Abstract
Objectives
To investigate the landscape of cognitive impairment (CI) screening for adults with age‐related hearing loss (ARHL) among otolaryngologists and audiologists. To identify provider factors and patient characteristics that impact rates of CI screening and referral.
Methods
A 15 question online survey was sent to members of the Georgia Society of Otolaryngology (GSO), Georgia Academy of Audiology (GAA), American Otological Society and American Neurotology Society (AOS/ANS), and posted on the web forum for two hearing disorders special interest groups within the American‐Speech‐Language‐Hearing Association (ASHA). Responses were collected anonymously. Chi‐square tests were used to compare responses.
Results
Of the 66 included respondents, 61% (n = 40) were otolaryngologists and 35% (n = 23) were audiologists. Respondents were significantly more likely to refer patients for CI assessment than to screen (64% vs 21%, respectively, P < .001). The complaint of a neurological symptom, such as memory loss, would prompt screening or referral for only 27.3% (n = 18) and 51.52% (n = 34) of respondents, respectively. Forty‐two percent (n = 28) of respondents suggested CI screening with the MMSE vs 20% (n = 13) with the Montreal Cognitive Assessment.
Conclusions
Despite recommendations for cognitive assessment in high‐risk populations, such as older adults with ARHL, the practice of CI screening and referral is not yet commonplace among otolaryngologists and audiologists. These providers have a unique opportunity to assess adults with ARHL for CI and ensure appropriate referral.
Level of Evidence
5
Keywords: cognition, hearing loss, screening
1. INTRODUCTION
Dementia affects ~46.8 million people worldwide and is projected to continue to grow to 131.5 million people by 2050.1 The estimated total worldwide cost in 2015 was US $818 billion and is predicted to increase as prevalence increases.2, 3, 4 Despite the increasing prevalence and cost, the screening recommendations for dementia and cognitive impairment (CI) are ill‐defined. For example, the Affordable Care Act of 2010 mandates that Medicare recipients undergo yearly cognitive screening during their annual WellCare visit, but does not specify choice and use of screening tools.3, 4 In contrast, in 2013, the United States Preventative Services Task Force (USPTF) found no strong evidence for generalized CI screening of the public, as screening was not found to significantly increase quality of life or disease progression; however, it did find evidence for CI screening for patients who report subjective memory loss or exhibit risk factors for CI.4, 5 Additionally, it called for an increase in identification and understanding of potential risk factors for CI.
Disabling hearing loss (HL) affects 432 million adults worldwide and approximately two‐thirds of adults older than 70 years.6 In the last decade, mounting research has indicated an independent and linear relationship between CI and HL, and a recent meta‐analysis of 36 epidemiologic studies demonstrated that age‐related hearing loss (ARHL) was significantly associated with decline in all main cognitive domains and with increased risk for CI and incident dementia.7, 8, 9 Furthermore, the 2017 Lancet Commission on Dementia Prevention, Intervention and Care identified HL as the highest contributing modifiable risk factor for dementia.2
With HL now established as a strong risk factor for CI, investigation has begun on how to modify that risk. Several studies have measured and are measuring the effect of aural rehabilitation and cochlear implantation on cognition in patients with ARHL, with promising results.10, 11, 12, 13, 14, 15, 16 The future of continued research on the modification of CI in those with ARHL may depend on the early identification of those most at risk, and this in turn may depend on appropriate screening. Recent reports from the audiology and psychiatry literature have reviewed and summarized the two most well‐known and used cognitive screening tests for the general community and the subset of those with HL, the Montreal Cognitive Assessment (MOCA) or the Mini‐Mental Status Exam (MMSE); however, dozens more screening tools have been used in the general population.17, 18, 19, 20 Additionally, work is being put into the validation of CI screening tools made specifically for patients with ARHL, such as the HI‐MOCA.21 Still, there have been no published guidelines to assist providers in determining the appropriate clinical setting or presentation of a patient with ARHL that should prompt CI screening.
Thus, the objective of this study was to understand the current practices and perceptions of audiologists and otolaryngologists on the screening of CI for adults with ARHL. We hypothesized that the current rate of CI screening in this population would likely be low, but vary among provider profession, experience, time, and knowledge of current literature. Additionally, we hypothesized that there would be no consensus on the patient characteristics or presentations that would prompt screening. Secondarily, we aimed to understand the current practices and perceptions of referral for CI screening, to identify gaps in guideline adherence.
