Abstract
Objective
The objective is to clarify the effects of Notch/p38MAPK signaling pathway on articular cartilage defect recovery by BMSCs tissue and provide a basis for clinical treatments of articular cartilage defects.
Methods
A total of 96 healthy male rabbits (weighed 1.5–2.0 kg) that were fully-grown were selected and grouped as the no-treatment group, the model group, and the treatment group in a random manner. Each group included 32 rabbits in total. The no-treatment group was fed without any interventions. The model group and the treatment group were constructed into rabbit knee-joint articular cartilage defect models. In addition, rabbits in the treatment group were given intervention treatments with Notch inhibitor (DAPT) combined with p38MAPK inhibitor (SB203580). The general conditions of rabbits in each group and the conditions of the stained articular cartilage tissue samples were observed, the proliferation of chondrocytes of rabbits in each group was compared.
Results
(1) After drug interventions, in contrast to the rabbits in the model group, the general conditions and the chondrocyte recovering situations of rabbits in the treatment group were obviously improved; (2) 8 weeks after model construction, the articular cartilage empty bone lacuna rate of rabbits in the treatment group was (12.13 ± 1.81)%, which was obviously lower than the synchronous (21.55 ± 3.07)% articular cartilage empty bone lacuna rate of rabbits in the model group, and there was a statistical significance in the differences (P < 0.05); (3) the absorbance value (OD value) of chondrocytes in the treatment group was (0.34 ± 0.015), which was obviously higher than the (0.10 ± 0.020) OD value of chondrocytes in the model group, and there was a statistical significance in the differences (P < 0.05).
Conclusion
The inhibition of Notch/p38MAPK signaling pathway can promote the recovery of articular cartilage by BMSCs tissue, accelerate the proliferation of chondrocytes, and contribute to the recovery of knee-joint injuries in rabbits, which provides a reliable basis for clinical treatments of articular cartilage defects.
Keywords: Articular cartilage, Notch pathway, p38MAPK signaling pathway, Bone marrow mesenchymal stem cell, BMSCs
1. Introduction
Among the clinical treatments of orthopedic diseases, the effective treatments of cartilage defects have always been extremely difficult problems. Generally, of all the traditional treatments of cartilage defects, the therapeutic effect of autologous bone transplantation is the best; however, due to the limited sources of autologous bone grafts, new defects will occur to the donor bones; thus, the application of autologous bone transplantation is relatively limited (Tung et al., 2017). At present, the recovery of bone defects through tissue engineering technology has become a promising treatment clinically; in addition, due to their osteogenic differentiation abilities, the bone marrow mesenchymal stem cells (BMSCs) have become the research hot spot in the field of medical tissue engineering (Han et al., 2017). Besides, BMSCs not only have a wide range of sources but also have the abilities to differentiate into various cells, such as osteoblasts and myocytes. Therefore, it is crucial to determine the active mechanism of BMSCs in osteogenic differentiation.
The cartilage matrix and chondrocytes together constitute the articular cartilage, and the function of the articular cartilage is maintained mainly by the normal metabolism of chondrocytes (Shang et al., 2017). Thus, the superfluous apoptosis of chondrocytes could be an important factor that leads to cartilage damages. Mitogen-activated protein kinases (MAPKs) signal transduction pathway (STP) is ctitical for the apoptosis of chondrocytes. Degeneration of articular cartilage and the occurrence of osteoarthritis are closely related to the MAPKs signaling pathway (Yang et al., 2018). MAPKs are a class of protein kinases widely found in mammals that affect cell proliferation, differentiation, and apoptosis by affecting the transcription of genes in animal cells. The MAPKs family includes 8 subfamilies, such as p38MAPK, ERK, and JNK. Under the stimulation of different external factors, each subfamily will form different conduction pathways, which will mediate different biological effects (Wu et al., 2017).
