Abstract
Biomarkers, also called biological markers, are indicators to identify a biological case or situation as well as detecting any presence of biological activities and processes. Proteins are considered as a type of biomarkers based on their characteristics. Therefore, proteomics approach is one of the most promising approaches in this field. The purpose of this review is to summarize the use of proteomics approach and techniques to identify proteins as biomarkers for different diseases. This review was obtained by searching in a computerized database. So, different researches and studies that used proteomics approach to identify different biomarkers for different diseases were reviewed. Also, techniques of proteomics that are used to identify proteins as biomarkers were collected. Techniques and methods of proteomics approach are used for the identification of proteins' activities and presence as biomarkers for different types of diseases from different types of samples. There are three essential steps of this approach including: extraction and separation of proteins, identification of proteins, and verification of proteins. Finally, clinical trials for new discovered biomarker or undefined biomarker would be on.
Keywords: Proteomics, Diseases biomarkers, Biological markers, Protein informatics, Bioinformatics
1. Introduction
Reliable biomarkers, also called reliable biological markers, are reliable indicators to identify a biological case or situation. These biomarkers can detect any presence biological activities and processes (Biomarkers Definitions Working Group, 2001, Strimbu and Tavel, 2010). In medicine, biological markers are used to detect a disease, discover drugs, and monitoring care of patients. According to that, biomarkers can be classified into four types including: predisposition biomarkers, diagnostic biomarkers, prognostic biomarkers, and predictive biomarkers (Mayeux, 2004, Medicine, 2019, Brody, 2016, Huss, 2015).
Also, biomarkers can be classified based on their characteristics such as molecular biomarkers. Molecular biomarkers can be divided based on their biophysical properties into nucleic acid, peptides, proteins, lipids, metabolites, and other small molecules (Medicine, 2019, Huss, 2015, Davis et al., 2013).
Also, reliable biomarkers can be hormones, different receptors, enzymes as well as genetic changes. In addition, they can be isoenzymes, carbohydrate epitopes, many products of oncogenes, and different oncofetal antigens. All these biomarkers can be used to diagnose a specific disease and differentiate between these diseases (Huss, 2015, DePrimo, 2007, Doustjalali, 2014).
Among all these types of biomarkers, proteins can be very sensitive to be detected in a very tiny amount of a sample to diagnose a specific type of diseases in its early diagnosis (Doustjalali, 2014, Shah and Misra, 2011). Therefore, novel approaches as well as new applications and techniques were necessary to accomplish the identification and discovery of reliable biomarkers for different types of diseases by using proteins as biomarkers. One of the most promising approach and techniques that are used for the identification of proteins as biomarkers for diseases are called proteomics (DePrimo, 2007, Doustjalali, 2014, Shah and Misra, 2011).
In order to understand proteomics, understanding what is proteome is necessary. According to American Medical Association (AMA) and Office of Cancer Clinical Proteomics Research at National Cancer Institute (NCI), the term of proteome was taken from two words; protein and genome, so prote- was taken from protein and -ome from genome. Therefore, proteomes are proteins that are expressed by different genomes, and other cells (Patel et al., 2016, Rodriguez, 2015, Maloy et al., 2013, Sabino and Hermes, 2017).
Thus, proteomics is a huge scale that studies different functions of proteins, structures of proteins, roles behind the appearance of specific proteins, and the principal of each protein. Biological and medical researchers and scientists applied the applications and techniques of proteomics approach to identify biomarkers to help them in diseases diagnosis, prognosis, and treatment (Patel et al., 2016, Rodriguez, 2015, Maloy et al., 2013, Sabino and Hermes, 2017, Kisluk et al., 2014).
Therefore, based on proteomics approach for reliable biomarkers’ identification for diagnostic and prognostic purposes for different types of diseases, this paper discusses the ability of discovering or identifying reliable biomarkers for different diseases with different types of samples by using different proteomics techniques.
As the goal of this paper is to describe different proteomics techniques to identify reliable biomarkers for different diseases with different types of samples. Thus, achieving this goal, different researches and studies that used proteomics approach to discover and identify different biomarkers for different diseases were reviewed. Therefore, the database of this review paper was selected to be recent studies from 2000 up to 2019 titled with proteomics approach, methods, and tools to diagnose many diseases and identify new diseases. Then, all the proteomics techniques that are included in all the steps of using proteomics approach would be discussed in this paper based on these techniques' advantages and disadvantages to achieve the goal.
