Abstract
Objectives
Postoperative respiratory adverse events (PRAEs) are known complications following adenotonsillectomy (AT). Clinical data at a single institution were reviewed to investigate the factors that may contribute to PRAEs in the postanesthesia care unit (PACU). The relationship between PRAEs in the PACU and escalation of care, defined as either an unplanned admission for outpatient surgery or unplanned pediatric intensive care unit (PICU) admission, was investigated.
Methods
The perioperative records for all patients who underwent AT from 2016 to 2018 were reviewed. The surgical procedure was performed at both the main campus and the ambulatory surgery center in accordance with the institutional obstructive sleep apnea (OSA) guidelines. Patient characteristics and intraoperative medications were compared. Categorical variables were summarized as counts with percentages and compared using chi‐square tests or Fisher's exact tests. Continuous variables were summarized as medians with interquartile ranges and compared using rank‐sum tests. Multivariable logistic regression was performed to evaluate the association of intraoperative dosing with the occurrence of PRAEs.
Results
The study cohort included 6110 patients. Ninety‐three patients (2%) experienced PRAEs in the PACU. Of these 93 patients, 14 (15%) resulted in an escalation of care, nearly all of which were unplanned PICU admissions. PRAEs tended to occur in younger patients, non‐Hispanic black patients, and those with a higher American Society of Anesthesiologists (ASA) status.
Conclusions
PRAEs are infrequent after AT at a tertiary institution with OSA guidelines in place. However, when PRAEs do occur, escalation of care may be required. Risk factors include age, ethnic background, and ASA physical status.
Level of Evidence
III.
Keywords: adenotonsillectomy, hypoxemia, laryngospasm, respiratory, tonsillectomy
1. INTRODUCTION
In children with obstructive sleep apnea (OSA) or sleep‐disordered breathing (SDB), adenotonsillectomy (AT) is a frequently chosen intervention to improve clinical and behavioral symptoms and overall quality of life.1 As surgical intervention does not result in immediate resolution of symptoms, postoperative respiratory adverse events (PRAEs) still may occur.2, 3 The incidence of PRAEs is higher in children having otolaryngological procedures than the general surgical population and can require escalation of care.4 Various factors may increase the incidence of PRAEs including patient comorbid conditions, age, patient anatomical features, and perioperative choice of medications.1, 5, 6
Opioid medications are the mainstay of analgesia for AT perioperatively, although they impact postoperative respiratory function. Thus, efforts to control pain with opioids may result in adverse respiratory events.1 Children with OSA or SDB are more sensitive to opioids, which increases the potential for adverse respiratory events.7, 8 Previous studies have demonstrated an increased risk of PRAEs with higher opioid doses.9, 10, 11, 12 To limit opioid use during these procedures, nonopioid medications, such as acetaminophen and dexmedetomidine, are being employed more commonly to help control pain and agitation, while potentially minimizing the incidence of PRAEs.13, 14 The American Academy of Otolaryngology—Head and Neck Surgery has provided recommendations concerning postoperative pain management in their clinical practice guidelines.15 While many institutions have implemented protocols for caring for patients after AT, these protocols may vary, and reporting of outcomes remains limited.
Previous investigations have also sought to identify patient characteristics which are associated with PRAEs following AT. The most commonly identified risk factor is age, with patients <3 years of age at greater risk than older patients.5, 16 Comorbid conditions may be contributory as sicker patients, defined by American Society of Anesthesiologists (ASA) physical status, tend to have an increased incidence of events.5 One study identified African‐American patients as having a higher likelihood of PRAEs relative to other ethnic groups.17 Data on the role of weight/body mass index (BMI) are conflicting, with some sources citing weight/BMI as an independent risk factor while others have failed to prove its significance.5, 16, 18
In the current study, clinical data from a single institution were retrospectively reviewed to investigate the factors which may contribute to PRAEs in the postanesthesia care unit (PACU), specifically intraoperative dosing of opioids and adjunctive medications and patient characteristics (age, gender, race/ethnicity, BMI, and ASA status). Additionally, we investigated the relationship of PRAEs in the PACU and subsequent events after admission as well as the need for escalation of care, defined as either an unplanned admission for outpatient surgery or an unplanned pediatric intensive care unit (PICU) admission for planned overnight admissions. We hypothesize that both patient characteristics and intraoperative medication administration will impact the incidence of PRAE; furthermore, we hypothesize that PRAE is typically confined to the PACU, with only the occasional occurrence after discharge from the PACU.
