Abstract
Saffron plant (Crocus sativus L.) is being used as a source of saffron spice and medicine to cure or prevent different types of diseases including cancers. We report the isolation, characterization of bioactive small molecule ([crocetin (β-d-glucosyl) ester] from the leaf biowastes of saffron plant of Kashmir, India. MTTC assay and Bio-autography aided approach were used to assess anti-oxidant activity and anti-cancer properties of crocin (s) against DPPH free radical and breast cancer cell line respectively. Crocetin beta-d-glucosyl ester restrained proliferation of human breast adeno-carcinoma cell model (MCF-7) without significantly affecting normal cell line (L-6). Further studies involving molecular mechanics generalized born surface area and molecular docking showed that crocetin beta-d-glucosyl ester exhibits strong affinity for estrogen receptor alpha and histone deacetylase 2 (crucial receptors involved in breast cancer signalling) as evidenced by the negative docking score and binding free energy (BFE) values. Therefore, crocetin beta-d-glucosyl ester from Crocus sativus biowastes showed antiproliferative effect possibly by inhibiting estrogen receptor alpha and HDAC2 mediated signalling cascade.
Keywords: Saffron, Floral biowastes, Antioxidant, Crocetin beta-d-glucosyl ester, Breast cancer, Molecular docking
Abbreviations: DPPH, 2,2-diphenyl-1-picrylhydrazyl; MTT, 3-(4,5–dimethyl thiazol–2–yl)–5–diphenyltetrazolium bromide; FBS, Fetal Bovine serum; DMEM, Dulbecco’s Modified Eagle’s Medium; UV, Ultra violet; TLC, Thin layer chromatography; FTIR, Fourier-transform infrared spectroscopy; NMR, Nuclear magneticresonance; LC-MS/MS, Liquid chromatography–mass spectrometry
1. Introduction
Breast cancer has implications in excess women mortality rates, the main reasons of it being lack of safer, effective, non-toxic and potent treatment options (Latosińska and Latosińska, 2013). In the United States, there were 40,290 breast cancer related deaths seen during 2015, more than 2.8 million breast cancer women cases were identified during 2016, among which 40,610 were estimated to die at the end of 2017, and around 255,180 new cases will be diagnosed (Bray et al., 2018). Breast cancer majorly occurs due to genetic mutations (85%) specifically mutations of BRCA1 and BRCA2 genes. There are mounting evidences that natural products that are currently used in traditional medicinal system, possess a wider range of chemical diversity and potential to be the source for modern drug discovery (Gilbert et al., 1997), because natural bioactive molecules are curative as compared to inherently destructive chemotherapy and cytotoxic drugs (Sodde et al., 2015). This has lead deep passion among scientists towards identification of pharmaceutically important novel plant based compounds as compared to synthetic drugs that could intervene to modulate different signalling cascades so as to circumvent breast cancers (Bishayee et al., 2011, Sodde et al., 2015). Although, breast cancer related mortality incidences have declined to some extent due to currently employed therapies such as selective radiotherapy, chemotherapy, estrogen receptor modulators (or SERMs) in the form of tamoxifen, raloxifene and class of aromatase inhibitors (letrozole, exemestane, anastrozole). However, not all races are getting benefitted from such aggressive therapies because, the patients generally relapse or suffer from side effects such as menopausal complications, blood clots, osteoporosis, etc (Cuzick et al., 2013). It is an established fact that an alternative mode of treatment is vital either singly or in combinatorial drug regimen against breast cancers (Bray et al., 2018). These grim facts and figures tempt scientists to desperately look for newer, safer, cheaper and potent natural sources of anticancer drugs that were being used in different ethno-medicinal systems from past several centuries due to their better tolerability (Tiwari, 2011). These measures if proved scientifically will result to tackle this unmet medical condition using alternative natural drug therapy.
Crocus sativus L. (Crocus sativus var. Cashmerianus Royle) is considered as a legendary, highly remunerative cash crop, being source of luxury spice known as saffron. Among the world’s total saffron production of 205 tons, Iran contributes 160 tons (~80%), Jammu and Kashmir, India contributes around 8–10 tons (~5%), Greek 4–6 tons (~3%), Morocco 0.8–1 ton (~0.5%), Spain 0.3 to 0.5 ton (~0.25%) and rest is contributed by other countries (Fernández, 2004). It is economically very important medicinal spice, possessing fabulous ethno-pharmacological potential. Saffron plant has a rich history of being used in various folk medicinal systems (Traditional Indian, Iranian and Azerbaijani) to cure or prevent different types of diseases including cancer (Samarghandian and Borji, 2014, Mollazadeh et al., 2015, Hire et al., 2017, Khorasanchi et al., 2018). The production of small quantity of saffron leave behind huge quantities of least priced bio-wastes in the form of tepals, leaves, stamen etc. These bio-wastes could play source of lead compounds for food and pharmaceutical industries, as they have potential medicinal properties including cytotoxic, antioxidant, antifungal etc. (Mir et al., 2014). The outcome of proper scientific evaluation of different organs of this species is anticipated to find new bioactive molecules against various cancer types, as they possess different types of yellowish carotenoids (Crocins). Crocin molecules being the main constituent of saffron extract is a family of carotenoids that constitutes 6–30% in terms of saffron total dry matter, the concentration of which depends upon growing conditions, variety and processing methods (Melnyk et al., 2010, Mollazadeh et al., 2015). Apart from stigmatic portion of C. sativus which is an important source of bioactive constituents, there is a growing zeal among natural product researchers to study other least explored tissue types of this plant species including leaves so as to isolate the potential phytocompounds responsible for anticancer properties (Mousavi et al., 2009, Lu et al., 2015).
There are marvellous biological properties attributed towards crocin(s) such as antioxidant, as it increases glutathione peroxidase and superoxide dismutase activity that helps in the detoxification of free radicals (Bathaie and Sajjadi, 2017) also these molecules act as chemo preventive agents (Bathaie and Sajjadi, 2017). Moreover, crocins reported much effective against wide range of malignant cells both in-vitro and in-vivo e.g. HeLa, HL60 (Ashrafi et al., 2005, Jalili et al., 2015, Jiang et al., 2018), MCF-7, MDA-MB-231, adenocarcinoma HT-29 (Melnyk et al., 2010), AGS-gastric adenocarcinoma (Bhandari, 2015), HT-29, Caco-2, CEM/ADR5000, HepG2, LAPC-4, CWR22, DU145, L1210/P3888 Leukemia, Sarcoma S-180, HT-29, C26 colon carcinoma, BxPC-3, TC-1, SKOv3, A549 (Bathaie et al., 2014, Bathaie and Sajjadi, 2017). Hence, crocins molecules possess important biological properties such as antioxidant, anticancer, chemo prevention etc. (Liakopoulou-Kyriakides and Kyriakidis, 2002, Melnyk et al., 2010, Bathaie et al., 2014, Kim et al., 2014). Crocins do not compromise safety of normal cells as they are non-toxic and non-mutagenic agents with potential to treat different cancer types by modulating various signalling pathways (Abdullaev and Espinosa-Aguirre, 2004, Bhandari, 2015, Milajerdi et al., 2016).
