Abstract
Background
One of the world's leading causes of death among females is breast cancer. Oncolytic viruses are promising anticancer therapy that can overcome resistance to current conventional therapies. Measles virus replicates in and destroys malignant cells without affecting healthy cells. The study aimed to evaluate the lives attenuated Measles virus vaccine against Iraqi patient derived breast cancer cells that have functional BRCA1/BRCA2 genes and compare its activity against international breast cancer MCF-7 and CAL-51 cell lines.
Methods
The virus was propagated in VERO-hSLAM slam cells. The MTT cytotoxicity assay used to test the virus's ability to kill three human breast cell lines (AMJ13), (MCF-7), and (CAL-51). The cytopathic effect of the measles virus was determined using an H&E stain. Immunocytochemistry assay using specific anti H protein monoclonal antibody for measles virus in the virally infected cells. Finally, apoptosis induction in the infected cells tested using double staining of acridine orange/propidium iodide.
Results
The result shown that breast cancer cells are effectively infected and destroyed by live attenuated measles virus vaccine, and it caused a significant cytopathic effect in the infected cell lines after 48–72 h of infection with remarkable effect on AMJ13 cells (IC50 was 3.527 for AMJ13, when it was 5.079 and 9.171 for MCF-7 and CAL-51 respectively). Measles virus treatment induces apoptosis significantly in breast cancer cell lines compared with control cells.
Conclusion
MeV vaccine is useful and safe as anticancer therapy with a notable impact on the local Iraqi breast cancer AMJ13 cells.
Keywords: Measles virus vaccine, Oncolytic activity, Breast cancer, AMJ13
1. Introduction
Breast cancer is a severe universal life-threatening disease. Annually, it accounts for more than two million cases (about 26% of all newly diagnosed cancers) and also causing the most significant amount of cancer-associated mortality in females. In 2018 it was calculated that 627,000 women died of breast cancer–approximately 15% of all women's cancer fatalities (WHO, 2019). Breast cancer is an aggressive tumor that is exceptionally resistant to present methods of therapy, like chemotherapy and radiotherapy, and radical surgical resection may be the alternative option (MacNeill and Karakatsanis, 2017, Yu, et al., 2015). The interest in oncolytic virotherapy (the using of replicating viruses as an anticancer therapy) has increased over the past decade (Gauvrit et al., 2008). Oncolytic viruses are anticancer therapy when oncolytic viruses proliferate in and destroy malignant cells without affecting healthy cells. Oncolytic viruses can get into and infect cancer cells by way of membrane fusion or attachment to their receptors that emerge from the surface of the target cell (Al-Khateeb and Muna’am Al-Hilli, 2018). Several viruses have been extensively studied in breast cancer research to assess their oncolytic activity like measles virus (MeV), vesicular stomatitis virus (VSV), herpes simplex virus (HSV), adenovirus, vaccinia (VACV) and reovirus (O'Bryan and Mathis, 2018). MeV is a member of the genus Morbillivirus of the Paramyxoviridae family under the order Mononegavirales (Cox and Plemper, 2015). MeV interacts with three types of host cell receptors via membrane cofactor protein (CD46), signaling lymphocytic activation molecule (SLAM)), or (CD150), and the poliovirus receptor-related 4 (PVRL4) (Lin and Richardson, 2016). Recently, nectin-4 has also been found to be a receptor for wild and measles virus vaccine strains (Noyce et al., 2011). As SLAM and CD46 are often overexpressed in tumor cells, attenuated MeV have been specifically targeting cancer cells, by reducing their development to oncogenic cells (Msaouel et al., 2018). MeV vaccine (Edmonston Strain) has been tested to treat many malignancies such as Glioblastoma (Al-Shammari et al., 2014, Ismaee et al., 2014), epithelial ovarian cancer (Peng et al., 2002), prostate cancer (Msaouel et al., 2009), and hepatocellular carcinoma (Blechacz et al., 2006). AMJ13 (Ahmed, Mahfoodha, Mortadha, Jabria-2013) is the first Iraqi breast cancer cell line which was established in 2014 and characterized from the primary tumor of Iraqi breast cancer patient (Alawsi et al., 2019). AMJ13 cells are positive for both BRCA1 and BRCA2, rather than for vimentin, and they are not express estrogen and progesterone receptors, but weakly positive for HER2/neu gene expression (Al-Shammari et al., 2015). Many previous researches investigated the effect of MeV against international breast cancer cell lines like MDA and MCF-7 and indicated its inhibitory effect at their growth (McDonald et al., 2006, Sugiyama et al., 2013). In this research, a comparison was made between the influence of measles virus vaccine on international breast cancer (MCF-7 and CAL-51) cell lines and the local breast cancer cell line (AMJ13) which is derived from Iraqi patient, and to evaluate the MeV vaccine strain oncolytic effect against local Iraqi breast cancer cells.