2. METHODS
This study was approved by the Institutional Review Board at Nationwide Children's Hospital. Retrospectively patients, 2‐18 years of age, were identified through a search of the electronic medical record database, who underwent AT between January 2016 and June 2018 at our institution. Given the infrequent occurrence of tonsillectomy in patients less than 2 years of age, the study did not include patients in that age group. At the time that the surgery was scheduled, admission status such as observational (2‐4‐hour postoperative stay) or overnight admission to either the inpatient ward or planned PICU admission was determined by the primary otolaryngologist and the pediatric anesthesiologist in alignment with institutional guidelines (see below).19
Patients were excluded who had a recorded allergy to acetaminophen, dexmedetomidine, fentanyl, hydromorphone, or morphine, or who were missing data on study covariates. The primary outcome was the occurrence of PRAEs in PACU, defined as bronchospasm, laryngospasm, apnea/hypopnea, or hypoxemia/prolonged oxygen (O2) requirement. An initial text search identified narrative text from the Electronic Medical Record (EMR) containing key phrases related to these events (Table 1). Each event had a series of key phrases, identified in Table 1, which were searched in the EMR. Positive search results were manually reviewed by the corresponding author and identified as either true positive or false positives. Event location was determined by the note's author (PACU nurse vs inpatient ward nurse), note's time relative to PACU discharge, or note type (PACU discharge note vs inpatient ward/PICU accept note). The significance of the event was then confirmed by the need for intervention or pharmacologic treatment. Bronchospasm was confirmed by the use of a bronchodilatory agent (albuterol, racemic epinephrine, ketamine). Laryngospasm was confirmed by the use of positive pressure for an oxygen saturation (SpO2) <90% or the administration of propofol or succinylcholine. Apnea/hypopnea was defined as need for bag‐valve‐mask ventilation. Hypoxemia/prolonged O2 requirement was defined as the need for supplemental O2 for >90 minutes to maintain an SpO2 > 90%.
Table 1.
Key phrases used to identify PRAEs in the narrative text of the EMR
| Event | Key phrasesa |
|---|---|
| Apnea | Apnea, hypopnea, bag valve mask, bag‐valve mask, bvm, mask ventilat |
| Bronchospasm | Bronchospasm, bronchodilat, albut, racemic epi, ketamine |
| Hypoxemia | Hypox, low blood oxygen, low blood O2, prolonged oxygen, sup O2, supplemental O2, sup oxygen, supp oxygen, supplemental oxygen, oxygen level, O2 level |
| Laryngospasm | Laryngospasm, positive pressure, propofol, diprivan, succinylcholine, anectine, SpO2, oxygen sat, O2 sat |
Abbreviations: EMR, electronic medical record; PRAE, postoperative respiratory adverse event.
Each key phrase was placed between wild card characters, so that the record would be flagged regardless of the text surrounding the phrase. For example, “bronchodilat” would flag instances of both “bronchodilatory” and “bronchodilation.”
Patient characteristics obtained for the study included age, gender, race/ethnicity (non‐Hispanic white, non‐Hispanic black, other), BMI percentile (<85%, 85%‐95%, ≥95%), ASA status (1, 2, ≥3), and location of surgery (main operating room [MOR] vs ambulatory surgery center). Intraoperative opioids included morphine, fentanyl, and hydromorphone, and the total intraoperative opioid dose was reported as intravenous (IV) morphine equivalents (ME). Adjunctive medications included IV acetaminophen and dexmedetomidine.
In the initial descriptive analysis, patient characteristics and intraoperative medications were compared according to incidence of PRAEs. Categorical variables were summarized as counts with percentages, and compared using chi‐square tests or Fisher's exact tests, as appropriate. Continuous variables were summarized as medians with interquartile ranges (IQRs) and compared using rank‐sum tests. In further analysis, multivariable logistic regression was performed to evaluate the association of intraoperative dosing with the occurrence of PRAEs, adjusting for the patient characteristics listed above. Analysis was performed using Stata/IC 14.2 (College Station, TX: StataCorp, LP), with two‐tailed P < 0.05 considered statistically significant.
