqueous | Cuts down level of blood glucose via boosting the level insulin and reducing lipogenesis | Alloxan-induced diabetic rats | [339] |
|
| ||||||
| 29 | Phoenix dactylifera | Leaves | 70% ethanol | Cuts down levels of blood glucose, TC, and TG levels and also causes an upsurge in the level of insulin when compared with the control group | Alloxan-induced diabetic rats | [340] |
|
| ||||||
| 30 | Phyllanthus niruri | Aerial parts | Methanol | Cuts down levels of blood glucose, TC, and TG levels | Alloxan-induced diabetic rats | [341] |
|
| ||||||
| 31 | Pongamia pinnata | Leaves | Petroleum ether, chloroform, ethanol, and water | Reduction in the level of blood glucose level | Streptozotocin- and alloxan-induced diabetic rats | [342, 343] |
|
| ||||||
| 32 | Psidium guajava | Fruits | Aqueous | Cuts down the levels of blood glucose and lipid profile Rejuvenate pancreatic β-cells, thus boosting insulin secretion Suppresses pancreatic nuclear factor kappa B expression |
Streptozotocin-induced diabetic rat | [344] |
|
| ||||||
| 33 | Sida cordifolia | Aerial parts | Methanol and aqueous | Cut down levels of serum glucose level, insulin, and cholesterol | Streptozotocin-induced diabetic rats | [345] |
|
| ||||||
| 33 | Sphaeranthus indicus | Roots and stolons | Ethanol | Cut down levels of blood glucose and boost levels of hepatic glycogen and plasma insulin | Streptozotocin-nicotinamide diabetic rats | [346] |
|
| ||||||
| 34 | Terminalia bellerica | Fruits | Methanol | Enhance insulin secretion via modulating levels of cAMP and intracellular calcium in the pancreatic β-cells | Streptozotocin-induced diabetic rats | [347] |
|
| ||||||
| 35 | Terminalia chebula | Seeds | Chloroform | Cuts down levels of blood glucose | Streptozotocin-induced diabetic rats | [348] |
|
| ||||||
| 36 | Trigonella foenum-graecum | Seeds | Ethanol | Cuts down levels of blood glucose | Alloxan-induced diabetic rats | [349] |
|
| ||||||
| 37 | Zaleya decandra | Roots | Ethanol | Cuts down levels of blood glucose, TC, TG, total proteins, urea, creatinine, and lipid peroxidation | Alloxan-induced diabetic rats | [350] |
Table 2.
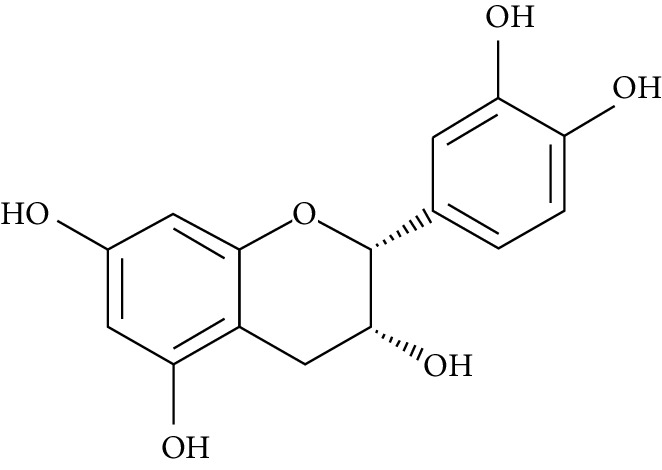
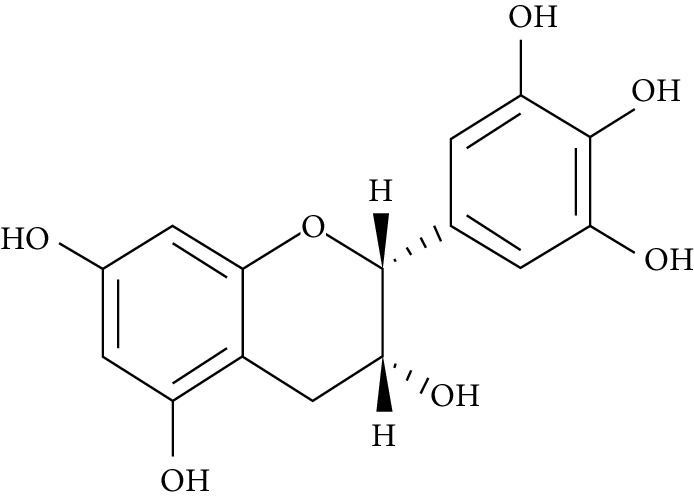
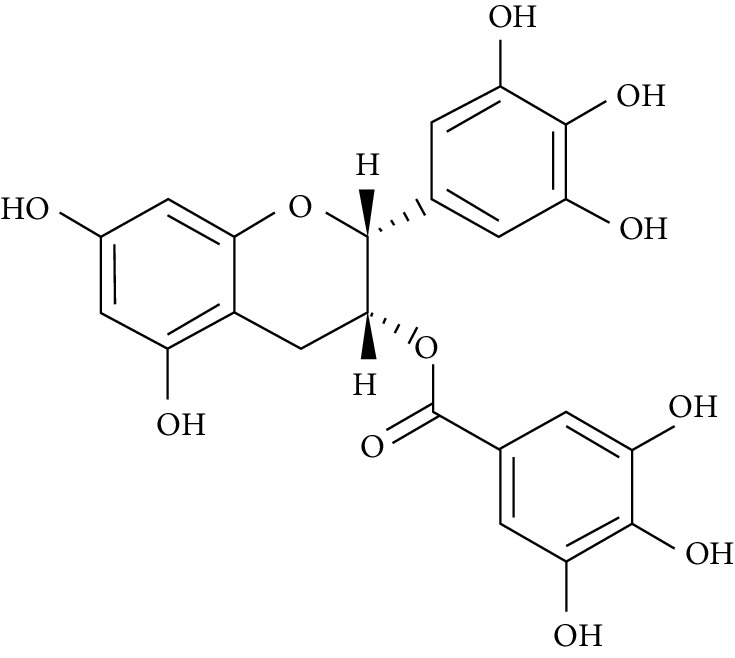
Plant sources, structures, and antidiabetic mechanisms of some potential antidiabetic phytochemicals on different cell lines.
| Number | Plant | Phytochemical isolated | Assay used | Antidiabetic mechanism | Structure | Reference |
|---|---|---|---|---|---|---|
| 1 | Black beans | Cyanidin 3-Glucoside |
Adipocyte 3T3-L1 | Upsurge in adipocyte glucose uptake, improvement in GLUT 4 expression and translocation, elevation in nuclear PPARγ activity, improvement in insulin resistance |
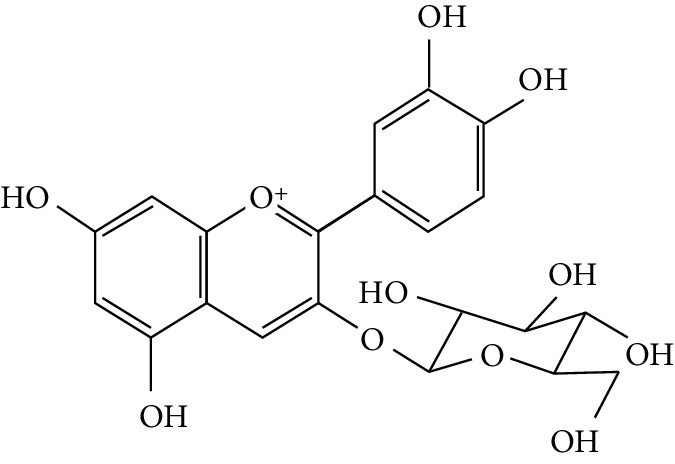
|
[351, 352] |
|
| ||||||
| 2 | Camellia sinensis (green tea), Acacia karroo, Harungana madagascariensis, and Prunus africana | (–)-Epicatechin (EP) | 3T3-L1 adipocytes | Promote the translocation of GLUT 4 through the activation of PI3K and elevation in the phosphorylation of PKCλ/ζ |
|
[353] |
| (-)-Epigallocatechin (EGC) | 3T3-L1 adipocytes | Upgrade the translocation of GLUT 4 by way of stimulation of PI3K and elevation in the phosphorylation of PKCλ/ζ |
|
[353] | ||
|
| ||||||
| 3 | (–)-Epigallocatechin-3-gallate (EGCG) | 3T3-L1 adipocytes | Weakens JNK phosphorylation and elevates GLUT 4 translocation |
|
[354–359] | |
| H4IIE cells | Accelerates the PI3K/aPKCλ, AMPK, and NF-κB pathways | |||||
| Insulin-resistant L6 myotubes | Quickens glucose uptake and accelerates translocation of GLUT 4 to plasma membrane in skeletal muscle | |||||
| HepG2 | Mitigates insulin signalling blockade by reducing IRS-1 Ser307 phosphorylation through the AMPK activation pathway | |||||
| L6 cells | Enhances glucose uptake by expanding GLUT 4 translocation to plasma membrane | |||||
| L6E9 myotubes and 3T3-L1 adipocytes | Accelerates glucose uptake as well as GLUT 4 expression and translocation | |||||
|
| ||||||
| 4 | Catharanthus roseus, Acalypha wilkesiana, and Elaeodendron croceum | Naringenin | L6 myotubes | Accelerated glucose uptake and enhanced AMPK activation |
|
[360] |
|
| ||||||
| 5 | Drynaria fortunei (Kunze) J. Sm., Citrus aurantium L., and Citrus medica L. | Naringin | L6 myotubes | Accelerated glucose uptake and enhanced AMPK activation |
|
[360] |
|
| ||||||
| 6 | Averrhoa carambola L | Apigenin-6-C-β-L-fucopyranoside | Rat soleus muscle | Acceleration of insulin secretion and glycogen synthesis and cutting down blood glucose level |
|
[361] |
|
| ||||||
| 7 | Citrus tangerine, Citrus reticulata, and Citrus depressa | Tangeritin | C2C12 myotubes | Phosphorylated AMPK and AS160 and enhanced glucose uptake and GLUT 4 translocation |
|
[362, 363] |
| 3T3-F442A adipocytes | Accelerated glucose uptake | |||||
|
| ||||||
| 8 | Justicia spicigera | Kaempferitrin | Rat soleus muscle | Acceleration of glucose uptake, GLUT 4 translocation, and glucose homeostasis |
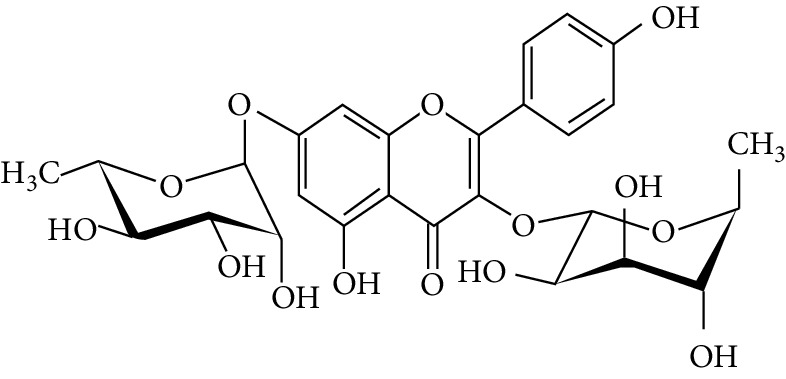
|
[364] |
|
| ||||||
| 9 | Euonymus alatus | Kaempferol | 3T3-L1 adipocytes | Enhanced glucose uptake and mitigated hyperglycemia and PPARγ agonist activity |
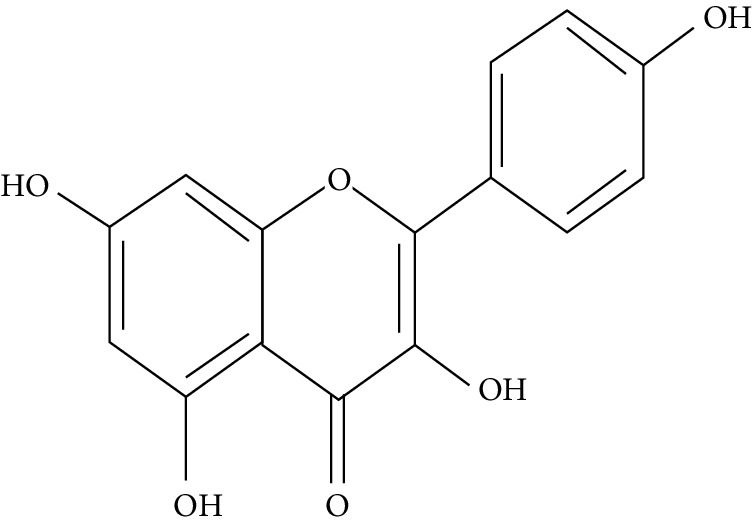
|
[365] |
|
| ||||||
| 10 | Beaumontia grandiflora | Kaempferol 3-neohesperidoside | Rat soleus muscle | Enhances glycogen synthesis |
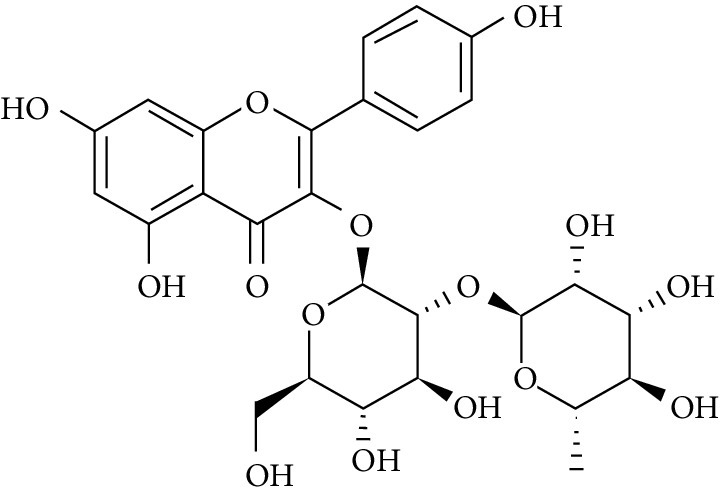
|
[366] |
|
| ||||||
| 11 | Maclura pomifera, Maclura tinctoria, and Psidium guajava | Morin | HepG2 | Enhances the phosphorylation of the insulin receptor, Akt, and FOXO1, hinders gluconeogenesis, and enhances glycogen synthesis |
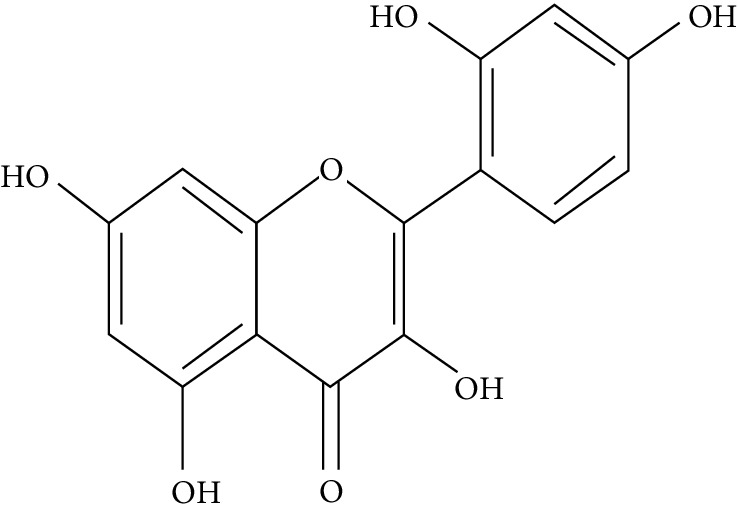
|
[367] |
|
| ||||||
| 12 | Black ginger (Kaempferia parviflora Wall.) | Pentamethyl quercetin | 3T3-L1 cell | Elevation of GLUT 4 and PPAR levels in mRNA |
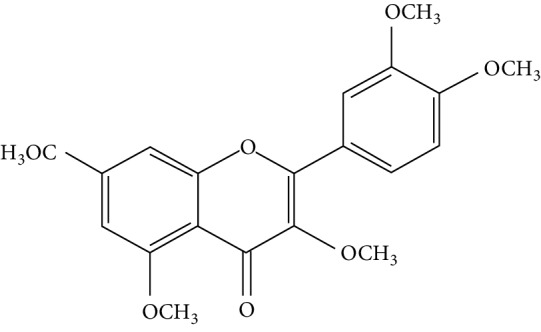
|
[368] |
|
| ||||||
| 13 | Curcuma domestica valeton, Cuscuta reflexa, and Daucus carota | Quercetin | 3T3-L1 adipocytes | Enhanced glucose uptake and mitigated hyperglycemia and PPARγ agonist activity |
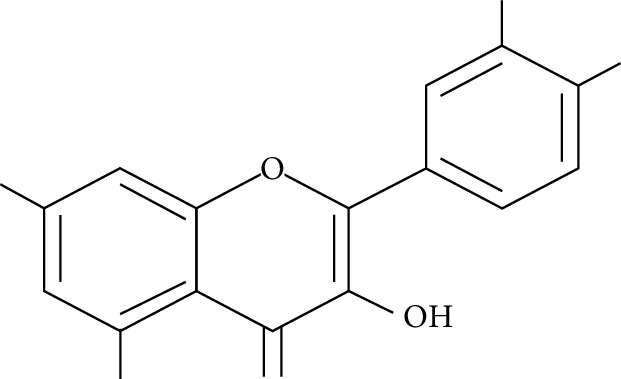
|
[365] |
|
| ||||||
| 14 | Solanum crinitum Lam | Tetramethylkaempferol | 3T3-L1 cell | Elevation of GLUT 4 and PPAR levels in mRNA |
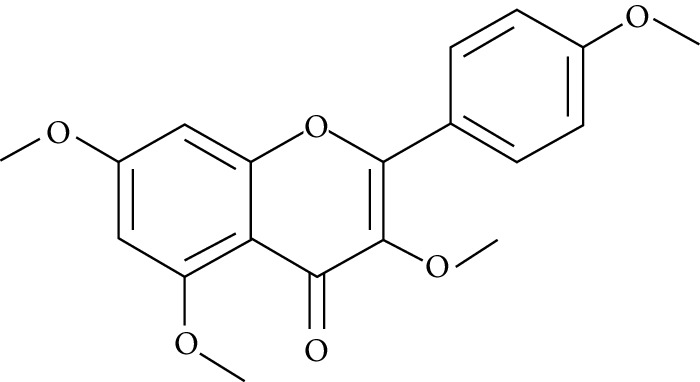
|
[368] |
|
| ||||||
| 15 | Tetracera scandens | Genistein | INS-1 rat insulinoma cells | Stimulated insulin secretion via activation of Ca2+/CaMK II |
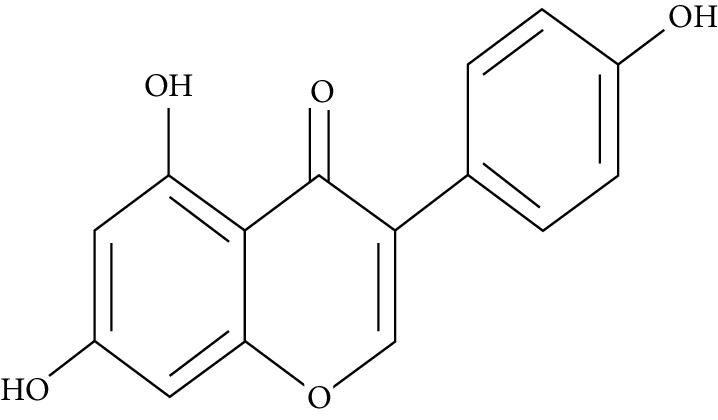
|
[369] |
6.2. Clinical Studies
In recent times, the use of conventional drugs for the treatment and management of diabetes has raised a lot concern from the general public because of their constitutive side effects, thus promoting the exploration of medicinal plants as alternative therapies [370, 371]. A number of medicinal plants used in the management or treatment of diabetes in folk medicine have been proven to possess a large amount of bioactive components which elicit antihyperglycemic or antidiabetic activity [371, 372]. In spite of all these great attributes and potentials ascribed to medicinal plants used for the management/treatment of diabetes in other models, there is scant information concerning their efficacy in clinical/human trials. Therefore, this section sheds light on a few medicinal plants that have been explored in clinical/human trials.
6.2.1. Allium cepa
Allium cepa L. (onion) is a perennial herb in which different products (extracts, essential oil, freeze-dried powder, and juice) from bulbs have been shown to exhibit antidiabetic activity [373–378]. Eldin et al. in 2010 assessed the hypoglycaemic potential of A. cepa in type 1 and type 2 diabetic patients whose were administered 100 g of the crude fresh slices of A. cepa per day. The result revealed a significant decrease in the levels of fasting blood glucose (FBG) by about 89 mg/dL in (type 1 diabetes patients) and 40 mg/dL in (type 2 diabetes patients) after 4 hours of administration. Also, a decrease in the levels in subgroup Ib (positive control) by 145 mg/dL was observed 4 hours later [379].
