Abstract
Objectives
The treatment of inner ear disorders remains challenging due to anatomic barriers intrinsic to the bony labyrinth. The purpose of this review is to highlight recent advances and strategies for overcoming these barriers and to discuss promising future avenues for investigation.
Data Sources
The databases used were PubMed, EMBASE, and Web of Science.
Results
Although some studies aimed to improve systemic delivery using nanoparticle systems, the majority enhanced local delivery using hydrogels, nanoparticles, and microneedles. Developments in direct intracochlear delivery include intracochlear injection and intracochlear implants.
Conclusions
In the absence of a systemic drug that targets only the inner ear, the best alternative is local delivery that harnesses a combination of new strategies to overcome anatomic barriers. The combination of microneedle technology with hydrogel and nanoparticle delivery is a promising area for future investigation.
Level of Evidence
NA
Keywords: inner ear disorders, intracochlear delivery, microneedles, nanoparticles
1. INTRODUCTION
The current management of inner ear disorders consists of systemic drug delivery, intratympanic injection, and surgical intervention. Despite the assortment of treatment options, current approaches have been associated with inconsistent efficacy and safety. Systemic therapy—often the first‐line approach for treating sudden sensorineural hearing loss and Ménière's disease—is noninvasive, but it is also linked to subtherapeutic inner ear drug concentrations and adverse effects.1 Intratympanic injection, which involves filling the middle ear with a drug and relying on diffusion into the inner ear, can attain higher inner ear drug concentrations.2, 3, 4 However, intratympanic injection suffers from unintentional clearance of the drug via the eustachian tube, inconsistent diffusion rate into the inner ear, and risk of damaging middle ear structures.5, 6 Direct microinjection of drugs through a cochleostomy is an alternative method that reduces the variability associated with intratympanic injection but carries the risk of significant trauma to the inner ear.7 Thus, there is an unmet need for new inner ear delivery methods that are both effective and safe. The challenges with current treatment options are primarily due to anatomic barriers intrinsic to the bony labyrinth. As such, recent advances in drug delivery techniques have focused on bypassing these barriers. The goal of this review is to discuss recent advances in inner ear drug delivery techniques in the framework of anatomic barriers.
2. ANATOMIC BARRIERS
Anatomic barriers of the inner ear affect all modalities of drug delivery to the inner ear. Encased within the otic capsule—a bony shell composed of the densest portion of temporal bone—the inner ear is an anatomically isolated structure. In the guinea pig, the otic capsule is thin and may permit a certain level of drug diffusion into the inner ear, whereas in humans, the otic capsule is considerably thicker, preventing drug diffusion within.8, 9 Challenges with systemic delivery stem primarily from the blood‐labyrinth barrier (BLB), whereas challenges with local delivery (eg, intratympanic injection) result from barriers such as the round window membrane (RWM), the oval window and stapes, and the loss of intratympanic drug solution through the eustachian tube (Figure 1). These communication pathways to and from the inner ear have been recently reviewed in detail.10 In what follows, the roles of these barriers—the BLB, the RWM, the oval window, and the eustachian tube—are discussed in the context of drug delivery to the inner ear.
Figure 1.
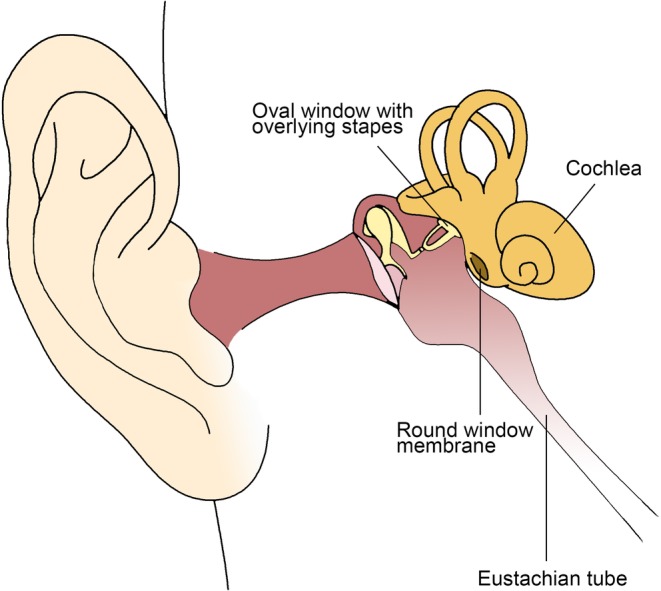
Schematic of ear anatomy. Anatomic barriers include the cochlea encased in bone, blood‐labyrinth barrier (not shown), round window membrane, oval window, and eustachian tube. Printed with permission from Jeffrey W. Kysar, PhD and Anil K. Lalwani, MD
2.1. Blood‐labyrinth barrier
The BLB divides the vascular compartment of the inner ear from the inner ear fluid spaces. The BLB consists of a network of vascular endothelial cells coupled together by tight junctions, basement membrane, pericytes, and perivascular resident macrophage‐like melanocytes that, combined, regulate exchange between the blood and surrounding perivascular space.11 In addition to maintaining normal fluid and ion physiology and preventing pathogens from entering the inner ear, the BLB also prevents therapeutic agents in systemic circulation from accessing the inner ear. It is estimated that the BLB limits diffusion into the scala tympani and scala vestibuli perilymph to 6.5% and 3.7% of total plasma concentration, respectively.12 Compared to 13.7% entry into cerebrospinal fluid, the BLB may be less permeable than the blood‐brain barrier. Therefore, attaining therapeutic levels of a drug within the inner ear requires high levels of a systemic drug, which can lead to ototoxicity from aminoglycoside antibiotics or wide‐ranging side effects from systemic corticosteroids.1
2.2. Round window membrane
The RWM is the communication between the scala tympani and the middle ear. It consists of three layers: an outer epithelial layer facing the middle ear, a central connective tissue layer, and an inner epithelial layer interfacing with perilymph (Figure 2). Composed of low cuboidal epithelial cells bound by extensive interdigitations and tight junctions, the outer layer is thought to play the largest role in controlling RWM permeability.13 This layer also likely has absorptive capabilities, suggested by the presence of microvilli and organelles with metabolic and transport capability. The connective tissue core consists of fibroblasts, collagen, and elastic fibers, which provide the RWM with strength and flexibility—the mechanical properties that allow it to bear perilymph pressure. The innermost layer is composed of squamous epithelial cells with large extracellular spaces that allow contact between the connective tissue matrix with the perilymphatic space. Long, overlapping, lateral extensions and cytoplasmic pinocytotic vesicles within this layer also suggest absorptive capabilities.
Figure 2.