3. RESULTS
The initial retrospective review identified 6392 patients, ranging in age from 2 to 18 years, who underwent AT during the study period. Thirty‐four patients were excluded due to allergies and 248 patients were excluded due to missing covariate information. The remaining 6110 patients included 3106 male and 3004 female patients, with a median age of 6 years (IQRs: 4 and 8). Preexisting institutional guidelines (Table 2) determined whether the surgical procedure took place in the MOR or ambulatory surgery center.19 Four patients were planned PICU admissions.
Table 2.
Institutional pediatric AT guidelinesa
| Age | Less than 3 years of age for AT |
| Less than 2 years of age for adenoidectomy (except for patients with eustachian tube dysfunction, serous otitis, sinusitis as the sole diagnosis) | |
| Sleep study | AHI >10 |
| Pulse oximetry reading <80% | |
| ETCO2 > 50 mm Hg | |
| Obesity | BMI >95% (percentile for age) |
| Craniofacial syndromes | Yes (ie, Downs syndrome, Pierre Robin, etc.) |
| Comorbid conditions | Asthma (moderate/severe), cystic fibrosis, CHD, diabetes mellitus, and hypotonia, and other significant medical conditions |
Abbreviations: AHI, apnea/hypopnea index; AT, adenotonsillectomy; BMI, body mass index; CHD, congenital heart disease; ETCO2, end‐tidal carbon dioxide.
Patients requiring postoperative admission or prolonged observation from reference 16.
Ninety‐three of 6110 (2%) experienced a PRAE in the PACU, including 14 (15%) cases of bronchospasm, 27 (29%) cases of laryngospasm, 23 (25%) cases of apnea, and 41 (44%) cases of hypoxemia. Nine of the 93 patients (10%) who experienced a PRAE in the PACU subsequently experienced a PRAE following discharge from the PACU, all of which were episodes of hypoxemia managed with supplemental oxygen via nasal cannula while the patient was asleep, but removed when the child was awake, as it was no longer needed. In comparison, 87 of 6017 (1%) patients who did not experience a PRAE in the PACU, experienced a PRAE following discharge from the PACU. Nearly all of these incidents were due to hypoxemia (94%), which was also managed with supplemental oxygen (P < .001). Fourteen of the 93 patients who experienced a PRAE in the PACU (15%) required escalation of care with 1 unplanned admission for a patient scheduled in the ambulatory surgery center and 13 unplanned PICU admissions for patients scheduled in the MOR. Overall, these patients tended to be younger (3 years of age or less) and African‐American ethnicity. More than half (8/14) of the patients who required escalated care were discharged the following day (Figure 1).
Figure 1.
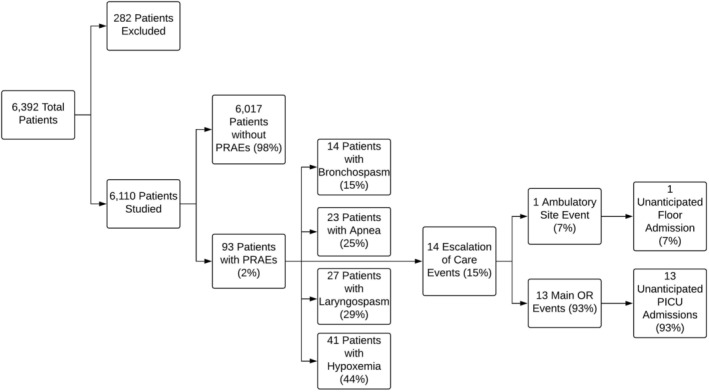
Division of patients with a postoperative respiratory adverse event (PRAE)
Intraoperative dosing of medications did not differ between those who experienced PRAEs and those who did not (Table 3 ). Both percentages of medications administered and the IQRs for the dose of the medication administered overlapped considerably. Neither gender (54% vs 49%, P = .371) nor BMI (P = .404) were statistically significant. Children who experienced PRAEs were younger than those who did not (4 vs 6 years of age, P < .001). Similarly, non‐Hispanic, African‐American patients (33% vs 17%, P < .001) and those with an ASA physical status ≥3 (P < .001) experienced PRAEs more often than other races/ethnicities or ASA physical statuses. Findings were similar with multivariable logistic regression (Table 4) as age, race/ethnicity, and ASA physical status influenced the number of PRAEs, while BMI and medications administered did not impact the incidence of PRAEs.