6.2.2. Aloe vera
Aloe vera extracts administered to three hundred and forty-eight prediabetic patients and T2DM patients for a period of 6-8 weeks revealed a substantial decrease in fasting blood glucose (FBG) [371]. It was also found that the administration of gliberclamide alone to 72 T2DM patients (49 men and 23 women) with elevated FBG levels did not ameliorate the blood glucose levels, whereas those administered 80% Aloe vera juice together with gliberclamide recorded a decline in the level of FBG in less than two weeks administration with no harm done in both the liver and kidney [371, 380].
In addition, Aloe QDM complex or placebo was orally administered during a randomized control trial (8 weeks) to one hundred and thirty-six (136) participants who were randomly assigned to the sixty-eight (68) participants from each group. The study revealed a considerable decrease in body weight, body fat mass, fasting blood glucose (FBG), fasting serum insulin (FSI), and Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR) after eight weeks of treatment [381].
6.2.3. Cinnamon
The antidiabetic potentials of cinnamon are highly appreciated in Ayurvedic and Chinese medicine [382] with an increase in the number of discoveries about its insulin boosting potentials [383, 384]. However, contradictory results have emerged from clinical trials using cinnamon as supplementation [371, 385, 386]. From the five clinical studies reviewed by Kirkham et al. [385], one study involves a randomized, placebo-controlled clinical trial that investigated the effect of cinnamon on blood glucose levels using a total 311 participants (eight studies) which were divided into five type 2 diabetic and three nondiabetic groups, respectively. The results revealed that only two of the diabetic groups had a significant decrease (p < 0.05) in FBG levels (18–29% and 10.3%), while the other three groups had no significant differences. In one of the nondiabetic groups, an 8.4% decrease was recorded (p < 0.01) in FBG vs. placebo when comparing with those administered with placebo, while another group recorded a significant decrease in glucose response employing oral glucose tolerance tests (p < 0.05). Another study by Mang et al. in 2006 examined the effects of oral administration of cinnamon extracts on 79 patients diagnosed with diabetes mellitus type 2 (T2DM) (44 men and 21 women, using oral antidiabetics or diet) using a randomized placebo-controlled clinical trial. Three grams of aqueous cinnamon extract was administered for 4 months after which a substantial decrease in fasting plasma glucose (FBG) level was recorded in the cinnamon group (10.3%) in comparison to the placebo group (3.4%); this finding corroborates the moderate hypoglycemic potential of cinnamon [387]. Gutierrez et al. in 2016 conducted a study using ten sedentary and obese females (22.7 ± 4 years; BMI 35.39 ± 5.36 kg/m2) who were administered 5 g of encapsulated Cassia cinnamon bark or 5 g of encapsulated placebo. They found out that there was a significant decrease in blood glucose levels with an improvement in glucose tolerance following OGTT by 10.1% in comparison with the placebo group [388]. However, there was no amelioration in insulin sensitivity (IS) and insulin resistance (IR) in young women. Furthermore, another double-blind, placebo-controlled study involved 72 juvenile diabetes subjects (diagnosis for ≥18 months prior to the study, age group 13–18 years) who were administered cinnamon (1 g/day) for a period of 90 days [389]. It was observed that all these studies spanned for a period of more than three months, but moderate antidiabetic effect was observed.
6.2.4. Juglans regia
The leaves of Juglans regia are used in folk medicine in Iran for the treatment of diabetes mellitus and hyperglycemia, and lipid profile potential was evaluated on 61 T2DM patients [390]. Subjects used for this study were diagnosed with T2DM and had FBG values ranging between 150 and 200 mg/dL, glycated hemoglobin (HbA1c) levels between 7% and 9%, and ages between 40 and 60 years. The subjects were selected and randomly distributed into two groups: the Juglans regia group and the placebo group. The J. regia group received 100 mg capsules twice a day for a period of three months, while the control group received 100 mg placebo capsules applying the same dosage. These dosage patterns for both groups was coadministered with the standard antidiabetic therapy which is made up of metformin, glibenclamide, and nutritional regimen. The results showed that J. regia-treated patients had a significant reduction in the levels of FBG, HbA1c, total cholesterol, and triglyceride in comparison with the baseline and placebo group after three months of administration [390].
6.2.5. Momordica charantia
Momordica charantia hypoglycemic and antihyperglycemic activities have been reported in whole plant, fruit pulp, seeds, and leaves in a number of in vivo studies because of the ability to reduce blood glucose levels and boost plasma insulin [391, 392].
A randomized, double-blind, active-control trial involving patients with ages between 35 and 70 years and who were recently diagnosed with type 2 diabetes (fasting plasma glucose (FPG) ≥126 mg/dL or 2 h postprandial glucose levels during 75 g oral glucose tolerance-test (OGTT) ≥200 mg/dL) was used for the study. The administration of 500 mg of dried fruit pulp (powder) which contained 0.04–0.05% charantin (2000 mg/day) to T2DM patients for a period of 4 weeks brought about a significant reduction in the levels of fructosamine without any side effects recorded [393].
6.2.6. Ocimum tenuiflorum
Ocimum tenuiflorum (Ocimum sanctum) is popularly known as Thulasi/Tulsi in India. The ethanolic extract and fixed oil of O. sanctum have shown a significant antidiabetic effect in vivo [394, 395]. O. tenuiflorum is indigenous to India and certain parts of north and eastern Africa, China, Hainan Island, and Taiwan where the fresh and dried leaves are used in herbal in medicine [396].
A study conducted by Agrawal et al. in 1996 explored and studied the effects of treatment with O. tenuiflorum and O. album leaves on fasting and postprandial blood glucose and serum cholesterol levels of non-insulin-dependent diabetes mellitus patients using a randomized, placebo-controlled, crossover single blind trial. The results pointed out a substantive reduction in levels of fasting (17.6%) and postprandial blood glucose levels (7.3%) with urine glucose levels revealing a similar result [397].
Another study investigated the effect of Ocimum sanctum (Tulsi) on 30 young overweight/obese subjects using a randomized, parallel group, open label pilot trial. A 250 mg capsule of Tulsi extract was administered twice daily, and it brought about significant reduction in plasma insulin and insulin resistance by 28.49% and 24.79%, respectively, upon 8 weeks of administration. In addition, serum lipid level was regularized with a reduction in body weight and BMI observed when compared to the control group [398].
6.2.7. Panax ginseng
Panax ginseng is a herb native to China, Japan, and Korea with distinctive branched roots [399]. The antidiabetic activity of ginseng has been documented by several authors. Kim et al. analysed data gathered from four dissimilar randomized clinical trials where the subjects were administered 0.78–6 g of ginseng per day for a period of 12 weeks. Their findings revealed that ginseng had no significantly modulated blood glucose level in T2DM patients [400]. On the contrary, findings from Shergis et al. in 2013 showed some encouragement as ginseng boosted glucose metabolism in a survey of six clinical trials [401]. In addition, another study by Shishtar et al. assessed sixteen trials where subjects with and without diabetes were subjected to the intake of different ginseng preparations (0.1–20 g/day) for a period of 4–24 weeks. The findings showed that both groups (diabetic and nondiabetic) had a significant decrease in fasting blood sugar levels [402].