2. Materials and methods
2.1. Cell lines
Four cell lines (VERO-hSLAM) (MCF-7), (AMJ13), and (CAL-51), were provided by the cell bank unit of the Iraqi Center for Cancer and Medical Genetics Research (ICCMGR), Mustansiriyah University. VERO-hSLAM, MCF-7, and CAL-51 cells were retained as monolayer cultures in MEM medium containing 10% FCS, whereas AMJ13 cells were cultivated in RPMI-1640 supplement with 10% FCS and regularly assessed for standard growth features, and they are always confirmed, the passage used in this study was 33.
2.2. Virus
Attenuated measles virus vaccines (Serum Institute of India PVT. LTD., India) were obtained from Al Anbar Health Directorate – Division of vaccination. The virus was propagated in VERO-hSLAM cells (4.5 × 106 cells / T75 flasks) in 5 ml medium of serum-free RPMI-1640 at 37 °C. After two hours, the medium was pulled up and replaced with new serum-free media. After 3–7 days incubation at 37 °C, the cells were harvested when syncytia reached 80% to 90%. Eventually, the proliferating viruses were collected through 3 cycles of freezing and thawing. Determination of titer of virus stocks by 50% endpoint dilution assay using VERO-hSLAM cells in 96-well plates.
2.3. Cytotoxicity of the attenuated measles vaccine strain
Breast cancer (MCF-7, AMJ13, and CAL-51) cells viability were examined with the 3- (4,5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide (MTT) assay (Bio-world, USA), a water-soluble yellow color dye which is easily absorbed by viable cells and decreased by mitochondrial dehydrogenase action. The water-insoluble reduction product is a blue formazan, then dissolved using a DMSO solution for colorimetric measurement. Cells at (10,000 cells/well for each) were cultured onto 96-well plates and infected with various MOIs (20, 10, 5, 3, 1, 0.5, and 0.1) of the measles vaccine. After 72 h, Cells in PBS have been washed, and 0.5 μg/ml MTT-solution added for up to 2 h. Once the supernatant was discarded and the cells dried, 100 μL DMSO was added per well. The extinction values at 570 nm were determined photometrically using spectrophotometric analysis (Wang, 2006).
The rate of growth inhibition (cytotoxicity percentage) was calculated using the following formula: G.I% = (A − B)/A X100, where A is the mean optical density of the control wells and B is the mean optical density of the treated wells (Alsaedi et al., 2019). Graphpad prism 7.0 (version 2016), software has been used to determine the inhibiting MOI destroying 50% of infected cells. (IC50).
2.4. Cytopathology study
Formalin-fixed cells were flooded with hematoxylin stain for 3–5 min, then rinsed using distilled water. After that, slides with adherent cells were flooded with eosin stain for 1–2 min. Ethanol 90% was used to dehydrate stained cells, and afterward, slides flooded in absolute alcohol. Finally, the xylene-based mounting medium was used to maintain cell sections.
2.5. Detection of MeV hemagglutinin protein
Immunoperoxidase kit was used for the detection of hemagglutinin protein of MeV in breast cancer cell lines using anti-Measles hemagglutinin (H; 6016) primary antibody (Santacruz Biotechnology, USA). The test's concept is that the primary antibody (anti-measles monoclonal antibody) links to MeV's H protein, afterward the secondary antibody links to an antigenic epitope on the primary antibody. Finally, the avidin, including the horseradish peroxidase enzyme binds to the molecule of biotin connected to the secondary antibody. This procedure of staining was done according to the manufacturer's recommendation (Santa Cruz Biotechnology, Dallas, TX, USA).
2.6. Quantitative image analysis
ImageJ Fiji software was used to quantitatively immunocytochemistry (ICC) digital image analysis. Three different ICC images for each slide were analyzed by this protocol. Initially, color, de-convolution technicality was used to un-mix the stained regions of pure DAB and hematoxylin, leaving a ˈgratis picture. Separate DAB or hematoxylin pixel intensities range from 0 to 255. The value 0 is the darkest color shade, while 255 is the lightest color shade in the picture. Then, we quantify the image of the DAB. Using the ImageJ software, the numeral of pixels of a particular intensity value versus their intensity was increased (Chatterjee et al., 2013). The following formula has been used to estimate Optical Density: “OD = log (max intensity / mean intensity)” where for 8-bit pictures max intensity = 255 (Mustafa et al., 2015).
2.7. Apoptosis assay
Acridine orange-propidium iodide (AO-PI) stain used to detect apoptosis caused due to growing MeV in breast cancer cell lines. Cell lines were cultivated in 96-well plates. After 80% growth, cancer cells treated with MEV (according to IC50 for each cell line) for 72hs. Control wells left without treating. AO-PI stain prepared by adding 1 µL of acridine orange and 1 µL of propidium iodide with a proportion of 1:1 in 1 ml of phosphate buffer saline. Afterward, the media were discarded, followed by staining with 50 µL/well AO-PI stain. After twenty seconds, the stain was withdrawn from the wells. Morphological variations in live and apoptotic cells examined and photographed using the inverted fluorescent microscope (Assayaghi and Alabsi, 2016). AO dye stains viable and defunct cells. PI is stained cells that have ruptured membrane. Living cells will show a uniform green color. Apoptotic cells will integrate PI, so they stain orange color (Kasibhatla et al., 2006). Image J version 1.47 software was used to calculate the results by counting apoptotic cells.