Crocin and crocetin exist in Crocus. They are the chemical components with antioxidative properties primarily responsible for the color of the stigmas of Crocus sativus L. (Saffron). Crocetin is a carotenoid dicarboxylic acid with 20 carbon atoms and it is the core of crocin. Crocin, in general term, includes Crocin-I (Crocetin-di-beta -D-gentiobiosyl ester), Crocin-II (Crocetin-beta-D-gentiobiosyl-beta-d-glucosyl ester), Crocin-III (Crocetin-mono-beta-D-gentiobiosyl ester), Crocin-IV (beta-D-monoglucoside ester of monomethyl alpha-crocetin), Crocetin-di-(beta-d-glucosyl) ester, Crocetin-mono-beta -d-glucosyl ester. Crocin mainly exists in trans-form, but can also present in cis-form in minor amount. Crocetin beta-d-glucosyl ester has antioxidant properties, (Bathaie and Sajjadi, 2017) also these molecules act as chemo preventive agents (Bathaie and Sajjadi, 2017). Moreover, Crocetin have been reported to act against malignant cells both in-vitro and in-vivo e.g. HeLa, HL60 (Ashrafi et al., 2005, Jalili et al., 2015, Jiang et al., 2018),
Estrogen receptor alpha has potent implications breast cancer which is quite evident from the fact that it is over-represented in majority of such cancers. Estrogen provoked breast cancer signalling is mediated by estrogen receptor alpha and due to these reasons this receptor has remained the central focus in anti-estrogen breast cancer therapy. Besides breast cancer signalling is epigenetically fuelled by HDAC2 over activity. On the whole these findings suggest that estrogen receptor alpha and HDAC2 are the promising targets for breast cancer drugs and therapy. Taking these grim facts into account we selected these two receptors for our molecular docking and binding affinity studies.
It is important to validate the ethno-medicinal properties of plant derived drugs, because there is a huge scientific and industrial interest mushrooming within pharmaceutical domain to isolate and characterize plant based bioactive compounds including carotenoids (Crocins) that could open up novel ways to treat and prevent cancer incidences specifically breast cancers (Sajjadi and Bathaie, 2017). Saffron plant has a rich history of being used as a folklore medicine to prevent and treat different diseases including cancers which has been recently studied through various scientific evidences (Melnyk et al., 2010, Khorasanchi et al., 2018). Therefore, the current study attempted to isolate and characterize crocin(s) from leaves of Crocus sativus which are considered to be the major and cheaper biowastes of saffron industry. The antioxidant and antiproliferative properties were undertaken using in-vitro and in-silico based combinatorial approach.
2. Materials and methods
2.1. Plant material collection, authentication and processing
The leaves of Crocus sativus var. Cashmerianus were collected from saffron karewas of Pampore Kashmir, India (1574 m above sea level) during March 2019 and were shade dried. The authentication of sample was done by an expert taxonomist and the voucher specimen of the same was deposited at Kashmir university herbarium (KASH) under accession number KASH-1733. The dried samples were subjected to grinding into fine powder and phytochemical extraction through maceration was carried out using petroleum ether as an organic solvent (Tiwari, 2011).
2.2. Chemicals and solvents
The chemicals and reagents including 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(4,5–dimethyl thiazol–2–yl)–5–diphenyltetrazolium bromide (MTT) were procured from Sigma Aldrich, USA. Pre-coated TLC aluminum sheets from Analtech, Inc, Germany. 60–120 mesh size silica gel, Fetal Bovine serum (FBS), Phosphate Buffered Saline (PBS), Dulbecco’s Modified Eagle’s Medium (DMEM), Trypsin. EDTA, Glucose, MHA medium, PDB medium, solvents and antibiotics were procured from Hi-Media Laboratories Ltd., Mumbai. UV–Vis spectrophotometer (Chemito Technologies, India), FT-IR spectrums (Perkin Elmer, MA, USA), NMR spectrometer (Bruker) and mass spectra’s were recorded using WATERS-Q-TOF Micromass.
2.3. Biological materials and culture medium
MCF-7 (Human breast carcinoma cell line) and L-6 (Normal rat muscle cell line) were obtained from National Centre for Cell Sciences (NCCS), Pune, India. DMEM medium containing 10% inactivated Fetal Bovine Serum (FBS), streptomycin (100 g/ml), amphotericin B (5 g/ml) and penicillin (100 IU/ml) with humidified atmosphere of 5% CO2 was maintained at 37 °C until confluent and was used for the culture of cell stock. Later, the dissociation of the cells was done using TPVG (Trypsin Phosphate Versene Glucose) solution containing 0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS and 25 cm2 culture flasks were used to grow the stock cultures.
2.4. Localization, isolation and purification of Crocin (s)
Bioautography: The identification of biologically active compounds was localized by DPPH/antioxidant TLC bioautography assay (Cannell, 1998, Sarker et al., 2006). Briefly extract was applied on TLC plates as a spot using capillary tube at the concentration of 100 µg/ml. The plates were dried, immersed in 0.2% DPPH solution in methanol and left for half an hour. The appearance of white, yellow spots against a purple background indicates antioxidant activity. The isolation of crocetin beta-d-glucosyl ester using column chromatography, followed by preparative thin layer chromatography on pre-coated TLC plates of 20 × 20 cm (Kalimuthu et al., 2011). Briefly, a glass column of 5 cm diameter and 70 cm length was packed with activated 400 g silica gel slurry (Silica gel was dried at 100 C with mesh size of 60–120; Merk India) dissolved in petroleum ether. The crude extract (10 g) was dissolved in minimum quantity of toluene, followed by adsorption onto 20 g of silica gel, the solvent was allowed to evaporate and then the silica bound sample was placed at the top of the already packed silica gel column. The mobile phase toluene: acetone: water: acetic acid (16:2:2:2) was allowed to elute the column using increase in polarity in different ratios at fraction of 5 ml volume were collected, evaporated using rotary evaporator at controlled temperature of 40–50 °C. The identity of the fractions was examined by TLC on silica gel coated aluminum sheets (UV 254, Macherey/Nagel GmbH & co. Duren Germany). The developed plate was dried, exposed to iodine vapors (spots marked) and finally derivatized with anisaldehyde reagent (10 ml sulphuric acid + ice cooled mixture of methanol & 20 ml acetic acid + 1 ml anisaldehyde). The fractions that showed the same UV/Vis spectrum (Cannell, 1998) as well as same TLC development profiles (Color and RF Value) were pooled together and concentrated to dryness under reduced pressure using rotary evaporator. The column sub fractions were purified using preparative pre-coated TLC plates via bioautography approach. The experiment was repeated several times till the purity of the compound was assured by aiming that compound is present as a single spot.
The identification of biologically active compounds were localized by DPPH-Antioxidant TLC Bioautography assay (Cannell, 1998, Sarker et al., 2006). The isolation of crocetin beta-d-glucosyl ester was carried out using preparative thin layer chromatography on pre-coated TLC plates of 20 × 20 cm and also by silica gel based column chromatography (Kalimuthu et al., 2011). All the scrapped spots were collected and dissolved in highly soluble solvents (ethanol, methanol and DMSO). The solution was subjected to centrifugation to remove silica gel pellet, supernatant was collected and solvent evaporated using rotary evaporator. The physical properties of purified compounds were noted down e.g. color, solubility &Rf values.
2.5. Structural elucidation of Crocin(s)
The purified molecule was characterized for structural elucidation using various spectroscopic hyphenated techniques such as FT-IR (Assimiadis et al., 1998), UV–Vis (Cossignani et al., 2014), NMR (Yilmaz et al., 2010), MS-MS (Kalimuthu et al., 2011, Montoro et al., 2012) and the data was compared with previously published literature.
2.6. Free radical scavenging (DPPH) bioautography assay
The antioxidant activity of isolated compound was further tested using TLC based qualitative assay described by Sarker et al. (2006) with minute modifications. Briefly, respective compounds were applied on TLC plates as a spots using capillary tubes at the concentration of 100 µg/ml. The plates were dried, immersed in 0.2% of DPPH solution in methanol and left for half an hour. The appearance of white-yellow spots against a purple background indicates antioxidant activity.