6.2.8. Sauropus androgynus
Sauropus androgynus is one of the most popular herbs in Asia because of its slimming potential, and it has also been shown to possess an antidiabetic potential [403]. Its antidiabetic potential was validated in a clinical trial using 18 type 1 diabetes mellitus subjects between 50 and 65 years and having body weights of 70-85 kg. The results revealed a substantive decline in blood glucose levels as reflected in glycemic index (GI) scores (GI = 55) which were far less than the glucose level in the control group (GI = 100) [404].
6.2.9. Vitis vinifera
Hokayem et al. in 2013 investigated the clinical efficacy of nutritional doses of grape polyphenols (PPs) capable of abating fructose-induced oxidative stress and insulin resistance in thirty-eight (38) healthy overweight/obese first-degree relatives of T2DM patients (18 men and 20 women, aged 30–65 years, BMI between 25 and 35 kg/m2, waist circumference > 94 cm for men and >80 cm for women, FBG < 110 mg/dL) using a randomized, double-blind controlled trial between a grape PP (2 g/day) group and a placebo (PCB) group [405].
After 8 and 9 weeks of supplementation, the mixture of grape PPs at tested nutritional doses abated fructose-induced oxidative stress and insulin resistance, thus making it a vital agent in the area of preventive nutrition [405].
A new insight into the field of phytochemicals in diabetes mellitus is in their ability to act as a subordinate for allopathic drugs used for diabetes management and treatment due to their antioxidant potential which helps in combating oxidative stress and other cellular damages or side effects associated with the intake of allopathic medicine during diabetes treatment [406]. It is also worth mentioning that vitamin supplementation in T2DM subjects aids in improving the antioxidant status through a lot of ways such as causing a surge in the levels of glutathione peroxidase (GPx), superoxide dismutase enzyme (SOD), and total antioxidant capacity (TAC) and also mitigating malondialdehyde (MDA) and thiobarbituric acid reactive substance (TBARS) products. Results obtained from Balbi et al. (2018) revealed that postdiagnosis supplementation of vitamin E (alone or in combination) in T2DM promoted health benefits such as enhancement of plasma antioxidant capacity and the concentration of enzymes (GPx and SOD) and lower levels of MDA and TBARS products [406]. Another study revealed that vitamins C and E are the most frequently administered antioxidants used as supplements in subjects with type 2 DM. They also noted that the supplementation period was between 3 and 12 weeks [407]. T2DM patients are more susceptible to micro- and macrovascular complications; thus, daily intake of vitamins as supplements offers a new approach for metabolic control, as well as summing up the effect of diet, exercise, and medication.
7. Conclusion
Reactive oxygen species produced either exogenously or endogenously are one of major contributors to the development/initiation of T2DM, an ailment that is causing increasing morbidity and mortality in humans all over the globe. Researchers in different parts of the world are exploring natural sources (medicinal plants and natural products) for the management and treatment of diabetes mellitus because the available allopathic drugs sold over the counter are very expensive and come with a number of worrisome side effects. As a result, the exploration of medicinal plants with antidiabetic potential is gaining greater attention on a daily basis because of the presence of potent phytochemicals. In recent times, research findings have revealed that these plant chemicals possess the ability of mitigating diabetes mellitus via a number of mechanisms such as the regulation of insulin signalling, which induces gene and protein expression; the promotion of insulin secretion; the improvement of β-cell function; and the (re)absorption of glucose in both in vitro and in vivo models. However, only a few of these active compounds from natural sources have been translated to clinical use. An example of such is metformin derived from Galega officinalis. Hence, there is a need for more scientific progress in the area of converting phytochemicals with antidiabetes activity to clinical drugs as a means of reforming the management/treatment of T2DM in years to come.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 2017. Eighth. 2017. [PubMed]
- 2.Cho N. H., Shaw J. E., Karuranga S., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Ogurtsova K., da Rocha Fernandes J. D., Huang Y., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation (IDF) IDF Diabetes Altas 2017. 8th. 2017. [PubMed]
- 5.Du Toit L., Biesman-Simons T., Levy N., Dave J. A. A practical approach to managing diabetes in the perioperative period. South African Medical Journal. 2018;108(5):p. 369. doi: 10.7196/samj.2018.v108i5.13311. [DOI] [Google Scholar]
- 6.IDF, International Diabetes Federation. IDF Diabetes Atlas. 7th. Brussels, Belgium: International Diabetes Federation; 2015. https://www.diabetesatlas.org. [PubMed] [Google Scholar]
- 7.World Bank. World Health Organization Global Health Expenditure Database, World Bank Group - Open Knowledge Repository; 2016. [Google Scholar]
- 8.Zimmet P. Z., Magliano D. J., Herman W. H., Shaw J. E. Diabetes: a 21st century challenge. The Lancet Diabetes and Endocrinology. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 9.Tuomi T., Santoro N., Caprio S., Cai M., Weng J., Groop L. The many faces of diabetes: a disease with increasing heterogeneity. The Lancet. 2014;383(9922):1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- 10.Kahn S. E., Cooper M. E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmachari G. Bio-flavonoids with promising anti-diabetic potentials: a critical survey. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. 2011. pp. 187–212.
- 12.Johansen J., Harris A. K., Rychly D. J., Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical pratice. Cardiovascular Diabetology. 2005;4(1):p. 5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forcados G. E., James D. B., Sallau A. B., Muhammad A., Mabeta P. Oxidative stress and carcinogenesis: potential of phytochemicals in breast cancer therapy. Nutrition and Cancer. 2017;69(3):365–374. doi: 10.1080/01635581.2017.1267777. [DOI] [PubMed] [Google Scholar]
- 14.Pisoschi A. M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. European Journal of Medicinal Chemistry. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Drummond G. R., Selemidis S., Griendling K. K., Sobey C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Reviews Drug Discovery. 2011;10(6):453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacher P., Beckman J. S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D. X., Gutterman D. D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292(5):H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 18.Pitocco D., Tesauro M., Alessandro R., Ghirlanda G., Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. International Journal of Molecular Sciences. 2013;14(11):21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lazo-de-la-Vega-Monroy M.-L., Fernndez-Mej C. Oxidative Stress and Chronic Degenerative Diseases - a Role for Antioxidants. IntechOpen; 2013. Oxidative stress in diabetes mellitus and the role of vitamins with antioxidant actions. [DOI] [Google Scholar]
- 21.Höhn A., Jung T., Grune T. Pathophysiological importance of aggregated damaged proteins. Free Radical Biology and Medicine. 2014;71:70–89. doi: 10.1016/j.freeradbiomed.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Weber D., Davies M. J., Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biology. 2015;5:367–380. doi: 10.1016/j.redox.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liou G. Y., Storz P. Reactive oxygen species in cancer. Free Radical Research. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai E. C., Hirsch I. B., Brunzell J. D., Chait A. Reduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDM. Diabetes. 1994;43(8):1010–1014. doi: 10.2337/diabetes.43.8.1010. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura M., Heinecke J. W., Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. Journal of Clinical Investigation. 1994;94(2):771–778. doi: 10.1172/JCI117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher A. B., Zhang Q. Encyclopedia of Respiratory Medicine. Academic Press; 2006. NADPH and NADPH oxidase; pp. 77–84. [DOI] [Google Scholar]
- 27.Yadav N., Kumar S., Marlowe T., et al. Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death & Disease. 2015;6(11):e1969–e1969. doi: 10.1038/cddis.2015.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill M. M., Adrain C., Duriez P. J., Creagh E. M., Martin S. J. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. The EMBO Journal. 2004;23(10):2134–2145. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans J. L., Goldfine I. D., Maddux B. A., Grodsky G. M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrine Reviews. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya S., Sil P. Role of plant-derived polyphenols in reducing oxidative stress-mediated diabetic complications. Reactive Oxygen Species. 2018;5 doi: 10.20455/ros.2018.811. [DOI] [Google Scholar]
- 31.Martín-Gallán P., Carrascosa A., Gussinyé M., Domínguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radical Biology and Medicine. 2003;34(12):1563–1574. doi: 10.1016/S0891-5849(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 32.Rains J. L., Jain S. K. Oxidative stress, insulin signaling, and diabetes. Free Radical Biology and Medicine. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimsrud P. A., Xie H., Griffin T. J., Bernlohr D. A. Oxidative stress and covalent modification of protein with bioactive aldehydes. Journal of Biological Chemistry. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muellenbach E. A., Diehl C. J., Teachey M. K., et al. Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant α-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism. 2008;57(10):1465–1472. doi: 10.1016/j.metabol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierce A., Mirzaei H., Muller F., et al. GAPDH is conformationally and functionally altered in association with oxidative stress in mouse models of amyotrophic lateral sclerosis. Journal of Molecular Biology. 2008;382(5):1195–1210. doi: 10.1016/j.jmb.2008.07.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Matute P., Zulet M. A., Martínez J. A. Reactive species and diabetes: counteracting oxidative stress to improve health. Current Opinion in Pharmacology. 2009;9(6):771–779. doi: 10.1016/j.coph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Tangvarasittichai S., Poonsub P., Tangvarasittichai O., Sirigulsatien V. Serum levels of malondialdehyde in type 2 diabetes mellitus Thai subjects. Siriraj Medical Journal. 2009;61(1):p. 26. [Google Scholar]
- 38.Moreira P. I., Sayre L. M., Zhu X., Nunomura A., Smith M. A., Perry G. Detection and localization of markers of oxidative stress by in situ methods: application in the study of Alzheimer disease. Methods in Molecular Biology. 2010;610:419–434. doi: 10.1007/978-1-60327-029-8_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahmanto A. S., Morgan P. E., Hawkins C. L., Davies M. J. Cellular effects of peptide and protein hydroperoxides. Free Radical Biology and Medicine. 2010;48(8):1071–1078. doi: 10.1016/j.freeradbiomed.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54 doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 41.Yan L. J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. Journal of Diabetes Research. 2014;2014:11. doi: 10.1155/2014/137919.137919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg H., Baron A. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45(5, article 800):623–634. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- 43.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 44.Aiello L. P., Cahill M. T., Cavallerano J. D. Growth factors and protein kinase C inhibitors as novel therapies for the medical management diabetic retinopathy. Eye. 2004;18(2):117–125. doi: 10.1038/sj.eye.6700585. [DOI] [PubMed] [Google Scholar]
- 45.Geraldes P., King G. L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation Research. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett S. P., Griffiths G. D., Schor A. M., Leese G. P., Schor S. L. Growth factors in the treatment of diabetic foot ulcers. British Journal of Surgery. 2003;90(2):133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 47.Pathak D., Gupta A., Kamble B., Kuppusamy G., Suresh B. Oral targeting of protein kinase C receptor: promising route for diabetic retinopathy? Current Drug Delivery. 2012;9(4):405–413. doi: 10.2174/156720112801323080. [DOI] [PubMed] [Google Scholar]
- 48.Shi G.-J., Shi G.-R., Zhou J.-y., et al. Involvement of growth factors in diabetes mellitus and its complications: a general review. Biomedicine and Pharmacotherapy. 2018;101:510–527. doi: 10.1016/j.biopha.2018.02.105. [DOI] [PubMed] [Google Scholar]
- 49.Naidu P. B., Uddandrao V. V. S., Naik R. R., et al. Effects of S-allylcysteine on biomarkers of the polyol pathway in rats with type 2 diabetes. Canadian Journal of Diabetes. 2016;40(5):442–448. doi: 10.1016/j.jcjd.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Sharavana G., Joseph G. S., Baskaran V. Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. European Journal of Nutrition. 2017;56(8):2475–2485. doi: 10.1007/s00394-016-1283-0. [DOI] [PubMed] [Google Scholar]
- 51.Koya D., Jirousek M. R., Lin Y. W., Ishii H., Kuboki K., King G. L. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. Journal of Clinical Investigation. 1997;100(1):115–126. doi: 10.1172/jci119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang P., Zhang Y. J., Huang Y., Zhao J. J., Jiang T., Zhang N. Effect of PKC signalling pathway and aldose reductase on expression of fibronectin induced by transforming growth factor-β1 in human mesangial cells. Chinese Journal of Pathology. 2010;39(6):405–409. [PubMed] [Google Scholar]
- 53.Degenhardt T. P., Thorpe S. R., Baynes J. W. Chemical modification of proteins by methylglyoxal. Cellular and Molecular Biology (Noisy-le-Grand, France) 1998;44(7):1139–1145. [PubMed] [Google Scholar]
- 54.Sun K., Wang W., Wang C., et al. AGEs trigger autophagy in diabetic skin tissues and fibroblasts. Biochemical and Biophysical Research Communications. 2016;471(3):355–360. doi: 10.1016/j.bbrc.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Bhat S., Mary S., Giri A. P., Kulkarni M. J. Mechanisms of Vascular Defects in Diabetes Mellitus. Springer; 2017. Advanced Glycation End Products (AGEs) in diabetic complications; pp. 423–449. [DOI] [Google Scholar]
- 56.Piperi C., Adamopoulos C., Dalagiorgou G., Diamanti-Kandarakis E., Papavassiliou A. G. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. Journal of Clinical Endocrinology and Metabolism. 2012;97(7):2231–2242. doi: 10.1210/jc.2011-3408. [DOI] [PubMed] [Google Scholar]
- 57.Thomson M., al-Qattan K. K., JS D., Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine. 2015;16(1, article 992) doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yiu W. H., Wong D. W. L., Wu H. J., et al. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE-induced oxidative stress. Kidney International. 2016;89(2):386–398. doi: 10.1038/ki.2015.331. [DOI] [PubMed] [Google Scholar]