2.8. Statistical analysis
Data were displayed as mean ± SD for all triplicate observation readings. Three images were used for ICC testing. Multiple comparisons of one-way variance assessment (ANOVA) was performed to demonstrate differences among organizations using statistical significance assessment (GraphPad Prism version 7.0 for Windows, GraphPad Software, San Diego, CA, USA), to assess the cytotoxicity and identification apoptosis of MeV effect on MCF-7, AMJ13, and CAL-51cell lines in vitro, and statistically significant was considered to be p < 0.05.
3. Results
3.1. Cytotoxicity of MeV on breast cancer cell lines
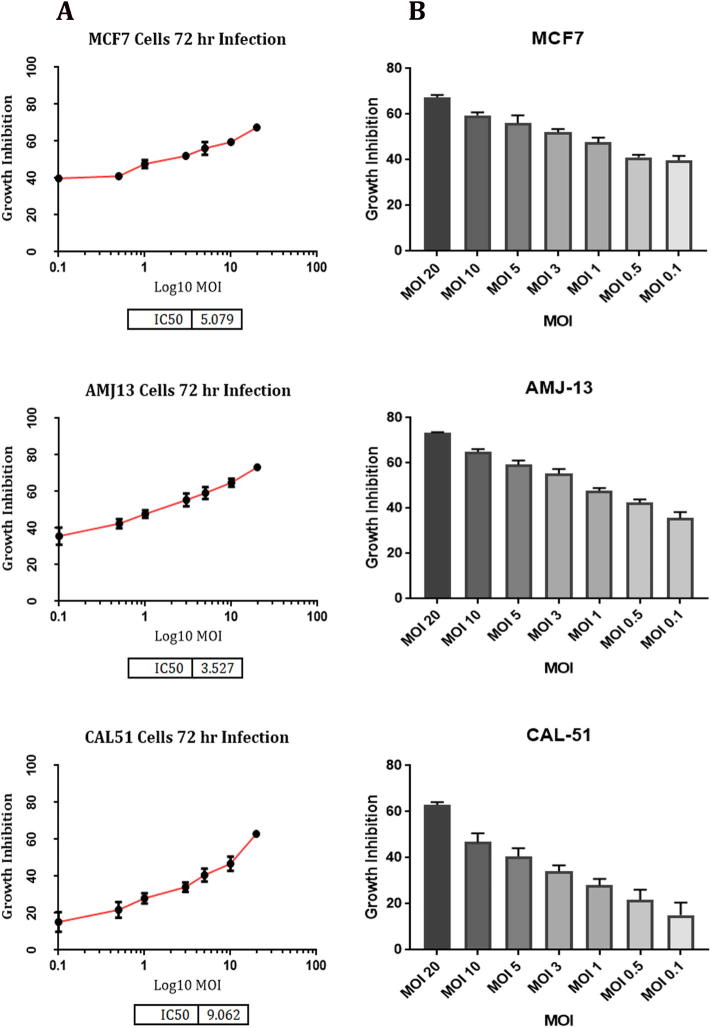
We inoculated various MOIs (0.1, 0.5, 1, 3, 5, 10, 15, 20) of the measles virus vaccine on breast cell lines to calculate the inhibitory dose 50 (IC50). After an incubation period of 72 h, the MTT assay was performed. The growth inhibition (GI) and IC50 determined as a proportion of viable cells following infection with measles virus compared to control cells that were not exposed to the virus. The results revealed that MeV vaccine has cytotoxic effects on different breast cancer cell lines (MCF-7, AMJ13, and CAL-51), with IC50 of (5.079, 3.527, and 9.062) respectively, and the inhibitory effect of a virus was increased by increasing the virus MOI (Fig. 1).
Fig. 1.
In vitro anti-cancer activity of the MeV vaccine on breast cancer (MCF-7, AMJ13, and CAL-51) cell lines. (A) IC50 of the MeV vaccine. (B) The growth inhibition at different MOIs of MeV vaccine.
The results showed that the local cell line (AMJ13) was the most affected by the virus and the IC50 value was the lower (3.527) in comparison to international cell lines (MCF-7 and CAL-51), that meaning it needed a less number of viruses to kill half the number of cells.