2. Proteomics approaches to identify reliable biomarkers
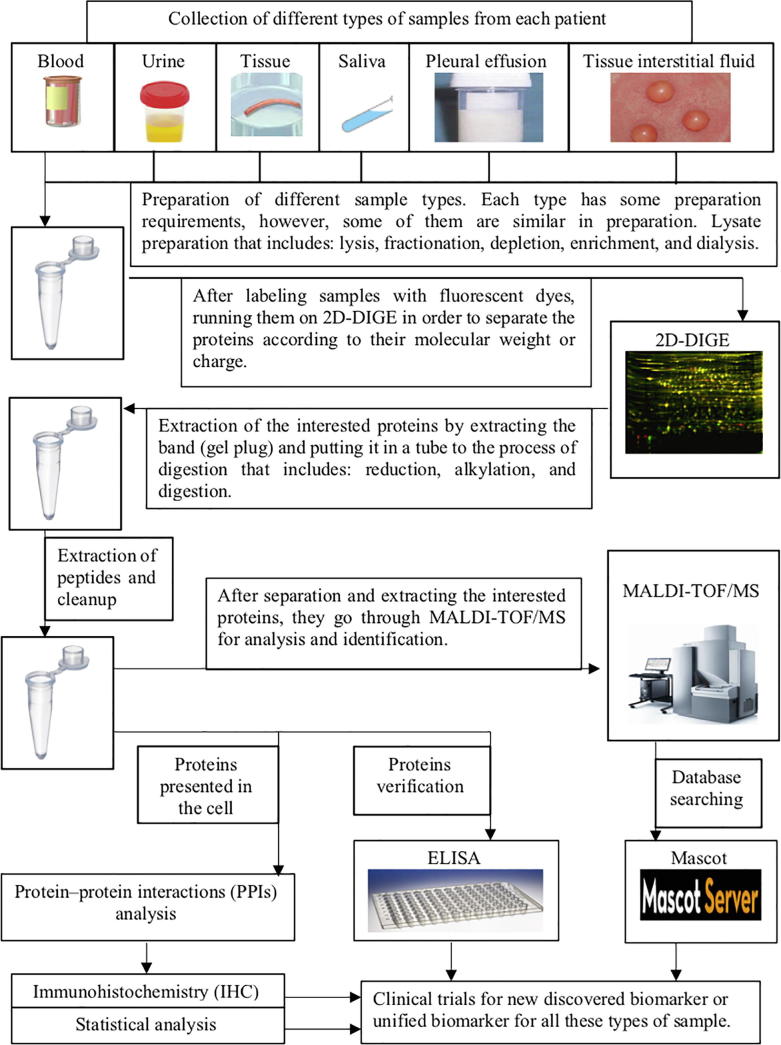
The goal of this paper is to describe use of proteomics approach and techniques to identify reliable biomarkers for the diagnosis of different diseases form different types of samples. After collecting the samples from patients, types of samples include blood samples, tissue samples, tissue interstitial fluid samples, saliva and urine samples (Kisluk et al, 2014). There are three main steps in proteomic analysis in order to identify a biomarker in a specific disease. These steps including; (1) extraction and separation of proteins, (2) identification of proteins, and (3) verification of proteins (Fig. 1) (Liu et al, 2014). Then, the final steps include: (1) Database searching, (2) Protein–protein interactions (PPIs) analysis, and (3) Statistical analysis (Fig. 1) (Swiss Institute of Bioinformatics (SIB), 2019, IBM, 2019, Delwiche and The, 2012).
Fig. 1.
The process of methods of proteomics approach to identify reliable biomarkers.
2.1. Collection, pretreatment, and preparation of the samples
First, collecting different types of samples from a group of patients. So, getting from each patient a sample of pleural effusion, saliva, blood, urine, tissue, and tissue interstitial fluid (TIF). Secondly, pretreatment and preparation of samples depends on the nature of the sample such as; tissue samples pretreatment and preparation are different from blood samples pretreatment and preparation. Therefore, using different lysis buffers, salines, and different digestive processes are required from sample to sample. Also, the environments and conditions that are required to save the samples helpful for the tests are different.
2.2. Extraction and separation of proteins
There are several proteomics techniques to extract and separate proteins involving: 2-DE, LCM, and 2D-DIGE (Liu et al., 2014, Roy and Shukla, 2008, Wang et al., 2015).
2-DE is the most common used proteomics tool in proteomics research because it has the ability to compare the quantity of proteins as well as showing the isoforms of these proteins on the same exact gel. So, in this technique, separation of proteins would be based on two principles; first, in 2-DE, separation of proteins would be based on isoelectric points, then; two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) would separate proteins according to the molecular weights of these proteins (Liu et al., 2014, Marín-García, 2007).
LCM is a technique that is used in proteomics researches to extract the exact needed cells from a sample. So, by using LCM, researchers ensure that the isolation of these isolated cells is clean from other cells of the sample as well as keeping the molecular characteristics of the cells from being changed. This technique is able to detect signals of proteins that have low presence by dilution the signals of these proteins. Integration between LCM and 2-DE gives a powerful technique that is used by proteomics researches in order to look for new targets and biomarkers (Roy and Shukla, 2008, Zhao and Sun, 2015, Oyejide et al., 2017).