A study has found that the Notch pathway functions in the differentiation of BMSC into osteoblasts, which needs to cooperate with other signaling pathways to play the physiological functions. Currently, it has been clarified that the Notch pathway is an evolutionarily-preserved STP that participates in developing innumerable tissues and organs, which is vital for regulating the development, growth, and apoptosis of all kinds of cells. Therefore, based on the above theories, the rabbit knee-joint articular cartilage defect models were constructed to explore the effects of inhibiting Notch/p38MAPK signaling pathway on articular cartilage recovery by BMSCs tissue and hopefully to lay a foundation for the medical treatments.
2. Materials and methods
2.1. Experimental materials and grouping
Ninety-six healthy male rabbits (weighed 1.5–2.0 kg) that were fully-grown were purchased from Qingdao Kangda Biotechnology Co., Ltd., China, whose animal quarantine was qualified, and the cleaning level was level-II. Fed with standard bar rabbit feed, each rabbit was raised in a separate cage, with free access to water. All the rabbits were fed adaptively for 1 week. Excretion of rabbits was cleaned every day, and the rabbit cages were disinfected once a week. All experimental rabbits were exposed to sunlight at a daily light-dark time ratio of 12:12. The requirements on the surrounding environment were 22–24 °C of ambient temperature and 45–65% of relative humidity. The room temperature remained comfortable and constant. The experiment was started until the rabbits were in good conditions.
All rabbits were grouped as the no-treatment group, the model group, and the treatment group in a random manner. Each group included 32 rabbits in total. Rabbits in the no-treatment group were fed without any interventions. Rabbits in the treatment group and the model group were constructed into rabbit knee-joint articular cartilage defect models. In addition, rabbits in the treatment group were given intervention treatments with Notch inhibitor combined with p38MAPK inhibitor. The Notch pathway inhibitor was γ-secretase inhibitor DAPT, and the p38MAPK pathway inhibitor was SB203580. The dosage of DAPT was 10 mg/kg, which was injected through the abdomen of each rabbit per day for 7 consecutive days; the dosage of SB203580 was 50 mg/kg, which was injected through the abdomen of each rabbit per day for 3 consecutive weeks.
2.2. The rabbit model construction of knee-joint articular cartilage defects and the isolated culture of chondrocytes
Based on the previous studies, the optimized joint scoring method was selected to construct the knee-joint articular cartilage defect models. Rabbits were injected with 3% sodium pentobarbital from the ear veins at a dosage of 1 mL/kg for anesthesia. Then, the fur of the right lower limbs was shaved, and the exposed skin was disinfected in routine operations. Next, the inner edge of the patella was sliced open, and the subcutaneous tissues were separated layer-wise. After the inner articular capsule was cut, the joint cavity was fully exposed. The knee-joint was over-extended by gentle pull, and the patella was dislocated outward. Then, a small hole with a diameter of about 2.5 mm in the center of the medial condyle of the femur was drilled by using a 2.0 mm Kirschner wire hand drill to construct the cartilage defect model of medial condyle of the femur. The treatments of animals and the experimental processes were conducted in conformity with the Chinese Experimental Animal Protection and Management Regulations. All the animal experiment plans and schemes were agreed by the ethic committee of laboratory animal protection.
Respective at the 3rd week and the 8th week of the experiment, 4 white rabbits were randomly taken from each experiment group and put to deaths through air embolization after blood sample collection. The bilateral femurs of the rabbits were taken through the posterolateral hip joints and fixed in 10% aqueous formaldehyde solution. The specific experimental procedures were as follows: (1) The anesthesia was performed by injecting 3% sodium pentobarbital into the ear vein; then, the skin of the right lower limb was disinfected, and the sterile surgical cloth was laid. (2) Along the middle horizontal line of the knee joint, the skin was cut open layer-wise, and the subcutaneous tissues were separated; once the patella was exposed, the patellar ligament was cut to fully expose the knee joint cavity. (3) The hyaline cartilage on the surface of femoral medial condyle of rabbits was taken and placed on a glass dish, which was then labeled for the primary culture of chondrocytes.