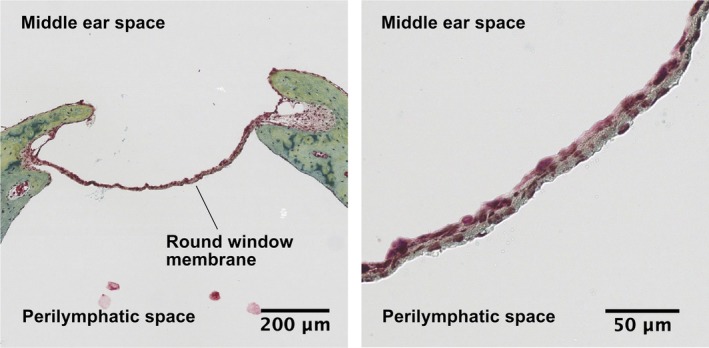
Cross section of the guinea pig RWM, pentachrome stain. The RWM is suspended between bone, which is stained in green, shown on the left at ×8 magnification. The three layers of the RWM can be appreciated at ×40 magnification, shown on the right. The outer epithelial layer, in contact with the middle ear space, is composed of low cuboidal epithelial cells connected by tight junctions. The central connective tissue layer is stained beige due to its composition of collagen (yellow) and elastic fibers (black). The inner epithelial layer is composed of squamous epithelial cells with large extracellular spaces allowing contact between the connective tissue matrix and the perilymph. RWM, round window membrane. Printed with permission from Jeffrey W. Kysar, PhD and Anil K. Lalwani, MD
Importantly, the RWM is semipermeable, allowing passage of a wide range of materials including antibiotics, local anesthetics, toxins, and albumin.14 RWM permeability is affected by many factors, including the size, configuration, concentration, liposolubility, and electrical charge of the diffusing molecules, as well as properties of the RWM itself such as its thickness.14, 15, 16 RWM permeability also decreases in pathological states such as otitis media due to the inflammatory process. For these reasons, diffusion across the RWM is often inconsistent and associated with unreliable therapeutic dosing, particularly during intratympanic injection.17, 18
2.3. Oval window
The oval window represents the communication between the scala vestibuli and the middle ear. It is covered by the stapes footplate, which is attached to the perimeter of the oval window by the annular ligament.19 To date, the oval window has received less attention as a portal for drug delivery to the inner ear due to its physical obstruction by the stapes footplate. However, studies show that diffusion across the oval window does occur via intratympanic administration.20, 21, 22 Diffusion across the oval window is also thought to be well suited for the treatment of vestibular disorders due to its proximity to vestibular tissue.20 In guinea pigs, horse peroxidase‐labeled granules diffuse across the oval window after intratympanic administration and can be visualized in the chondrocytes of the stapediovestibular joint and on the scala vestibuli surface of Reissner's membrane using electron microscopy.21, 22 Some studies suggest that the oval window may even play a larger role than the RWM with inner ear diffusion as higher levels of gadolinium are detected in the scala vestibuli than in the scala tympani after intratympanic administration, and higher levels of chitosan nanoparticles are detected at the stapes footplate compared to the RWM.23, 24 However, it remains unclear what fraction of inner ear delivery can be attributed to diffusion across the RWM and across the oval window, with other studies suggesting up to 35% diffusion across the oval window.25, 26
2.4. Eustachian tube clearance
The eustachian tube is a passageway between the middle ear space and the nasopharynx. It serves to equalize middle ear pressure but also presents a barrier to inner ear delivery during intratympanic injection because drugs can exit through the eustachian tube into the nasopharynx. Limited drug residence time in the middle ear decreases the ability of the drug to diffuse into the inner ear, leading to subtherapeutic drug levels.10 For this reason, intratympanic injection in the clinic often requires the patient to remain supine for several minutes before standing to allow for longer drug residence time in the middle ear.27 However, this routine is time‐consuming and impractical in the clinic with unclear benefits.
3. RECENT ADVANCES IN INNER EAR DELIVERY
Recent studies have focused on overcoming various aspects of these anatomic barriers to improve inner ear delivery. Some studies aimed to enhance systemic delivery using nanoparticles to increase blood circulation time of drugs, but the majority of studies addressed local delivery, using novel methods to increase RWM permeability, overcome eustachian tube drug clearance, and increase cellular uptake of drug using nanoparticles. There has also been interest in developing new, minimally invasive methods for intracochlear delivery.
3.1. Enhancing systemic delivery
Systemic delivery of drugs offers several advantages over local delivery, including noninvasiveness and clinical ease of use, but is associated with subtherapeutic inner ear drug concentrations and adverse, off‐target effects.1 Recent studies have used nanoparticle systems to overcome the difficulties associated with systemic delivery. The mechanisms by which nanoparticles can increase inner ear delivery via systemic administration are numerous, but they primarily focus on increasing blood circulation time of drugs. Coating polylactic acid nanoparticles with polyethylene glycol (PEG) can reduce opsonization and prevent interactions with the reticuloendothelial system in the liver or spleen, allowing for improved systemic drug retention.28 PEG‐coated polylactic acid nanoparticles have been shown to result in sustained delivery of rhodamine, a dye with molecular weight comparable to that of gentamicin, to the cochlea through systemic administration. They also facilitate the delivery of betamethasone to the cochlea, allowing for significant reductions in histological and functional damage after noise‐induced hearing loss.28 Poly(lactic‐co‐glycolic acid) (PLGA) nanoparticles demonstrate improved inner ear delivery with systemic administration; systemic administration of rhodamine does not result in cochlear uptake at 10 minutes, whereas systemic administration of PLGA nanoparticles carrying rhodamine does.29 Systemic administration of dexamethasone‐loaded PEG‐coated PLGA particles can also attenuate cisplatin ototoxicity in guinea pigs, whereas the same effect is not observed with dexamethasone alone.30 As such, nanoparticles have a variety of uses in systemic inner ear delivery, ranging from stabilizing delicate drug structures and preventing premature drug elimination to increasing drug diffusion into the inner ear. As described in Section 3.4, they can also be conjugated with targeting peptides that promote uptake within selective cells in the inner ear.
3.2. Increasing RWM permeability
A primary shortcoming of intratympanic injection is inconsistent diffusion of the drug across the RWM. As such, several recent studies have attempted to increase the permeability of the RWM to enhance drug diffusion into the inner ear. This can be achieved by (a) applying chemical agents to the RWM to alter its permeability or (b) introducing microperforations in a controlled and precise manner to the RWM.