2D-DIGE is a novel technique that is used proteomics researches that based on using different fluorescent dyes in order to label proteins, without affecting the isoelectric points and molecular weight proteins, before running them on a 2-DE gel. So, when the signals of proteins are received, these signals can be identified even if the proteins are in the same spot. 2D-DIGE in proteomics researches gives reliable results because of the labeling of proteins gives different signals of different fluorescence showing specific proteins (Liu et al., 2014, Wang et al., 2004).
1-DE and 2-DE techniques are considering as powerful proteomic techniques in order to extract and separate proteins. 1-DE separates proteins depending on these proteins charge while 2-DE separates proteins depending on these proteins molecular weight. However, these techniques have some limitations in proteins separation. This paper describes 2D-DIGE that separates proteins depending on proteins molecular weight or proteins charge with fluorescent labeling (Liu et al., 2014, Marín-García, 2007, Wang et al., 2004). There is a need for rehydration for the gel by using some solutions (e.g. isoelectric focusing (IEF) that separate proteins based on their charges) or using different type of gel such as SDS-PAGE for separation of proteins based on their molecular weight. Then, use of different types of stains (e.g. coomassie blue or silver) to visualize proteins (Yang et al., 2014, Liu et al., 2014).
2.3. Identification of proteins
There are several proteomics techniques to identify proteins involving; tissue array, and mass spectrometry (MS) and its different forms (Liu et al., 2014, Roy and Shukla, 2008, Wang et al., 2015, Mellon, 2003, Cao and Limbach, 2017).
Tissue array is not the tool that is preferred by proteomics researchers because it identifies a very huge number of proteins in rapid way. Thus, it causes a challenge and difficult work to choose and prove the best target. On the other hand, MS save that time and makes the work easier than tissue array does. (Roy and Shukla, 2008, Aulbach and Amuzie, 2017).
Because of the accuracy and sensitivity of mass measurements, MS became the most important basic technique that is used to identify proteins in proteomics approach especially in the application of tumor marker identification (Wang et al., 2015, Mellon, 2003, Cao and Limbach, 2017). There are many types of MS, however, this paper describes the types of MS that are used in proteomics approach in order to identify biological markers. Types of proteomics-based mass spectrometry that are used to identify biological markers including: LC-MS/MS, SELDI-TOF/MS, two-dimensional gel electrophoresis–mass spectrometry (2-DE/MS), and MALDI-TOF/MS (Wang et al., 2015, Mellon, 2003, Cao and Limbach, 2017, Roy et al., 2019, Byrum et al., 2010).
LC–MS/MS is an effective technique to identify biomarkers in proteomics researches. This technique allows for identifying several proteins at the same time, and for this reason these techniques are called high-throughput (Wang et al., 2015). However, this technique has some disadvantages such as; incompletion of proteins digestion, and facing some difficulty in peptides chromatographic separation. LC–MS/MS has high selectivity as well as high sensitivity that enables it to analyze complex biological substances even if in low amounts. However, LC–MS/MS generally needs sample preparation process in order to remove unwanted materials for clear analysis (Wang et al., 2015).
SELDI-TOF/MS is one of the most important techniques to identify reliable biomarkers in proteomics researches. In SELDI-TOF/MS, there is chromatographic surface that can bind to a specific part of the wanted protein and the rest will be removed away (Wang et al., 2015, Byrum et al., 2010). So, this specific part of protein would bind with that surface based on electrostatic interaction, absorption, or biochemical affinity. Also, in this technique, there are different chips are used in order to isolate proteins from the entire sample. SELDI-TOF/MS has two advantages including; testing proteins that are not digested, and it analyzes rapidly. Also, this technique allows for identifying several proteins at the same time, and for this reason these techniques are called high-throughput (Wang et al., 2015, Byrum et al., 2010).
Moreover, SELDI-TOF/MS is high selectivity as well as high sensitivity that enables it to analyze complex biological substances even if in low amounts more than other techniques of proteomics requirements (Wang et al., 2015, Byrum et al., 2010). However, SELDI-TOF/MS has some disadvantages including; results reproducibility of this technique is poor due to many different chips are used, and noise. Moreover, this technique is not very accurate as other proteomics-based mass spectrometry technique as well as it cannot be suitable for proteins that have large molecular weight (Wang et al., 2015, Byrum et al., 2010).
The most important advantage of 2-DE/MS is the availability in a lot of laboratories. In fact, this technique is easy and simple to use (Wang et al., 2015). Moreover, it is commonly use in separation and identification of proteins because it is high throughput that identify and separate a huge number of proteins in one time on one gel. This technique allows proteomics researchers to monitor any change that may occur during the disease process and that because of the probing with antibodies that can be performed on transfer blots. However, 2-DE/MS has some disadvantages that it takes very long time and it cannot deal with proteins that have very low molecular weights. Also, it has limitations in repeatability as well as limitations in reproducibility (Wang et al., 2015).