The secondary culture of rabbit chondrocytes was performed on a clean bench. The specific procedures were as follows: (1) The residual debris on the tissue was rinsed with pH7.3 sterile D-hanks solution, and the cartilage tissue was then washed thrice. (2) The rabbit cartilage tissue was cut into small pieces of about 1 mm3 with a pair of ophthalmic scissors and placed in a culture dish. (3) The culture dish was added with 5 mL of 0.25% trypsin and shaken for about 30 min; then, the trypsin was pipetted out. (4) The culture dish was added with about 5 mL of 0.2% type II collagenase and shaken for 14 h, and the cells were collected every 6 h. The method of collecting cells was as follows: (1) The culture dish was taken out, the type II collagenase solution was pipetted out, and the solution was filtered with a strainer before being centrifuged in a glass centrifuge tube. (2) The supernatant was abandoned, the cytes were rinsed with 10% fetal bovine serum (FBS), and the obtained cytes were put into in a culture dish that contained 20% FBS DMEM culture medium. The method of secondary culture was as follows: Once 80% of the culture dish bottom was overspread with chondrocytes, the trypsin was added for digestion, and the ratio of secondary culture was 1:2. The medium in the culture dish was renewed once every 2 days since the secondary culture of cells was started, and the culture medium was 10% FBS DMEM culture medium.
Once the number of chondrocytes in the culture dish exceeded 85%, the culture medium was discarded, and the serum-free culture medium was added to rinse thrice; then, the chondrocytes were added with trypsin digestive juice before being observed under a microscope; once the edges of the cells were observed to be gradually brightened and the gaps between the cells were observed to be widened, the digestion was terminated; afterward, the cells were mixed evenly, transferred into a centrifuge tube with a capacity of 15 mL, and centrifuged for 5 min. The supernatant was abandoned; the cryopreservation solution and the cell culture medium mixture were added, the cells were pipetted out into a cryopreservation tube, stood at −20 °C for 2 h, and stored in liquid nitrogen.
In terms of cell resuscitation, the cryopreservation tube was taken out from liquid nitrogen and stood at room temperature; then, it was shaken in a 37 °C water bath to make it be evenly heated and quickly dissolved; the supernatant was discarded after centrifugation; next, the cells were resuspended by adding 5 mL of cell culture medium before the supernatant was discarded again; afterward, the cells were transferred to a culture dish and cultured in a cell culture incubator, and the culture medium was changed the next day.
2.3. Detection index and observation method
(I) The general condition of rabbits in each group and the recovery of knee-joint articular cartilage defects of rabbits were observed. During the experiment, the body weight of each rabbit was measured once a week, and the mental conditions, the gloss of fur, activities, intake of water and food, and the urine and feces of all rabbits were observed and recorded.
(II) The bone tissue samples of rabbits were observed and detected. The articular cartilage tissues of rabbits in each experiment group were histologically stained with Hematoxylin-Eosin (HE) and Masson. The samples were placed under an optical microscope and an electron microscope to observe changes in bone cells, and the rate of empty bone lacuna was calculated. (1) Optical microscopy observation: The obtained articular cartilage tissue samples were rinsed, dehydrated, decolored, and infiltrated in paraffin; then, the samples were embedded, sectioned, decolored, and sealed for microscopy. (2) Electron microscopic observation: The stationary liquid was discarded after the articular cartilage tissue samples were obtained; then, each sample was cleaned by phosphate buffer saline (PBS) for 2 times, with 10 min for each time; next, the samples were washed once with 4% sucrose solution for 5 min; afterward, the samples were dehydrated successively in gradient alcohol of 70%, 80%, 90%, and 100%, with 10 min for each gradient; the appropriate observation surface was chosen after the samples were dried; the samples were attached to the electrically conductive adhesive for cladding, and were then observed under the microscope at appropriate magnification.
(III) The proliferation of chondrocytes was observed. After being treated with interventions for 72 h, the Cell Counting Kit-8 (CCK-8) method was utilized to detect the effects on the chondrocyte proliferation after articular cartilage defects in rabbits. In each group, the culture dish of chondrocytes was added with 10 uL of drugs. After 72 h, the absorbance value (OD value) of the cells was determined at λ = 480 nm to indicate the proliferation level of chondrocytes.