- 59.Kay A. M., Simpson C. L., Stewart J. A. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. Journal of Diabetes Research. 2016;2016:8. doi: 10.1155/2016/6809703.6809703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suchal K., Malik S., Khan S. I., et al. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: role of AGE-RAGE/MAPK pathways. Scientific Reports. 2017;7(1) doi: 10.1038/srep42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu Y. Y., Tang L. Q., Wei W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Molecular and Cellular Endocrinology. 2017;443:89–105. doi: 10.1016/j.mce.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Ram M., Singh V., Kumawat S., Kant V., Tandan S. K., Kumar D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. International Immunopharmacology. 2016;30:137–149. doi: 10.1016/j.intimp.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 63.Goldfarb S., Ziyadeh F. N., Kern E. F. O., Simmons D. A. Effects of polyol-pathway inhibition and dietary myo-inositol on glomerular hemodynamic function in experimental diabetes mellitus in rats. Diabetes. 1991;40(4):465–471. doi: 10.2337/diab.40.4.465. [DOI] [PubMed] [Google Scholar]
- 64.Wilson D., Bohren K., Gabbay K., Quiocho F. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992;257(5066):81–84. doi: 10.1126/science.1621098. [DOI] [PubMed] [Google Scholar]
- 65.Shaw S., Wang X., Redd H., Alexander G. D., Isales C. M., Marrero M. B. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. Journal of Biological Chemistry. 2003;278(33):30634–30641. doi: 10.1074/jbc.m305008200. [DOI] [PubMed] [Google Scholar]
- 66.Khanra R., Bhattacharjee N., Dua T. K., et al. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomedicine & Pharmacotherapy. 2017;94:726–741. doi: 10.1016/j.biopha.2017.07.112. [DOI] [PubMed] [Google Scholar]
- 67.Engerman R. L., Kern T. S., Larson M. E. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia. 1994;37(2):141–144. doi: 10.1007/s001250050084. [DOI] [PubMed] [Google Scholar]
- 68.Garcia Soriano F., Virág L., Jagtap P., et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nature Medicine. 2001;7(1):108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 69.Soltesova Prnova M., Ballekova J., Gajdosikova A., Gajdosik A., Stefek M. A novel carboxymethylated mercaptotriazinoindole inhibitor of aldose reductase interferes with the polyol pathway in streptozotocin-induced diabetic rats. Physiological Research. 2015;64(4):587–591. doi: 10.33549/physiolres.933034. [DOI] [PubMed] [Google Scholar]
- 70.Boesten D. M. P. H. J., Von Ungern-Sternberg S. N. I., Den Hartog G. J. M., Bast A. Protective pleiotropic effect of flavonoids on NAD+ levels in endothelial cells exposed to high glucose. Oxidative Medicine and Cellular Longevity. 2015;2015:7. doi: 10.1155/2015/894597.894597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Dam P. S., Gispen W.-H., Bravenboer B., van Asbeck B. S., Erkelens D. W., Marx J. J. M. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes/Metabolism Reviews. 1995;11(3):181–192. doi: 10.1002/dmr.5610110303. [DOI] [PubMed] [Google Scholar]
- 72.Shakeel M. Recent advances in understanding the role of oxidative stress in diabetic neuropathy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(4):373–378. doi: 10.1016/j.dsx.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 73.Li F., Zhao Y. B., Wang D. K., Zou X., Fang K., Wang K. F. Berberine relieves insulin resistance via the cholinergic anti-inflammatory pathway in HepG2 cells. Journal of Huazhong University of Science and Technology [Medical Sciences] 2016;36(1, article 1543):64–69. doi: 10.1007/s11596-016-1543-5. [DOI] [PubMed] [Google Scholar]
- 74.Jagdale A. D., Bavkar L. N., More T. A., Joglekar M. M., Arvindekar A. U. Strong inhibition of the polyol pathway diverts glucose flux to protein glycation leading to rapid establishment of secondary complications in diabetes mellitus. Journal of Diabetes and its Complications. 2016;30(3):398–405. doi: 10.1016/j.jdiacomp.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Sheela D. L., Nazeem P. A., Narayanankutty A., et al. Coconut phytocompounds inhibits polyol pathway enzymes: implication in prevention of microvascular diabetic complications. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2017;127:20–24. doi: 10.1016/j.plefa.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beriault D., Shi Y., Werstuck G. Investigating the role of the hexosamine biosynthesis pathway in diabetic atherosclerosis. Canadian Journal of Cardiology. 2013;29(10, article S254) doi: 10.1016/j.cjca.2013.07.417. [DOI] [Google Scholar]
- 78.Marsh S. A., Dell’Italia L. J., Chatham J. C. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40(3):819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taparra K., Tran P. T., Zachara N. E. Hijacking the hexosamine biosynthetic pathway to promote EMT-mediated neoplastic phenotypes. Frontiers in Oncology. 2016;6 doi: 10.3389/fonc.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiaradonna F., Ricciardiello F., Palorini R. The nutrient-sensing hexosamine biosynthetic pathway as the hub of cancer metabolic rewiring. Cells. 2018;7(6):p. 53. doi: 10.3390/cells7060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin C. X., Sleaby R., Davidoff A. J., et al. Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Pharmacological Research. 2017;116:45–56. doi: 10.1016/j.phrs.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 82.Virkamäki A., Daniels M. C., Hämäläinen S., Utriainen T., Mcclain D., Yki-Järvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance in multiple insulin sensitive tissues. Endocrinology. 1997;138(6):2501–2507. doi: 10.1210/endo.138.6.5172. [DOI] [PubMed] [Google Scholar]
- 83.Schleicher E. D., Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney International. 2000;58:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 84.Sayeski P. P., Kudlow J. E. Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-α gene transcription. Journal of Biological Chemistry. 1996;271(25):15237–15243. doi: 10.1074/jbc.271.25.15237. [DOI] [PubMed] [Google Scholar]
- 85.Kolm-Litty V., Sauer U., Nerlich A., Lehmann R., Schleicher E. D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. Journal of Clinical Investigation. 1998;101(1):160–169. doi: 10.1172/jci119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pang Y., Bounelis P., Chatham J. C., Marchase R. B. Hexosamine pathway is responsible for inhibition by diabetes of phenylephrine-induced inotropy. Diabetes. 2004;53(4):1074–1081. doi: 10.2337/diabetes.53.4.1074. [DOI] [PubMed] [Google Scholar]
- 87.Yaribeygi H., Atkin S. L., Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. Journal of Cellular Physiology. 2019;234(2):1300–1312. doi: 10.1002/jcp.27164. [DOI] [PubMed] [Google Scholar]
- 88.Fakhruddin S., Alanazi W., Jackson K. E. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. Journal of Diabetes Research. 2017;2017:30. doi: 10.1155/2017/8379327.8379327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. Journal of Neurochemistry. 2002;80(5):780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 90.Turrens J. F. Mitochondrial formation of reactive oxygen species. The Journal of Physiology. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venditti P., Di Stefano L., Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion. 2013;13(2):71–82. doi: 10.1016/j.mito.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology & Medicine. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 93.Raza H., Prabu S. K., John A., Avadhani N. G. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. International Journal of Molecular Sciences. 2011;12(5):3133–3147. doi: 10.3390/ijms12053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kakkar R., Kalra J., Mantha S. V., Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Molecular and Cellular Biochemistry. 1995;151(2):113–119. doi: 10.1007/bf01322333. [DOI] [PubMed] [Google Scholar]
- 95.Chatuphonprasert W., Lao-Ong T., Jarukamjorn K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharmaceutical Biology. 2014;52(4):419–427. doi: 10.3109/13880209.2013.839714. [DOI] [PubMed] [Google Scholar]
- 96.Hagglof B., Marklund S. L., Holmgren G. CuZn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase in lymphocytes and erythrocytes in insulin-dependent diabetic children. Acta Endocrinologica. 1983;102(2):235–239. doi: 10.1530/acta.0.1020235. [DOI] [PubMed] [Google Scholar]
- 97.Sindhu R. K., Koo J. R., Roberts C. K., Vaziri N. D. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clinical and Experimental Hypertension. 2004;26(1):43–53. doi: 10.1081/ceh-120027330. [DOI] [PubMed] [Google Scholar]
- 98.Turkseven S., Kruger A., Mingone C. J., et al. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289(2):H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 99.Shailey S., Basir S. F. Protective role of Azadirachta indica against oxidative damage in skeletal and cardiac muscle of alloxan diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4 [Google Scholar]
- 100.Kakkar R., Mantha S. V., Radhi J., Prasad K., Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clinical Science. 1998;94(6):623–632. doi: 10.1042/cs0940623. [DOI] [PubMed] [Google Scholar]
- 101.Simonyan M. A., Gevorkyan D. M., Mkhitaryan V. G. Quantitative changes in Cu, Zn-activated superoxide dismutase and catalase in the liver of rats with alloxan diabetes. Bulletin of Experimental Biology and Medicine. 1987;103(3):339–341. doi: 10.1007/bf00840555. [DOI] [Google Scholar]
- 102.Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of “autoxidative glycosylation” in diabetes. Biochemical Journal. 1987;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hunt J. V., Dean R. T., Wolff S. P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing, Biochemical Journal. 1988;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sjöholm Å., Nyström T. Inflammation and the etiology of type 2 diabetes. Diabetes/Metabolism Research and Reviews. 2006;22(1):4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 107.Donath M. Y., Böni-Schnetzler M., Ellingsgaard H., Ehses J. A. Islet inflammation impairs the pancreatic β-cell in type 2 diabetes. Physiology. 2009;24(6):325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 108.Sandberg M., Jansson L. Effects of cyclooxygenase inhibition on insulin release and pancreatic islet blood flow in rats. Upsala Journal of Medical Sciences. 2014;119(4):316–323. doi: 10.3109/03009734.2014.964381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. Journal of Biological Chemistry. 1990;265(28):16737–16740. [PubMed] [Google Scholar]
- 110.Crofford L. J. COX-1 and COX-2 tissue expression: implications and predictions. The Journal of Rheumatology. 1997;49:15–19. [PubMed] [Google Scholar]
- 111.Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annual Review of Pharmacology and Toxicology. 1998;38(1):97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 112.Chandrasekharan N. V., Dai H., Roos K. L. T., et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Warner T. D., Mitchell J. A. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proceedings of the National Academy of Sciences. 2002;99(21):13371–13373. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shehata H. Drugs and Drug Therapy. 4th. Elsevier Ltd; 2010. [Google Scholar]
- 115.Zurier R. B. Kelley’s Textbook of Rheumatology. Elsevier; 2013. Prostaglandins, leukotrienes, and related compounds; pp. 340–357. [DOI] [Google Scholar]
- 116.Pop-Busui R., Kellogg A., Cheng H. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Current Drug Targets. 2008;9(1):68–76. doi: 10.2174/138945008783431691. [DOI] [PubMed] [Google Scholar]
- 117.Kirkby N. S., Zaiss A. K., Wright W. R., et al. Differential COX-2 induction by viral and bacterial PAMPs: consequences for cytokine and interferon responses and implications for anti-viral COX-2 directed therapies. Biochemical and Biophysical Research Communications. 2013;438:249–256. doi: 10.1016/j.bbrc.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Font-Nieves M., Sans-Fons M. G., Gorina R., et al. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E 2 production in astrocytes. Journal of Biological Chemistry. 2012;287(9):6454–6468. doi: 10.1074/jbc.m111.327874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song H., Park J., Bui P. T. C., et al. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environmental Research. 2017;158:490–498. doi: 10.1016/j.envres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Verma S., Chandra H., Banerjee M. Cyclooxygenase 1 (COX1) expression in type 2 diabetes mellitus: a preliminary study from North India. Egyptian Journal of Medical Human Genetics. 2016;17(1):41–45. doi: 10.1016/j.ejmhg.2015.07.003. [DOI] [Google Scholar]
- 121.Guo Z., Su W., Allen S., et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic / mice. Cardiovascular Research. 2005;67(4):723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Szerafin T., Erdei N., Fülöp T., et al. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circulation Research. 2006;99(5):e12–e17. doi: 10.1161/01.res.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 123.Redondo S., Ruiz E., Gordillo-Moscoso A., et al. Overproduction of cyclo-oxygenase-2 (COX-2) is involved in the resistance to apoptosis in vascular smooth muscle cells from diabetic patients: a link between inflammation and apoptosis. Diabetologia. 2011;54(1, article 1947):190–199. doi: 10.1007/s00125-010-1947-x. [DOI] [PubMed] [Google Scholar]
- 124.Cheng H., Fan X., Moeckel G. W., Harris R. C. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. Journal of the American Society of Nephrology. 2011;22(7):1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng H. F., Wang C. J., Moeckel G. W., Zhang M. Z., McKanna J. A., Harris R. C. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney International. 2002;62(3):929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 126.Nasrallah R., Robertson S. J., Hébert R. L. Chronic COX inhibition reduces diabetes-induced hyperfiltration, proteinuria, and renal pathological markers in 36-week B6-Ins2Akita mice. American Journal of Nephrology. 2009;30(4):346–353. doi: 10.1159/000229304. [DOI] [PubMed] [Google Scholar]
- 127.Quilley J., Santos M., Pedraza P. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300(6):H2316–H2322. doi: 10.1152/ajpheart.01259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abdelrahman A. M., Al Suleimani Y. M. Four-week administration of nimesulide, a cyclooxygenase-2 inhibitor, improves endothelial dysfunction in the hindlimb vasculature of streptozotocin-induced diabetic rats. Archives of Pharmacal Research. 2008;31(12, article 2155):1584–1589. doi: 10.1007/s12272-001-2155-5. [DOI] [PubMed] [Google Scholar]
- 129.Guengerich F. P. Cytochrome P450: Structure, Mechanism, and Biochemistry. Fourth. Springer; 2015. Human cytochrome P450 enzymes; pp. 473–535. [DOI] [Google Scholar]
- 130.Wang Z., Hall S. D., Maya J. F., Li L., Asghar A., Gorski J. C. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. British Journal of Clinical Pharmacology. 2003;55(1):77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ortiz de Montellano P. R. Cytochrome P450: Structure, Mechanism, and Biochemistry. Third. Springer; 2005. [DOI] [Google Scholar]