The permeability of the RWM can be increased by exotoxins and endotoxins; desiccation by suctioning near the round window niche; and benzyl alcohol, a common preservative in commercially available drug formulations.17, 31, 32 The clinical application of these methods is unclear, limited by intersubject variability affecting the reliability of these techniques. Of note, benzyl alcohol causes a burning sensation with intratympanic injection and can require anesthesia during use.32 Other solvents and detergents, such as dimethyl sulfoxide, N‐methylpyrrolidone, and saponin, may also increase RWM permeability, but studies investigating the toxicity of these agents to the inner ear or middle ear are lacking.32 Histamine has been shown to increase the absorption of dexamethasone phosphate, enhancing RWM permeability through its vasoactive effects.33, 34 When dexamethasone is administered with both histamine and hyaluronic acid as adjuvants, the inner ear concentration of dexamethasone phosphate is increased further.34 Pretreatment of the RWM with hyaluronic acid has been shown to significantly enhance gene delivery with a viral vector.35, 36 Although chemical agents can enhance RWM permeability, their use is generally hampered by high interindividual variability due to discrepancies in intrinsic properties of the RWM.
Creating microperforations in the RWM is a novel technique for mechanically increasing the permeability of the RWM. Small perforations have been shown to increase the diffusion of small molecules by more than 35‐fold across the RWM.37, 38 Moreover, the increase in RWM permeability depends not on the variable intrinsic properties of the RWM but on characteristics of the microperforations themselves, such as the size of perforation.38 Thus, the ability to introduce microperforations in a controlled manner leads to the precise and reliable enhancement of RWM permeability. Microneedles are designed with the microanatomic and mechanical properties of the RWM in mind, to create controlled and precise perforations in the membrane with minimal trauma (Figure 3).40, 41 To date, several needle designs have been fabricated with an assortment of unique characteristics.39, 41, 42, 43, 44, 45 Microneedles fabricated using two‐photon polymerization (2PP) are able to perforate guinea pig RWM reliably.41, 45 In the guinea pig, these perforations are not associated with any notable lasting effects on hearing, and the perforations also heal within a week after perforation.45 Microneedles can also create precise perforations in the human RWM.39 One of the challenges of microneedle use on the RWM is ensuring that the needle is inserted far enough to create a perforation but not so far as to cause damage to structures behind the RWM.39 Due to the anatomic constraints of RWM access in vivo, confirmation of microneedle perforation can be difficult; silver chloride‐plated microneedles can detect contact with perilymph based on changes in voltage.44
Figure 3.
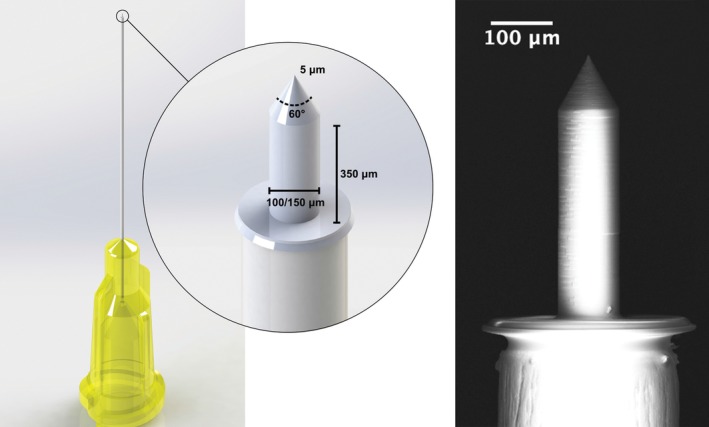
Microneedles for cochlear delivery. Microneedles are designed to create precise perforations in the round window membrane to enhance diffusion after intratympanic injection. The figure shows a microneedle design used for the human round window membrane, fabricated using two‐photon polymerization. Printed with permission from Jeffrey W. Kysar, PhD and Anil K. Lalwani, MD
3.3. Overcoming eustachian tube clearance
Another shortcoming of intratympanic injection is unintentional drug clearance via the eustachian tube. Thus far, efforts to reduce drug clearance have focused on developing (a) devices that allow for repeated dosing to maintain adequate drug solution within the middle ear and (b) viscous gel formulations that directly impede leakage, thereby increasing drug residence time. Devices that allow for repeat dosing or continuous infusion include the Silverstein Microwick, the μCath, and numerous other micropumps and continuous infusion devices in various stages of development.46, 47, 48, 49, 50, 51, 52, 53 The Silverstein Microwick is a polyvinyl acetate wick that is placed through a myringotomy tube and rested in the round window niche. With the Microwick, the patient self‐administers medication, which the wick absorbs and transports from the external canal to the RWM, localizing to its vicinity for improved diffusion. Gelfoam, a gelatin sponge that can be placed during certain surgical procedures, can accomplish a similar goal (Figure 4).54 The μCath is an implantable catheter with a bulbous tip designed to lock in place within the round window niche and an opposing end with two lumens—for fluid injection and aspiration—that can be attached to a variety of pumps. The most common method for implantation of the μCath is through a tympanomeatal flap under general anesthesia. As these techniques are associated with exposure of the middle ear space, recent studies have explored alternative, less invasive methods to improve drug residence time—namely, using hydrogel drug formulations.
Figure 4.
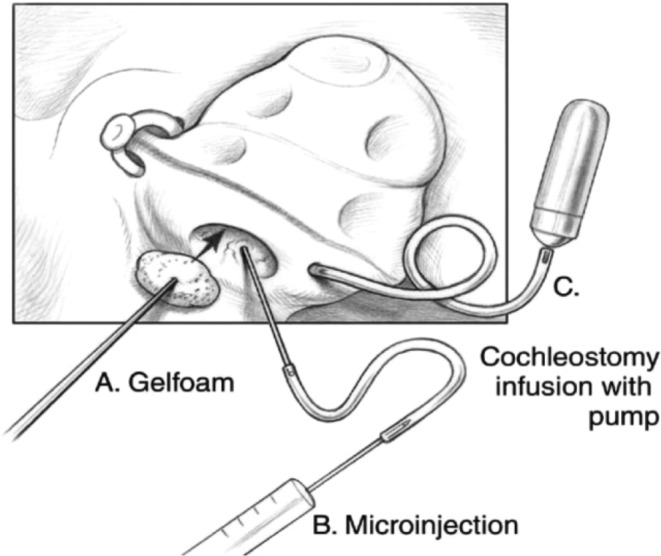
Several routes of cochlear delivery. The delivery of a therapeutic agent to the cochlea can be performed by: diffusion of the agent across an intact round window membrane with a gelatin sponge (A), intracochlear injection through the round window membrane (B), and infusion with osmotic minipump through cochleostomy (C). Printed with permission from Anil K. Lalwani, MD
Thermosensitive hydrogels are particularly suitable for intratympanic injection because they can remain in liquid form for injection at room temperature but quickly transform into solid gel at body temperature.55 A solid gel is resistant to leakage through the eustachian tube, thus dramatically increasing drug residence time in the middle ear. Prolonged contact with the RWM facilitates drug diffusion to the inner ear. Poloxamer 407 (P407), a triblock copolymer, is one example of a thermosensitive hydrogel that has been extensively studied for inner ear drug delivery via intratympanic injection.8, 56, 57 In guinea pigs, a single dose of dexamethasone/P407 can extend the duration of drug in perilymph to at least 10 days, compared to less than 24 hours with a single dose of dexamethasone in aqueous solution.57 Moreover, P407 decreases the variability in dexamethasone concentrations along the length of the cochlea.8 P407 is safe for use in humans; filling the middle ear space with gel causes a transient conductive hearing loss but does not cause any lasting structural or functional change in the ear.57, 58 Of note, P407 has been the subject of two completed Phase 3 clinical trials in OTO‐104, a single‐dose dexamethasone/P407 formulation for the treatment of unilateral Meniere's disease. However, further development was suspended after OTO‐104 failed to demonstrate benefit over placebo. A variety of other hydrogels, including chitosan glycerophosphate and hyaluronic acid, has been studied extensively with similar safety and efficacy.59, 60, 61, 62
3.4. Nanoparticle‐assisted local delivery
In addition to improving systemic delivery to the inner ear, nanoparticles can also assist local drug administration by stabilizing drug structures, enhancing drug diffusion across the RWM, and increasing cellular uptake of drugs. Phospholipid‐based nanoparticles are of particular interest because their bilayer structure enables them to encapsulate and transport both hydrophobic and hydrophilic molecules across the RWM.63 Dexamethasone encapsulated in four types of phospholipid‐based nanoparticles—neutral, anionic, cationic, and cationic‐PEG—have been tested in a mouse model of ototoxicity induced by kanamycin and furosemide.64 Although all four types of nanoparticles with encapsulated dexamethasone were superior to pure dexamethasone sodium phosphate solution in recovering hearing in a mouse model of ototoxicity, cationic‐PEG nanoparticles obtained the best therapeutic results. Only cationic‐PEG nanoparticles exhibited significant cellular uptake within the organ of Corti, likely due to the positively charged particles easily binding to the negatively charged cell surface glycoproteins.