MALDI-TOF/MS is the most powerful technique in proteomics researches to identify proteins. It is the most appropriate MS technique for analyzing proteins that have high molecular weight (Wang et al., 2015, Byrum et al., 2010, Sandalakis et al., 2017). As any technique, MALDI-TOF/MS has some advantages and some disadvantages. MALDI-TOF/MS is high throughput because it allows for identifying several proteins at the same time. Also, this technique is easy to use and has high selectivity as well as high sensitivity. Disadvantages of this technique include: limitations in development, mass window range, preparation of sample is variable, and different preferential peaks for identical samples (Wang et al., 2015, Byrum et al., 2010, Sandalakis et al., 2017).
So, MS is the best method for this purpose because it is accurate and sensitive (Wang et al., 2015, Mellon, 2003, Cao and Limbach, 2017, Roy et al., 2019, Byrum et al., 2010). There are many types of proteomics-based MS including; LC-MS/MS, SELDI-TOF/MS, 2-DE/MS, and MALDI-TOF/MS. MALDI-TOF/MS is the most powerful technique in proteomics researches to identify proteins. It is the most appropriate proteomics-based MS technique for proteins identification that has high molecular weight. MALDI-TOF/MS is high throughput because it allows for identifying several proteins at the one time. Also, this technique is easy to use and has high selectivity as well as has high sensitivity. So, mixing MALDI with TOF/MS would identify proteins that were isolated by 2D-DIGE (Wang et al., 2015, Mellon, 2003, Cao and Limbach, 2017, Roy et al., 2019, Byrum et al., 2010).
2.4. Verification of proteins
There are several proteomics techniques to verify proteins involving; ELISA, and western blot (Liu et al., 2014, Wang et al., 2015).
ELISA is a powerful technique that is used in proteomics researches in order to verify biomarkers (Wang et al., 2015, He, 2013, Immer and Lacorn, 2015). ELISA analysis is an immunochemical test includes enzymes. It quantifies the level of targeted proteins. ELISA has antigens, antibodies, as well as capture antibodies. In this technique, there is a hard surface that has been attached with capture antibodies in order to bind the biomarker onto that hard surface. ELISA is a rapid sensitive technique, and it is very accurate (Wang et al., 2015, He, 2013, Immer and Lacorn, 2015).
Western blot is another powerful technique that is used in proteomics researches in order to verify biomarkers. In this technique, polyclonal antibodies or monoclonal antibodies are used for detecting proteins. So, in western blot, proteins would be separated by using SDS-PAGE to get an idea about molecular weight of separated proteins and their isoforms (Wang et al., 2015, Ray et al., 2013, Gershwin, 2008).
There are several proteomics techniques to verify proteins involving: ELISA, and western blot (Liu et al., 2014, Wang et al., 2015, He, 2013, Ray et al., 2013). However, ELISA is better for proteins verification because it is a rapid sensitive technique, and it is very accurate. ELISA analysis is an immunochemical test includes enzymes. It quantifies the level of targeted proteins. ELISA has antigens, antibodies, as well as capture antibodies. In this technique, there is a hard surface that has been attached with capture antibodies in order to bind the biomarker onto that hard surface (Wang et al., 2015, He, 2013, Gershwin, 2008).
2.5. Database searching
There are many different kinds of servers to search for proteins by their MS/MS data looking for biomarkers that have been discovered or identified. These servers include Mascot, MS-Tag, PepProb, Phenyx, Sonar, and Poptiam (Corthell, 2011).
Mascot is considered as the most used server (Yang et al, 2014). In Mascot, there is a search for proteins by their MS/MS data. Also, proteins can be searched according to peptide mass fingerprint and sequence query (Matrix Science, 2019).
2.6. Protein–protein interactions (PPIs) analysis
The purpose of this step is to monitor the physical connection between identified proteins as biomarkers and their interactions. PPI can be performed by STRING software (SIB, 2019).
2.7. Immunohistochemistry (IHC)
IHC is performed to monitor any proteins presented in the cell or tissue and that occurred by antibodies-antigens binding principle. In fact, it is usually used in cancer cases to diagnose the abnormal cells that present in normal tissue (Drew and Shieh, 2015, Corthell, 2014).
2.8. Statistical analysis
This statistical analysis can be accomplished by Statistical Analysis System (SAS) software or Statistical package for social sciences (SPSS) (IBM, 2019, Delwiche and The, 2012, Lafler and Basic, 2001). These softwares are powerful analytic technique that can save time instead of searching by old tools that take a lot of time. These softwares can find any novel insights in data provided in accurate way. SAS is a software that has various procedures to analyze data and it is divided into four categories including: statistical procedures, scoring procedures, reporting procedures, and utility procedures (Delwiche and The, 2012, Lafler and Basic, 2001).
As Fig. 1 describes the methods of proteomics approach to identify reliable biomarkers, in the first step there are a collection of different types of sample from patients to discover a new biomarker for the diagnosis of specific type of diseases or identify a unified biomarker for all these types of sample. Then, these samples were prepared by using the same procedure with some differences in some requirements for each type of samples. However, as general preparation, samples were prepared by lysate preparation that includes: lysis buffers to facilitate the isolation of proteins, fractionation process for protein size reducing, abstraction of the protein that have high expression from the sample, depletion to separate the high abundance proteins, as well as enrichment and dialysis to regulate the concentrations of proteins.