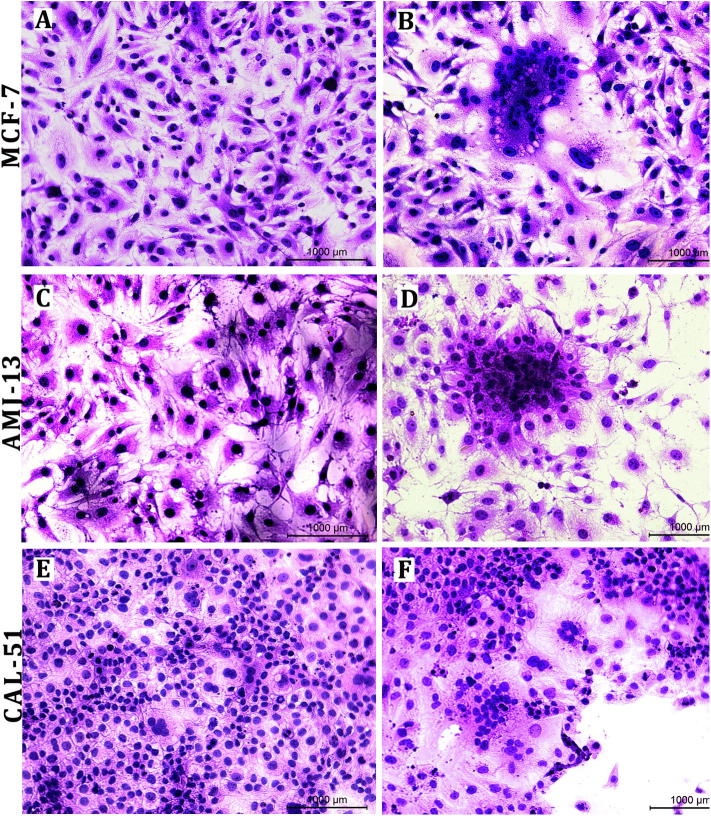
The typical CPE of MeV was the of multinucleated giant cell (syncytia) formation due to cell-cell fusion. The live attenuated measles virus showed high efficiency for replicating in and killing of tumor cells. Many alterations in morphological characteristics of cancer cells after 72 h of exposure represented by cell rounded shrinkage, cell aggregation, and hollow spaces with cell debris due to cell lysis and death, whereas uninfected cells shoed no morphological alterations (Fig. 2).
Fig. 2.
CPE of MeV on breast cancer cell lines. A. MCF-7 cells were uninfected with MeV. B. Showing MCF-7 cell fusion after 48 h of infection. C. Showing AMJ13 uninfected with MeV. D. Syncytia formation in AMJ13 after 48 h. E. CAL-51 cells are uninfected with MeV. F. Syncytia formation in CAL5 with large flatulent space with cell debris after 48 h, (H&E) 20×.
3.2. Detection of H-protein in cell lines infected with measles virus
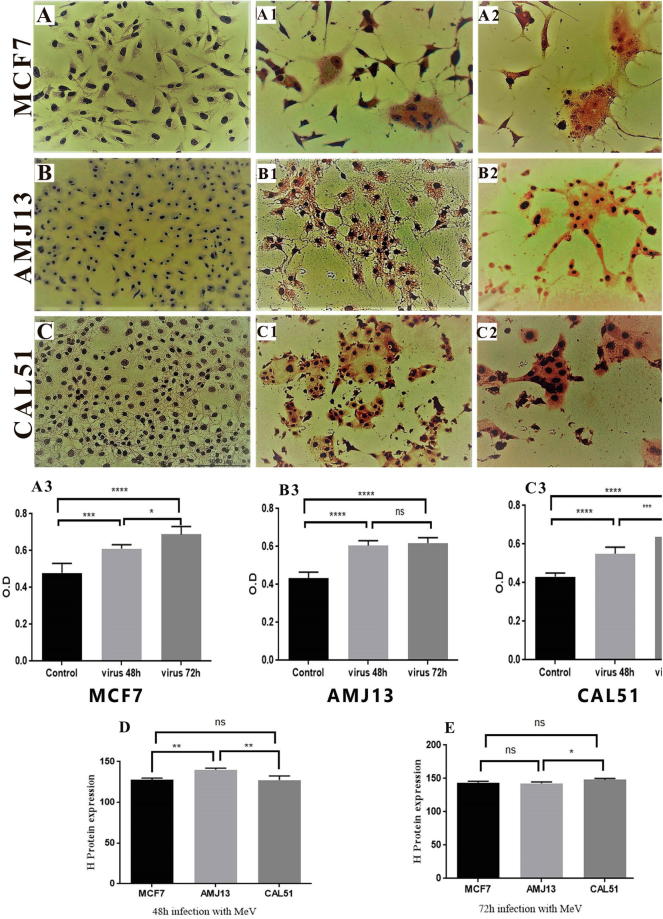
Immunocytochemistry stain using monoclonal antibody specific for an H protein of measles virus showed that hemagglutinin proteins appeared in the cytoplasm of MCF-7, AMJ13, and CAL-51 cell lines after 48 h and 72 h of virus infection as it stained brown color. While uninfected cell cytoplasm was negatively stained. Furthermore, the nuclei of both infected and uninfected cells were stained blue color (Fig. 3).
Fig. 3.
Detection of MeV H protein in breast cancer cell line. (A, B and C) control cells showed no color in the cytoplasm of uninfected cells with blue nuclei 20×. (A1, B1, and C1) show brown color in the cytoplasm of cells infected with MeV and blue nuclei after 48 h of infection 20×. (A2, B2, and C2) show brown color in the cytoplasm of cells infected with MeV and blue nuclei after 72 h of infection with more substantial space than cells infected for 48 h 20×. (A3, B3, and C3) Digital Image Scoring was demonstrating the existence of MeV H protein in infected cells (MCF-7, AMJ13, and CAL-51 respectively) when stained with MeV H-specific relative mAbs that were analyzed using ImageJ. (D) Relative expression of MeV after 48 h of infection. (E) Relative expression of MeV after 72 h of infection.