PLGA, a biodegradable polymer, is another type of nanoparticle that has been extensively studied for local inner ear delivery. PLGA nanoparticles carrying rhodamine have been shown to permeate through the RWM when applied locally, with more cochlear uptake than with systemic delivery.29 PLGA nanoparticles also distribute more evenly throughout the inner ear compared to other delivery methods. When applied on the RWM in chinchillas, PLGA‐encapsulated iron oxide nanoparticles were well distributed in the inner ear fields, inner ear membranes, hair cells, supporting cells of the organ of Corti, spiral ligament, and stria vascularis.65 They have also been shown to effectively deliver a number of drugs into the inner ear, including lidocaine and dexamethasone.30, 66 Local administration of dexamethasone‐loaded PEG‐coated PLGA particles can attenuate cisplatin ototoxicity in guinea pigs.30, 67
In addition to improved RWM permeation, increased cellular uptake, and sustained release properties, a major advantage of nanoparticles is their ability to target specific cell types when conjugated to cell‐penetrating peptides. Nanoparticles conjugated with a SS‐31 peptide cause mitochondrial‐specific accumulation in hair cells.68 This effect has been used to confer protective effects against aminoglycoside‐induced hair cell damage in a zebrafish model. Likewise, Tet1 peptide conjugated to polymersomes targets the cochlear nerve.69 However, improved cochlear uptake was only seen when the polymersomes were delivered via cochleostomy and not after transtympanic injection, suggesting that RWM permeability still limited the efficacy of nanoparticles.
Another unique characteristic of nanoparticles is the ability to exploit forces generated by magnetic fields to pull superparamagnetic iron oxide nanoparticles (SPIONs) across the RWM. This technique has been demonstrated in vivo in rat and guinea pig models, as well as in vitro in human temporal bone70; intratympanic injection of magnetic nanoparticles loaded with prednisolone mitigate cisplatin‐induced hearing loss more than an injection of methylprednisolone alone.71 These SPIONs cause minimal inflammatory response. Application of nanoparticles with iron oxide cores in a chitosan matrix loaded with prednisolone was not associated with a difference in hearing loss at 2 and 30 days after treatment, compared to the application of prednisolone alone.72 Very mild inflammatory changes limited to the middle ear were observed at 2 and 30 days after treatment, with almost complete reversibility of changes by day 90.
Other types of nanoparticles that have been studied in the literature include lipid nanocapsules,73 polymersomes,74 hydroxyapatite nanoparticles,75 and silica nanoparticles,76 and were the subject of a recent review.77
3.5. Advances with direct intracochlear delivery
Direct delivery of therapeutics into the cochlea offers several advantages over intratympanic injection, including bypassing the variability imposed by the properties of the RWM, attaining higher peak concentrations of drug, and reducing basal‐apical gradients of a drug within the scala tympani compared to intratympanic application of a drug.7, 78, 79 Early models for intracochlear injection utilized cochleostomy for direct microinjection of drugs or infusion of drugs into the cochlea using osmotic minipumps (Figure 4).74, 80, 81, 82, 83, 84, 85 Newer ideas include introducing a PLGA‐based implant into the scala tympani via a cochleostomy in the basal turn of the cochlea. Simulations predict that the implant can reach constant drug levels within a few hours and last for several weeks.86 Placement of this implant has been demonstrated ex vivo in guinea pig models and did not result in macroscopic traumatization of inner ear structures based on CT scans. However, methods involving cochleostomy are known to be traumatic to the inner ear and risk hearing loss; alternative approaches may reduce this risk.87 Two recent options are microinjection through the RWM and placement of intracochlear implants that avoids cochleostomy.
Intracochlear injection through the RWM demonstrates higher and more consistent drug levels compared to intratympanic injection.7, 88, 89 Intracochlear injection also provides greater control over inner ear delivery because therapeutics can be dosed in discrete units rather than relying on diffusion. Perilymph leakage from the inner ear due to intracochlear injection can be reduced by sealing the round window niche with a gel or adhesive prior to injection.89
Intracochlear implantation is another method for intracochlear delivery that avoids cochleostomy. Previously, sustained intracochlear release of drugs required the use of drug‐eluting cochlear implant electrodes. Recent developments in this area include the usage of a microscaffold cochlear electrode array for continual release of steroids to preserve residual hearing, coating electrodes with laminin to promote neuritic outgrowth from auditory neurons, and coating the cochlear implant with brain‐derived neurotrophic factor‐producing mesenchymal stem cells to protect spiral ganglion neurons.90, 91, 92 However, the usage of such methods are limited to those with profound hearing loss requiring cochlear implantation.