- 132.Bansal S., Liu C. P., Sepuri N. B. V., et al. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. Journal of Biological Chemistry. 2010;285(32):24609–24619. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shimojo N., Ishizaki T., Imaoka S., Funae Y., Fuji S., Okuda K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochemical Pharmacology. 1993;46(4):621–627. doi: 10.1016/0006-2952(93)90547-A. [DOI] [PubMed] [Google Scholar]
- 134.Bondoc F. Y., Bao Z., Hu W. Y., et al. Acetone catabolism by cytochrome P450 2E1: studies with CYP2E1-null mice. Biochemical Pharmacology. 1999;58(3):461–463. doi: 10.1016/S0006-2952(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 135.Brash A. R. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 136.Niedowicz D. M., Daleke D. L. The role of oxidative stress in diabetic complications. Cell Biochemistry and Biophysics. 2005;43(2):289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 137.Radmark O., Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. Journal of Lipid Research. 2009;50(Supplement):S40–S45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen Y., Gu J., Chakrabarti S. K., et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-α in macrophages. Endocrinology. 2007;148(3):1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 139.Nagelin M. H., Srinivasan S., Lee J., Nadler J. L., Hedrick C. C. 12/15-Lipoxygenase activity increases the degradation of macrophage ATP-binding cassette transporter G1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(10):1811–1819. doi: 10.1161/ATVBAHA.108.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stavniichuk R., Drel V. R., Shevalye H., et al. Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radical Biology and Medicine. 2010;49(6):1036–1045. doi: 10.1016/j.freeradbiomed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bleich D., Chen S., Zipser B., Sun D., Funk C. D., Nadler J. L. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. Journal of Clinical Investigation. 1999;103(10):1431–1436. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furtmüller P. G., Zederbauer M., Jantschko W., et al. Active site structure and catalytic mechanisms of human peroxidases. Archives of Biochemistry and Biophysics. 2006;445(2):199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 143.Lazarevic-Pasti T., Leskovac A., Vasic V. Myeloperoxidase inhibitors as potential drugs. Current Drug Metabolism. 2015;16(3):168–190. doi: 10.2174/138920021603150812120640. [DOI] [PubMed] [Google Scholar]
- 144.Van Der Veen B. S., De Winther M. P. J., Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxidants and Redox Signaling. 2009;11(11):2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 145.Wiersma J. J., Meuwese M. C., van Miert J. N. I., et al. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Medical Science Monitor. 2008;14(8):CR406–CR410. [PubMed] [Google Scholar]
- 146.Yaribeygi H., Farrokhi F. R., Rezaee R., Sahebkar A. Oxidative stress induces renal failure: a review of possible molecular pathways. Journal of Cellular Biochemistry. 2018;119(4):2990–2998. doi: 10.1002/jcb.26450. [DOI] [PubMed] [Google Scholar]
- 147.Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 148.Babior B. M. NADPH oxidase: an update. Blood. 1999;93(5):1464–1476. doi: 10.1182/blood.V93.5.1464. [DOI] [PubMed] [Google Scholar]
- 149.Chen F., Haigh S., Barman S., Fulton D. J. R. From form to function: the role of Nox4 in the cardiovascular system. Frontiers in Physiology. 2012;3 doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serrander L., Cartier L., Bedard K., et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochemical Journal. 2007;406(1):105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yaribeygi H., Mohammadi M. T., Rezaee R., Sahebkar A. Crocin improves renal function by declining Nox-4, IL-18, and p53 expression levels in an experimental model of diabetic nephropathy. Journal of Cellular Biochemistry. 2018;119(7):6080–6093. doi: 10.1002/jcb.26806. [DOI] [PubMed] [Google Scholar]
- 152.Eid A. A., Ford B. M., Block K., et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. Journal of Biological Chemistry. 2010;285(48):37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee H. J., Lee D. Y., Mariappan M. M., et al. Hydrogen sulfide inhibits high glucose-induced NADPH oxidase 4 expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. Journal of Biological Chemistry. 2017;292(14):5665–5675. doi: 10.1074/jbc.M116.766758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Förstermann U., Sessa W. C. Nitric oxide synthases: regulation and function. European Heart Journal. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Faria A. M., Papadimitriou A., Silva K. C., Lopes De Faria J. M., Lopes De Faria J. B. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes. 2012;61(7):1838–1847. doi: 10.2337/db11-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xia N., Förstermann U., Li H. Implication of eNOS uncoupling in cardiovascular disease. Reactive Oxygen Species. 2017;3 doi: 10.20455/ros.2017.807. [DOI] [Google Scholar]
- 157.Chen C. A., Wang T. Y., Varadharaj S., et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zweier J. L., Chen C. A., Druhan L. J. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxidants and Redox Signaling. 2011;14(10):1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen C. A., Lin C. H., Druhan L. J., Wang T. Y., Chen Y. R., Zweier J. L. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. Journal of Biological Chemistry. 2011;286(33):29098–29107. doi: 10.1074/jbc.m111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luo S., Lei H., Qin H., Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Current Pharmaceutical Design. 2014;20(22):3548–3553. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 161.Heitzer T., Krohn K., Albers S., Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43(11):1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 162.Satoh M., Fujimoto S., Haruna Y., et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. American Journal of Physiology-Renal Physiology. 2005;288(6):F1144–F1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 163.Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiologica Scandinavica Supplementum. 1986;548:87–99. [PubMed] [Google Scholar]
- 164.Azenabor A., Erivona R., Adejumo E., Ozuruoke D., Azenabor R. Xanthine oxidase activity in type 2 diabetic Nigerians. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13(3):2021–2024. doi: 10.1016/j.dsx.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 165.Desco M. C., Asensi M., Marquez R., et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51(4):1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 166.Henriksen E. J., Diamond-Stanic M. K., Marchionne E. M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radical Biology and Medicine. 2011;51(5):993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Feillet Coudray C. Xanthine oxidase is variably involved in nutritional and physio-pathologic oxidative stress situations. Journal of Physiobiochemical Metabolism. 2013;2(1) doi: 10.4172/2324-8793.1000104. [DOI] [Google Scholar]
- 168.Domingueti C. P., Sant’Ana Dusse L. M., das Graças Carvalho M., de Sousa L. P., Gomes K. B., Fernandes A. P. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and its Complications. 2016;30(4):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 169.Liu J., Wang C., Liu F., Lu Y., Cheng J. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Analytical and Bioanalytical Chemistry. 2015;407(9):2569–2579. doi: 10.1007/s00216-015-8481-0. [DOI] [PubMed] [Google Scholar]
- 170.Tuomilehto J., Lindström J., Eriksson J. G., et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 171.Fève B., Bastard J. P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nature Reviews Endocrinology. 2009;5(6):305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 172.Elmarakby A. A., Sullivan J. C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics. 2012;30(1):49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 173.Aggarwal B. B., Gupta S. C., Kim J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sprague A. H., Khalil R. A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical Pharmacology. 2009;78(6):539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Imai Y., Dobrian A. D., Weaver J. R., et al. Interaction between cytokines and inflammatory cells in islet dysfunction, insulin resistance and vascular disease. Diabetes, Obesity and Metabolism. 2013;15(s3):117–129. doi: 10.1111/dom.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guilherme A., Virbasius J. V., Puri V., Czech M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Olson A. L. Regulation of GLUT4 and insulin-dependent glucose flux. ISRN Molecular Biology. 2012;2012:12. doi: 10.5402/2012/856987.856987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Akash M. S. H., Shen Q., Rehman K., Chen S. Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. Journal of Pharmaceutical Sciences. 2012;101(5):1647–1658. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 179.Akash M. S. H., Rehman K., Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. Journal of Cellular Biochemistry. 2013;114(3):525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 180.Rehman K., Akash M. S. H. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? Journal of Biomedical Science. 2016;23(1, article 303):p. 87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Swaroop J. J., Naidu J. N., Rajarajeswari D. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. The Indian Journal of Medical Research. 2012;135(1, article 93435):127–130. doi: 10.4103/0971-5916.93435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hu G., Lindström J., Valle T. T., et al. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Archives of Internal Medicine. 2004;164(8):p. 892. doi: 10.1001/archinte.164.8.892. [DOI] [PubMed] [Google Scholar]
- 183.Morohoshi M., Fujisawa K., Uchimuraa I., Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45(7):954–959. doi: 10.2337/diab.45.7.954. [DOI] [PubMed] [Google Scholar]
- 184.Iida K. T., Shimano H., Kawakami Y., et al. Insulin up-regulates tumor necrosis factor-α production in macrophages through an extracellular-regulated kinase-dependent pathway. Journal of Biological Chemistry. 2001;276(35):32531–32537. doi: 10.1074/jbc.m009894200. [DOI] [PubMed] [Google Scholar]
- 185.Chung H. Y., Kim H. J., Kim J. W., Yu B. P. The inflammation hypothesis of aging. Annals of the New York Academy of Sciences. 2001;928(1):327–335. doi: 10.1111/j.1749-6632.2001.tb05662.x. [DOI] [PubMed] [Google Scholar]
- 186.Ye J. Mechanisms of insulin resistance in obesity. Frontiers of Medicine in China. 2013;7(1, article 262):14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koulmanda M., Bhasin M., Awdeh Z., et al. The role of TNF-α in mice with type 1- and 2-diabetes. PLoS One. 2012;7(5):p. e33254. doi: 10.1371/journal.pone.0033254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Belwal T., Nabavi S., Nabavi S., Habtemariam S. Dietary anthocyanins and insulin resistance: when food becomes a medicine. Nutrients. 2017;9(10):p. 1111. doi: 10.3390/nu9101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hotamisligil G. S., Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nature Reviews Immunology. 2008;8(12):923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Solinas G., Karin M. JNK1 and IKKβ: molecular links between obesity and metabolic dysfunction. FASEB Journal. 2010;24(8):2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 191.Baker R. G., Hayden M. S., Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metabolism. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Prentki M., Nolan C. J. Islet cell failure in type 2 diabetes. Journal of Clinical Investigation. 2006;116(7):1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim H., Lee M.-S. Role of innate immunity in triggering and tuning of autoimmune diabetes. Current Molecular Medicine. 2009;9(1):30–44. doi: 10.2174/156652409787314471. [DOI] [PubMed] [Google Scholar]
- 194.Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 195.Wolf G., Ziyadeh F. N., Stahl R. A. K. Atrial natriuretic peptide stimulates the expression of transforming growth factor-beta in cultured murine mesangial cells: relationship to suppression of proliferation. Journal of the American Society of Nephrology. 1995;6:224–233. doi: 10.1681/ASN.V62224. [DOI] [PubMed] [Google Scholar]
- 196.Wolf G., Ziyadeh F. N., Zahner G., Stahl R. A. K. Angiotensin II-stimulated expression of transforming growth factor beta in renal proximal tubular cells: attenuation after stable transfection with the c-mas oncogene. Kidney International. 1995;48(6):1818–1827. doi: 10.1038/ki.1995.480. [DOI] [PubMed] [Google Scholar]
- 197.Forbes J. M., Thallas V., Thomas M. C., et al. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. The FASEB Journal. 2003;17(12):1762–1764. doi: 10.1096/fj.02-1102fje. [DOI] [PubMed] [Google Scholar]
- 198.Sharma K., Jin Y., Guo J., Ziyadeh F. N. Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45(4):522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 199.Ziyadeh F. N., Hoffman B. B., Han D. C., et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hill C., Flyvbjerg A., Rasch R., Bak M., Logan A. Transforming growth factor-beta2 antibody attenuates fibrosis in the experimental diabetic rat kidney. Journal of Endocrinology. 2001;170(3):647–651. doi: 10.1677/joe.0.1700647. [DOI] [PubMed] [Google Scholar]
- 201.Hinerfeld D., Traini M. D., Weinberger R. P., et al. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. Journal of Neurochemistry. 2004;88(3):657–667. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- 202.Wang B., Koh P., Winbanks C., et al. MiR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes. 2010;60(1):280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iwano M., Kubo A., Nishino T., et al. Quantification of glomerular TGF-β1 mRNA in patients with diabetes mellitus. Kidney International. 1996;49(4):1120–1126. doi: 10.1038/ki.1996.162. [DOI] [PubMed] [Google Scholar]
- 204.Lizotte F., Denhez B., Guay A., Gévry N., Côté A. M., Geraldes P. Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes. 2016;65(12):3705–3717. doi: 10.2337/db16-0254. [DOI] [PubMed] [Google Scholar]
- 205.Dagher Z., Gerhardinger C., Vaz J., Goodridge M., Tecilazich F., Lorenzi M. The increased transforming growth factor-β signaling induced by diabetes protects retinal vessels. The American Journal of Pathology. 2017;187(3):627–638. doi: 10.1016/j.ajpath.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guay C., Roggli E., Nesca V., Jacovetti C., Regazzi R. Diabetes mellitus, a microRNA-related disease? Translational Research. 2011;157(4):253–264. doi: 10.1016/j.trsl.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 207.Cheng R., Dang R., Zhou Y., Ding M., Hua H. MicroRNA-98 inhibits TGF-β1-induced differentiation and collagen production of cardiac fibroblasts by targeting TGFBR1. Human Cell. 2017;30(3, article 163):192–200. doi: 10.1007/s13577-017-0163-0. [DOI] [PubMed] [Google Scholar]
- 208.Ghosh A. K., Rai R., Flevaris P., Vaughan D. E. Epigenetics in reactive and reparative cardiac fibrogenesis: the promise of epigenetic therapy. Journal of Cellular Physiology. 2017;232(8):1941–1956. doi: 10.1002/jcp.25699. [DOI] [PubMed] [Google Scholar]