2.4. The regulation of bone formation by the Notch pathway
Bone is formed by the proliferation, migration, and differentiation of stem cells derived from the sclerotome or neural crest. The development of bone stem cells is controlled by innumerable STPs. Researches suggested that the Notch pathway is vital for the formation of bone and cartilage, which not only maintains the pluripotency of bone and cartilage precursor cells but also prevents the premature differentiation of pluripotent stem cells into osteoblasts (Chen et al., 2017). The over-activation of the Notch pathway inhibits the differentiation and maturation of osteoblasts. In addition, the Notch pathway is vital for the proliferation of bone and cartilage precursor cells and the inhibition of cell terminal differentiation.
Notch pathway not only functions in the development of the embryonic central axis skeletal system but also participates in the development of bone and cartilage and the regulation of their homeostasis. For example, the consecutive activation of Notch caused by the sudden Notch2 mutation will lead to the idiopathic extremity osteolysis syndrome, which is an autosomal genetic disease. Therefore, the Notch pathway is important for the formation of the skeletal system during embryonic development and is equally valuable for the normal development of the skeletal system after birth. It also provides a theoretical basis for the clinical utilization of the Notch pathway as a target for gene regulation and combined with bone tissue engineering to treat clinical diseases such as bone defects.
2.5. The regulation of chondrocytes by MAPK STP
The MAPK STP is a type of serine and threonine protein kinase that is widely present in eukaryotic cells. Activating the MAPK STP is dependent on extracellular signals or stimulatory cytokines. When the MAPK STP is activated, the extracellular signals are amplified into the nuclei, which regulates the transcription factor activity and the gene expression. The MAPKs family is divided into 8 subfamilies, which are respectively extracellular regulated protein kinases (ERK), p38 MAPK, ERK3, JNK, BMK1/ERK5, ERK27, ERK8, and NLK (Wang et al., 2017). It has been found that the pathogenesis of osteoarthritis is closely related to JNK, p38MAPK, and ERK pathways. When the ERK1/2 STP is stimulated by external factors, it can promote cell proliferation and determine the terminal differentiation of cells.
Besides, p38MAPK belongs to the serine and threonine protein kinases and can be divided into 4 subtypes, which are respectively p38α, p38β, p38δ, and p38γ, in which p38α and p38β can be expressed in almost all tissue cells while p38δ and p38γ have certain specificity. Various extracellular stressors such as stimulating cytokines can activate p38MAPK, produce a phosphorylation cascade, and regulate the gene expression activities of various downstream enzymes and transcription factors, thereby regulating the cell function. In addition, p38MAPK is also involved in the regulation of chondrocyte anabolism. Studies have found that the JNK STP and p38MAPK STP mediate the inflammatory responses and are involved in the degradation of the extracellular matrix of cartilage, while ERK STP promotes cell hypertrophy (Frank et al., 2017). The activation of MAPK STP is associated with certain pathological changes in bone and joint. The ERK STP is involved in the process of apoptosis and proliferation of chondrocytes. It is also found that the major factor that activates the p38MAPK STP in chondrocytes is the changes in the intra-articular environment and bio-stress.
2.6. Statistical method
The SPSS 20.0 statistics software was utilized to analyze the resulted data. The measurement data were expressed in the form of x ± s (mean number ± standard deviation), which were submitted to the homogeneity test of variance. P > 0.05 indicated the homogeneity of variance, and the pair comparisons between each mean number were analyzed by one-way ANOVA. P < 0.05 suggested inhomogeneity of variance, and the one-way ANOVA should be corrected by Welch. The pair comparisons of multivariate analysis were performed by the Least Significant Difference (LSD) method. The differences were proved to be significant in terms of statistics if P < 0.05.