3.3. Apoptosis induction by the measles virus
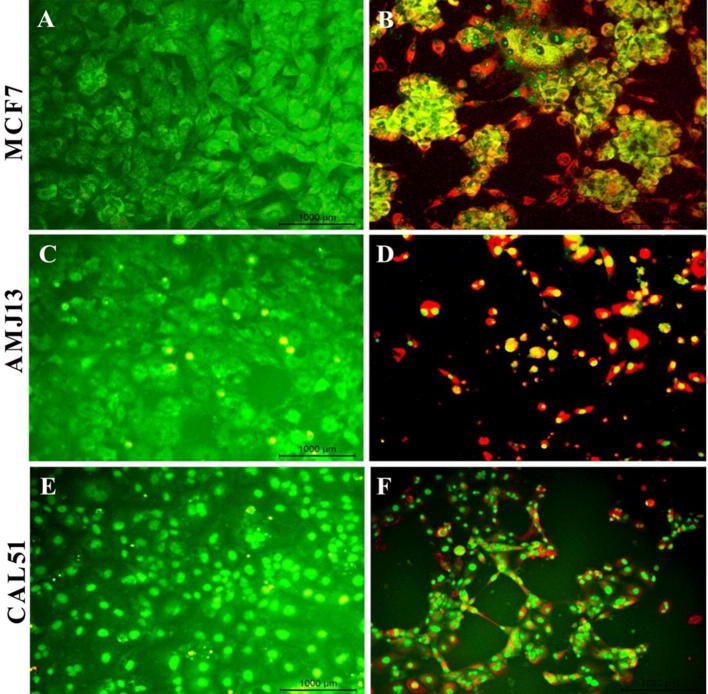
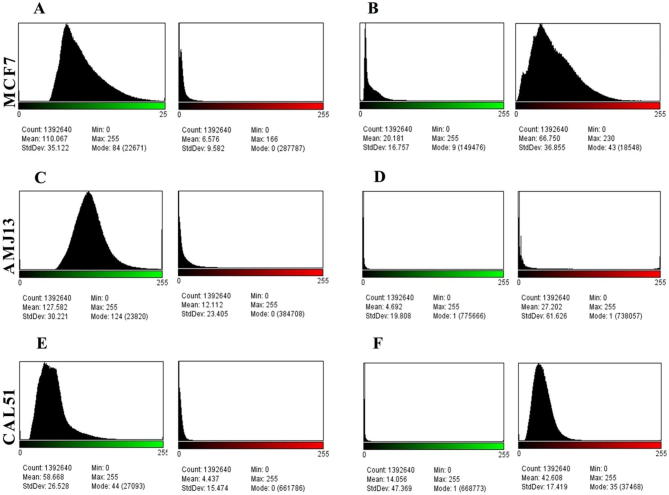
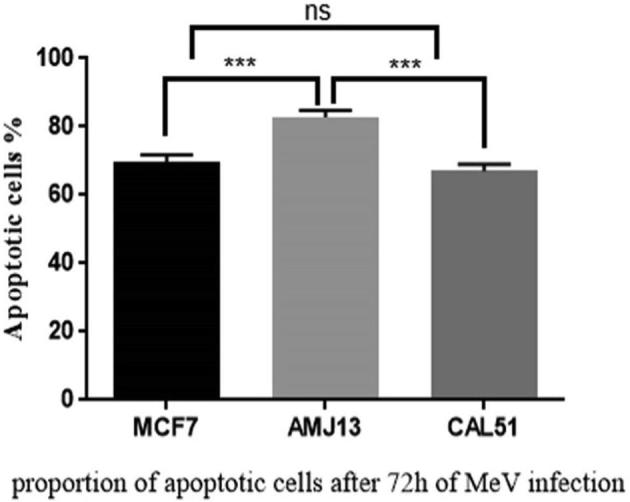
Acridine orange/propidium iodide (AO/PI) staining was used to conceptualize the apoptosis distinctive nuclear modifications and apoptotic body formation. Cells were examined using a fluorescence microscope and have been counted for apoptosis quantification. In live cells, the nucleus and cytoplasm fluorescence green. The apoptotic cells incorporated propidium iodide and therefore exhibited orange and red fluorescence as well as displayed condensed nuclei, often fragmented. Necrotic cells stain orange but, without condensed chromatin, have a nuclear morphology similar to that of the viable cells (Fig. 4). In Fig. 5, the amounts of non-apoptotic cells (green color) and apoptotic cells (red color) were statically analysis to histogram in the images by using ImageJ software. The mean percent of apoptotic cells was significantly lower (5.974) % in uninfected MCF-7 cells (A), while it was (69.767) % in infected cells (B). In AMJ-13, the mean of apoptotic cells in uninfected cells (C) was (9.493) %, whereas it was (82.75) % in infected cells (D). Finally, the mean of apoptotic CAL-51 cells in infected cells (E) was (7.562) %, while it was (67.01) % in infected cells (F). The results showed there is a significant difference in the means of apoptotic cells (P < 0.05) among the three tested cell lines (Fig. 6).