Table 3.
Patient characteristics and intraoperative medications according to the incidence of PRAEs in the PACU following AT (N = 6110)
| Characteristics | PRAE (N = 93) | No PRAE (N = 6017) | P value |
|---|---|---|---|
| Intraoperative medications | |||
| Received IV acetaminophen | 16 (17%) | 800 (13%) | .271 |
| IV acetaminophen (mg/kg)a | 15 (12, 15) | 14 (11, 15) | .499 |
| Received dexmedetomidine | 21 (23%) | 1286 (21%) | .778 |
| Dexmedetomidine (mg/kg)a | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | .559 |
| Received opioids | 92 (99%) | 6006 (99.8%) | .168 |
| Opioids (IV ME/kg)a | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | .098 |
| Patient characteristics | |||
| Age (years) | 4 (2, 6) | 6 (4, 8) | <.001 |
| Female | 50 (54%) | 2954 (49%) | .371 |
| Race/ethnicity | |||
| Non‐Hispanic white | 53 (57%) | 4172 (69%) | <.001 |
| Non‐Hispanic black | 31 (33%) | 997 (17%) | |
| Other | 9 (10%) | 848 (14%) | |
| BMI percentile | |||
| <85% | 54 (58%) | 3775 (63%) | .404 |
| 85–95% | 11 (12%) | 792 (13%) | |
| ≥95% | 28 (30%) | 1450 (24%) | |
| ASA status | |||
| 1 | 8 (9%) | 1151 (19%) | <.001 |
| 2 | 62 (63%) | 4345 (72%) | |
| ≥3 | 23 (25%) | 521 (9%) | |
| Ambulatory surgery center | 2 (2%) | 1809 (30%) | <.001 |
| Main operating room | 91 (98%) | 4208 (70%) |
Note: The data are listed as the median and IQR or number (N) and %.
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; IV, intravenous; IQR, interquartile range; ME, morphine equivalents; PACU, postanesthesia care unit; PRAE, postoperative respiratory event.
Intraoperative doses among patients who received the drug.
Table 4.
Multivariable logistic regression of the occurrence of a PRAE in the PACU following AT (N = 6110)
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Intraoperative medications received | |||
| IV acetaminophen (mg/kg) | 1.0 | (0.9, 1.0) | .482 |
| Dexmedetomidine (mg/kg) | 0.4 | (0.1, 1.8) | .245 |
| Intraoperative opioids (IV ME/kg) | 4.9 | (0.8, 28.6) | .078 |
| Patient characteristics | |||
| Age (years) | 0.9 | (0.8, 0.95) | .002 |
| Female | 1.4 | (0.9, 2.1) | .104 |
| Race/ethnicity | |||
| Non‐Hispanic white | Ref. | ‐ | ‐ |
| Non‐Hispanic black | 2.1 | (1.3, 3.3) | .001 |
| Other | 0.8 | (0.4, 1.5) | .437 |
| BMI percentile | |||
| <85% | Ref. | ‐ | ‐ |
| 85–95 | 1.2 | (0.6, 2.4) | .529 |
| ≥95% | 1.5 | (0.9, 2.5) | .136 |
| ASA status | |||
| 1 | Ref. | ‐ | ‐ |
| 2 | 1.2 | (0.6, 2.6) | .586 |
| ≥3 | 3.3 | (1.4, 7.7) | .006 |
| Ambulatory surgery center | 0.1 | (0.02, 0.3) | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; IV, intravenous; ME, morphine equivalents; OR, odds ratio; PACU, postanesthesia care unit; PRAE, postoperative respiratory event.