2. MATERIALS AND METHODS
2.1. Survey design
We developed an online survey to assess the practices of screening for CI in adults with ARHL among providers who regularly see adults with ARHL. The survey was distributed to three faculty and three resident members of the Otolaryngology Department at Emory University to test understanding of the content and intent of survey questions. The final version was a 15‐question web‐based survey maintained by Google Forms (Google LLC, Mountain View, California). The survey contained questions on demographics, screening and referral habits, and screening and referral knowledge (Supporting Information A).
Three questions assessed the screening and referral habits of all respondents. Providers were asked if they ever carry out cognitive screening or ever refer patients in their practice for cognitive screening. Providers who responded that they did perform screening or did provide referral were then directed to a set of three questions regarding patient characteristics that would their prompt screening or referral. Additionally, providers were asked to which type of specialist they would refer their patients for cognitive screening.
Providers who indicated that they did not perform screening or referral were directed to sets of three questions that assessed knowledge of patient characteristics that should prompt screening or referral. These respondents were also asked to which type of specialist should they refer their patients for cognitive screening. Finally, all respondents were asked which cognitive screening tool was the best to use for adults with ARHL.
Response choices assessing patient characteristics focused on age, degree of HL, and presentation on initial consultation. Choices were the same for providers who indicated that they would or would not screen or refer; therefore, questions assessing habits and knowledge were uniform. These response choices were designed to represent typical clinical scenarios that could be seen in a general otolaryngology clinic.
2.2. Survey distribution
To assess practices and perceptions of providers who regularly see adults with ARHL, the survey was distributed to general otolaryngologists, neurotologists/otologists and audiologists. The final instrument was sent to administrative representatives from the Georgia Society of Otolaryngology (GSO) (estimated 146 members), Georgia Academy of Audiology (GAA) (estimated 60 members), American Otological Society and American Neurotology Society (AOS/ANS) (estimated 364 members) for approval. Once approval was obtained, the survey was distributed to the email LISTSERVs for the GSO, GAA, and AOS/ANS by each respective administrator. Additionally, the survey was sent to two leadership members of the special interest groups (SIGs), “Aural Rehabilitation and Its Instrumentation” and “Audiology and Public Health” within the American‐Speech‐Language‐Hearing Association (ASHA) (estimated 255 and 129 members, respectively) for approval from their respective committees. When approval was obtained, the survey link was posted to each respective SIG's web‐based forum. A request for a reminder to complete the survey was also sent out ~3 weeks after the initial posting. Through the survey introduction (Supporting Information A), respondents were assured anonymous data collection and reporting, as approved by the Emory Institutional Review Board. No incentives were provided for survey completion.
2.3. Statistical analysis
Responses were tabulated by Google Forms and transferred to Excel. Summary statistics (frequency and percentages) were developed from providers' survey responses. To identify provider characteristics and perceptions significantly associated with screening and referral, chi‐square and Fisher's exact tests were used for non‐ordinal variables, and Cochran‐Mantel‐Haenszel's test was used for ordinal variables. Pairwise comparisons were done for any variables where P < .15 in the overall categorical analysis. Additionally, any variables with P < .15 from these univariate analyses were considered for multivariable logistic regression.
Two multiple logistic regression models were fit, one for screen and one for refer. “Profession,” “time allotted for new patient visit,” “new patients with ARHL seen weekly,” and “degree of HL as a factor for screening” were included a priori in the screening logistic regression model. “Profession,” “new patients with ARHL seen weekly,” and “degree of HL as a factor for referral” were included a priori in the referral logistic regression model. Only a priori variables and variables with P < .15 from univariate regression were included in the final multivariable logistic regression models in to generate a model that had potential to capture all relevant factors.
All analyses were conducted in SAS 9.4 TS Level 1 M5 (SAS Institute Inc, Cary, North Carolina). An alpha level of .05 was utilized to determine statistical significance for the univariate analyses and multivariable logistic regression.
3. RESULTS
Of the 70 respondents, 66 were providers who see adult patients with HL regularly. The four excluded participants identified as head and neck surgeons or pediatric otolaryngologists.
3.1. Study demographics
The study demographics are listed in Table 1. Neurotologists made up the largest group of respondents, followed by audiologists and general otolaryngologists. Three respondents identified as speech‐language pathologists (SLPs) who work with cochlear implant patients regularly. No other providers identified themselves as experts in cochlear implant or cognitive evaluations. The majority (72.7%, n = 48) of respondents completed training before 2010. Just over half (56.1%, n = 37) practice in the private setting. A minority of providers (13.6%, n = 9) see <10 patients with ARHL weekly, and just over two‐thirds of providers (69.7%, n = 46) allot 30 minutes or more for a new patient visit for HL.