3. Results
3.1. Comparison of general conditions between rabbits in each group
During the experiment, in the no-treatment group, rabbits showed no obvious changes; besides, the body weights were gradually increased, the body weights of rabbits in the model group decreased from (1835.8 ± 105.6) g before modeling to (1388.7 ± 108.6) g.The mental conditions were good, the daily activities were normal, the fur gloss was excellent, the food and water intake was normal, and no other symptoms appeared.
In the model group, rabbits showed obvious weight losses after the completion of the knee defect model construction; besides, the spirits were sluggish, the fur color was rough, the food and water intake were obviously decreased, and the daily activities were obviously reduced; rabbits even had no movements for a long time. In most cases, obvious symptoms of diarrhea were seen.
In the treatment group, after the completion of the knee defect model construction and drug administration, the body weights of rabbits were slightly decreased, the mental states were poor, the fur gloss was good, the diet was slightly reduced, and some rabbits had the symptoms of mild diarrhea during the experimental process.
3.2. Microscopic results of articular cartilage tissues of rabbits in each group
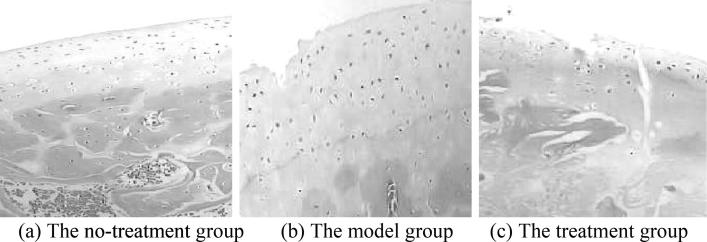
In the no-treatment group, under the optical microscope, the rabbit bone tissue samples were observed: the surface of articular cartilage was smooth, the distribution was neat, the structure was clear, the tide line was intact, the morphology and structure of bone trabecula were intact, the structure of bone cells was clearly visible, and the surrounding osteoblasts were densely distributed. The hematopoietic cells in the medullary cavity were abundant, the ratio of adipocytes to hematopoietic cells was uniform, and no empty bone lacuna was seen. Under the electron microscope, it was observed that the bone tissue structure was normal, the sample surface was smooth and covered with oval protrusions, and no collapse or crack was seen. The HE staining results of the articular cartilage tissues of the no-treatment group were shown in Fig. 2(a).
Fig. 2.
HE staining results of articular cartilage tissues (×100).
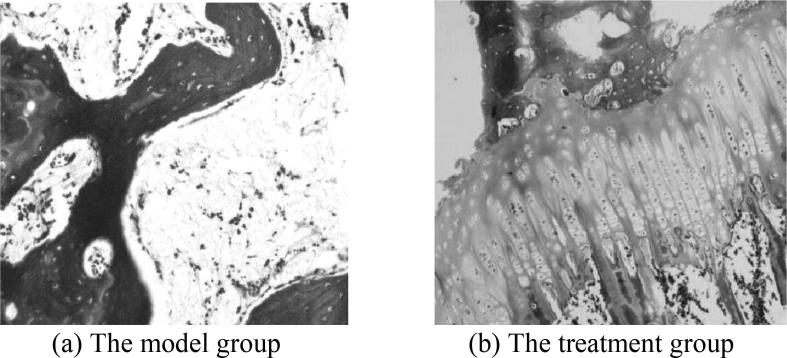
In the model group, under the optical microscope, the rabbit bone tissue samples were observed: the surface of the articular cartilage was rough and irregular, the damage and erosion were severe, the obvious cracks were observed and had penetrated into the transition layer; in addition, some rabbits showed complete peeling and the structure was blurred. The tide line was broken, the bone trabecula was obviously thin, and the gap was enlarged, the adipocytes in the medullary cavity were obviously increased, and the hematopoietic cells were obviously reduced. Even some of the hematopoietic cells of the rabbits were completely disappeared, and the bone cells were contracted and aggregated. A large number of empty bone lacunae were seen. Under the electron microscope, it was observed that most of the bone trabeculae were broken and collapsed, and no bone cells were seen on the surface. The shape of the bone lacuna was irregular, and a large number of adipocytes were found in the medullary cavity. The Masson staining results of the articular cartilage tissues of the model group were shown in Fig. 1(a), and the HE staining results were shown in Fig. 2(b).