4. DISCUSSION
Our data suggest that the incidence of PRAEs is low (2%) following AT in pediatric patients. In line with our hypothesis, patients who did not have problems in the PACU were unlikely to have events after discharge from the PACU. When PRAEs did occur, the spectrum of events included bronchospasm, laryngospasm, apnea, and hypoxemia, with the latter being most common. As previously reported, specific demographic features predicative of PRAEs included age, ASA physical status, and ethnic background. Intraoperative dosing and choice of medications did not impact the incidence of PRAE. Although the majority of patients who experience a PRAE in the PACU will have recovered completely prior to PACU discharge, 10% have a PRAE after PACU discharge. However, the majority of these events were persistent hypoxemia while sleeping, only requiring the administration of oxygen. Most of these hypoxemic events were short‐lived and resolved within 24 hours, thereby allowing discharge the following day. Additionally, in the majority of cases, the acuity of such patients was such that they could be cared for on the inpatient ward with continuous pulse oximetry monitoring.
In a smaller subset of patients, the severity of the PRAE was significant enough to warrant escalation of care with admission to the PICU for monitoring of respiratory status. However, none of these patients required endotracheal intubation, and most were discharged home the following day. In many cases, this included a discharge home from the PICU. These findings underscore the need for ongoing close loop communication between providers (ie, pediatric otolaryngologist and pediatric anesthesiologist, pediatric anesthesiologist and PACU nurse, PACU nurse and floor/PICU nurse) regarding the events that occurred in PACU and what to be aware of while managing the patient beyond the PACU.
These unplanned admissions or escalations of care increase the cost of the hospital encounter and may not be reimbursed. At our institution, the charge of a PICU bed is roughly $6000 a night. While guidelines at our institution have successfully helped us to stratify which patients are better suited for an ambulatory setting vs the inpatient setting, future adjustments in these guidelines may be required as we are better able to stratify risk and identify patients at risk for PRAEs.19 Currently, the presence of any one characteristic from the guidelines (Table 2) requires inpatient surgery; however, neither race/ethnicity nor ASA status are currently fully accounted for in the guidelines. One possibility for better stratification of patients to account for both operative and postoperative locations would be to our preexisting guidelines with the findings from this study (Figure 2).
Figure 2.
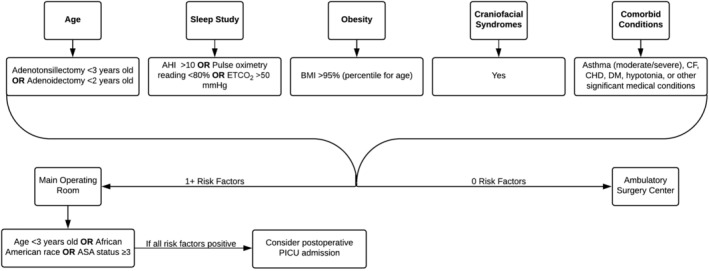
Risk stratification for patients undergoing adenotonsillectomy (AT)
The 13 patients who required PICU admission received supplemental oxygen with a nasal cannula (n = 8) or noninvasive positive pressure ventilation (n = 5) along with more intensive monitoring as the escalations of care. None of the patients required endotracheal intubation. Hypoxemia in the PACU was the most common reason for escalation of care to the PICU; however, there were also three incidences of laryngospasm, two of hypopnea, and two of upper airway obstruction. One episode of hypopnea was the result of respiratory depression due to opioid administration in a morbidly obese patient (BMI for age ≥ 99th percentile.) This patient received a single dose of fentanyl (0.5 μg/kg) for pain. Although this dose was appropriate based on μg/kg, it may have been more appropriate to use ideal body weight and not actual body weight for dose calculation.
These 13 patients mirrored the independent risk factors identified by our data and previous studies. Eleven patients had at least one risk factor for PRAE, and 5 had 2 risk factors. More than half (7/13) were 2 years of age at the time of surgery and nearly half (6/13) were African American. None exhibited more than 2 risk factors. Most (9/13) were identified as ASA physical 2 in the preoperative assessment, with SDB and adenotonsillar hypertrophy being the most common comorbid conditions. Other notable comorbid conditions included one patient with moderate persistent asthma and another with a history of prematurity and bronchopulmonary dysplasia. Not all PRAEs resulting in PICU admission can be predictive based on preexisting risk factors. Other factors such as coexisting upper respiratory tract infections, which do not preclude proceeding with the case, may on an individual basis result in a PRAE which requires escalation of care. Given these concerns, ongoing observation and evaluation of these patients is mandatory to allow for early identification of PRAEs and interventions, which may prevent their progression to postoperative respiratory failure and the need for more aggressive interventions, such as endotracheal intubation.