- 209.Gustafson B. Adipose tissue, inflammation and atherosclerosis. Journal of Atherosclerosis and Thrombosis. 2010;17(4):332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 210.Conde J., Scotece M., Gómez R., et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors. 2011;37(6):413–420. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 211.Tamura Y., Kawao N., Okada K., et al. Plasminogen activator inhibitor-1 is involved in streptozotocin-induced bone loss in female mice. Diabetes. 2013;62(9):3170–3179. doi: 10.2337/db12-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ashcroft F. M., Rorsman P., Europe PMC Funders Group Diabetes mellitus and the β-cell: the last ten years. Cell. 2018;148(6):1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Okon E. B., Chung A. W. Y., Rauniyar P., et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54(8):2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 214.Gil L. T., Roselló A. M., Torres A. C., Moreno R. L., Orihuela J. A. F. Modulation of soluble phases of endothelial/leukocyte adhesion molecule 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 with interleukin-1β after experimental endotoxic challenge. Critical Care Medicine. 2001;29(4):776–781. doi: 10.1097/00003246-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 215.Noda K., Nakao S., Ishida S., Ishibashi T. Leukocyte adhesion molecules in diabetic retinopathy. Journal of Ophthalmology. 2012;2012:6. doi: 10.1155/2012/279037.279037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Okada S., Shikata K., Matsuda M., et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52(10):2586–2593. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- 217.Chow F. Y., Nikolic-Paterson D. J., Ozols E., Atkins R. C., Tesch G. H. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. Journal of the American Society of Nephrology. 2005;16(6):1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- 218.Miyamoto K., Khosrof S., Bursell S. E., et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proceedings of the National Academy of Sciences. 1999;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hirano Y., Sakurai E., Matsubara A., Ogura Y. Suppression of ICAM-1 in retinal and choroidal endothelial cells by plasmid small-interfering RNAs in vivo. Investigative Opthalmology & Visual Science. 2010;51(1):p. 508. doi: 10.1167/iovs.09-3457. [DOI] [PubMed] [Google Scholar]
- 220.Leinonen E., Hurt-Camejo E., Wiklund O., Hultén L. M., Hiukka A., Taskinen M. R. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166(2):387–394. doi: 10.1016/S0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 221.Jude E. B., Abbott C. A., Young M. J., Anderson S. G., Douglas J. T., Boulton A. J. M. The potential role of cell adhesion molecules in the pathogenesis of diabetic neuropathy. Diabetologia. 1998;41(3):330–336. doi: 10.1007/s001250050911. [DOI] [PubMed] [Google Scholar]
- 222.Doupis J., Lyons T. E., Wu S., Gnardellis C., Dinh T., Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. The Journal of Clinical Endocrinology & Metabolism. 2009;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Spranger J., Kroke A., Mohlig M., et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 224.Luotola K., Pääkkönen R., Alanne M., et al. Association of variation in the interleukin-1 gene family with diabetes and glucose homeostasis. The Journal of Clinical Endocrinology & Metabolism. 2009;94(11):4575–4583. doi: 10.1210/jc.2009-0666. [DOI] [PubMed] [Google Scholar]
- 225.Brown B. D., Nsengimana J., Barrett J. H., et al. An evaluation of inflammatory gene polymorphisms in sibships discordant for premature coronary artery disease: the GRACE-IMMUNE study. BMC Medicine. 2010;8(1, article 264) doi: 10.1186/1741-7015-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stegger J. G., Schmidt E. B., Tjønneland A., et al. Single nucleotide polymorphisms in IL1B and the risk of acute coronary syndrome: a Danish case-cohort study. PLoS ONE. 2012;7(6):p. e36829. doi: 10.1371/journal.pone.0036829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsimikas S., Duff G. W., Berger P. B., et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a) Journal of the American College of Cardiology. 2014;63(17):1724–1734. doi: 10.1016/j.jacc.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Donath M. Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nature Reviews Drug Discovery. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 229.Gothai S., Ganesan P., Park S. Y., Fakurazi S., Choi D. K., Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 2016;8(8):p. 461. doi: 10.3390/nu8080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Anuradha C. V. Phytochemicals targeting genes relevant for type 2 diabetes. Canadian Journal of Physiology and Pharmacology. 2013;91(6):397–411. doi: 10.1139/cjpp-2012-0350. [DOI] [PubMed] [Google Scholar]
- 231.Kim S., Shin B. C., Lee M. S., Lee H., Ernst E. Red ginseng for type 2 diabetes mellitus: a systematic review of randomized controlled trials. Chinese Journal of Integrative Medicine. 2011;17(12):937–944. doi: 10.1007/s11655-011-0937-2. [DOI] [PubMed] [Google Scholar]
- 232.Priya Rani M., Padmakumari K. P., Sankarikutty B., Lijo Cherian O., Nisha V. M., Raghu K. G. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. International Journal of Food Sciences and Nutrition. 2011;62(2):106–110. doi: 10.3109/09637486.2010.515565. [DOI] [PubMed] [Google Scholar]
- 233.Unuofin J. O., Otunola G. A., Afolayan A. J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon. 2018;4(9):p. e00810. doi: 10.1016/j.heliyon.2018.e00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang L.-p., Sun H.-l., Wu L.-m., et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investigative Opthalmology & Visual Science. 2009;50(5):p. 2319. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 235.Ohno M., Shibata C., Kishikawa T., et al. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Scientific Reports. 2013;3(1) doi: 10.1038/srep02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ahad A., Mujeeb M., Ahsan H., Siddiqui W. A. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie. 2014;106:101–110. doi: 10.1016/j.biochi.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 237.Prabhakar O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 2013;386(8, article 871):705–710. doi: 10.1007/s00210-013-0871-2. [DOI] [PubMed] [Google Scholar]
- 238.Guo R., Liu B., Wang K., Zhou S., Li W., Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diabetes and Vascular Disease Research. 2014;11(2):92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 239.Choi J., Yokozawa T., Oura H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Medica. 1991;57(3):208–211. doi: 10.1055/s-2006-960075. [DOI] [PubMed] [Google Scholar]
- 240.Tsai S.-J., Huang C.-S., Mong M.-C., Kam W.-Y., Huang H.-Y., Yin M.-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. Journal of Agricultural and Food Chemistry. 2012;60(1):514–521. doi: 10.1021/jf203259h. [DOI] [PubMed] [Google Scholar]
- 241.Li J. J., Lee S. H., Kim D. K., et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. American Journal of Physiology-Renal Physiology. 2009;297(1):F200–F209. doi: 10.1152/ajprenal.90649.2008. [DOI] [PubMed] [Google Scholar]
- 242.Ahad A., Ganai A. A., Mujeeb M., Siddiqui W. A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicology and Applied Pharmacology. 2014;279(1):1–7. doi: 10.1016/j.taap.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 243.Kumar B., Gupta S. K., Srinivasan B. P., et al. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvascular Research. 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 244.Chang C. L. T., Lin Y., Bartolome A. P., Chen Y. C., Chiu S. C., Yang W. C. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evidence-based Complementary and Alternative Medicine. 2013;2013:33. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim S., Jwa H., Yanagawa Y., Park T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. Journal of Medicinal Food. 2012;15(6):527–534. doi: 10.1089/jmf.2011.2008. [DOI] [PubMed] [Google Scholar]
- 246.Liu M., Wu K., Mao X., Wu Y., Ouyang J. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: regulation of PKB/GLUT4 signaling in skeletal muscle. Journal of Ethnopharmacology. 2010;127(1):32–37. doi: 10.1016/j.jep.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 247.Zhao M., Zhang Z. F., Ding Y., Wang J. B., Li Y. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-κB in C2C12 myotubes. Molecules. 2012;17(6):7083–7092. doi: 10.3390/molecules17067083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu Y., Ou-Yang J. P., Wu K., Wang Y., Zhou Y. F., Wen C. Y. Hypoglycemic effect of Astragalus polysaccharide and its effect on PTP1B. Acta Pharmacologica Sinica. 2005;26(3):345–352. doi: 10.1111/j.1745-7254.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- 249.Mae T., Kishida H., Nishiyama T., et al. A licorice ethanolic extract with peroxisome proliferator-activated receptor-γ ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. The Journal of Nutrition. 2003;133(11):3369–3377. doi: 10.1093/jn/133.11.3369. [DOI] [PubMed] [Google Scholar]
- 250.Weidner C., de Groot J. C., Prasad A., et al. Amorfrutins are potent antidiabetic dietary natural products. Proceedings of the National Academy of Sciences. 2012;109(19):7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Park S., Kim D. S., Kang S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. European Journal of Nutrition. 2011;50(2):107–118. doi: 10.1007/s00394-010-0120-0. [DOI] [PubMed] [Google Scholar]
- 252.Qin B., Panickar K. S., Anderson R. A. Cinnamon: potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. Journal of Diabetes Science and Technology. 2010;4(3):685–693. doi: 10.1177/193229681000400324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rafehi H., Ververis K., Karagiannis T. C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes, Obesity and Metabolism. 2012;14(6):493–499. doi: 10.1111/j.1463-1326.2011.01538.x. [DOI] [PubMed] [Google Scholar]
- 254.Couturier K., Qin B., Batandier C., et al. Cinnamon increases liver glycogen in an animal model of insulin resistance. Metabolism. 2011;60(11):1590–1597. doi: 10.1016/j.metabol.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 255.Li J., Liu T., Wang L., et al. Antihyperglycemic and antihyperlipidemic action of cinnamaldehyde in C57BLKS/J db/db mice. Journal of Traditional Chinese Medicine. 2012;32(3):446–452. doi: 10.1016/S0254-6272(13)60053-9. [DOI] [PubMed] [Google Scholar]
- 256.Valette G., Sauvaire Y., Baccou J., Ribes G. Hypocholesterolaemic effect of Fenugreek seeds in dogs. Atherosclerosis. 1984;50(1):105–111. doi: 10.1016/0021-9150(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 257.Mohan V., Balasubramanyam M. Fenugreek and insulin resistance. The Journal of the Association of Physicians of India. 2001;49:1055–1056. [PubMed] [Google Scholar]
- 258.Puri D., Prabhu K. M., Murthy P. S. Mechanism of action of a hypoglycemic principle isolated from fenugreek seeds. Indian Journal of Physiology and Pharmacology. 2002;46(4):457–462. [PubMed] [Google Scholar]
- 259.Gupta A., Gupta R., Lal B. Effect of Trigonella foenum-graecum (Fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. Journal of the Association of Physicians of India. 2001;49:1057–1061. [PubMed] [Google Scholar]
- 260.Guo J., Li L., Pan J., et al. Pharmacological mechanism of semen litchi on antagonizing insulin resistance in rats with type 2 diabetes. Journal of Chinese Medicinal Materials. 2004;27:435–438. [PubMed] [Google Scholar]
- 261.Zhang H., Teng Y. Effect of li ren (semen litchi) anti-diabetes pills in 45 cases of diabetes mellitus. Journal of Traditional Chinese Medicine. 1986;6(4):277–278. [PubMed] [Google Scholar]
- 262.Al-Romaiyan A., King A. J., Persaud S. J., Jones P. M. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytotherapy Research. 2013;27(7):1006–1011. doi: 10.1002/ptr.4815. [DOI] [PubMed] [Google Scholar]
- 263.Fujii M., Takei I., Umezawa K. Antidiabetic effect of orally administered conophylline-containing plant extract on streptozotocin-treated and Goto-Kakizaki rats. Biomedicine & Pharmacotherapy. 2009;63(10):710–716. doi: 10.1016/j.biopha.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 264.Sabet F., Asgary S., Rahimi P., Mahzouni P., Madani H. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan-induced diabetic rats. Journal of Research in Medical Sciences. 2012;17:386–392. [PMC free article] [PubMed] [Google Scholar]
- 265.Yun S. N., Moon S. J., Ko S. K., Im B. O., Chung S. H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Archives of Pharmacal Research. 2004;27(7):790–796. doi: 10.1007/BF02980150. [DOI] [PubMed] [Google Scholar]
- 266.Yoo K. M., Lee C., Lo Y. M., Moon B. The hypoglycemic effects of American red ginseng (Panax quinquefolius L.) on a diabetic mouse model. Journal of Food Science. 2012;77(7):H147–H152. doi: 10.1111/j.1750-3841.2012.02748.x. [DOI] [PubMed] [Google Scholar]
- 267.Lee H. J., Lee Y. H., Park S. K., et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2009;58(8):1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 268.Chen F., Chen Y., Kang X., Zhou Z., Zhang Z., Liu D. Anti-apoptotic function and mechanism of ginseng saponins in Rattus pancreatic β-cells. Biological & Pharmaceutical Bulletin. 2012;35(9):1568–1573. doi: 10.1248/bpb.b12-00461. [DOI] [PubMed] [Google Scholar]
- 269.Kim H. Y., Kim K. Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic β-cell mass in the hypoglycemic effects of Korean red ginseng in Goto-Kakizaki rats. Journal of Ethnopharmacology. 2012;142(1):53–58. doi: 10.1016/j.jep.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 270.Kim H. Y., Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic β-cells. Journal of Agricultural and Food Chemistry. 2007;55(8):2816–2823. doi: 10.1021/jf062577r. [DOI] [PubMed] [Google Scholar]
- 271.Jong-Anurakkun N., Bhandari M. R., Hong G., Kawabata J. α-Glucosidase inhibitor from Chinese aloes. Fitoterapia. 2008;79(6):456–457. doi: 10.1016/j.fitote.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 272.Lee J. H., Zhou H. Y., Cho S. Y., Kim Y. S., Lee Y. S., Jeong C. S. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Archives of Pharmacal Research. 2007;30(10):1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- 273.Bhathena S. J., Velasquez M. T. Beneficial role of dietary phytoestrogens in obesity and diabetes. The American Journal of Clinical Nutrition. 2002;76(6):1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 274.Lee Y. S., Kim W. S., Kim K. H., et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 275.Jeong H. W., Hsu K. C., Lee J. W., et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(4):E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 276.Chen C., Zhang Y., Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochemical and Biophysical Research Communications. 2010;397(3):543–547. doi: 10.1016/j.bbrc.2010.05.153. [DOI] [PubMed] [Google Scholar]
- 277.Yin J., Gao Z., Liu D., Liu Z., Ye J. Berberine improves glucose metabolism through induction of glycolysis. American Journal of Physiology-Endocrinology and Metabolism. 2008;294(1):E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Meng B., Li J., Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Current Pharmaceutical Design. 2013;19(11):2101–2113. doi: 10.2174/1381612811319110011. [DOI] [PubMed] [Google Scholar]