Table 1.
Study demographics, total n = 66
| % | n | |
|---|---|---|
| Profession | ||
| Audiologist | 34.8 | 23 |
| Neurotologist/otologist | 39.4 | 26 |
| General otolaryngologist | 21.2 | 14 |
| Other (speech‐language pathologists) | 4.6 | 3 |
| Year of training completion | ||
| 1980s or earlier | 16.7 | 11 |
| 1990s | 15.1 | 10 |
| 2000s | 40.9 | 27 |
| 2010s | 27.3 | 18 |
| Practice type | ||
| Academic | 43.9 | 29 |
| Private | 56.1 | 37 |
| New patients with ARHL seen weekly | ||
| <10 | 13.6 | 9 |
| 10‐20 | 37.9 | 25 |
| 20‐30 | 22.7 | 15 |
| >30 | 25.8 | 17 |
| Time allotted for a new patient visit for ARHL | ||
| <30 min | 30.3 | 20 |
| 30 min | 37.9 | 25 |
| 45 min | 4.6 | 3 |
| 1 h | 7.6 | 5 |
| >1 h | 19.7 | 13 |
Abbreviation: ARHL, age‐related hearing loss.
3.2. Practice patterns
Just under a quarter (21.21%, n = 14) of providers reported screening for CI, whereas nearly two‐thirds (63.63%, n = 42) reported referring for CI (P < .001) (Table 2). Profession was the only factor significantly associated with screening. Providers in the “other” category, three SLPs, were much more likely to screen (100%, n = 3) than audiologists (21.74%, n = 5, P = .0215), neurotologists (15.38%, n = 4, P = .0096), and general otolaryngologists (14.29%, n = 2, P = .0147). The association between screening and the numbers of visits weekly for a new patient with ARHL and the time allotted for a visit for a new patient with ARHL approached significance (P = .0558 and P = .068, respectively). Providers seeing <10 patients with ARHL weekly were more likely to screen (44.44%, n = 4) than those seeing 10 to 20 (24%, n = 6), 20 to 30 (13.33%, n = 2) and >30 patients (11.76%, n = 2). Additionally, providers allotting more than 1 hour for a visit were more likely to screen (46.15%, n = 6) than those allotting <30 minutes (15%, n = 3) or 30 minutes to 1 hour (15.15%, n = 5). Year of training completion and practice type were not significantly associated with cognitive screening.
Table 2.
Provider characteristics associated with screening and referral
| Do not screen | Do screen | P value | Do not refer | Do refer | P value | |
|---|---|---|---|---|---|---|
| Totals | 78.78% (n = 52) | 21.21% (n = 14) | 36.36% (n = 24) | 63.63% (n = 42) | ||
| Profession | .0253(f) | .0056(f) | ||||
| Audiologist | 78.26% (n = 18) | 21.74% (n = 5) | 39.13% (n = 9) | 60.87% (n = 14) | ||
| General otolaryngologist | 85.71% (n = 12) | 14.29% (n = 2) | 71.43% (n = 10) | 28.57% (n = 4) | ||
| Neurotologist | 84.62% (n = 22) | 15.38% (n = 4) | 19.23% (n = 5) | 80.77% (n = 21) | ||
| Other | 0% (n = 0) | 100% (n = 3) | 0% (n = 0) | 100% (n = 3) | ||
| Year of training completion by decade | .3177(f) | .7940 | ||||
| Before 2010s | 75% (n = 36) | 25% (n = 12) | 35.42% (n = 17) | 64.58% (n = 31) | ||
| During 2010s | 88.89% (n = 16) | 11.11% (n = 2) | 38.89% (n = 7) | 61.11% (n = 11) | ||
| Practice type | .2621 | .4256 | ||||
| Private | 83.78% (n = 31) | 16.22% (n = 6) | 40.54% (n = 15) | 59.46% (n = 22) | ||
| Academic | 72.41% (n = 21) | 27.59% (n = 8) | 31.03% (n = 9) | 68.97% (n = 20) | ||
| New patients with ARHL seen weekly | .0558(c) | .6985(c) | ||||
| <10 | 55.56% (n = 5) | 44.44% (n = 4) | 33.33% (n = 3) | 66.67% (n = 6) | ||
| 10‐20 | 76% (n = 19) | 24% (n = 6) | 44% (n = 11) | 56% (n = 14) | ||
| 20‐30 | 86.67% (n = 13) | 13.33% (n = 2) | 26.67% (n = 4) | 73.33% (n = 11) | ||
| >30 | 88.24% (n = 15) | 11.76% (n = 2) | 35.29% (n = 6) | 64.71% (n = 11) | ||
| Time allotted for new patient visit | .0553(c) | .8429(c) | ||||
| <30 min | 85% (n = 17) | 15% (n = 3) | 40% (n = 8) | 60% (n = 12) | ||
| 30 min‐1 h | 84.85% (n = 28) | 15.15% (n = 5) | 30.3% (n = 10) | 69.7% (n = 23) | ||
| >1 h | 53.85% (n = 7) | 46.15% (n = 6) | 46.15% (n = 6) | 53.85% (n = 7) |
Notes: Fisher's exact and Cochran‐Mantel‐Haenszel used in place of chi‐square when appropriate. Values reported in the format % (n). Chi‐square by default; (f)‐Fishers; (c)‐CMH.