New developments have focused on intracochlear implants that can be used independent of cochlear implants for a wider patient population. One option is the Hybrid Ear Cube, a miniaturized silicone‐based implant.93 This implant consists of a cuboid portion placed in the middle ear adjacent to the oval window or round window and a cylindrical structure that passes through the oval window or round window that remains in contact with perilymph (Figure 5). The cuboid portion acts as a drug reservoir, so the drug can diffuse through the cylindrical portion into the perilymph. The cuboid has two halves that can be loaded with two different drug formulations, offering increased flexibility compared to a previously proposed monoblock system.94 The Hybrid Ear Cube has been shown to retain its structure and size, even when immersed for up to 100 days in artificial perilymph, and has demonstrated very slow release rates, with <0.5% of loaded dexamethasone, or 0.5 μg, released after 2 months. It has yet to be tested in vivo in animal models. A second option that has been investigated is the implantation of polyvinyl alcohol‐polymer‐coated drug particles that can be delivered by a single injection through the RWM.95 In this study, polymer‐coated fluticasone propionate was implanted into guinea pig cochlea in vivo, providing stable drug release for at least 90 days with good hearing preservation. However, a 32‐gauge needle was used to puncture the RWM for injection of this polymer‐based delivery system, and the authors of the study believe that a surgical insertion tool would be required to provide consistent hearing preservation.
Figure 5.
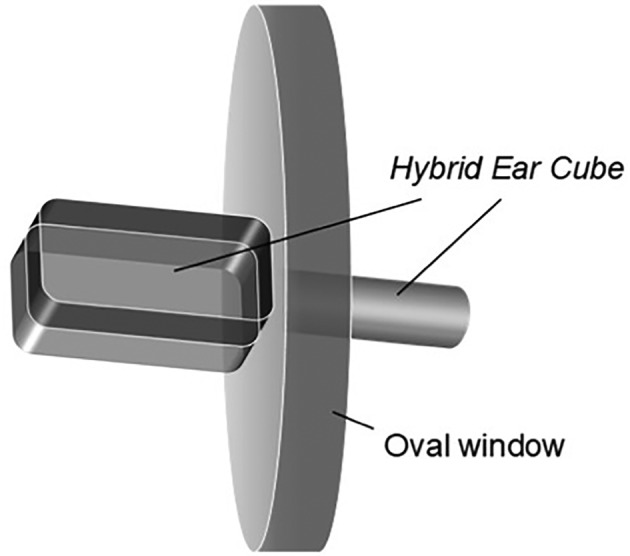
Hybrid ear cube. The hybrid ear cube is a silicone‐based implant that consists of a cuboid portion placed in the middle ear adjacent to the oval window (shown) or round window and a cylindrical structure that passes through the oval window or round window that remains in contact with the perilymph.93 The cuboid structure has two halves that can be loaded with two different drug formulations and acts as a drug reservoir such that drugs can diffuse through the cylindrical portion into the perilymph. Reprinted from European Journal of Pharmaceutical Sciences, Volume 126, Gehrke et al., Hybrid Ear Cubes for local controlled dexamethasone delivery to the inner ear, pages 23‐32, 2019, with permission from Elsevier
4. OPTIMAL INNER EAR DELIVERY METHOD
Recent advances in drug delivery to the inner ear have focused on bypassing anatomic barriers that limit the efficacy of current clinical practices. Although some studies have explored the utility of nanoparticles to enhance inner ear drug delivery by systemic administration, most studies have focused on augmenting the delivery of therapeutics via local approach. Improved intratympanic techniques include increasing RWM permeability by applying chemical agents or introducing microperforations, localizing drugs to the RWM using microcatheters and hydrogels, and enhancing cellular uptake of drugs with drug targeting by local injection of nanoparticles. Advances in intracochlear delivery include microinjections through the RWM and insertion of intracochlear implants for sustained delivery of drugs.
The ideal inner ear delivery method is a noninvasive, yet targeted, approach. Of all the reviewed methods, systemic delivery distinguishes itself with a clear advantage: its ease of administration and noninvasiveness. However, systemic delivery is associated with subtherapeutic drug concentrations in the inner ear and adverse off‐target effects—limitations that could theoretically be overcome by an optimal therapeutic: one that is systemically delivered, can bypass the BLB to reach the inner ear, and activates only within the inner ear. Although this optimal therapeutic currently eludes clinical medicine, several ideas have been proposed to help reach this goal. The unique chemical composition of endolymph within the inner ear has been suggested as a feature that can be exploited for inner ear‐specific drug activation.96 High‐intensity acoustic signals, which can be further amplified by the ossicles, are another proposed unique feature of the ear that may be used.96 However, in the absence of such an optimal therapeutic in medicine today, local delivery is a more feasible option for an efficacious inner ear delivery technique with minimal adverse effects.
Recent advances in local inner ear delivery techniques have been successful in overcoming anatomic barriers. Microneedles introduce perforations that directly increase RWM permeability and reduce the effects of RWM intervariability. Hydrogels increase RWM contact time and reduce eustachian tube clearance. Nanoparticles enhance cellular uptake and can target drug particles to specific cell types. However, independently, each technique remains susceptible to anatomic barriers. Microneedle‐assisted delivery is still limited by eustachian tube leakage. Hydrogel‐assisted delivery cannot overcome the intrinsic variation in RWM permeability. Nanoparticle delivery is hindered by both poor RWM permeability and leakage through the eustachian tube. Only by combining multiple strategies can the anatomic barriers be addressed comprehensively.
Various combinations of local inner ear delivery strategies have already been demonstrated in the literature. Nanoparticles are particularly well suited for use in conjunction with other delivery techniques. Loading PLGA nanoparticles onto a chitosan/glycerophosphate‐based thermosensitive hydrogel increases drug residence time within the cochlea compared to administration of PLGA nanoparticles alone.60 Surface modification of PLGA nanoparticles with hydrophilic molecules such as poloxamer can further improve cellular uptake.97 Liposomes loaded with the prodrug dexamethasone phosphate and deposited into hyaluronic acid gel also demonstrated sustained delivery of dexamethasone with reduced eustachian tube clearance.59 These liposomes remain in hyaluronic acid adjacent to the RWM, creating a local reservoir that allowed sustained release of dexamethasone into the perilymph over 30 days, with increased conversion of dexamethasone phosphate to active dexamethasone. Likewise, nanohydrogel consisting of liposomal nanoparticles loaded onto a chitosan‐based hydrogel acts as a reservoir to release nanoparticles in a controlled and sustained manner into the perilymphatic system.62 Nanoparticles have also been introduced directly into the inner ear via cochleostomy, with improved distribution over intratympanic injection alone.69, 74
More recently, microperforations have been studied in combination with the thermoreversible hydrogel poloxamer 407.38 Microperforations used in conjunction with a poloxamer increased the diffusion rate compared to the poloxamer alone. However, these effects were limited; increased diffusion was only seen with microperforations of a certain size. Further investigation is necessary to clarify the effect of microperforations when used with hydrogel carriers. Microneedle technology is also likely to be compatible with nanoparticle delivery. Besides creating perforations that increase diffusion of nanoparticles across the RWM, they may play a role in the future of direct intracochlear injection across the RWM. Small and precise microneedle perforations in the RWM may induce less trauma than cochleostomy and heal more quickly. Thus, the combination of microneedle technology with hydrogel delivery and nanoparticle delivery is an important next step in the research of local delivery techniques to the inner ear.