Our data demonstrate that patient demographic features are more associated with PRAEs than the intraoperative medications administered. However, these findings should be taken in the context of the fact that this care was provided in a large, tertiary care children's hospital with fellowship trained pediatric anesthesiologists. As such, intraoperative opioid administration was generally limited with the frequent use of adjunctive medication. Our data support what multiple studies have shown. Age, race/ethnicity, and ASA physical status are independent risk factors for PRAEs.5, 16, 17 Conversely, while there is literature which suggests that BMI also plays a role in PRAEs, our data did not find support this finding.5, 16, 18
Although it did not reach statistical significance, the odds ratio (OR) associated with dexmedetomidine administration was 0.4, suggesting that it may decrease the incidence of PRAEs. Dexmedetomidine is an α2‐adrenergic agonist that is commonly used perioperatively for sedation, analgesia, and anxiolysis. Given its mechanism of action, it may decrease emergence agitation allowing for a smoother emergence from anesthesia as well as decreasing perioperative opioid requirements.
As a retrospective study, there are specific limitations to the study. The study should be considered in the context of our institutional guidelines for caring for these patients which are in accordance with guidelines set forth by various organizational and surgical societies. We postulated that variations in the perioperative care of patients presenting for AT may impact the incidence of PRAEs. As the study was not meant to evaluate the impact of these guidelines, we did not collect data on patients before and after the institution of these guidelines. Additionally, we relied on a complete and accurate medical record when reviewing the patient records for PRAEs. Incomplete or inaccurate data in the record could potentially skew the results. The most likely inaccuracy would be the omission or downplaying of an event, especially if it was short lived or no harm came to the patient. Variance in reliability, while it can be controlled partially in a study's methodology, is still unavoidable. Two people may judge a potential event differently, based off their understanding of the event as provided by the medical record. Alternatively, an individual may judge a potential event differently when rating the event depending on when the event is reviewed.
Additionally, there is not a universal definition of a PRAE, thus allowing for variance in results, depending on the criteria and its interpretation. As there is no universal definition for PRAE, a better alternative may be to investigate each patient's specific respiratory parameters that necessitated interventions (SpO2, nadir and average; mode of oxygen therapy required; other therapies/interventions required, such as albuterol administration). However, such detail may not be possible in a retrospective review thereby necessitating a prospective evaluation which might limit patient numbers in the study cohort.
5. CONCLUSIONS
In summary, PRAEs are a concerning but rare post‐anesthetic complication in pediatric patients undergoing AT. For those patients experiencing PRAEs in the PACU, continued vigilance is suggested, as 15% had subsequent events that required escalation of postoperative care, to either an unplanned inpatient admission or the intensive care unit. However, in most cases, recurrence of PRAEs other than the need for ongoing oxygen administration was uncommon. As noted in previous studies, specific demographic features were associated with PRAEs. Future studies to further define these predictive features may be helpful in the development or refinement of institutional guidelines for postoperative care of such patients.
CONFLICT OF INTERESTS
T.B.H., A.T., J.D.T., and V.T.R. declare no potential conflict of interest. K.R.J. receives royalties from Marpac, Inc. for a patented tracheostomy collar design, commercially available, and from a product safety medical consultant, Intertek, Inc.
ACKNOWLEDGMENTS
The authors would like to thank Rebecca Miller for her help with the statistics for this manuscript.