2.7. Antiproliferative assay
Cytotoxicity studies were performed by MTT assay as described in the previous literature (Sarker et al., 2006). Briefly, Crocetin beta-d-glucosyl ester small molecule was dissolved in distilled DMSO (0.1%) and stock solution of 1 mg/ml concentration was made using DMEM supplemented with 2% inactivated FBS. The solution so obtained was sterilized by filtration. The serial dilutions were made to prepare different concentrations of the extract (31.25, 62.5, 125, 250, 500, 1000 µg/ml) to carry out cytotoxic studies. The monolayer cell culture was trypsinized and using DMEM containing 10% FBS, the cell count was adjusted to 1.0 × 105 cells/ml. 0.1 ml of the diluted cell suspension (approximately 10,000 cells) was added to each well of the 96 well microtitre plate, and DMSO (0.1%) without crocetin beta-d-glucosyl ester was considered as negative control, the cell suspension was kept at 37 °C for 24 h. The supernatant was flicked off and monolayer so obtained was once washed with medium followed by addition of 100 µl of different test concentrations to the partial monolayer in microtitre plates (Tarsons India Pvt. Ltd., Kolkata, India). The plates were then incubated at 37 °C for 72 h in 5% CO2 atmosphere and observations were noted every 24 h interval using microscopic examination (20×). The solution in the wells was discarded after 3 days of incubation and to each well 50 µl of MTT in PBS was added. The plates were then gently shaken and incubated for 3 h at 37 °C in 5% CO2 atmosphere. The supernatant obtained was discarded first and then 100 µl of propanol was added. In order to solubilise the formed formazan, the plates were gently shaken and absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and the concentration of test sample needed to inhibit cell growth by 50% (IC50) values was generated from the dose–response curves for both the cell lines (MCF-7 and L-6). All the experiments were carried out in triplicates (n = 3)
% Growth inhibition = 100 − (Mean OD of individual test group × 100)
Mean OD of control group
2.8. Ligand preparation
All the ligand molecules were retrieved from PubChem with PubChem CID of 449,459 for 4-hydroxytamoxifen and10368299 for crocetin beta-d-glucosyl ester. The ligand molecules were prepared using the LigPrep of Maestro v11.0 of Schrodinger tool (Van Den Driessche and Fourches, 2017, Van Den Driessche and Fourches, 2018). The ligands were minimized and protonation states were generated using the Epik (Shelley et al., 2007). Moreover, the ligand molecules were desalted and specified chiralities were retained (Ganai et al., 2015). For ligands going to be docked against metalloenzyme HDAC2, metal binding states were also generated.
2.9. Protein preparation
The crystal structures of estrogen receptor alpha and HDAC2 were retrieved from Protein Data Bank with PDB ID 3ERT (Shiau et al., 1998) and 4LY1 respectively (Lauffer et al., 2013). The protein preparation was done by Shrodinger package (Protein Preparation wizard) to certify correctness of the structure (Kalyaanamoorthy and Chen, 2013, Sastry et al., 2013). The hydrogen atoms which were missing were added to these structures and bond orders were properly assigned. The Shrodinger package was utilized to fill the side chains and loops which were missing in the protein (Mir et al., 2014, Lu et al., 2015). Every molecule of water beyond 5 Å was deleted. In the subsequent steps, the redundant protein chains and heteroatoms were also removed. However, the cocrystallized ligands were retained in both structures for subsequent use in grid generation (Mir et al., 2014). Moreover, in HDAC2, the heteroatom Zinc was retained as it serves as a cofactor for the given enzyme. In the next step the structures were optimized, water molecules making less than 3 hydrogen bonds with non-waters were deleted and the structures were subjected to minimization where heavy atoms were converged to 0.30 Å (Harder et al., 2015). In case of both structures grid was specified using the co-crystallized ligand as centroid.
2.10. Pose validation by self-docking
The Protein preparation wizard was employed to prepare estrogen receptor alpha along with its cocrystallized ligand until minimization. Then the separation of the ligand was done and the ligand was redocked to estrogen alpha receptor using the protocol of extra precision flexible docking and the calculation of the root mean square deviation (RMSD) between the native and docked pose was carried out (Sándor et al., 2010).
2.11. Molecular docking
The ligands that were prepared were docked against the grid specified receptors using the extra precision flexible docking protocol. Glide (Grid-based Ligand Docking with Energetics) program of Schrödinger package (Glide v7.3) was used to perform molecular docking (Friesner et al., 2006). The docked scores were obtained from the pose viewer files of docked complexes.
2.12. Molecular mechanics generalized born surface area (MMGBSA)
The binding free energy (BFE) of inhibitors was calculated by using an implicit solvation model, MMGBSA. The docked complexes were used as input and no flexibility was given to receptor (frozen condition. Prime MM-GBSA makes five important energy calculations namely optimized free receptor (Receptor), optimized free ligand (Ligand), optimized complex (complex), receptor from optimized complex, ligand from optimized complex (Lyne et al., 2006). From these energies, MMGBSA free energy of binding is calculated as:
MMGBSA dG Bind = Complex − Receptor − Ligand.
3. Results
3.1. Isolation and characterization of [crocetin (β-d-glucosyl) ester]/Crocetin beta-d-glucosyl ester
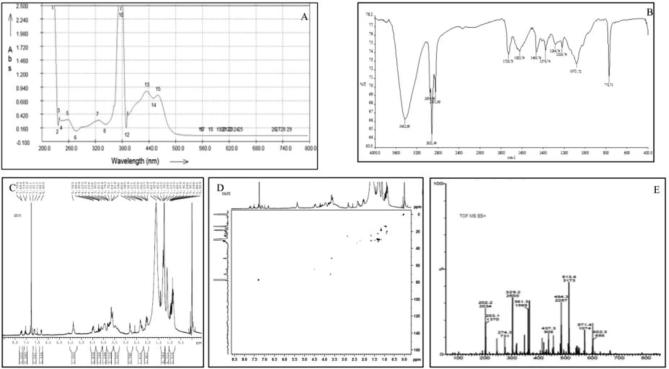
Analytical thin layer chromatography (TLC) lead identification of ideal mobile phase (Toluene: acetone: water: acetic acid in the ratio of 16:2:2:2) which gave maximum separation of bands in the crude leaf extracts of Crocus sativus (Fig. 1A). The crude petroleum ether extract was subjected to column chromatography using solvents of differential polarities starting from non-polar towards molar polarity index. The polarity of the solvent was increased step up step by 10% towards more polarity index. Seven different fractions so obtained were tested for antioxidant activity, however only one fraction showed marked antioxidant activity against DPPH free radical (Fig. 1B) Post preparative TLC, the study ended up with isolation of a yellow coloured compound from the leaves of C. sativus. The weight of compound so obtained was found as 17 mg with Rf value as 0.53. The solubility of purified compound was verified using ethanol, methanol and DMSO, it dissolves completely in all these solvents.
Fig. 1.