Fig. 1.
Masson staining results of articular cartilage tissues (×100).
In the treatment group, under the optical microscope, the rabbit bone tissue samples were observed: the surface of the articular cartilage was slightly irregular, the structure was basically clear, the cartilage was mildly degenerated, the tide line was blurred, and the blood vessels were visible under the cartilage. In addition, the arrangement of bone trabecula was more regular and larger than that of the model group. The number of hematopoietic cells in the bone medullary cavity was large, the proliferation of adipocytes was not obvious, the bone cells were mostly normal, and the empty bone lacunae were less. Under the electron microscope, the structure of bone trabecula was tight, the number of osteoblasts was large, the bone lacuna was abundant, the bone surface was smooth, the bone cells and matrix collagen fibers were normal, and the medullary cavity was regular. The Masson staining results of the articular cartilage tissues of the treatment group were shown in Fig. 1(b), and the HE staining results were shown in Fig. 2(c).
3.3. Results of femur empty bone lacuna rates of rabbits in each group
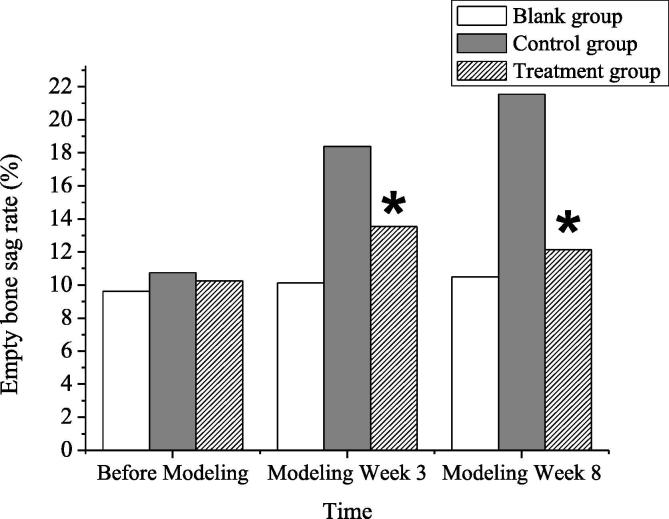
The empty bone lacunae of all rabbits were observed before modeling, at the 3rd week of modeling, and at the 8th week of modeling, respectively. Before the modeling, no significant differences were found in the rate of empty bone lacuna between rabbits in each group (P > 0.05), which was comparable. After the modeling, the no-treatment group was contrasted to the model group and the treatment group and the same groups before modeling, and the rate of empty bone lacuna was obviously increased, and there was a statistical significance in the differences (P < 0.05). Thus, it indicated that the knee-joint articular cartilage defects would increase the rate of empty bone lacuna, suggesting that the models were successfully constructed. At the 3rd week and the 8th week after modeling, in contrast to the model group during simultaneous period, the rate of the empty bone lacuna of rabbits in the treatment group was apparently diminished, and there was a statistical significance in the differences (P < 0.05). Thus, it indicated that by inhibiting the Notch/p38MAPK STP, the rate of empty lacunae could be reduced, and the further development of knee-joint articular cartilage defects could be delayed. The comparison results of empty bone lacuna rates between rabbits in each group were shown in Table 1 and Fig. 3.
Table 1.
Comparison of empty bone lacuna rates between rabbits in each group (%).
| Groups | Before Modeling | Modeling Week 3 | Modeling Week 8 |
|---|---|---|---|
| The no-treatment group | 9.63 ± 1.21 | 10.13 ± 1.33 | 10.50 ± 1.86 |
| The control group | 10.75 ± 1.28 | 18.38 ± 2.30 | 21.55 ± 3.07 |
| The treatment group | 10.25 ± 1.83 | 13.54 ± 1.60 | 12.13 ± 1.81 |
Fig. 3.
Comparison of empty bone lacuna rates between rabbits in each group (Note: * indicated that in contrast to rabbits in the model group in simultaneous period, P < 0.05.)