Then, labeling samples with fluorescent dyes, running them on 2D-DIGE in order to separate the proteins according to their molecular weight or charge. After visualizing 2D-DIGE, extraction of the interested proteins is the next step. So, extracting the band (gel plug) and putting it in a tube to the process of digestion to cut the proteins as well as reduction and alkylation to prevent proteins folding due to the broken bond of proteins. Following by extraction of peptides and cleanup in the purpose of separating proteins with low abundance (Fig. 1).
After all these preparations and extractions of these samples, they go through MALDI-TOF/MS for analysis and identification. MALDI is used in ionization proteins that give MS a chance to identify small molecules as well as large molecules. So, combining among MALDI and TOF and MS would give a powerful proteomic based mass spectrometry that called MALDI-TOF/MS to identify proteins with different sizes.
Then, ELISA is performed to verify proteins as well as PPIs analysis to detect proteins presented in the cells. Also, IHC would be performed as well as statistical analysis. Then, after MALDI-TOF/MS, the data of the proteins would be taken for data search in Mascot server that is available online (Fig. 1).
Finally, all the data that were collected from 2D-DIGE, MALDI-TOF/MS, PPIs analysis, immunohistochemistry, ELISA, data search and analysis would get together for clinical trial for new discovered biomarker or a unified biomarker for all these types of sample (Fig. 1).
3. Examples of resent studies
There are many recent studies used proteomics approach in the purpose of identifying reliable biomarkers for different types of diseases. They accomplished their researches with the same approach and somehow similar process steps but different techniques (Yang et al., 2014, Karagiannis et al., 2014, Cordeiro et al., 2015, Njunge et al., 2017, Bonnet et al., 2019, Bharucha et al., 2019).
In 2014, a study has been done by Dr. Jing Yang and her team by using proteomics approach and techniques to identify new biomarkers to diagnose a basic neck and head cancer (Nasopharyngeal carcinoma (NPC)) in its early stage. They used 2D-DIGE combined with MALDI-TOF-MS analysis to identify Enolase 1 (ENO1) and Cyclophilin A (CYPA) as biomarkers for the diagnosis of NPC (Yang et al, 2014).
Also, in 2014, a study has been done by Dr. George Karagiannis and his team by using proteomics approach and techniques to identify new biomarkers to diagnose colorectal cancer. They used Liquid Chromatography Tandem Mass Spectrometry (LC–MS/MS) to identify Olfactomedin-4 as biomarkers for the diagnosis of colorectal cancer (Karagiannis et al., 2014).
In 2015, Prof. Ana Paula Cordeiro and her team used proteomics approach and techniques to discover an evidence of involvement of Kallikrein-kinin system in the bacterial meningitis pathophysiology. Also, by studying sequent model of candidate biomarkers, they improved differential diagnosis of enteroviral meningitis, pneumococcal meningitis, and meningococcal meningitis (Cordeiro et al, 2015).
A study has been accomplished by Dr. James Njunge and his team in (2017) by using proteomics approach and techniques to discover biomarkers for acute bacterial meningitis (ABM) and cerebral malaria (CM). They have found two proteins as biomarkers including: myeloperoxidase (MPO) and lactotransferrin (LTF), which have also the ability to be used in diagnoses for monitoring the care of these diseases′ patients (Njunge et al., 2017).
In 2018, Dr. Julien Bonnet and his team used proteomics approach and techniques to discover new biomarkers for Human African trypanosomiasis (HAT). These biomarkers include; Neogenin, Intelectin 2, Secretogranin 2, Neuroserpin, and Moesin (Bonnet et al., 2019).
A study has been accomplished by Dr. Tehmina Bharucha and her team in (2019) by using different proteomics techniques for diagnosing suspected central nervous system (CNS). They have found that ELISA can detect 89–94% of these biomarkers and Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight (MALDI-ToF) is a potintial technique to detect diseases' biomarkers (Bharucha et al, 2019).
The biological significances of all of these studies are similar that all types of diseases that can be life threatening. So early detection of these diseases can help patients to get early treatment and the quality of patients’ life would be better. Also, having as much as possible of proteomic data in order to help patients with these types of diseases to be early diagnosed in the future (Yang et al., 2014, Karagiannis et al., 2014, Cordeiro et al., 2015, Njunge et al., 2017, Bonnet et al., 2019, Bharucha et al., 2019).
As methodology process of these researches are different, there are basic or essential steps remain the same with using different techniques. Methodology of these studies were based on different proteomics techniques including; preparation of sample and extracting proteins as well as determination of proteins concentration and molecular weight and proteins labeling were performed in all researches with considering the differences in materials that were used to reach that goal.