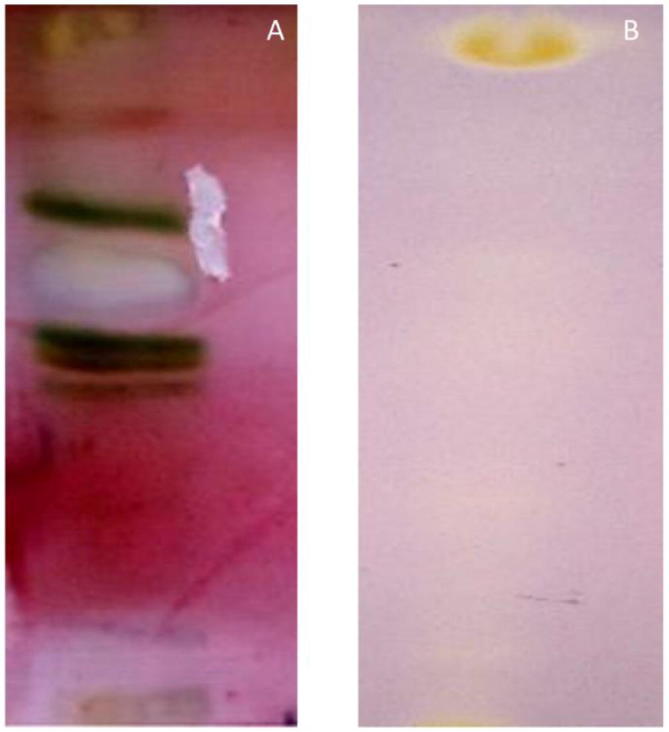
(A) TLC separation of petroleum ether leaf extract of Crocus sativus using mobile phase Toluene: acetone: water: acetic acid (16:2:2:2), (B) Antioxidant TLC Bio autography assay of crocetin beta-d-glucosyl ester using DPPH as free radical.
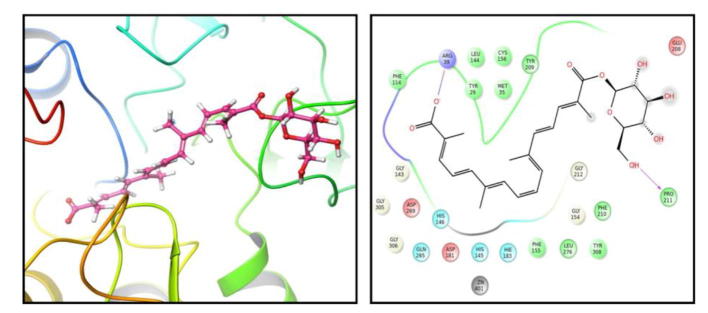
UV–Vis-spectral data has showed various characteristics absorption bands (Fig. 2A) between 226 and 500 nm i.e. λmax at 260 nm and 437 corresponds to glucosyl ester bonds and all-trans-carotenoids respectively. The FT-IR showed bands at wavelength of 3432 cm−1 assigned to hydroxyl groups (—OH), 2921, 1463 and 1376 cm−1 for C—H, 1728 cm−1 for carbonyl group (C O), 1623 cm−1 for C C group, 1071 cm−1 for C—O sugar groups (Fig. 2B). The 1H NMR spectrum of molecule in DMSO solution showed the presence of terminal methyl groups by exhibiting signals at δ 0.89, methylene groups at δ 1.29 and methyl groups attached to the double bonds by exhibiting signals at δ 1.56. Further the bunch of signals between δ 3.00 and 4.00 are due to protons present in the sugar residue. The signals at δ 4.5 and 5.5 are due to the anomeric protons. The couple of signals between δ 6.0 and 7.5 are due to unsaturated protons of crocins. In 1H–13C heteronuclear single quantum coherence (HSQC), signals at δ 14.00 for methyl carbon, between δ 20 and 40 for the carbon atoms attached to the double bond, between δ 50 and 80 for the sugar carbons (Fig. 2C and D). The scan for mass spectrum was run in positive ion mode and molecular mass of isolated compounds was found as 490. Molecular ion EI-MS+ (Mass spectroscopy) was observed at m/z = 513 ([M+Na]+) along with an additional signal at m/z 328 [(M+H)-Glc]+ (Fig. 2E). The molecule (Fig. 3-PubChem CID-10368299) is identified as crocetin beta-d-glucosyl ester/[crocetin (β-d-glucosyl)ester].
Fig. 2.
A Spectrums of crocetin beta-d-glucosyl ester based on hyphenated spectroscopic techniques (A) UV–Vis (B) FT-IR (C) 1H NMR (D) 13C NMR (E) Mass spectrum (ESI+). The interpretation of the obtained data along with its comparison with literature data has characterized the compound as [crocetin (β-d-glucosyl) ester] (Molecular formula = C26H34O9).
Fig. 3.
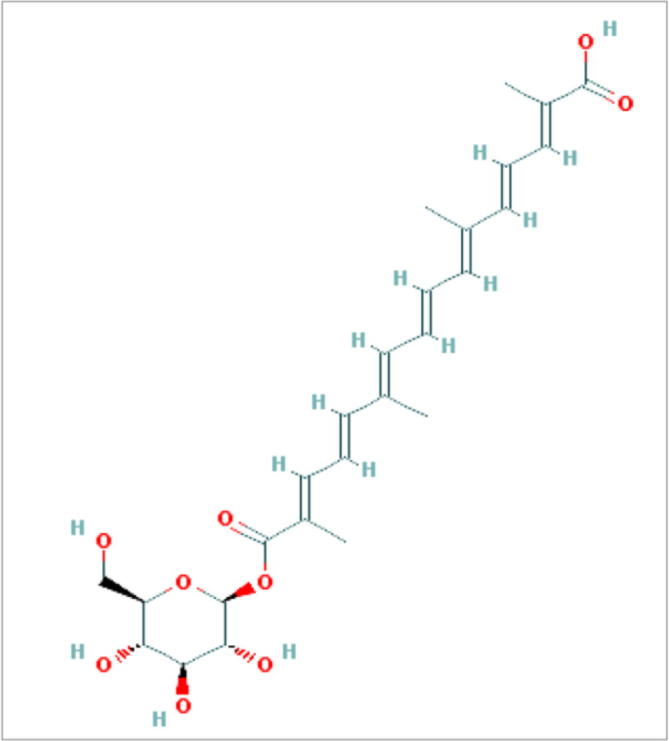
Structure of crocetin (β-d-glucosyl) ester retrieved from Pubchem (CID-10368299).