5. CONCLUSION
Various anatomic barriers pose a challenge to safe and effective inner ear drug delivery. In the absence of a systemically administered therapeutic that targets only the inner ear, promising new strategies have focused on methods of local delivery that overcome these anatomic challenges. The optimal method for local drug delivery to the inner ear likely uses a combination of novel strategies to increase the efficacy and precision of the delivered drug. Combining microneedle technology with hydrogel and nanoparticle delivery of drugs to the inner ear is a promising avenue for future research.
CONFLICT OF INTEREST
Dr Anil K. Lalwani serves on the Medical Advisory Board of Advanced Bionics and on the Surgical Advisory Board of MED‐EL. For the remaining authors, no conflicts of interest were declared.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge support by the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders (NIDCD) with award number R01DC014547.
Szeto B, Chiang H, Valentini C, Yu M, Kysar JW, Lalwani AK. Inner ear delivery: Challenges and opportunities. Laryngoscope Investigative Otolaryngology. 2020;5:122–131. 10.1002/lio2.336
Funding information National Institute on Deafness and Other Communication Disorders, Grant/Award Number: R01DC014547
REFERENCES
- 1. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161:S1‐s45. [DOI] [PubMed] [Google Scholar]
- 2. Silverstein H, Lewis WB, Jackson LE, Rosenberg SI, Thompson JH, Hoffmann KK. Changing trends in the surgical treatment of Meniere's disease: results of a 10‐year survey. Ear Nose Throat J. 2003;82:185‐187, 191‐194. [PubMed] [Google Scholar]
- 3. Diamond C, O'Connell DA, Hornig JD, Liu R. Systematic review of intratympanic gentamicin in Meniere's disease. J Otolaryngol. 2003;32:351‐361. [DOI] [PubMed] [Google Scholar]
- 4. Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol. 2007;28:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 5. Martin E, Perez N. Hearing loss after intratympanic gentamicin therapy for unilateral Meniere's disease. Otol Neurotol. 2003;24:800‐806. [DOI] [PubMed] [Google Scholar]
- 6. Bremer HG, van Rooy I, Pullens B, et al. Intratympanic gentamicin treatment for Meniere's disease: a randomized, double‐blind, placebo‐controlled trial on dose efficacy ‐ results of a prematurely ended study. Trials. 2014;15:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hahn H, Salt AN, Biegner T, et al. Dexamethasone levels and base‐to‐apex concentration gradients in the scala tympani perilymph after intracochlear delivery in the guinea pig. Otol Neurotol. 2012;33:660‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salt AN, Hartsock J, Plontke S, Lebel C, Piu F. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel‐based formulation. Audiol Neurootol. 2011;16:323‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol Neurotol. 2009;30:131‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018;362:25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi X. Pathophysiology of the cochlear intrastrial fluid‐blood barrier (review). Hear Res. 2016;338:52‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inamura N, Salt AN. Permeability changes of the blood‐labyrinth barrier measured in vivo during experimental treatments. Hear Res. 1992;61:12‐18. [DOI] [PubMed] [Google Scholar]
- 13. Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc Res Tech. 1997;36:201‐211. [DOI] [PubMed] [Google Scholar]
- 14. Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437‐447. [DOI] [PubMed] [Google Scholar]
- 15. Goycoolea MV, Muchow D, Schachern P. Experimental studies on round window structure: function and permeability. Laryngoscope. 1988;98:1‐20. [DOI] [PubMed] [Google Scholar]
- 16. Salt AN, Hartsock JJ, Piu F, Hou J. Dexamethasone and dexamethasone phosphate entry into perilymph compared for middle ear applications in guinea pigs. Audiol Neurootol. 2018;23:245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeda K, Morizono T. Changes of the permeability of round window membrane in otitis media. Arch Otolaryngol Head Neck Surg. 1988;114:895‐897. [DOI] [PubMed] [Google Scholar]
- 18. Schachern PA, Paparella MM, Goycoolea MV, Duvall AJ 3rd, Choo YB. The permeability of the round window membrane during otitis media. Arch Otolaryngol Head Neck Surg. 1987;113:625‐629. [DOI] [PubMed] [Google Scholar]
- 19. Brunner H. Attachment of the stapes to the oval window in man. AMA Arch Otolaryngol. 1954;59:18‐29. [DOI] [PubMed] [Google Scholar]
- 20. King E, Salt A, Kel G, Eastwood H, O'Leary S. Gentamicin administration on the stapes footplate causes greater hearing loss and vestibulotoxicity than round window administration in guinea pigs. Hear Res. 2013;304:159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka K, Motomura S. Permeability of the labyrinthine windows in guinea pigs. Arch Otorhinolaryngol. 1981;233:67‐75. [DOI] [PubMed] [Google Scholar]
- 22. Saijo S, Kimura RS. Distribution of HRP in the inner ear after injection into the middle ear cavity. Acta Otolaryngol. 1984;97:593‐610. [DOI] [PubMed] [Google Scholar]
- 23. King E, Salt A, Eastwood H, O'Leary S. Direct entry of gadolinium into the vestibule following intratympanic applications in guinea pigs and the influence of cochlear implantation. J Assoc Res Otolaryngol. 2011;12:741‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding S, Xie S, Chen W, et al. Is oval window transport a royal gate for nanoparticle delivery to vestibule in the inner ear? Eur J Pharm Sci. 2019;126:11‐22. [DOI] [PubMed] [Google Scholar]
- 25. Salt AN, King EB, Hartsock JJ, Gill RM, O'Leary SJ. Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hear Res. 2012;283:14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salt AN, Hartsock JJ, Gill RM, King E, Kraus FB, Plontke SK. Perilymph pharmacokinetics of locally‐applied gentamicin in the guinea pig. Hear Res. 2016;342:101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bear ZW, Mikulec AA. Intratympanic steroid therapy for treatment of idiopathic sudden sensorineural hearing loss. Mo Med. 2014;111:352‐356. [PMC free article] [PubMed] [Google Scholar]
- 28. Horie RT, Sakamoto T, Nakagawa T, Ishihara T, Higaki M, Ito J. Stealth‐nanoparticle strategy for enhancing the efficacy of steroids in mice with noise‐induced hearing loss. Nanomedicine. 2010;5:1331‐1340. [DOI] [PubMed] [Google Scholar]
- 29. Tamura T, Kita T, Nakagawa T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000‐2005. [DOI] [PubMed] [Google Scholar]