- 279.Chuengsamarn S., Rattanamongkolgul S., Luechapudiporn R., Phisalaphong C., Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–2127. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wongeakin N., Bhattarakosol P., Patumraj S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 Expressions. BioMed Research International. 2014;2014:10. doi: 10.1155/2014/161346.161346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jain D., Bansal M. K., Dalvi R., Upganlawar A., Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. Journal of Integrative Medicine. 2014;12(1):35–41. doi: 10.1016/S2095-4964(14)60001-7. [DOI] [PubMed] [Google Scholar]
- 282.Zhang X., Zhang R., Lv P., et al. Emodin up-regulates glucose metabolism, decreases lipolysis, and attenuates inflammation in vitro. Journal of Diabetes. 2015;7(3):360–368. doi: 10.1111/1753-0407.12190. [DOI] [PubMed] [Google Scholar]
- 283.Sabu M. C., Smitha K., Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. Journal of Ethnopharmacology. 2002;83(1-2):109–116. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 284.Iso H., Date C., Wakai K., Fukui M., Tamakoshi A., and the JACC Study Group The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Annals of Internal Medicine. 2006;144(8):554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 285.Hayashino Y., Fukuhara S., Okamura T., Tanaka T., Ueshima H., for the HIPOP-OHP Research Group High oolong tea consumption predicts future risk of diabetes among Japanese male workers: a prospective cohort study. Diabetic Medicine. 2011;28(7):805–810. doi: 10.1111/j.1464-5491.2011.03239.x. [DOI] [PubMed] [Google Scholar]
- 286.Ortsäter H., Grankvist N., Wolfram S., Kuehn N., Sjöholm Å. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutrition & Metabolism. 2012;9(1):p. 11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wolfram S., Raederstorff D., Preller M., et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents. The Journal of Nutrition. 2006;136(10):2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 288.Fu Z., Zhang W., Zhen W., et al. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fu Z., Gilbert E. R., Pfeiffer L., Zhang Y., Fu Y., Liu D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Applied Physiology, Nutrition, and Metabolism. 2012;37(3):480–488. doi: 10.1139/h2012-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Leiherer A., Mündlein A., Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascular Pharmacology. 2013;58(1-2):3–20. doi: 10.1016/j.vph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 291.Arunkumar E., Anuradha C. V. Genistein promotes insulin action through adenosine monophosphate-activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutrition Research. 2012;32(8):617–625. doi: 10.1016/j.nutres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 292.Chen A. Y., Chen Y. C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry. 2013;138(4):2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Abo-Salem O. M. Kaempferol attenuates the development of diabetic neuropathic pain in mice: possible anti-inflammatory and anti-oxidant mechanisms. Open Access Macedonian Journal of Medical Sciences. 2014;2(3, article 424) doi: 10.3889/oamjms.2014.073. [DOI] [Google Scholar]
- 294.Zang Y., Zhang L., Igarashi K., Yu C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Function. 2015;6(3):834–841. doi: 10.1039/c4fo00844h. [DOI] [PubMed] [Google Scholar]
- 295.Abuohashish H. M., Al-Rejaie S. S., Al-Hosaini K. A., Parmar M. Y., Ahmed M. M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetology & Metabolic Syndrome. 2013;5(1):p. 5. doi: 10.1186/1758-5996-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Vanitha P., Uma C., Suganya N., et al. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin- induced diabetic rats. Environmental Toxicology and Pharmacology. 2014;37(1):326–335. doi: 10.1016/j.etap.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 297.Tzeng T.-F., Liou S.-S., Liu I.-M. Myricetin ameliorates defective post-receptor insulin signaling via β-endorphin signaling in the skeletal muscles of fructose-fed rats. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1093/ecam/neq017.neq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chang C. J., Tzeng T. F., Liou S. S., Chang Y. S., Liu I. M. Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/787152.787152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sandeep M. S., Nandini C. D. Influence of quercetin, naringenin and berberine on glucose transporters and insulin signalling molecules in brain of streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy. 2017;94:605–611. doi: 10.1016/j.biopha.2017.07.142. [DOI] [PubMed] [Google Scholar]
- 300.Pu P., Gao D. M., Mohamed S., et al. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Archives of Biochemistry and Biophysics. 2012;518(1):61–70. doi: 10.1016/j.abb.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 301.Szkudelska K., Szkudelski T. Resveratrol, obesity and diabetes. European Journal of Pharmacology. 2010;635(1-3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 302.Do G. M., Jung U. J., Park H. J., et al. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Molecular Nutrition & Food Research. 2012;56(8):1282–1291. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- 303.Mishra R., Sellin D., Radovan D., Gohlke A., Winter R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem. 2009;10(3):445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- 304.Breen D. M., Sanli T., Giacca A., Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochemical and Biophysical Research Communications. 2008;374(1):117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 305.Kar P., Laight D., Rooprai H. K., Shaw K. M., Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabetic Medicine. 2009;26(5):526–531. doi: 10.1111/j.1464-5491.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 306.Brasnyó P., Molnár G. A., Mohás M., et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 307.Patil R. N., Patil R. Y., Ahirwar B., Ahirwar D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pacific Journal of Tropical Medicine. 2011;4(1):20–23. doi: 10.1016/S1995-7645(11)60025-4. [DOI] [PubMed] [Google Scholar]
- 308.Hegazy G. A., Alnoury A. M., Gad H. G. The role of Acacia arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Medical Journal. 2013;34(7):727–733. [PubMed] [Google Scholar]
- 309.Omara E. A., Nada S. A., Farrag A. R. H., Sharaf W. M., El-Toumy S. A. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine. 2012;19(12):1059–1067. doi: 10.1016/j.phymed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 310.Geetha G., Kalavalarasariel Gopinathapillai P., Sankar V. Anti diabetic effect of Achyranthes rubrofusca leaf extracts on alloxan induced diabetic rats. Pakistan Journal of Pharmaceutical Sciences. 2011;24(2):193–199. [PubMed] [Google Scholar]
- 311.Ahmed D., Kumar V., Verma A., et al. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complementary and Alternative Medicine. 2014;14(1, article 1848) doi: 10.1186/1472-6882-14-243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 312.Patel P., Parikh M., Johari S., Gandhi T. Antihyperglycemic activity of Albizzia lebbeck bark extract in streptozotocin-nicotinamide induced type II diabetes mellitus rats. AYU (An International Quarterly Journal of Research in Ayurveda) 2015;36(3):335–340. doi: 10.4103/0974-8520.182752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kumar R., Sharma B., Tomar N. R., Roy P., Gupta A. K., Kumar A. In vivo evaluation of hypoglycemic activity of Aloe spp. and identification of its mode of action on GLUT-4 gene expression in vitro. Applied Biochemistry and Biotechnology. 2011;164(8):1246–1256. doi: 10.1007/s12010-011-9210-6. [DOI] [PubMed] [Google Scholar]
- 314.Noor A., Gunasekaran S., Soosai Manickam A., Vijayalakshmi M. A. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin-induced diabetic rats. Current Science. 2008;94:1070–1076. [Google Scholar]
- 315.Manjunath K., Bhanu G., Subash K. R., Tadvi N. A., Manikanta M., Umamaheswara K. Effect of Aloe vera leaf extract on blood glucose levels in alloxan induced diabetic rats. National Journal of Physiology, Pharmacy and Pharmacology. 2016;6(5):p. 471. doi: 10.5455/njppp.2016.6.0718613072016. [DOI] [Google Scholar]
- 316.Afolayan A. J., Sunmonu T. O. Artemisia afra Jacq. ameliorates oxidative stress in the pancreas of streptozotocin-induced diabetic Wistar rats. Bioscience, Biotechnology, and Biochemistry. 2011;75(11):2083–2086. doi: 10.1271/bbb.100792. [DOI] [PubMed] [Google Scholar]
- 317.Dheer R., Bhatnagar P. A study of the antidiabetic activity of Barleria prionitis Linn. Indian Journal of Pharmacology. 2010;42(2):69–73. doi: 10.4103/0253-7613.64493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pari L., Amarnath Satheesh M. Antidiabetic effect of Boerhavia diffusa: effect on serum and tissue lipids in experimental diabetes. Journal of Medicinal Food. 2004;7(4):472–476. doi: 10.1089/jmf.2004.7.472. [DOI] [PubMed] [Google Scholar]
- 319.Baquer N. Z., Gupta D., Raju J. Regulation of metabolic pathways in liver and kidney during experimental diabetes: effects of antidiabetic compounds. Indian Journal of Clinical Biochemistry. 1998;13(2):63–80. doi: 10.1007/BF02867866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bhat M., Zinjarde S. S., Bhargava S. Y., Kumar A. R., Joshi B. N. Antidiabetic Indian plants: a good source of potent amylase inhibitors. Evidence-Based Complementary and Alternative Medicine. 2011;2011:6. doi: 10.1093/ecam/nen040.nen040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Geethan P. K. M. A., Prince P. S. M. Antihyperlipidemic effect of D-pinitol on streptozotocin-induced diabetic Wistar rats. Journal of Biochemical and Molecular Toxicology. 2008;22(4):220–224. doi: 10.1002/jbt.20218. [DOI] [PubMed] [Google Scholar]
- 322.Perez-Gutierrez R. M., Muñiz-Ramirez A., Gomez Y. G., Ramírez E. B. Antihyperglycemic, antihyperlipidemic and antiglycation effects of Byrsonima crassifolia fruit and seed in normal and streptozotocin-induced diabetic rats. Plant Foods for Human Nutrition. 2010;65(4):350–357. doi: 10.1007/s11130-010-0181-5. [DOI] [PubMed] [Google Scholar]
- 323.Vasconcelos C. F. B., Maranhão H. M. L., Batista T. M., et al. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea Martius bark extract on streptozotocin-induced diabetes in Wistar rats. Journal of Ethnopharmacology. 2011;137(3):1533–1541. doi: 10.1016/j.jep.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 324.Prakasam A., Sethupathy S., Pugalendi K. V. Influence of Casearia esculenta root extract on protein metabolism and marker enzymes in streptozotocin-induced diabetic rats. Polish Journal of Pharmacology. 2004;56:587–593. [PubMed] [Google Scholar]
- 325.Agnihotri A., Singh V. Effect of Tamarindus indica Linn. and Cassia fistula Linn. stem bark extracts on oxidative stress and diabetic conditions. Acta Poloniae Pharmaceutica. 2013;70(6):1011–1019. [PubMed] [Google Scholar]
- 326.Singh S. N., Vats P., Suri S., et al. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. Journal of Ethnopharmacology. 2001;76(3):269–277. doi: 10.1016/S0378-8741(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 327.Nabeel M. A., Kathiresan K., Manivannan S. Antidiabetic activity of the mangrove species Ceriops decandra in alloxan-induced diabetic rats. Journal of Diabetes. 2010;2(2):97–103. doi: 10.1111/j.1753-0407.2010.00068.x. [DOI] [PubMed] [Google Scholar]
- 328.Orhan N., Aslan M., Şüküroǧlu M., Deliorman Orhan D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC-TOF-MS analysis. Journal of Ethnopharmacology. 2013;146(3):859–865. doi: 10.1016/j.jep.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 329.Agarwal V., Sharma A. K., Upadhyay A., Singh G., Gupta R. Hypoglycemic effects of Citrullus colocynthis roots. Acta Poloniae Pharmaceutica. 2012;69:75–79. [PubMed] [Google Scholar]
- 330.Dechandt C. R. P., Siqueira J. T., de Souza D. L. P., et al. Combretum lanceolatum flowers extract shows antidiabetic activity through activation of AMPK by quercetin. Revista Brasileira de Farmacognosia. 2013;23(2):291–300. doi: 10.1590/s0102-695x2012005000140. [DOI] [Google Scholar]
- 331.Nain P., Saini V., Sharma S., Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. Journal of Ethnopharmacology. 2012;142(1):65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 332.Hassan Z., Yam M. F., Ahmad M., Yusof A. P. M. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules. 2010;15(12):9008–9023. doi: 10.3390/molecules15129008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Venkatesh S., Reddy B. M., Reddy G. D., Mullangi R., Lakshman M. Antihyperglycemic and hypolipidemic effects of Helicteres isora roots in alloxan-induced diabetic rats: a possible mechanism of action. Journal of Natural Medicines. 2010;64(3):295–304. doi: 10.1007/s11418-010-0406-9. [DOI] [PubMed] [Google Scholar]
- 334.Maheshwari P., Baburao B., Pradeep Kumar C., Reddy A. R. N. Antidiabetic activity of methanolic extract of Hiptage bengalensis leaves in alloxan induced diabetic models. Pakistan Journal of Biological Sciences. 2013;16(17):844–851. doi: 10.3923/pjbs.2013.844.851. [DOI] [PubMed] [Google Scholar]
- 335.Mishra S. B., Verma A., Mukerjee A., Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Medicine. 2011;4(9):689–693. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 336.Kumar S., Kumar V., Prakash O. M. Antidiabetic and hypolipidemic activities of Kigelia pinnata flowers extract in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine. 2012;2(7):543–546. doi: 10.1016/s2221-1691(12)60093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ma C., Yu H., Xiao Y., Wang H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharmaceutical Biology. 2017;55(1):2170–2177. doi: 10.1080/13880209.2017.1396350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kesari A. N., Gupta R. K., Watal G. Hypoglycemic effects of Murraya koenigii on normal and alloxan-diabetic rabbits. Journal of Ethnopharmacology. 2005;97(2):247–251. doi: 10.1016/j.jep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 339.Saba A. B., Oyagbemi A. A., Azeez O. I. Antidiabetic and haematinic effects of Parquetina nigrescens on alloxan induced type-1 diabetes and normocytic normochromic anaemia in Wistar rats. African Health Sciences. 2010;10 [PMC free article] [PubMed] [Google Scholar]
- 340.Mard S. A., Jalalvand K., Jafarinejad M., Balochi H., Naseri M. K. G. Evaluation of the antidiabetic and antilipaemic activities of the hydroalcoholic extract of Phoenix dactylifera palm leaves and its fractions in alloxan-induced diabetic rats. The Malaysian Journal of Medical Sciences. 2010;17:4–13. [PMC free article] [PubMed] [Google Scholar]
- 341.Okoli C. O., Ibiam A. F., Ezike A. C., Akah P. A., Okoye T. C. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic rats. African Journal of Biotechnology. 2010;9 [Google Scholar]