3.2. Crocetin beta-d-glucosyl ester as a significant antioxidant and anticancer agent
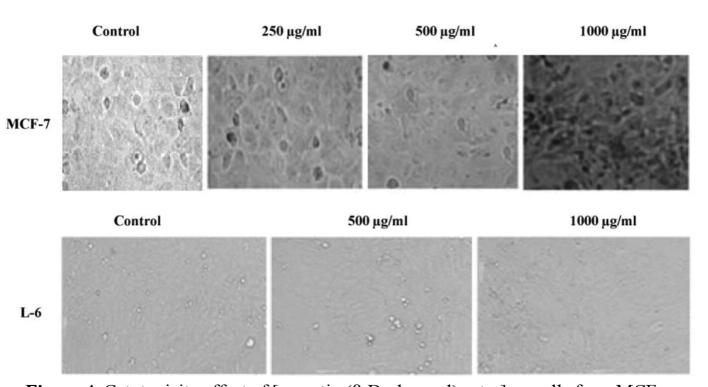
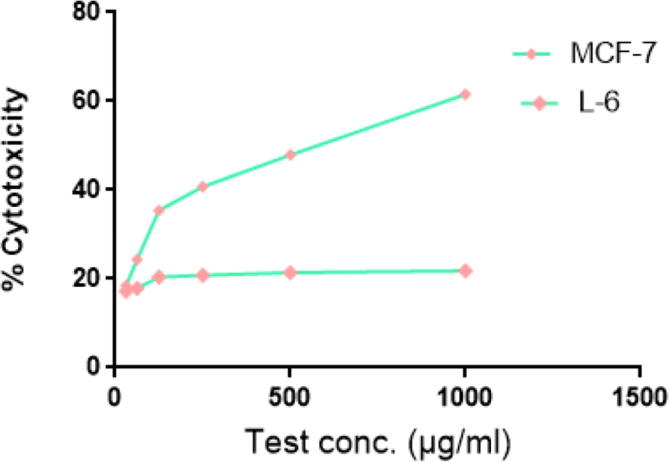
The antioxidant activity has revealed appearance of white-yellow spots against purple background on TLC antioxidant bioautographic plates, indicating significant antioxidant property (Sarker et al., 2005). The antiproliferative activity of crocetin beta-d-glucosyl ester against MCF-7 cell line has showed statistically significant inhibitory effect in a dose dependent way with IC50 value of 628.36 ± 15.52 µg/ml (P < 0.001) (Table 1). The percentage cytotoxicity effect ranged from 18.5 ± 0.8% to 61.57 ± 1.90% at different concentrations of crocetin beta-d-glucosyl ester molecule (31.25–1000 µg/ml). The microscopic examination has revealed that crocetin beta-d-glucosyl ester treated MCF-7 cells have showed cellular apoptotic characteristics such as cell shrinkage, reduced cytoplasm, cytoplasmic vacuole like areas, pyknotic nuclei, nuclear condensation and fragmentation (altered nuclear morphology), while as L-6 cell line has intact cell distribution along with no-marked apoptotic or anti-proliferative characteristics (Fig. 4, Fig. 5).
Table 1.
Cytotoxicity effect of Crocus sativus derived Crocetin beta-d-glucosylesteron on MCF-7 cell line.
| Compound name | Test conc. (µg/ml) | % Cytotoxicity* MCF-7 | IC50(µg/ml)* MCF-7 | % Cytotoxicity* L-6 | IC50(µg/ml)* L-6 |
|---|---|---|---|---|---|
| [crocetin (β-d-glucosyl) ester] | 1000 | 61.57 ± 1.90 | 628.36 ± 15.52 | 21.80 ± 0.90 | >1000 |
| 500 | 47.89 ± 2.0 | 21.45 ± 1.2 | |||
| 250 | 40.78 ± 0.95 | 20.8 ± 0.75 | |||
| 125 | 35.46 ± 1.2 | 20.45 ± 0. 5 | |||
| 62.5 | 24.34 ± 1.0 | 17.89 ± 1.32 | |||
| 31.25 | 18.5 ± 0.8 | 17.20 ± 0.45 |
% Cytotoxicity: The statistically significant percentage of cell toxicity (p < 0.05) in MCF-7 cells upon progressive increase of dose (31.25, 62.5, 125, 250, 500, 1000 µg/ml). IC50: Effective concentration of molecules in µg/ml required to achieve 50% growth inhibition of breast cancer cells (MCF-7). Data are representative of three independent experiments (mean ± SD).
Fig. 4.
Cytotoxicity effect of [crocetin (β-d-glucosyl) ester] on cells from MCF-7 cell line. The cells were seeded in 96-well plate (10,000 cells/well) for 24 h, then exposed to 0.1% DMSO based extract of [crocetin (β-d-glucosyl) ester] 1 for 72 h at different concentrations (31.25, 62.5, 125, 250, 500, 1000 µg/ml). The statistically significant concentration dependent cytotoxicity (P value = 0.0019) was observed with IC50 as 628.36 µg/ml. Data are representative of three independent experiments (mean ± SD). Representative slides of MTT assay for MCF-7 cell line upon treatment with [crocetin (β-d-glucosyl) ester]. The prominent morphological changes are seen in treated cells at concentration gradient of 250, 500, 1000 µg/ml, representing apoptosis. L-6 cell line has not demonstrated any significant levels of cytotoxicity upon treatment by [crocetin (β-d-glucosyl) ester] at 500, 1000 µg/ml concentrations with IC50 > 1000 µg/ml. Untreated MCF-7 and L-6 cells act as negative control without any morphological variations. Data are representative of three independent experiments (mean ± SD).
Fig. 5.
Relationship between dose of [crocetin (β-d-glucosyl) ester] and % cytotoxicity against MCF and L-6 cell lines at different concentrations (31.25, 62.5, 125, 250, 500, 1000 µg/ml). The statistically significant concentration dependent cytotoxicity (P value = 0.0019) was observed with IC50 as 628.36 µg/ml. Non-significant levels of cytotoxicity was observed in L-6 cell line.
3.3. Molecular docking simulation studies showed crocetin beta-d-glucosyl ester shows stronger affinity against estrogen receptor alpha
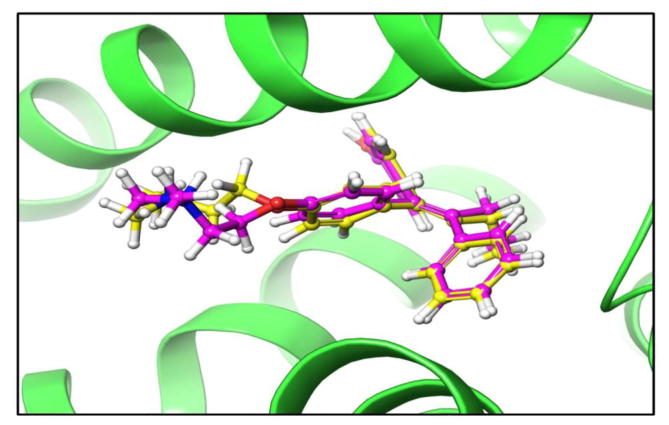
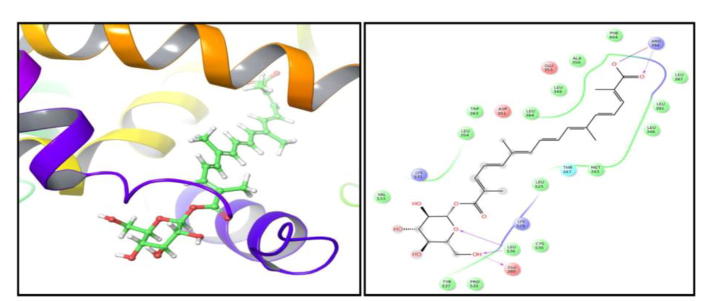
Before performing molecular docking studies, it is mandatory to validate algorithm for reproducing the crystal pose of ligand. We performed self-docking as described in methods and computational details. The redocked and native pose presented and RMSD of 1.9341 Å thus validating the accuracy of our algorithm to generate correct pose (Ganaiet al., 2015) (Fig. 6). Molecular docking is regarded as the central facet in drug discovery. Our studies showed crocetin beta-d-glucosylester inhibits the defined receptor as evidenced by the docking score of −6.9 against the defined receptor (Table 2). Crocin −1 showed affinity towards HDAC2 receptor as determined by a docking score of −6.61.
Fig. 6.
Pose validation by self-docking. The native ligand of crystal structure was redocked to its host receptor using extra precision flexible docking protocol. The RMSD between native (pink) and redocked (yellow) pose of ligand was found to be 1.9341 Å clearly suggesting that docking algorithm is working correctly.
Table 2.