Fig. 4.
Fluorescence microscopy images for apoptosis test for treated and untreated breast cancer cells. The uninfected MCF-7 green cells (A), AMJ13 (C), and CAL-51 (E) stained green color while the infected MCF-7 (B), AMJ13 (D), and CAL-51 (F) with measles virus stained red after 72 h 20×.
Fig. 5.
Histogram of image assessment of (MCF-7, AMJ13, andCAL-51) cell lines apoptosis test, 20×. (A) Uninfected MCF-7 cells with MeV. The mean of non-apoptotic cell green color was 110.067. The mean apoptotic cell red color was 6.576, so the apoptotic cell proportion was 5.974 percent. (B) MCF-7cell line 72 h after infection with MeV. For non-apoptotic cells, the mean of green color was 20.181. For apoptotic cells, the red color means was 66.750, so the proportion of live green cells was 30.233 percent. (C) Uninfected AMJ13 cells with MeV. The mean non-apoptotic cell green color was 127.582. The mean apoptotic cell red color was 12.112, so the apoptotic cell proportion was 9.493 percent. (D) AMJ13 cell line 72 h after infection with MeV. For non-apoptotic cells, the mean green color was 4.692. For apoptotic cells, the red color means was 27.202, so the proportion of live green cells was 17.248 percent. (E) Uninfected CAL-51 cells with MeV. The mean non-apoptotic cell green color was 58.668. The mean apoptotic cell red color was 4.437, so the apoptotic cell proportion was 7.562 percent. (F) CAL-51 cell line 72 h after infection with MeV. For non-apoptotic cells, the mean green color was 14.056. For apoptotic cells, the red color means was 42.608, so the proportion of live green cells was 32.989 percent.
Fig. 6.
The proportion of apoptotic cells after 72 h infection with measles virus.
4. Discussion
The data in the present study showed that measles virus vaccine productively infected and destroyed breast cancer cells, especially the newly patient-derived cancer cells and presence of significant CPE in the infected breast cancer cells after 48 to 72 h of infection. Pre-clinical studies, including basic cancer research needs patient-derived cancer models because these patient-derived cancer models faithfully reflect the therapeutic response of human cancers and shown to be promising in clinical applications (Oppel et al., 2019).
Like other paramyxoviruses, the infections with measles virus (MeV) are established by the adhesion of MeV to its host cell receptor (CD46) using the haemagglutinin (H) protein, while the penetration into the susceptible cell-mediated through the interaction between the fusion (F) protein and the cell membrane (Laksono et al., 2016). The newly synthesized glycoproteins pile up at the membrane of infected cell triggering fusion with the neighboring cells give rise to multinucleated giant cell (syncytia) production. In the subsequent stages, syncytia are normally lysed after a few days of infection. Therefore, the virus can spread from cell to others with no need for the complete production of viral particles. Syncytial infected cells lose their ability to replicate, so do not participate in the growth of further cancerous cells. Furthermore, any syncytia were destroyed, might liberate free viruses which can infect the other cells (Bajzer et al., 2008).
The results of a cytotoxicity test of MeV vaccine on (MCF-7, AMJ13, and CAL-51) cell lines after 72 h of infection, in the present study, showed that the rates of inhibition were dose-dependent, where the highest inhibition rate was at multiplicity of infection (MOI = 20), and the lowest inhibition rate was at (MOI = 0.1). Previous studies have indicated that it is not possible to infect extra cells with minimal levels of viruses that have infected cells, leading to a potential virotherapy imbalance (Al-Shammari et al., 2014). Moreover, this study revealed that the number of MeV particles that required to infect target cells was differences among different breast cancer cell lines, and they were typed dependencies. Our data appeared that the local breast cancer cell line (AMJ13) required the least number of viruses (IC50 = 3.527) for 50% inhibition, whereas the IC50s of MCF-7 and CAL-51 were 5.079 and 9.062 respectively. This result indicates that the local breast cancer cell line (AMJ13) was more sensitive to the MeV vaccine among the three types used in our study. Furthermore, the CAL-51 cell line was more resistance to the other. The infection with the measles virus depends on the types and numbers of MeV receptors distributed on the cell membrane surface of the target cell. Although the locally AMJ13 cell line is triple-negative (negative for ER, PR, and HER2) like the CAL-51 cell line, it is positive for BRCA1/2 (Alawsi et al., 2019). Ectopic expression of BRCA1 in breast and ovarian cancer cell lines promoted apoptosis induction. (Harkin et al., 1999) Have shown that the expression of BRCA1 can induce apoptosis by activating c-Jun N-terminal kinase / stress-activated protein kinase (JNK / SAPK). Moreover, (Thangaraju et al., 2000) demonstrated that HBRCA1 modulates stress-induced apoptotic signaling utilizing a pathway that involves sequentially H-Ras proto-oncogene, MEKK4, JNK, Fas (CD95)/FasL interactions, and procaspase-8 activation.