3.4. Effects of inhibiting Notch/p38MAPK STP on chondrocyte proliferation
Compared with rabbits in the no-treatment group, the absorbance values of chondrocytes in the treatment group and the model group were obviously decreased, the OD values were respectively 0.10 ± 0.020 and 0.34 ± 0.015, and there was a statistical significance in the differences (P < 0.05). In contrast to rabbits in the model group, the OD value of the treatment group was obviously greater than the OD value of the model group, and there was a statistical significance in the differences (P < 0.05), indicating that the inhibition of Notch/p38MAPK STP had a proliferative effect on rabbit chondrocytes. The comparison results of cartilage tissue absorbance values between rabbits in each group were demonstrated in Table 2.
Table 2.
A comparison of cartilage tissue absorbance values between rabbits in each group.
| Groups | OD |
|---|---|
| The no-treatment group | 0.40 ± 0.021 |
| The control group | 0.10 ± 0.020 |
| The treatment group | 0.34 ± 0.015 |
4. Discussion
Since the articular cartilage lacks blood supply, after injuries, the self-recovery ability of chondrocytes will be reduced; besides, if the range of articular cartilage injury is large, the injured cartilage may be completely unable to be recovered, which may cause the pathological changes of some joints and eventually the loss of normal mobile and physiological functions of joints (Cui et al., 2018, Gao et al., 2017, Johnson et al., 2017). When the articular cartilage full layer is damaged, the lower layer of the articular cartilage will initiate self-recovery, which will produce a large amount of fibrous tissue to fill the defect and compensate for the normal movement of the joint. However, the physical structure and physiological functional characteristics of these nascent fibrous tissues are far from normal articular cartilage, thereby the recovery of articular cartilage damage cannot achieve the fundamental therapeutic effects (Nikitin et al., 2018).
A large number of chondrocytes is found in the cartilage matrix, which mainly maintains the normal physiological functions and cartilage integrity, as well as the balance of cartilage damage and remodeling. As an important branch of MAPK signal, the p38 pathway is critical for the growth, apoptosis, and inflammatory reactions of chondrocytes. Through inhibiting p38 pathway, not only the apoptosis of chondrocytes was inhibited, and the further cartilage recovery was promoted but also the generation of inflammatory factors was inhibited and the progression of the disease was delayed (Zhou et al., 2017, Shunzhi et al., 2017, Deng et al., 2017). Both the Notch pathway and the chondrocyte apoptosis have played key roles in the development of cartilage defects, which regulate multiple procedures in the development of osteoblasts, including the differentiation and proliferation of osteoblasts and the functions of osteoclasts; in addition, they also regulate cartilage production during the process of development and homeostasis.
In the experiment, the rabbit knee-joint articular cartilage defect models were constructed to explore the effects of inhibiting Notch/p38MAPK STP on articular cartilage recovery by BMSCs tissue. The results elaborated that in the treatment group, after drug intervention, the general conditions of rabbits and the recovery of chondrocytes were significantly improved in contrast to the rabbits in, and the rate of the empty bone lacuna of articular cartilage was significantly reduced. Thus, it can be concluded that the inhibition of Notch/p38MAPK STP can promote the recovery of articular cartilage by BMSCs tissue and contribute to the recovery of knee-joint injuries in rabbits, which provides a reliable basis for clinical treatments of articular cartilage defects. In the recovering process after articular cartilage defects, other key STPs in MAPK were not discussed in the experiment, which needed further analysis in subsequent research.
Acknowledgement
This work was supported by Seed Fund of Shanghai Medical College of Health (State-level Project Cultivation Project) (SFP-18-20-14-004).
Footnotes
Peer review under responsibility of King Saud University.