Docking scores of Crocetin beta-d-glucosyl esteragainst estrogen receptor alpha and HDAC2.
| Ligand | Receptor | Docking score | dGBind(Kcal/mol) |
|---|---|---|---|
| 4-hydroxytamoxifen | Estrogen receptor alpha | −14.58 | −134.1 |
| Crocin-1 | −6.53 | −80.3 | |
| Crocin-1 | HDAC2 | −6.61 | −61.7441 |
3.4. Binding free energy calculation confirmed the predictions of molecular docking
The relative binding affinity of ligands especially of a congeneric series is calculated by MMGBSA, an implicit solvation model (Ganai et al., 2015, Kalyaanamoorthy and Chen, 2013). Crocin −1 showed BFE value of −80.3 Kcal/mol towards estrogen receptor alpha. The binding free energy value of (−61.7441 Kcal/mol) against HDAC2 was shown by the defined inhibitor (Table 2).
3.5. Crocetin beta-d-glucosyl ester shows similar but not identical interaction profile as that of 4-hydroxytamoxifen
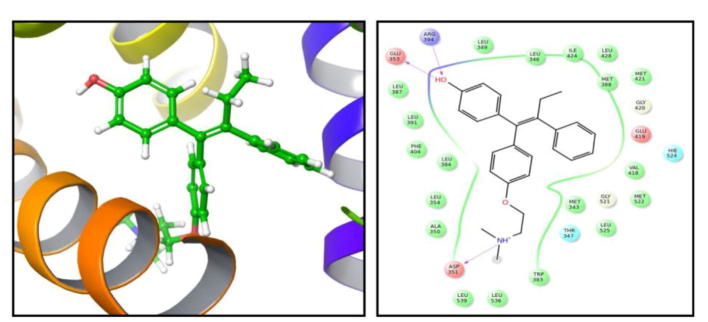
In order to gain insights regarding the interaction profile of crocetin beta-d-glucosyl ester against estrogen receptor alpha we generated ligand–protein contacts up to 4 Å from docked complexes. While 4-hydroxytamoxifen showed 3 hydrogen bonding interactions with Arg 394, Glu 353, Asp 351, crocetin beta-d-glucosyl ester portrayed 4 hydrogen bonding interactions with Arg 394, Leu 536 and Glu 380 (Fig. 6, Fig. 7, Fig. 8). In case of HDAC2 crocetin beta-d-glucosyl ester formed one hydrogen bonding interaction with Pro 211 and one salt bridge with Arg 39 (Fig. 9).
Fig. 7.
Residues of estrogen alpha receptor targeted by 4-Hydroxytamoxifen. This inhibitor targets Arg 394, Glu 353 and Asp 351 by forming hydrogen bonding interactions with them.
Fig. 8.
Residues targeted by Crocetin beta-d-glucosyl ester in estrogen receptor alpha. [crocetin (β-d-glucosyl) ester]-1 forms hydrogen bonding interaction with residues Glu 380, Leu 536 and Arg 394. Moreover, the defined inhibitor forms a salt bridge with Arg 394.
Fig. 9.
Crocetin beta-d-glucosyl ester targets HDAC2 residue Pro 211 by forming hydrogen bonding interaction with it and Arg 39 by forming salt bridge.
4. Discussion
Globally, breast cancer is the most common cancer type causing cancer related deaths in huge numbers among women and this is the second most cancer type in general. Since 2008 onwards breast cancer induced mortality rate has increased by 14%. Although several dozens of synthetic anticancer drugs have been discovered till date like tamoxifen, raloxifene and class of aromatase inhibitors. Nevertheless, with these treatments patients generally relapse or suffer from side effects such as menopausal complications, blood clots, osteoporosis etc (Cuzick et al., 2013). In contrary, natural products are generally seen as lesser toxic, effective and cheap. The screening of such potential bioactive molecules against breast cancer can open up novel gateway to tackle such therapeutically challenging disease.
There are many plant based potential molecules currently being used in the pharmaceutical industry and about 60% of anti-tumor and anti-infectious drugs which are prescribed worldwide come from plants like vincristrine/vinblastine from Catharanthus roseus (Rates, 2001). Crocus sativus is source of saffron which is a repository of complex molecules like carotenoids (Crocin, picrocrocin and safranal), glycosides, monoterpenes, aldehydes, anthocyanins, flavonoids, vitamins, amino acids, starch, mineral matter, proteins, gums (Fernández, 2006), chitinase Safchi A (Castillo et al., 2007) and other compounds present in saffron could be responsible for different properties including antioxidant, anticancer, anti-bacterial, antidiabetic, analgesic, aphrodisiac, sedative, anti-alzheimer’s, anti-tussive, anti-convulsant (Bhargava, 2011, Mir et al., 2012, Alhakmani et al., 2013). There are no toxicity reports associated with proper usage of saffron or its extracts because hematological and biochemical studies on the toxicity of saffron has showed that there are no signs of any toxicity found in kidney, liver or bladder (Hamidpour et al., 2014). The previous studies have specified the importance of Crocus sativus in traditional and modern therapy including its major components in the form of crocin(s) (Sajjadi and Bathaie, 2017, Khorasanchi et al., 2018, Mykhailenko et al., 2019). However, the biologically active crocin (s) have been isolated from stigmatic portion of saffron plant which is economically a costlier source. The current study therefore attempted to isolate and characterize a bioactive molecule from leaves of saffron plant in the form of crocetin beta-d-glucosyl ester which is a cheaper source. UV–Vis, FT-IR, NMR and mass spectrum of isolated molecule were compared with previous studies which confirmed this molecule as Crocetin beta-d-glucosyl ester (Cossignani et al., 2014, Cagliani et al., 2015). Based on the interpretation of obtained spectral data along with its comparison with literature data, we proposed the molecular formula of isolated compound as C26H34O9 (Fig. 2). This small molecule of crocetin beta-d-glucosyl ester is identified and characterized for the first time from cheaper sources (leaves) of C. sativus using hyphenated spectroscopic techniques (Fig. 3). Crocetin beta-d-glucosyl ester has demonstrated significant inhibitory activity against MCF-7 cell line without affecting normal cell line (L-6). Strong strong inhibitory effect of saffron crude extracts against MCF-7 cell lines has been observed earlier with IC50 value between 350 and 400 µg/ml (Chryssanthi et al., 2007) which could be due to synergistic effect of multiple compounds present in crude extract. The current investigation has successfully been able to prove that the main chemical player responsible against breast cancer cell lines is Crocetin beta-d-glucosyl ester. The intricate molecular mechanism of crocin molecules against breast cancer is globally still enigmatic, but the most accepted mechanism is that the sugar moiety of crocin(s) plays a vital role in its chemical activities due to in-tense electrostatic potential terminals which makes it as a trap for free radicals. There are other proposed mechanisms of crocin acting against different malignant human cell lines e.g. it strongly binds to histone H1 which induce unknown conformational changes that decreases the interaction between H1 protein with DNA (Ashrafi et al., 2005). Crocin increases tubulin polymerization and microtubule nucleation rate in a concentration dependent manner and it showed downregulation of cyclin D1, p21 and p53 which occurs in breast tumor induced by NMU injection in female rat. They also induce tumor cell apoptosis by down regulating the expression of B-cell lymphoma/leukemia-2 (Bcl-2), survivin, cyclin D1 and up regulating the expression of Bax (decrease Bcl-2/Bax ratio). The action of saffron extract on breast cancer cell line MCF-7 could be by induction of caspase-dependent pathway and exerts proapoptotic effects (Friesner et al., 2006). The evidence of antiproliferative activity of saffron derived crocin molecule against breast cancer cell lines indicates its ethno- pharmacological potential to prevent and treat cancer owing its strong antioxidant potential (Rates, 2001, Samarghandian and Borji, 2014, Harder et al., 2015). Crocin as a candidate chemopreventive agent against HCC. Crocin exhibited anti-inflammatory properties where NF-κB, among other inflammatory markers, was inhibited (Amin, et al., 2016). The folklore medicinal properties of saffron could be thus attributed due to strong radical scavenging activities incoherence with proapoptotic effects. The further evaluation of its clinical use would ameliorate saffron demand which will motivate farmers or traditional healers to promote its cultivation at a large scale, an opportunistic approach would lead its constructive conservation and usage sustainably, a way to assure biodiversity preservation. Structure based drug designing studies showed that crocetin beta-d-glucosyl ester has stronger affinity against estrogen receptor alpha, while as binding free energy calculation confirmed the predictions of molecular docking. Molecular docking is regarded as the central facet in drug discovery. It predicts the conformation and orientation of ligand within a target binding site (Ganai et al., 2015). Moreover, accurate structural modelling and correct prediction of activity are its two main aims. We performed molecular docking simulations against estrogen alpha receptor using 4-hydroxytamoxifen as positive control and crocetin beta-d-glucosyl ester as experimental molecule. Moreover, HDAC-2 over-expression is also seen in breast cancer so performed docking studies against this enzyme also (Müller et al., 2013). Thus our molecular docking simulations and binding free energy calculations clearly suggest that crocetin beta-d-glucosyl ester indeed shows affinity towards estrogen receptor alpha and HDAC2 as determined by the negative values of docking score and BFE (Kalyaanamoorthy and Chen, 2013). Crocetin beta-d-glucosyl ester shows similar but not identical interaction profile as that of 4-hydroxytamoxifen and thus it is quite evident that crocetin beta-d-glucosyl ester shows similar but not identical interaction profile against estrogen receptor alpha. The results of this study could act as a base to provide an alternative natural therapy for mitigation of breast cancer incidences.