Hamilton TB, Thung A, Tobias JD, Jatana KR, Raman VT. Adenotonsillectomy and postoperative respiratory adverse events: A retrospective study. Laryngoscope Investigative Otolaryngology. 2020;5:168–174. 10.1002/lio2.340
REFERENCES
- 1. Cohen N, Sommer DD. Post‐tonsillectomy pain control: consensus or controversy? Pain Manage. 2016;6:31‐37. [DOI] [PubMed] [Google Scholar]
- 2. De AJ, Waltuch TP, Gonik NR, et al. Sleep and breathing the first night after adenotonsillectomy in obese children with obstructive sleep apnea. J Clin Sleep Med. 2017;13(06):805‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nixon GM, Kermack AS, Mcgregor CD, et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulm. 2005;39(4):332‐338. [DOI] [PubMed] [Google Scholar]
- 4. Arambula AM, Xie DX, Whigham AS. Respiratory events after adenotonsillectomy requiring escalated admission status in children with obstructive sleep apnea. Int J Pediatr Otorhinol. 2018;107:31‐36. [DOI] [PubMed] [Google Scholar]
- 5. Subramanyam R, Yeramaneni S, Hossain MM, Anneken AM, Varughese AM. Perioperative respiratory adverse events in pediatric ambulatory anesthesia. Anesth Analg. 2016;122:1578‐1585. [DOI] [PubMed] [Google Scholar]
- 6. Tweedie DJ, Bajaj Y, Ifeacho SN, et al. Peri‐operative complications after adenotonsillectomy in a UKpediatric tertiary referral centre. Int J Pediatr Otorhi. 2012;76(6):809‐815. [DOI] [PubMed] [Google Scholar]
- 7. Brown KA, Laferrière A, Moss IR. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806‐810. [DOI] [PubMed] [Google Scholar]
- 8. Brown KA, Laferrière A, Lakheeram I, Moss IR. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;105:665‐669. [DOI] [PubMed] [Google Scholar]
- 9. Nafiu OO, Shanks A, Abdo S, Taylor E, Tremper TT. Association of high body mass index in children with early post‐tonsillectomy pain. Int J Pediatr Otorhinolaryngol. 2013;77:256‐261. [DOI] [PubMed] [Google Scholar]
- 10. Sadhasivam S, Chidambaran V, Olbrecht VA, et al. Opioid‐related adverse effects in children undergoing surgery: unequal burden on younger girls with higher doses of opioids. Pain Med. 2015;16:985‐997. [DOI] [PubMed] [Google Scholar]
- 11. Kako H, Tripi J, Walia H, et al. Utility of screening questionnaire and polysomnography to predict postoperative outcomes in children. Int J Pediatr Otorhinolaryngol. 2017;102:71‐75. [DOI] [PubMed] [Google Scholar]
- 12. Hadden SM, Burke CN, Skotcher S, Voepel‐Lewis T. Early postoperative outcomes in children after adenotonsillectomy. J Perianesth Nurs. 2011;26:89‐95. [DOI] [PubMed] [Google Scholar]
- 13. Zhuang PJ, Wang X, Zhang XF, Zhou ZJ, Wang Q. Postoperative respiratory and analgesic effects of dexmedetomidine or morphine for adenotonsillectomy in children with obstructive sleep apnoea. Anaesthesia. 2011;66:989‐993. [DOI] [PubMed] [Google Scholar]
- 14. Bellon M, Bot AL, Michelet D, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for postoperative pain management: a meta‐analysis of published studies. Pain Ther. 2016;5:63‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryng Head Neck. 2019;160:S1‐S42. [DOI] [PubMed] [Google Scholar]
- 16. Lawlor CM, Riley CA, Carter JM, Rodriguez KH. Association between age and weight as risk factors for complication after tonsillectomy in healthy children. JAMA Otolaryngol. 2018;144:399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tariq S, Syed M, Martin T, Zhang X, Schmitz M. Rates of perioperative respiratory adverse events among Caucasian and African American children undergoing general anesthesia. Anesth Analg. 2018;127:181‐187. [DOI] [PubMed] [Google Scholar]
- 18. Lavin JM, Shah RK. Postoperative complications in obese children undergoing adenotonsillectomy. Int J Pediatr Otorhinol. 2015;79:1732‐1735. [DOI] [PubMed] [Google Scholar]
- 19. Raman VT, Jatana KR, Elmaraghy CA, Tobias JD. Guidelines to decrease unanticipated hospital admission following adenotonsillectomy in the pediatric population. Int J Ped Otorhinol. 2014;78:19‐22. [DOI] [PubMed] [Google Scholar]