So, lysis buffer, fluorescent dyes, cell cultures, and phosphate buffered saline (PBS) were used in methods. In these researches, proteins were extracted and separated by using one-dimensional electrophoresis (1-DE) and two-dimensional differential gel electrophoresis (2D-DIGE) while some other researchers used two-dimensional electrophoresis (2-DE) and two-dimensional gel electrophoresis–mass spectrometry (2-DE/MS). Strong cation exchange liquid chromatography (SCX-LC) were applied in order to fractionate the sample and get the fractions following by reverse phase liquid chromatography (RP-LC) for separation purpose (Yang et al., 2014, Karagiannis et al., 2014, Cordeiro et al., 2015, Njunge et al., 2017, Bonnet et al., 2019, Bharucha et al., 2019).
For identification purpose of proteins, these studies used different identifications methods including: mass spectrometry (MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), liquid chromatography tandem mass spectrometry (LC-MS/MS), linear ion trap quadrupole orbitrap (LTQ-Orbitrap), and surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF/MS) (Yang et al., 2014, Karagiannis et al., 2014, Cordeiro et al., 2015, Njunge et al., 2017, Bonnet et al., 2019, Bharucha et al., 2019).
Then, all of that were followed by western blot, immunohistochemistry assay (IHC), and enzyme-linked immunosorbent assay (ELISA) in order to verify different expression of proteins. Also, Laser Capture Microdissection (LCM) was used to purify interested diseases markers from tissues. Also, analysis of protein–protein interaction (PPI) can be performed for exploring networks of proteins interactions. Some of these studies used MASCOT for database searching after the steps of identification and verification of proteins (Yang et al., 2014, Karagiannis et al., 2014, Cordeiro et al., 2015, Njunge et al., 2017, Bonnet et al., 2019, Bharucha et al., 2019).
4. Conclusion
In conclusion, biomarkers have the ability to detect any presence of biological activities and processes. In medicine, biological markers are used to detect a disease, discover drugs, and monitoring care of patients. Among all these types of biomarkers, proteins can be very sensitive to be detected in a very tiny amount of a sample to diagnose a specific type of diseases in its early diagnosis. Therefore, novel approaches as well as new applications and techniques were necessary to accomplish the identification and discovery of reliable biomarkers for different types of diseases by using proteins as biomarkers. Thus, proteomics approach is one of the most promising approaches in identification of proteins as biomarkers for these diseases.
This paper reviews techniques and methods of proteomics approach in purpose of identification reliable biomarkers for different types of diseases from different types of samples. So, starting with collection of different types of samples from patients including; blood, urine, tissue, saliva, pleural effusion, and tissue interstitial fluid. Then, preparation of different sample types including; lysis, fractionation, depletion, enrichment, and dialysis. Labeling samples with fluorescent dyes would be the next step before running the on gels to separate the proteins according to their molecular weight or charge. Next step would be extraction of peptides and cleanup before analysis and identification. Finally, clinical trials for new discovered biomarker or unified biomarker for would be on.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
The author would like to thank Deanship of Scientific Research at Majmaah University, Al Majmaah, 11952, Saudi Arabia for supporting this work under the Project Number R-1441-65.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aulbach A., Amuzie C. Second ed. Academic Press; 2017. Chapter 17 - Biomarkers in Nonclinical Drug Development. A Comprehensive Guide to Toxicology in Nonclinical Drug Development; pp. 447–471. ISBN 9780128036204. [Google Scholar]
- Bharucha Tehmina, Gangadharan Bevin, Kumar Abhinav, de Lamballerie Xavier, Newton Paul N., Winterberg Markus, Dubot-Pérès Audrey, Zitzmann Nicole. Mass spectrometry-based proteomic techniques to identify cerebrospinal fluid biomarkers for diagnosing suspected central nervous system infections. A systematic review. J. Infect. 2019;79(5):407–418. doi: 10.1016/j.jinf.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. Review PubMed PMID: 11240971. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Garcia C., Leger T., Couquet M.P., Vignoles P., Vatunga G., Ndung'u J., Boudot C., Bisser S., Courtioux B. Proteome characterization in various biological fluids of Trypanosoma brucei gambiense-infected subjects. J. Proteomics. 2019;30(196):150–161. doi: 10.1016/j.jprot.2018.11.005. Epub 2018 Nov 7 PubMed PMID: 30414516. [DOI] [PubMed] [Google Scholar]
- Brody Tom. Second ed. Academic Press; 2016. Biomarkers Clinical Trials; pp. 377–419. [Google Scholar]
- Byrum Stephanie, Siegel Eric R., Bhattacharyya Sudeepa, Suva Larry J. Academic Press; 2010. Proteomics of Bone Cancer. Bone Cancer; pp. 171–180. [Google Scholar]
- Cao X., Limbach P.A. 2017. Mass Spectrometry: Nucleic Acids and Nucleotides Studied Using MS Encyclopedia of Spectroscopy and Spectrometry; pp. 764–771. (Elsevier). [Google Scholar]
- Cordeiro Ana, Pereira Rosiane, Chapeaurouge Alex, Coimbra Clarice, Perales Jonas, Oliveira Guilherme, Candiani Talitah, Coimbra Roney. Comparative proteomics of cerebrospinal fluid reveals a predictive model for differential diagnosis of pneumococcal, meningococcal, and enteroviral meningitis, and novel putative therapeutic targets. BMC Genomics. 2015;16(Suppl 5):S11. doi: 10.1186/1471-2164-16-S5-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthell J. Protein identification using MS/MS data. J. Proteomics. 2011;74(10):1842–1845. doi: 10.1016/j.jprot.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Corthell John T. Immunohistochemistry Basic Molecular Protocols in Neuroscience: Tips, Tricks, and Pitfalls. Academic Press. 2014:91–103. [Google Scholar]
- Davis Myrtle A., Eldridge Sandy, Louden Calvert. Third ed. Academic Press; 2013. Chapter 10 - Biomarkers: Discovery, Qualification and Application. Haschek and Rousseaux's Handbook of Toxicologic Pathology; pp. 317–352. [Google Scholar]
- Delwiche L, Slaughter S. The Little SAS® Book: A Primer, Fifth ed. Chapter 8 and chapter9. 2012 Oct. SAS Institute. ISBN# 978-1-61290-343-9.