This study involved the combinatorial stragey for proving the cytotoxic effect of Crocin-1 against breast cancer model along with the possible underlying mechanism being involved. However, this study shedded no light on the higher order clinical studies which will further support the promsing effect of defined molecules. Thus capacious clinical studies are required to facilitate this molecule from bench to bedside.
5. Conclusions
Based on the facts and information provided in the current research article, it is concluded that Crocus sativus leaf derived crocetin beta-d-glucosyl ester small molecule is effective against DPPH free radical and against MCF-7 breast cancer cell line. The results of this study could also offer an opportunity to use leaves of Crocus sativus as cheap source of crocetin beta-d-glucosyl ester for pharmaceutical industry, this would open up new dimensions to prevent and tackle therapeutically challenging breast cancer cases naturally, economically and elegantly compared to conventional drug therapies. It is recommended here that further in-vitro and in-vivo vigorous studies regarding dose, safety and toxicity of crocetin beta-d-glucosyl ester molecule needs to be undertaken before they could enter into different phases of clinical trials, as a potential alternative against breast cancer.
Acknowledgments
Acknowledgments
Shabir Ahmad Ganai thanks DST-SERB for financial help in the form of Start Up Grant (YSS/2015/001267). The authors would also like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2019/116), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
All the authors confirm that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mudasir A. Mir, Email: mudasirbt@gmail.com.
Parvaiz Ahmad, Email: parvaizbot@yahoo.com.
References
- Abdullaev F., Espinosa-Aguirre J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Alhakmani F., Kumar S., Khan S.A. Estimation of total phenolic content, in–vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pacific J. Trop. Biomed. 2013;3:623–627. doi: 10.1016/S2221-1691(13)60126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A., Hamza A.A., Daoud S., Khazanehdari K., Al Hrout A., Baig B., Chaiboonchoe A., Adrian T.E., Zaki N., Salehi-Ashtiani K. Saffron-based crocin prevents early lesions of liver cancer: in vivo, in vitro and network analyses. Recent Patents Anti-Cancer Drug Discovery. 2016;11(1):121–133. doi: 10.2174/1574892810666151102110248. [DOI] [PubMed] [Google Scholar]
- Ashrafi M., Bathaie S., Taghikhani M., Moosavi-Movahedi A. The effect of carotenoids obtained from saffron on histone H1 structure and H1–DNA interaction. Int. J. Biol. Macromol. 2005;36:246–252. doi: 10.1016/j.ijbiomac.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Assimiadis M.K., Tarantilis P.A., Polissiou M.G. UV-Vis, FT-Raman, and 1H NMR spectroscopies of cis-trans carotenoids from saffron (Crocus sativus L.) Appl. Spectrosc. 1998;52:519–522. [Google Scholar]
- Bathaie S., Sajjadi M. Comparative study on preventive effect of saffron carotenoids, crocin and crocetin, in NMU-induced breast cancer in rat. Cell J. 2017;19:94. doi: 10.22074/cellj.2016.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathaie S.Z., Bolhassani A., Tamanoi F. The Enzymes. Elsevier; 2014. Anticancer effect and molecular targets of saffron carotenoids; pp. 57–86. [DOI] [PubMed] [Google Scholar]
- Bhandari P.R. Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. J. Trad. Complement. Med. 2015;5:81–87. doi: 10.1016/j.jtcme.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava V. Medicinal uses and pharmacological properties of Crocus sativus Linn (Saffron) Int. J. Pharm. Pharm. Sci. 2011;3:22–26. [Google Scholar]
- Bishayee A., Ahmed S., Brankov N., Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci.: J. Virtual Library. 2011;16:980. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cagliani L.R., Culeddu N., Chessa M., Consonni R. NMR investigations for a quality assessment of Italian PDO saffron (Crocus sativus L.) Food Control. 2015;50:342–348. [Google Scholar]
- Cannell R.J. Springer Science & Business Media; 1998. Natural Products Isolation. [Google Scholar]
- Castillo R., Gómez-Gómez L., Fernandez J. SafchiA is a new class of defence chitinase from saffron (Crocus sativus L.) Acta Hortic. 2007;739:195. [Google Scholar]
- Chryssanthi D.G., Lamari F.N., Iatrou G., Pylara A., Karamanos N.K., Cordopatis P. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007;27:357–362. [PubMed] [Google Scholar]
- Cossignani L., Urbani E., Simonetti M.S., Maurizi A., Chiesi C., Blasi F. Characterisation of secondary metabolites in saffron from central Italy (Cascia, Umbria) Food Chem. 2014;143:446–451. doi: 10.1016/j.foodchem.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Cuzick J., Sestak I., Bonanni B., Costantino J.P., Cummings S., DeCensi A., Dowsett M., Forbes J.F., Ford L., LaCroix A.Z. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J.-A. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2004;2:127–159. [Google Scholar]
- Fernández J.-A. Anticancer properties of saffron, Crocus sativus Linn. Adv. Phytomed. 2006;2:313–330. [Google Scholar]
- Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Ganai S.A., Shanmugam K., Mahadevan V. Energy-optimised pharmacophore approach to identify potential hotspots during inhibition of Class II HDAC isoforms. J. Biomol. Struct. Dyn. 2015;33:374–387. doi: 10.1080/07391102.2013.879073. [DOI] [PubMed] [Google Scholar]
- Gilbert B., Ferreira J.L., Almeida M.B.S., Carvalho E.S., Cascon V., Rocha L.M. The official use of medicinal plants in public health. Ciênc. cult. (Säo Paulo) 1997;49:339–344. [Google Scholar]
- Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M. Chemistry, pharmacology and medicinal property of sage as a viable agent in the treatment of prostate, pancreatic or other types of cancer. Global J. Med. Res. 2014 [Google Scholar]
- Harder E., Damm W., Maple J., Wu C., Reboul M., Xiang J.Y., Wang L., Lupyan D., Dahlgren M.K., Knight J.L. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2015;12:281–296. doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- Hire R.R., Srivastava S., Davis M.B., Konreddy A.K., Panda D. Antiproliferative activity of crocin involves targeting of microtubules in breast cancer cells. Sci. Rep. 2017;7:44984. doi: 10.1038/srep44984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili C., Tabatabaei H., Kakaberiei S., Roshankhah S., Salahshoor M.R. Protective role of Crocin against nicotine-induced damages on male mice liver. Int. J. Preventive Med. 2015;6 doi: 10.4103/2008-7802.165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Gu M., Liu J., Li H., Peng J., Zhang Y. Anticancer activity of crocin against cervical carcinoma (HeLa cells): Bioassessment and toxicity evaluation of crocin in male albino rats. J. Photochem. Photobiol., B. 2018;180:118–124. doi: 10.1016/j.jphotobiol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Kalimuthu S., Latha S., Selvamani P., Rajesh P., Balamurugan B., Chandrasekar T. Isolation, characterization and antibacterial evaluation on long chain fatty acids from Limnophila polystachya Benth. Asian J. Chem. 2011;23:791. [Google Scholar]