Our data by immunocytochemistry assay (Fig. 3) demonstrated that the presence of specific hemagglutinin protein expressed in the measles virus by using monoclonal Abs from whole infected cells. All cell lines showed positive results as brown color in the cytoplasmic portion of the infected cells. That indicates that breast cancer cells were successfully infected by MeV vaccine. The result showed a significant increase (P ≤ 0.05) in the percentage of the H protein in MCF-7 and CAL-51 cell lines after 72 h of infection as measured by the image - j software, and there is no significant increase in AMJ13 cell line after 72 h. This outcome spots the competition between viral replication, and the proliferation of tumor cells, and mortality rates of infected tumor cells (Friedman et al., 2006).
In vitro, the result of MeV's cytotoxic impact and apoptosis appears that the treatment with MeV significantly reduced the viability and increased the apoptosis of cancer cells compared with control cells (Fig. 5). The mechanism of MeV causes malignant cell apoptosis may be due to virus replication. This viral reproduction feature provides a continuous input dose that continues until it is stopped by an immune response or a dearth of susceptive cells (Mullen and Tanabe, 2002).
Morphological modifications identified during apoptosis include condensation of chromatin that can be imaged by the fluorescence of acridine orange. That appears from MeV cytopathic effect (CPE) by merging infected cells with adjacent cells, creating multinucleated giant cells, leading to cell death through apoptotic or non-apoptotic processes to a range of human malignant cells (Gauvrit et al., 2008). This specifically may attribute to the mechanism of entry of MeV. MeV as lytic virus, its replication in cancerous cells leads to killing and lysis of these cells. (Zhou et al., 2012) outlined the capacity of the live-attenuated measles virus vaccine to cause apoptosis in ovarian cancer cells because the MeV vaccine has oncolytic action against ovarian malignancies in vitro through an aberrant ROS activation-mediated epigenetic disability of e-cadherin and provides the theoretical foundation for the clinical implementation of the live-attenuated measles virus vaccine in vivo.
The current study used ImageJ software to analyze apoptosis assay fluorescent images (Fig. 6), which showed that after 72 h of MeV infection, the proportion of AMJ13 apoptotic cells was 82.75%, while for MCF-7 and CAL-51 were 69.767% and 67.01% respectively.
These results indicate that the local breast cancer cell line was susceptible to treatment by oncolytic MeV. Therefore, the live attenuated measles virus vaccine represents effective therapy that can be used in clinical breast cancer management.
5. Conclusion
The current study proved that MeV vaccine has robust oncolytic activity against breast cancer cells, especially new patient-derived breast cancer cells. These results of oncolytic activity and apoptosis induction provide evidence for the therapeutic value of this virus in the treatment of breast cancer, which indicates promising clinical outcomes.
Acknowledgments
Acknowledgments
The authors would like to acknowledge the Iraqi center for cancer and medical genetic research, Mustansiriyah University for the support during the study.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Khateeb, M.M., Muna’am Al-Hilli, L.A., 2018. A study of the prevalence of Estrogen & Progesterone Receptor markers positivity in female breast cancer cases in Al-Yarmouk Teaching Hospital. Mustansiriya Med. J. 12, 8–8.
- Al-Shammari A.M., Alshami M.A., Umran M.A., Almukhtar A.A., Yaseen N.Y., Raad K., Hussien A.A. Establishment and characterization of a receptor-negative, hormone-nonresponsive breast cancer cell line from an Iraqi patient. Breast Cancer: Targets and Therapy. 2015;7:223. doi: 10.2147/BCTT.S74509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shammari A.M., Ismaeel F.E., Salih S.M., Yaseen N.Y. Live attenuated measles virus vaccine therapy for locally established malignant glioblastoma tumor cells. Oncol. Virother. 2014;3:57. doi: 10.2147/OV.S59037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawsi D.F., Alshammari A.M., Majeed° M.F. The Significance of p53, Bcl-2, and HER-2/neu Protein Expression in Iraqi Females Breast Cancer Cell Line (AMJ13) Indian J. Public Health. 2019;10:181. [Google Scholar]
- Alsaedi I.I., Taqi Z.J., Hussien A.M.A., Sulaiman G.M., Jabir M.S. Graphene nanoparticles induces apoptosis in MCF-7 cells through mitochondrial damage and NF-KB pathway. Mater. Res. Express. 2019;6 [Google Scholar]
- Assayaghi R.M., Alabsi A.M. Apoptosis induction of newcastle disease virus strains (AF 2240 & V4-UPM) on HT-29Human colorectal adenocarcinoma cells. J. Cancer Res. Ther. Oncol. 2016;1:1. [Google Scholar]