- DePrimo S.E. Biomarkers. Comprehensive Medicinal Chemistry II. Elsevier. 2007:69–85. [Google Scholar]
- Doustjalali S. Two dimensional gel electrophoresis: an overview of proteomic technique in cancer research. J. Proteom. Bioinformat. 2014;07 [Google Scholar]
- Drew C, Shieh W. Chapter 10 - Immunohistochemistry. Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention. Pages 109-115. Academic Press. 2015 Feb. ISBN 9780128019191. doi: 10.1016/b978-0-12-801919-1.00010-5.
- Clinical Gershwin L. Sixth ed. Academic Press; 2008. Veterinary Immunology. Clinical Biochemistry of Domestic Animals; pp. 157–172. [Google Scholar]
- He Jianwen. Fourth ed. Elsevier; 2013. Chapter 5.1 – Practical Guide to ELISA Development. The Immunoassay Handbook; pp. 381–393. [Google Scholar]
- Huss Ralf. Academic Press; 2015. Chapter 19 – Biomarkers Translational Regenerative Medicine; pp. 235–241. [Google Scholar]
- IBM. SPSS Statistics, Put the power of advanced statistical analysis in your hands. 2019. Retrieved from https://www.ibm.com/products/us-en/offers/spss/spss-statistics-try-buy.html.
- Immer U., Lacorn M. Handbook of Food Allergen Detection and Control. Elsevier; 2015. Enzyme-linked immunosorbent assays (ELISAs) for detecting allergens in food; pp. 199–217. [Google Scholar]
- Karagiannis G.S., Pavlou M.P., Saraon P., Musrap N., Xie A., Batruch I., Prassas I., Dimitromanolakis A., Petraki C., Diamandis E.P. In-depth proteomic delineation of the colorectal cancer exoproteome: Mechanistic insight and identification of potential biomarkers. J. Proteomics. 2014;30(103):121–136. doi: 10.1016/j.jprot.2014.03.018. Epub 2014 Mar 27 PubMed PMID: 24681409. [DOI] [PubMed] [Google Scholar]
- Kisluk Joanna, Ciborowski Michal, Niemira Magdalena, Kretowski Adam, Niklinski Jacek. Proteomics biomarkers for non-small cell lung cancer. J. Pharm. Biomed. Anal. 2014;101:40–49. doi: 10.1016/j.jpba.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Lafler K. Basic SAS PROCedures for Generating Quick Results. Paper 256-29.SAS. 2001. SAS Alliance Partner, and SAS Certified Professional are registered trademarks of SAS Institute Inc. in the USA and other countries.
- Liu Wentao, Yang Qiumeng, Liu Bingya, Zhu Zhenggang. Serum proteomics for gastric cancer. Clin. Chim. Acta. 2014;431:179–184. doi: 10.1016/j.cca.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Maloy S., Brenner's Hughe K. 2nd ed. Academic Press, Elsevier; London: 2013. Encyclopedia of Genetics. [Google Scholar]
- Marín-García José. Post-Genomic Cardiology. Elsevier; 2007. Molecular and Biochemical Methodology in the Post-Genomic Era; pp. 11–25. [Google Scholar]
- Mayeux Richard. Biomarkers: potential uses and limitations. Neurotherapeutics. 2004;1(2):182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrix Science. Moscat Server. 2019. Retrieved from http://www.matrixscience.com.