- Kalyaanamoorthy S., Chen Y.P.P. Energy based pharmacophore mapping of HDAC inhibitors against class I HDAC enzymes. Biochim. Biophys. Acta (BBA)-Proteins Proteomics. 2013;1834:317–328. doi: 10.1016/j.bbapap.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Khorasanchi Z., Shafiee M., Kermanshahi F., Khazaei M., Ryzhikov M., Parizadeh M.R., Kermanshahi B., Ferns G.A., Avan A., Hassanian S.M. Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine. 2018;43:21–27. doi: 10.1016/j.phymed.2018.03.041. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Lee J.M., Kim S.C., Park C.B., Lee P.C. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem. Cell Biol. 2014;92:105–111. doi: 10.1139/bcb-2013-0091. [DOI] [PubMed] [Google Scholar]
- Latosińska J.N., Latosińska M. Drug Discovery. IntechOpen; 2013. Anticancer drug discovery—from serendipity to rational design. [Google Scholar]
- Lauffer B.E., Mintzer R., Fong R., Mukund S., Tam C., Zilberleyb I., Flicke B., Ritscher A., Fedorowicz G., Vallero R. Histone deacetylase (HDAC) inhibitor kinetic rate constants correlate with cellular histone acetylation but not transcription and cell viability. J. Biol. Chem. 2013;288:26926–26943. doi: 10.1074/jbc.M113.490706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakopoulou-Kyriakides M., Kyriakidis D. Studies in Natural Products Chemistry. Elsevier; 2002. Croscus sativus-biological active constitutents; pp. 293–312. [Google Scholar]
- Lu P., Lin H., Gu Y., Li L., Guo H., Wang F., Qiu X. Antitumor effects of crocin on human breast cancer cells. Int. J. Clin. Exp. Med. 2015;8:20316. [PMC free article] [PubMed] [Google Scholar]
- Lyne P.D., Lamb M.L., Saeh J.C. Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring. J. Med. Chem. 2006;49:4805–4808. doi: 10.1021/jm060522a. [DOI] [PubMed] [Google Scholar]
- Melnyk J.P., Wang S., Marcone M.F. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res. Int. 2010;43:1981–1989. [Google Scholar]
- Milajerdi A., Djafarian K., Hosseini B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J. Nutrit. Intermed. Metabol. 2016;3:23–32. [Google Scholar]
- Mir M., Rameashkannan M., Raj J., Malik A., Rajesh T. Vol. 1200. 2012. Phytochemical and pharmacological profile of Crocus sativus L. by-products found in Kashmir; pp. 213–226. (IV International Symposium on Saffron Biology and Technology). [Google Scholar]
- Mir M.A., Rameashkannan M., Pala R.A. Screening of Crocus sativus L.(Saffron) Bio-residues from kashmir as a source of phenols and flavonoids with antioxidant potential. Adv. Biotechnol. Patent. 2014;303 [Google Scholar]
- Mollazadeh H., Emami S.A., Hosseinzadeh H. Razi’s Al-Hawi and saffron (Crocus sativus): a review. Iranian J. Basic Med. Sci. 2015;18:1153. [PMC free article] [PubMed] [Google Scholar]
- Montoro P., Maldini M., Luciani L., Tuberoso C.I., Congiu F., Pizza C. Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J. Food Sci. 2012;77:C893–C900. doi: 10.1111/j.1750-3841.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- Mousavi S.H., Tavakkol-Afshari J., Brook A., Jafari-Anarkooli I. Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chem. Toxicol. 2009;47:1909–1913. doi: 10.1016/j.fct.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Müller B.M., Jana L., Kasajima A., Lehmann A., Prinzler J., Budczies J., Winzer K.-J., Dietel M., Weichert W., Denkert C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer-overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013;13:215. doi: 10.1186/1471-2407-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykhailenko O., Kovalyov V., Goryacha O., Ivanauskas L., Georgiyants V. Biologically active compounds and pharmacological activities of species of the genus Crocus: a review. Phytochemistry. 2019;162:56–89. doi: 10.1016/j.phytochem.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Rates S.M.K. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Sajjadi M., Bathaie Z. Comparative study on the preventive effect of saffron carotenoids, crocin and crocetin, in NMU-induced breast cancer in rats. Cell J. (Yakhteh) 2017;19:94. doi: 10.22074/cellj.2016.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014;6:99. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sándor M., Kiss R., Keserű G.r.M. Virtual fragment docking by Glide: a validation study on 190 protein− fragment complexes. J. Chem. Inf. Model. 2010;50:1165–1172. doi: 10.1021/ci1000407. [DOI] [PubMed] [Google Scholar]
- Sarker S.D., Latif Z., Gray A.I. Natural products isolation. Springer; 2006. Natural product isolation; pp. 1–25. [Google Scholar]
- Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Shelley J.C., Cholleti A., Frye L.L., Greenwood J.R., Timlin M.R., Uchimaya M. Epik: a software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Shiau A.K., Barstad D., Loria P.M., Cheng L., Kushner P.J., Agard D.A., Greene G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Sodde V.K., Lobo R., Kumar N., Maheshwari R., Shreedhara C. Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7) Pharmacognosy Mag. 2015;11:S156. doi: 10.4103/0973-1296.157719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker S.D., Kumarasamy Y., Shoeb M., Celik S., Yucel E., Middleton M., Nahar L. Antibacterial and antioxidant activities of three Turkish species of the genus. Centaurea, Oriental Pharmacy and Experimental Medicine. 2005;5:246–250. [Google Scholar]
- Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int. Pharm. Sci. 2011;1:98–106. [Google Scholar]
- Van Den Driessche G., Fourches D. Adverse drug reactions triggered by the common HLA-B* 57: 01 variant: a molecular docking study. J. Cheminf. 2017;9:13. doi: 10.1186/s13321-017-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Driessche G., Fourches D. Adverse drug reactions triggered by the common HLA-B* 57: 01 variant: virtual screening of DrugBank using 3D molecular docking. J. Cheminf. 2018;10:3. doi: 10.1186/s13321-018-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A., Nyberg N.T., Mølgaard P., Asili J., Jaroszewski J.W. 1 H NMR metabolic fingerprinting of saffron extracts. Metabolomics. 2010;6:511–517. [Google Scholar]