- 342.Tamrakar A. K., Jaiswal N., Yadav P. P., Maurya R., Srivastava A. K. Pongamol from Pongamia pinnata stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells. Molecular and Cellular Endocrinology. 2011;339(1-2):98–104. doi: 10.1016/j.mce.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 343.Goli V., Kanakam V., Macharala S., Gowrishankar N. L., Jimmidi B., Dhanalakshmi C. H. Antidiabetic activity of Pongamia pinnata flower extracts on alloxan induced diabetic rats. Journal of Global Pharma Technology. 2012;4(2):13–17. [Google Scholar]
- 344.Huang C. S., Yin M. C., Chiu L. C. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food and Chemical Toxicology. 2011;49(9):2189–2195. doi: 10.1016/j.fct.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 345.Kaur G., Kamboj P., Kalia A. N. Antidiabetic and anti-hypercholesterolemic effects of aerial parts of Sida cordifolia Linn. on streptozotocin-induced diabetic rats. Indian Journal of Natural Products and Resources. 2011;2:428–434. [Google Scholar]
- 346.Prabhu K. S., Lobo R., Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. Journal of Pharmacy and Pharmacology. 2008;60(7):909–916. doi: 10.1211/jpp.60.7.0013. [DOI] [PubMed] [Google Scholar]
- 347.Latha R. C. R., Daisy P. Therapeutic potential of octyl gallate isolated from fruits of Terminalia bellerica in streptozotocin-induced diabetic rats. Pharmaceutical Biology. 2013;51(6):798–805. doi: 10.3109/13880209.2013.766894. [DOI] [PubMed] [Google Scholar]
- 348.Rao N. K., Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine. 2006;6(1, article 91) doi: 10.1186/1472-6882-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mowl A., Alauddin M., Rahman M., Ahmed K. Antihyperglycemic effect of Trigonella foenum-graecum (fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: a brief qualitative phytochemical and acute toxicity test on the extract. African Journal of Traditional, Complementary and Alternative Medicines. 2010;6(3) doi: 10.4314/ajtcam.v6i3.57165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Meenakshi P., Bhuvaneshwari R., Rathi M. A., et al. Antidiabetic activity of ethanolic extract of Zaleya decandra in alloxan-induced diabetic rats. Applied Biochemistry and Biotechnology. 2010;162(4, article 8871):1153–1159. doi: 10.1007/s12010-009-8871-x. [DOI] [PubMed] [Google Scholar]
- 351.Martineau L. C., Couture A., Spoor D., et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9-10):612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 352.Inaguma T., Han J., Isoda H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proceedings. 2011;5(Supplement 8):p. P21. doi: 10.1186/1753-6561-5-s8-p21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ueda M., Furuyashiki T., Yamada K., et al. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes. Food & Function. 2010;1(2):167–173. doi: 10.1039/c0fo00105h. [DOI] [PubMed] [Google Scholar]
- 354.Yan J., Zhao Y., Suo S., Liu Y., Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radical Biology and Medicine. 2012;52(9):1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 355.Asano K., Takagi K., Haneishi A., Nakamura S., Yamada K. (−)-Epigallocatechin-3-gallate stimulates both AMP-activated protein kinase and nuclear factor-kappa B signaling pathways. Food Chemistry. 2012;134(2):783–788. doi: 10.1016/j.foodchem.2012.02.181. [DOI] [PubMed] [Google Scholar]
- 356.Ueda M., Nishiumi S., Nagayasu H., Fukuda I., Yoshida K.-i., Ashida H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochemical and Biophysical Research Communications. 2008;377(1):286–290. doi: 10.1016/j.bbrc.2008.09.128. [DOI] [PubMed] [Google Scholar]
- 357.Lin C. L., Lin J. K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Molecular Nutrition & Food Research. 2008;52(8):930–939. doi: 10.1002/mnfr.200700437. [DOI] [PubMed] [Google Scholar]
- 358.Murase T., Misawa K., Haramizu S., Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochemical Pharmacology. 2009;78(1):78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 359.Zhang Z. F., Li Q., Liang J., et al. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine. 2010;17(1):14–18. doi: 10.1016/j.phymed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 360.Zygmunt K., Faubert B., MacNeil J., Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and Biophysical Research Communications. 2010;398(2):178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 361.Cazarolli L. H., Folador P., Moresco H. H., Brighente I. M. C., Pizzolatti M. G., Silva F. R. M. B. Stimulatory effect of apigenin-6-C-β-l-fucopyranoside on insulin secretion and glycogen synthesis. European Journal of Medicinal Chemistry. 2009;44(11):4668–4673. doi: 10.1016/j.ejmech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 362.Kim M. S., Hur H. J., Kwon D. Y., Hwang J. T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Molecular and Cellular Endocrinology. 2012;358(1):127–134. doi: 10.1016/j.mce.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 363.Onda K., Horike N., Suzuki T. I., Hirano T. Polymethoxyflavonoids tangeretin and nobiletin increase glucose uptake in murine adipocytes. Phytotherapy Research. 2013;27(2):312–316. doi: 10.1002/ptr.4730. [DOI] [PubMed] [Google Scholar]
- 364.Cazarolli L. H., Pereira D. F., Kappel V. D., et al. Insulin signaling: a potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. European Journal of Pharmacology. 2013;712(1-3):1–7. doi: 10.1016/j.ejphar.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 365.Fang X. K., Gao J., Zhu D. N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sciences. 2008;82(11-12):615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 366.Cazarolli L. H., Folador P., Pizzolatti M. G., Silva F. R. M. B. Signaling pathways of kaempferol-3-neohesperidoside in glycogen synthesis in rat soleus muscle. Biochimie. 2009;91(7):843–849. doi: 10.1016/j.biochi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 367.Paoli P., Cirri P., Caselli A., et al. The insulin-mimetic effect of Morin: a promising molecule in diabetes treatment. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(4):3102–3111. doi: 10.1016/j.bbagen.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 368.Matsuda H., Kogami Y., Nakamura S., Sugiyama T., Ueno T., Yoshikawa M. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorganic & Medicinal Chemistry. 2011;19(9):2835–2841. doi: 10.1016/j.bmc.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 369.Lee M. S., Kim C. H., Hoang D. M., et al. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biological & Pharmaceutical Bulletin. 2009;32(3):504–508. doi: 10.1248/bpb.32.504. [DOI] [PubMed] [Google Scholar]
- 370.Sai K. S., Srividya N. Blood glucose lowering effect of the leaves of Tinospora cordifolia and Sauropus androgynus in diabetic subjects. Journal of Natural Remedies. 2002;2:28–32. [Google Scholar]
- 371.Salehi. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):p. 551. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Singh S., Gupta S. K., Sabir G., Gupta M. K., Seth P. K. A database for anti-diabetic plants with clinical/ experimental trials. Bioinformation. 2009;4(6):263–268. doi: 10.6026/97320630004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.El-Demerdash F. M., Yousef M. I., El-Naga N. I. A. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan- induced diabetic rats. Food and Chemical Toxicology. 2005;43(1):57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 374.Azuma K., Minami Y., Ippoushi K., Terao J. Lowering effects of onion intake on oxidative stress biomarkers in streptozotocin-induced diabetic rats. Journal of Clinical Biochemistry and Nutrition. 2007;40(2):131–140. doi: 10.3164/jcbn.40.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.El-Soud N. A., Khalil M. Antioxidative effects of Allium cepa essential oil in streptozotocin induced diabetic rats. Macedonian Journal of Medical Sciences. 2010;1(1):1–8. doi: 10.3889/MJMS.1857-5773.2010.0118. [DOI] [Google Scholar]
- 376.Ogunmodede O. S., Saalu L. C., Ogunlade B., Akunna G. G., Oyewopo A. O. An evaluation of the hypoglycemic, antioxidant and hepatoprotective potentials of onion (Allium cepa L.) on alloxan-induced diabetic rabbits. International Journal of Pharmacology. 2012;8(1):21–29. doi: 10.3923/ijp.2012.21.29. [DOI] [Google Scholar]
- 377.Akash M. S. H., Rehman K., Chen S. Spice plant Allium cepa: dietary supplement for treatment of type 2 diabetes mellitus. Nutrition. 2014;30(10):1128–1137. doi: 10.1016/j.nut.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 378.Governa P., Baini G., Borgonetti V., et al. Phytotherapy in the management of diabetes: a review. Molecules. 2018;23(1):105–122. doi: 10.3390/molecules23010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Eldin I. M. T., Ahmed E. M., Abd E. H. M. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environmental Health Insights. 2010;4 doi: 10.4137/ehi.s5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Bunyapraphatsara N., Yongchaiyudha S., Rungpitarangsi V., Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996;3(3):245–248. doi: 10.1016/S0944-7113(96)80061-4. [DOI] [PubMed] [Google Scholar]
- 381.Choi H.-C., Kim S.-J., Son K.-Y., Oh B.-J., Cho B.-L. Metabolic effects of Aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: randomized controlled trial. Nutrition. 2013;29(9):1110–1114. doi: 10.1016/j.nut.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 382.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T. P. A. Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition. 2007;40(3):163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Khan A., Bryden N. A., Polansky M. M., Anderson R. A. Insulin potentiating factor and chromium content of selected foods and spices. Biological Trace Element Research. 1990;24(2-3):183–188. doi: 10.1007/BF02917206. [DOI] [PubMed] [Google Scholar]
- 384.Sartorius T., Peter A., Schulz N., et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kirkham S., Akilen R., Sharma S., Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obesity and Metabolism. 2009;11(12):1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 386.Hasanzade F., Toliat M., Emami S. A., Emamimoghaadam Z. The effect of cinnamon on glucose of type II diabetes patients. Journal of Traditional and Complementary Medicine. 2013;3(3):171–174. doi: 10.4103/2225-4110.114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mang B., Wolters M., Schmitt B., et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. European Journal of Clinical Investigation. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 388.Gutierrez J. L., Bowden R. G., Willoughby D. S. Cassia cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. Journal of Dietary Supplements. 2016;13(4):461–471. doi: 10.3109/19390211.2015.1110222. [DOI] [PubMed] [Google Scholar]
- 389.Altschuler J. A., Casella S. J., MacKenzie T. A., Curtis K. M. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30(4):813–816. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]
- 390.Hosseini S., Jamshidi L., Mehrzadi S., et al. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. Journal of Ethnopharmacology. 2014;152(3):451–456. doi: 10.1016/j.jep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 391.Nkambo W., Anyama N. G., Onegi B. In vivo hypoglycemic effect of methanolic fruit extract of Momordica charantia L. African Health Sciences. 2014;13(4, article 933) doi: 10.4314/ahs.v13i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Jia S., Shen M., Zhang F., Xie J. Recent advances in Momordica charantia: functional components and biological activities. International Journal of Molecular Sciences. 2017;18(12):p. 2555. doi: 10.3390/ijms18122555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Fuangchan A., Sonthisombat P., Seubnukarn T., et al. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. Journal of Ethnopharmacology. 2011;134(2):422–428. doi: 10.1016/j.jep.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 394.Vats V., Yadav S. P., Grover J. K. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. Journal of Ethnopharmacology. 2004;90(1):155–160. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 395.Suanarunsawat T., Na Ayutthaya W. D., Songsak T., Thirawarapan S., Poungshompoo S. Antioxidant activity and lipid-lowering effect of essential oils extracted from Ocimum sanctum L. leaves in rats fed with a high cholesterol diet. Journal of Clinical Biochemistry and Nutrition. 2010;46 doi: 10.3164/jcbn.09-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pattanayak P., Behera P., Das D., Panda S. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: an overview. Pharmacognosy Reviews. 2010;4(7):95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Agrawal P., Rai V., Singh R. B. Randomized placebo-controlled, single blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. International Journal of Clinical Pharmacology and Therapeutics. 1996;34:406–409. [PubMed] [Google Scholar]
- 398.Satapathy S., Das N., Bandyopadhyay D., Mahapatra S. C., Sahu D. S., Meda M. Effect of Tulsi (Ocimum sanctum Linn.) supplementation on metabolic parameters and liver enzymes in young overweight and obese subjects. Indian Journal of Clinical Biochemistry. 2017;32(3):357–363. doi: 10.1007/s12291-016-0615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Xiang Y. Z., Shang H. C., Gao X. M., Zhang B. L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytotherapy Research. 2008;22(7):851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 400.Kim H. G., Yoo S. R., Park H. J., et al. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: a randomized, placebo-controlled clinical trial. Food and Chemical Toxicology. 2011;49(9):2229–2235. doi: 10.1016/j.fct.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 401.Shergis J. L., Zhang A. L., Zhou W., Xue C. C. Panax ginseng in randomised controlled trials: a systematic review. Phytotherapy Research. 2013;27(7):949–965. doi: 10.1002/ptr.4832. [DOI] [PubMed] [Google Scholar]
- 402.Shishtar E.'., Sievenpiper J. L., Djedovic V., et al. The effect of ginseng (the genus Panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One. 2014;9(9):p. e107391. doi: 10.1371/journal.pone.0107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Bunawan H., Bunawan S. N., Baharum S. N., Noor N. M. Sauropus androgynus (L.) Merr. induced bronchiolitis obliterans: from botanical studies to toxicology. Evidence-based Complementary and Alternative Medicine. 2015;2015:7. doi: 10.1155/2015/714158.714158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Suparmi S., Fasitasari M., Martosupono M., Mangimbulude J. C. Comparisons of curative effects of chlorophyll from Sauropus androgynus (L) Merr leaf extract and Cu-chlorophyllin on sodium nitrate-induced oxidative stress in rats. Journal of Toxicology. 2016;2016:7. doi: 10.1155/2016/8515089.8515089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hokayem M., Blond E., Vidal H., et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2013;36(6):1454–1461. doi: 10.2337/dc12-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Balbi M. E., Tonin F. S., Mendes A. M., et al. Antioxidant effects of vitamins in type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetology & Metabolic Syndrome. 2018;10(1) doi: 10.1186/s13098-018-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Choi S. W., Ho C. K. Antioxidant properties of drugs used in Type 2 diabetes management: could they contribute to, confound or conceal effects of antioxidant therapy? Redox Report. 2018;23(1):1–24. doi: 10.1080/13510002.2017.1324381. [DOI] [PMC free article] [PubMed] [Google Scholar]