- 30. Sun C, Wang X, Chen D, Lin X, Yu D, Wu H. Dexamethasone loaded nanoparticles exert protective effects against Cisplatin‐induced hearing loss by systemic administration. Neurosci Lett. 2016;619:142‐148. [DOI] [PubMed] [Google Scholar]
- 31. Mikulec AA, Hartsock JJ, Salt AN. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol Neurotol. 2008;29:1020‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li W, Hartsock JJ, Dai C, Salt AN. Permeation enhancers for intratympanically‐applied drugs studied using fluorescent dexamethasone as a marker. Otol Neurotol. 2018;39:639‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chandrasekhar SS, Rubinstein RY, Kwartler JA, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521‐528. [DOI] [PubMed] [Google Scholar]
- 34. Creber NJ, Eastwood HT, Hampson AJ, Tan J, O'Leary SJ. Adjuvant agents enhance round window membrane permeability to dexamethasone and modulate basal to apical cochlear gradients. Eur J Pharm Sci. 2019;126:69‐81. [DOI] [PubMed] [Google Scholar]
- 35. Kurioka T, Mizutari K, Niwa K, et al. Hyaluronic acid pretreatment for Sendai virus‐mediated cochlear gene transfer. Gene Ther. 2016;23:187‐195. [DOI] [PubMed] [Google Scholar]
- 36. Shibata SB, Cortez SR, Wiler JA, Swiderski DL, Raphael Y. Hyaluronic acid enhances gene delivery into the cochlea. Hum Gene Ther. 2012;23:302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelso CM, Watanabe H, Wazen JM, et al. Microperforations significantly enhance diffusion across round window membrane. Otol Neurotol. 2015;36:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santimetaneedol A, Wang Z, Arteaga D, et al. Small molecule delivery across a perforated artificial membrane by thermoreversible hydrogel poloxamer 407. Colloids Surf B Biointerfaces. 2019;182:110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiang H, Yu M, Aksit A, et al. 3D‐printed microneedles create precise perforations in human round window membrane in situ. Otol Neurotol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe H, Kysar JW, Lalwani AK. Microanatomic analysis of the round window membrane by white light interferometry and microcomputed tomography for mechanical amplification. Otol Neurotol. 2014;35:672‐678. [DOI] [PubMed] [Google Scholar]
- 41. Aksit A, Arteaga DN, Arriaga M, et al. In‐vitro perforation of the round window membrane via direct 3‐D printed microneedles. Biomed Microdevices. 2018;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watanabe H, Cardoso L, Lalwani AK, Kysar JW. A dual wedge microneedle for sampling of perilymph solution via round window membrane. Biomed Microdevices. 2016;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stevens JP, Watanabe H, Kysar JW, Lalwani AK. Serrated needle design facilitates precise round window membrane perforation. J Biomed Mater Res A. 2016;104:1633‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wazen JM, Stevens JP, Watanabe H, Kysar JW, Lalwani AK. Silver/silver chloride microneedles can detect penetration through the round window membrane. J Biomed Mater Res B Appl Biomater. 2017;105:307‐311. [DOI] [PubMed] [Google Scholar]
- 45. Yu M, Arteaga DN, Aksit A, et al. Anatomical and functional consequences of microneedle perforation of round window membrane. Otol Neurotol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silverstein H, Thompson J, Rosenberg SI, Brown N, Light J. Silverstein MicroWick. Otolaryngol Clin North Am. 2004;37:1019‐1034. [DOI] [PubMed] [Google Scholar]
- 47. Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plontke SK, Zimmermann R, Zenner HP, Lowenheim H. Technical note on microcatheter implantation for local inner ear drug delivery: surgical technique and safety aspects. Otol Neurotol. 2006;27:912‐917. [DOI] [PubMed] [Google Scholar]
- 49. Van Wijck F, Staecker H, Lefebvre PP. Topical steroid therapy using the Silverstein Microwick™ in sudden sensorineural hearing loss after failure of conventional treatment. Acta Otolaryngol. 2007;127:1012‐1017. [DOI] [PubMed] [Google Scholar]
- 50. Suryanarayanan R, Srinivasan VR, O'Sullivan G. Transtympanic gentamicin treatment using Silverstein MicroWick in Meniere's disease patients: long term outcome. J Laryngol Otol. 2009;123:45‐49. [DOI] [PubMed] [Google Scholar]
- 51. Schoendorf J, Neugebauer P, Michel O. Continuous intratympanic infusion of gentamicin via a microcatheter in Meniere's disease. Otolaryngol Head Neck Surg. 2001;124:203‐207. [DOI] [PubMed] [Google Scholar]
- 52. Hoffer ME, Kopke RD, Weisskopf P, et al. Use of the round window microcatheter in the treatment of Meniere's disease. Laryngoscope. 2001;111:2046‐2049. [DOI] [PubMed] [Google Scholar]
- 53. Pararas EE, Borkholder DA, Borenstein JT. Microsystems technologies for drug delivery to the inner ear. Adv Drug Deliv Rev. 2012;64:1650‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jero J, Mhatre AN, Tseng CJ, et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther. 2001;12:539‐548. [DOI] [PubMed] [Google Scholar]
- 55. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709‐2728. [DOI] [PubMed] [Google Scholar]
- 56. Wang XB, Dellamary L, Fernandez R, Ye QA, LeBel C, Piu F. Principles of inner ear sustained release following intratympanic administration. Laryngoscope. 2011;121:385‐391. [DOI] [PubMed] [Google Scholar]
- 57. Wang X, Dellamary L, Fernandez R, et al. Dose‐dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393‐401. [DOI] [PubMed] [Google Scholar]
- 58. Piu F, Wang X, Fernandez R, et al. OTO‐104: a sustained‐release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32:171‐179. [DOI] [PubMed] [Google Scholar]
- 59. El Kechai N, Mamelle E, Nguyen Y, et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J Control Release. 2016;226:248‐257. [DOI] [PubMed] [Google Scholar]
- 60. Dai J, Long W, Liang Z, Wen L, Yang F, Chen G. A novel vehicle for local protein delivery to the inner ear: injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev Ind Pharm. 2018;44:89‐98. [DOI] [PubMed] [Google Scholar]
- 61. Luo J, Xu L. Distribution of gentamicin in inner ear after local administration via a chitosan glycerophosphate hydrogel delivery system. Ann Otol Rhinol Laryngol. 2012;121:208‐216. [DOI] [PubMed] [Google Scholar]
- 62. Lajud SA, Nagda DA, Qiao P, et al. A novel chitosan‐hydrogel‐based nanoparticle delivery system for local inner ear application. Otol Neurotol. 2015;36:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang KJ, Son J, Jung SY, et al. Optimized phospholipid‐based nanoparticles for inner ear drug delivery and therapy. Biomaterials. 2018;171:133‐143. [DOI] [PubMed] [Google Scholar]