- Mellon F.A. Encyclopedia of Food Sciences and Nutrition. Elsevier; 2003. Mass Spectrometry | Principles and Instrumentation; pp. 3739–3749. [Google Scholar]
- Njunge J.M., Oyaro I.N., Kibinge N.K., Rono M.K., Kariuki S.M., Newton C.R., Berkley J.A., Gitau E.N. Cerebrospinal fluid markers to distinguish bacterial meningitis from cerebral malaria in children. Wellcome Open Res. 2017;26(2):47. doi: 10.12688/wellcomeopenres.11958.2. eCollection 2017. PubMed PMID: 29181450; PubMed Central PMCID: PMC5686508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyejide L., Mendes O.R., Mikaelian I. Second ed. Academic Press; 2017. Molecular Pathology: A Comprehensive Guide to Toxicology in Nonclinical Drug Development; pp. 407–445. [Google Scholar]
- Patel S., Ling J., Kim S.J., Schey K.L., Rose K., Kuchtey R.W. Proteomic analysis of macular fluid associated with advanced glaucomatous excavation. JAMA Ophthalmol. 2016;134(1):108–110. doi: 10.1001/jamaophthalmol.2015.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personalized Medicine Coalition (PMC). Advocates for Precision Medicine: Types of Biomarkers. 2019. Retrieved from http://www.personalizedmedicinecoalition.org/Education/Types_of_Biomarkers.
- Ray Animikh, Cholkar Kishore, Wang Zhiying, Mitra Ashim K. Woodhead Publishing; 2013. Characterization of ocular transporters. Ocular Transporters and Receptors; pp. 85–114. [Google Scholar]
- Rodriguez H. CPTAC, the Complementary Sibling of TCGA: An Interview with Dr. Henry Rodriguez about NCI’s Proteomics Program. National Cancer Institute at the National Institutes of Health. 2015 [Google Scholar]
- Roy Jyoti, Jain Neha, Singh Garima, Das Basudeb, Mallick Bibekanand. AGO-Driven Non-Coding RNAs. Elsevier; 2019. Small RNA proteome as disease biomarker: An incognito treasure of clinical utility; pp. 101–136. [Google Scholar]
- Roy P., Shukla Y. Applications of proteomic techniques in cancer research. Cancer Therapy. 2008;6(2):841–856. 16p. [Google Scholar]
- Sabino F, Hermes O, Auf dem Keller U. Body Fluid Degradomics and Characterization of Basic N-Terminome. Methods Enzymol. 2017 ;585:177-199. doi: 10.1016/bs.mie.2016.09.018. Epub 2016 Oct 19. PubMed PMID: 28109429. [DOI] [PubMed]
- Sandalakis Vassilios, Goniotakis Ioannis, Vranakis Iosif, Chochlakis Dimosthenis, Psaroulaki Anna. Use of MALDI-TOF mass spectrometry in the battle against bacterial infectious diseases: recent achievements and future perspectives. Expert Rev. Proteom. 2017;14(3):253–267. doi: 10.1080/14789450.2017.1282825. [DOI] [PubMed] [Google Scholar]
- Shah Tapan R., Misra Ambikanandan. Elsevier; 2011. Proteomics Challenges in Delivery of Therapeutic Genomics and Proteomics; pp. 387–427. [Google Scholar]
- Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. Review. PubMed PMID: 20978388; PubMed Central PMCID: PMC3078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiss Institute of Bioinformatics (SIB). NNF Center for Protein Research, and European Molecular Biology Laboratory, STRING program. 2019. Retrieved from http://string-db.org.
- Wang K, Ottens A, Haskins W, Liu M, Kobeissy F, Denslow N, Chen S, Hayes R. Proteomics Studies of Traumatic Brain Injury, International Review of Neurobiology. Pages 215-240. Academic Press. 2004 Oct. 0074-7742. ISBN 9780123668639. doi: 10.1016/s0074-7742(04)61009-9. [DOI] [PubMed]
- Wang Qihui, Yu Qiaoling, Lin Qingyu, Duan Yixiang. Emerging salivary biomarkers by mass spectrometry. Clin. Chim. Acta. 2015;438:214–221. doi: 10.1016/j.cca.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhou M., Zhao R., Peng S., Luo Z., Li X., Cao L., Tang K., Ma J., Xiong W., Fan S., Schmitt D.C., Tan M., Li X., Li G. Identification of candidate biomarkers for the early detection of nasopharyngeal carcinoma by quantitative proteomic analysis. J. Proteomics. 2014;23(109):162–175. doi: 10.1016/j.jprot.2014.06.025. Epub 2014 Jul 3 PubMed PMID: 24998431. [DOI] [PubMed] [Google Scholar]
- Zhao P, Sun M. Chapter 11 - The Maternal-to-Zygotic Transition in Higher Plants: Available Approaches, Critical Limitations, and Technical Requirements. Current Topics in Developmental Biology. Pages 373-398. Academic Press. 2015 Jul 0070-2153. ISBN 9780124095236. doi: 10.1016/bs.ctdb.2015.06.006. [DOI] [PubMed]