References
- Chen Z., Li Y.X., Fu H.J. Hepatitis B Virus Core Antigen Stimulates IL-6 Expression via p38, ERK and NF-κB Pathways in Hepatocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;41(1):91. doi: 10.1159/000455954. [DOI] [PubMed] [Google Scholar]
- Cui J., Wang Y., Dong B. Pharmacological inhibition of the Notch pathway enhances the efficacy of androgen deprivation therapy for prostate cancer. Int. J. Cancer. 2018;143(3):645. doi: 10.1002/ijc.31346. [DOI] [PubMed] [Google Scholar]
- Deng J., Shi Y., Zhang X. Role of p38 mitogen activated protein kinase signaling pathway in lung ischemia-reperfusion protection offered by HOE642. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(7):749–754. doi: 10.11817/j.issn.1672-7347.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Frank S.B., Berger P.L., Ljungman M. Human prostate luminal cell differentiation requires NOTCH3 induction by p38-MAPK and MYC. J. Cell Sci. 2017;130(11):1952–1964. doi: 10.1242/jcs.197152. [DOI] [PubMed] [Google Scholar]
- Gao S., Li C., Zhu Y. PEDF mediates pathological neovascularization by regulating macrophage recruitment and polarization in the mouse model of oxygen-induced retinopathy. Sci. Rep. 2017;7:42846. doi: 10.1038/srep42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Zhao J.Y., Wang W.T. Cdc42 promotes schwann cell proliferation and migration through Wnt/β-Catenin and p38 MAPK signaling pathway after sciatic nerve injury. Neurochem. Res. 2017;42(5):1–8. doi: 10.1007/s11064-017-2175-2. [DOI] [PubMed] [Google Scholar]
- Johnson B., Cooke L., Mahadevan D. Next generation sequencing identifies ‘interactome’ signatures in relapsed and refractory metastatic colorectal cancer. J. Gastrointest Oncol. 2017;8(1):20–31. doi: 10.21037/jgo.2016.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin D., Penzar D., Garazha A. Profiling of human molecular pathways affected by retrotransposons at the level of regulation by transcription factor proteins. Front. Immunol. 2018;9:30-. doi: 10.3389/fimmu.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y.Y., Yao M., Zhou Z.W. Alisertib promotes apoptosis and autophagy in melanoma through p38 MAPK-mediated aurora a signaling. Oncotarget. 2017;8(63):107076–107088. doi: 10.18632/oncotarget.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunzhi Y., Zhonghai L., Ning Y. Mechanical stress affects the osteogenic differentiation of human ligamentum flavum cells via the BMP-Smad1 signaling pathway. Mol. Med. Rep. 2017;16(5):7692. doi: 10.3892/mmr.2017.7543. [DOI] [PubMed] [Google Scholar]
- Tung C.W., Hsu Y.C., Cai C.J. Trichostatin A ameliorates renal tubulointerstitial fibrosis through modulation of the JNK-dependent Notch-2 signaling pathway. Sci. Rep. 2017;7(1):14495. doi: 10.1038/s41598-017-15162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Liu W., Tan T. Notch signaling negatively regulates BMP9-induced osteogenic differentiation of mesenchymal progenitor cells by inhibiting JunB expression. Oncotarget. 2017;8(65):109661–109674. doi: 10.18632/oncotarget.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.X., Zhang J.C., Liu Y. Effect of Chinese medicine of nourishing kidney and clearing liver on intermittent hypoxia induced injury model of HUVECs through p38MAPK/NF-ΰB signaling pathway. Zhongguo Zhong Yao Za Zhi. 2017;42(23):4661–4664. doi: 10.19540/j.cnki.cjcmm.20170928.001. [DOI] [PubMed] [Google Scholar]
- Yang L., Xu F., Zhang M. Role of LncRNA MALAT-1 in hypoxia-induced PC12 cell injury via regulating p38MAPK signaling pathway. Neurosci. Lett. 2018;670:41–47. doi: 10.1016/j.neulet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- Zhou Y., An Q., Guo R.X. miR424-5p functions as an anti-oncogene in cervical cancer cell growth by targeting KDM5B via the Notch signaling pathway. Life Sci. 2017;171(Complete):9–15. doi: 10.1016/j.lfs.2017.01.006. [DOI] [PubMed] [Google Scholar]