- 65. Ge XX, Jackson RL, Liu JZ, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137:619‐623. [DOI] [PubMed] [Google Scholar]
- 66. Horie RT, Sakamoto T, Nakagawa T, et al. Sustained delivery of lidocaine into the cochlea using poly lactic/glycolic acid microparticles. Laryngoscope. 2010;120:377‐383. [DOI] [PubMed] [Google Scholar]
- 67. Sun CL, Wang XL, Zheng ZZ, et al. A single dose of dexamethasone encapsulated in polyethylene glycol‐coated polylactic acid nanoparticles attenuates cisplatin‐induced hearing loss following round window membrane administration. Int J Nanomedicine. 2015;10:3567‐3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuang X, Zhou S, Guo W, Wang Z, Sun Y, Liu H. SS‐31 peptide enables mitochondrial targeting drug delivery: a promising therapeutic alteration to prevent hair cell damage from aminoglycosides. Drug Deliv. 2017;24:1750‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Zhang W, Johnston AH, Newman TA, Pyykko I, Zou J. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int J Nanomedicine. 2012;7:1015‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kopke RD, Wassel RA, Mondalek F, et al. Magnetic nanoparticles: inner ear targeted molecule delivery and middle ear implant. Audiol Neurootol. 2006;11:123‐133. [DOI] [PubMed] [Google Scholar]
- 71. Ramaswamy B, Roy S, Apolo AB, Shapiro B, Depireux DA. Magnetic nanoparticle mediated steroid delivery mitigates Cisplatin induced hearing loss. Front Cell Neurosci. 2017;11:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shimoji M, Ramaswamy B, Shukoor MI, et al. Toxicology study for magnetic injection of prednisolone into the rat cochlea. Eur J Pharm Sci. 2019;126:33‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scheper V, Wolf M, Scholl M, et al. Potential novel drug carriers for inner ear treatment: hyperbranched polylysine and lipid nanocapsules. Nanomedicine. 2009;4:623‐635. [DOI] [PubMed] [Google Scholar]
- 74. Zhang Y, Zhang W, Johnston AH, Newman TA, Pyykk I, Zou J. Comparison of the distribution pattern of PEG‐b‐PCL polymersomes delivered into the rat inner ear via different methods. Acta Otolaryngol. 2011;131:1249‐1256. [DOI] [PubMed] [Google Scholar]
- 75. Wu XW, Ding DL, Jiang HY, et al. Transfection using hydroxyapatite nanoparticles in the inner ear via an intact round window membrane in chinchilla. J Nanopart Res. 2012;14:13. [Google Scholar]
- 76. Schmidt N, Schulze J, Warwas DP, et al. Long‐term delivery of brain‐derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One. 2018;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mittal R, Pena SA, Zhu A, et al. Nanoparticle‐based drug delivery in the inner ear: current challenges, limitations and opportunities. Artif Cells Nanomed Biotechnol. 2019;47:1312‐1320. [DOI] [PubMed] [Google Scholar]
- 78. Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Plontke SK, Mynatt R, Gill RM, Borgmann S, Salt AN. Concentration gradient along the scala tympani after local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wareing M, Mhatre AN, Pettis R, et al. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61‐69. [DOI] [PubMed] [Google Scholar]
- 81. Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: introduction of adeno‐associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588‐592. [PubMed] [Google Scholar]
- 82. Lalwani AK, Han JJ, Walsh BJ, Zolotukhin S, Muzyczka N, Mhatre AN. Green fluorescent protein as a reporter for gene transfer studies in the cochlea. Hear Res. 1997;114:139‐147. [DOI] [PubMed] [Google Scholar]
- 83. Lalwani AK, Walsh BJ, Reilly PG, et al. Long‐term in vivo cochlear transgene expression mediated by recombinant adeno‐associated virus. Gene Ther. 1998;5:277‐281. [DOI] [PubMed] [Google Scholar]
- 84. Lalwani AK, Walsh BJ, Carvalho GJ, Muzyczka N, Mhatre AN. Expression of adeno‐associated virus integrated transgene within the mammalian vestibular organs. Am J Otol. 1998;19:390‐395. [PubMed] [Google Scholar]
- 85. Han JJ, Mhatre AN, Wareing M, et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus‐derived gene transfer vector. Hum Gene Ther. 1999;10:1867‐1873. [DOI] [PubMed] [Google Scholar]
- 86. Lehner E, Gündel D, Liebau A, Plontke S, Mäder K. Intracochlear PLGA based implants for dexamethasone release: challenges and solutions. Int J Pharm X. 2019;1:100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87‐90. [PubMed] [Google Scholar]
- 88. Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK. Marker retention in the cochlea following injections through the round window membrane. Hear Res. 2007;232:78‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Plontke SK, Hartsock JJ, Gill RM, Salt AN. Intracochlear drug injections through the round window membrane: measures to improve drug retention. Audiol Neurootol. 2016;21:72‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jang J, Kim J‐Y, Kim YC, et al. A 3D microscaffold cochlear electrode array for steroid elution. Adv Healthc Mater. 2019;8:e1900379. [DOI] [PubMed] [Google Scholar]
- 91. Bas E, Anwar MR, Goncalves S, et al. Laminin‐coated electrodes improve cochlear implant function and post‐insertion neuronal survival. Neuroscience. 2019;410:97‐107. [DOI] [PubMed] [Google Scholar]
- 92. Scheper V, Hoffmann A, Gepp MM, et al. Stem cell based drug delivery for protection of auditory neurons in a guinea pig model of cochlear implantation. Front Cell Neurosci. 2019;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gehrke M, Verin J, Gnansia D, et al. Hybrid ear cubes for local controlled dexamethasone delivery to the inner ear. Eur J Pharm Sci. 2019;126:23‐32. [DOI] [PubMed] [Google Scholar]
- 94. Gehrke M, Sircoglou J, Gnansia D, et al. Ear cubes for local controlled drug delivery to the inner ear. Int J Pharm. 2016;509:85‐94. [DOI] [PubMed] [Google Scholar]
- 95. Pierstorff E, Chen S, Chaparro MP, et al. A polymer‐based extended release system for stable, long‐term intracochlear drug delivery. Otol Neurotol. 2018;39:1195‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Devare J, Gubbels S, Raphael Y. Outlook and future of inner ear therapy. Hear Res. 2018;368:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wen X, Ding S, Cai H, et al. Nanomedicine strategy for optimizing delivery to outer hair cells by surface‐modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int J Nanomedicine. 2016;11:5959‐5969. [DOI] [PMC free article] [PubMed] [Google Scholar]