- Bajzer Ž., Carr T., Josić K., Russell S.J., Dingli D. Modeling of cancer virotherapy with recombinant measles viruses. J. Theor. Biol. 2008;252:109–122. doi: 10.1016/j.jtbi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Blechacz B., Splinter P.L., Greiner S., Myers R., Peng K.W., Federspiel M.J., Russell S.J., LaRusso N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Malhotra R., Varghese F., Bukhari A.B., Patil A., Budrukkar A., Parmar V., Gupta S., De A. Quantitative immunohistochemical analysis reveals association between sodium iodide symporter and estrogen receptor expression in breast cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Plemper R.K. The paramyxovirus polymerase complex as a target for next-generation anti-paramyxovirus therapeutics. Front. Microbiol. 2015;6:459. doi: 10.3389/fmicb.2015.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Tian J.P., Fulci G., Chiocca E.A., Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- Gauvrit A., Brandler S., Sapede-Peroz C., Boisgerault N., Tangy F., Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- Harkin D.P., Bean J.M., Miklos D., Song Y.-H., Truong V.B., Englert C., Christians F.C., Ellisen L.W., Maheswaran S., Oliner J.D. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- Ismaee F.E., Al-Shammari A.M., Salih S.M. Investigation of Live Attenuated Measles Virus Vaccine as Anti Tumor Agent. Al-Nahrain J. Sci. 2014;17:144–154. [Google Scholar]
- Kasibhatla, S., Amarante-Mendes, G.P., Finucane, D., Brunner, T., Bossy-Wetzel, E., Green, D.R., 2006. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harbor Protocols 2006, pdb. prot4493. [DOI] [PubMed]
- Laksono B.M., De Vries R.D., McQuaid S., Duprex W.P., De Swart R.L. Measles virus host invasion and pathogenesis. Viruses. 2016;8:210. doi: 10.3390/v8080210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-T., Richardson C.D. The host cell receptors for measles virus and their interaction with the viral hemagglutinin (H) protein. Viruses. 2016;8:250. doi: 10.3390/v8090250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill F., Karakatsanis A. Over surgery in breast cancer. The Breast. 2017;31:284–289. doi: 10.1016/j.breast.2016.10.023. [DOI] [PubMed] [Google Scholar]
- McDonald C.J., Erlichman C., Ingle J.N., Rosales G.A., Allen C., Greiner S.M., Harvey M.E., Zollman P.J., Russell S.J., Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- Msaouel P., Iankov I.D., Allen C., Morris J.C., Von Messling V., Cattaneo R., Koutsilieris M., Russell S.J., Galanis E. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 2009;69:82–91. doi: 10.1002/pros.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msaouel, P., Opyrchal, M., Dispenzieri, A., Whye Peng, K., J Federspiel, M., J Russell, S., Galanis, E., 2018. Clinical trials with oncolytic measles virus: current status and future prospects. Curr. Cancer Drug Targets 18, 177–187. [DOI] [PMC free article] [PubMed]
- Mullen J.T., Tanabe K.K. Viral oncolysis. Oncologist. 2002;7:106–119. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- Mustafa H.N., El Awdan S.A., Hegazy G.A., Jaleel G.A.A. Prophylactic role of coenzyme Q10 and Cynara scolymus L on doxorubicin-induced toxicity in rats: Biochemical and immunohistochemical study. Ind. J. Pharmacol. 2015;47:649. doi: 10.4103/0253-7613.169588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce R.S., Bondre D.G., Ha M.N., Lin L.-T., Sisson G., Tsao M.-S., Richardson C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan S.M., Mathis J.M. Oncolytic virotherapy for breast cancer treatment. Curr. Gene Ther. 2018;18:192–205. doi: 10.2174/1566523218666180910163805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppel F., Shao S., Schürmann M., Goon P., Albers A.E., Sudhoff H. An effective primary head and neck squamous cell carcinoma in vitro model. Cells. 2019;8:555. doi: 10.3390/cells8060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K.-W., TenEyck C.J., Galanis E., Kalli K.R., Hartmann L.C., Russell S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Sugiyama T., Yoneda M., Kuraishi T., Hattori S., Inoue Y., Sato H., Kai C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013;20:338. doi: 10.1038/gt.2012.44. [DOI] [PubMed] [Google Scholar]
- Thangaraju M., Kaufmann S.H., Couch F.J. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J. Biol. Chem. 2000;275:33487–33496. doi: 10.1074/jbc.M005824200. [DOI] [PubMed] [Google Scholar]
- Wang, F., 2006. Culture of animal cells: a manual of basic technique. In Vitro Cellular & Developmental Biology-Animal 42, 169–169.
- WHO 2019. Breast cancer.
- Yu, D.d., Wu, Y., Shen, H.y., Lv, M.m., Chen, W.x., Zhang, X.h., Zhong, S.l., Tang, J.h., Zhao, J.h., 2015. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci., 106, 959–964 [DOI] [PMC free article] [PubMed]
- Zhou S., Li Y., Huang F., Zhang B., Yi T., Li Z., Luo H., He X., Zhong Q., Bian C. Live-attenuated measles virus vaccine confers cell contact loss and apoptosis of ovarian cancer cells via ROS-induced silencing of E-cadherin by methylation. Cancer Lett. 2012;318:14–25. doi: 10.1016/j.canlet.2011.10.038. [DOI] [PubMed] [Google Scholar]