Profession was also significantly associated with referring for CI evaluation. General otolaryngologists were significantly less likely to refer for CI than neurotologists (28.57%, n = 4 vs 80.77%, n = 21, P = .002), and though not statistically significant, general otolaryngologists were also less likely to refer than audiologists and SLPs (60.87%, n = 14, P = .0911 and 100%, n = 3, P = .0515, respectively). Year of training completion, practice type, time allotted for a new patient visit for ARHL and number of new patients with ARHL seen weekly were not significantly associated with referral.
3.3. Knowledge and perceptions
Among providers who screen for CI, there was no consensus on which patient age group should prompt reflexive CI screening (Table 3); approximately one‐third would screen patients >60 years (29%, n = 4), one‐third would screen patients >70 years (36%, n = 5) and a third did not believe that age was a factor to be considered (36%, n = 5). There was similarly no consensus among providers who do not routinely screen as to what age should prompt screening, and over a third of these providers (40.4%, n = 21) believed that age is not a factor to be considered for screening. Likewise, there was no consensus among providers who do and do not screen as to which patient degree of HL should prompt CI screening, though nearly half of providers (43.94%, n = 29) believe that degree of HL should not be a factor. The majority of providers (84.85%, n = 56) would not screen a new patient with HL for CI or even patients presenting with a symptom of memory loss (69.7% n = 46). Interestingly, nearly three‐quarters of providers (72.73%, n = 48) would not screen a patient presenting with both HL and a symptom such as memory loss.
Table 3.
Patient characteristics/presentations prompting screening and referral
| Do not screen | Do screen | P value | Do not refer | Do refer | P value | |
|---|---|---|---|---|---|---|
| Totals | 78.78% (n = 52) | 21.21% (n = 14) | 36.36% (n = 24) | 63.63% (n = 42) | ||
| Patient age | .4115(f) | .422(f) | ||||
| >50 | 100% (n = 4) | 0% (n = 0) | 100% (n = 1) | 0% (n = 0) | ||
| >60 | 82.61% (n = 19) | 17.39% (n = 4) | 42.86% (n = 3) | 57.14% (n = 4) | ||
| >70 | 58.33% (n = 7) | 41.67% (n = 5) | 40% (n = 6) | 60% (n = 9) | ||
| >80 | 100% (n = 1) | 0% (n = 0) | 0% (n = 0) | 100% (n = 4) | ||
| Age not a factor | 80.77% (n = 21) | 19.23% (n = 5) | 35.9% (n = 14) | 64.1% (n = 25) | ||
| Patient degree of HL | .0009(f) | .2825(f) | ||||
| Mild or worse | 100% (n = 8) | 0% (n = 0) | 66.67% (n = 4) | 33.33% (n = 2) | ||
| Moderate or worse | 100% (n = 18) | 0% (n = 0) | 20% (n = 2) | 80% (n = 8) | ||
| Severe or worse | 45.45% (n = 5) | 54.55% (n = 6) | 50% (n = 5) | 50% (n = 5) | ||
| Profound of worse | NA (n = 0) | NA (n = 0) | 0% (n = 0) | 100% (n = 1) | ||
| Degree of HL is not a factor | 72.41% (n = 21) | 27.59% (n = 8) | 33.33% (n = 13) | 66.67% (n = 26) | ||
| Presentation of HL | .2005(f) | .4482(f) | ||||
| No | 82.14% (n = 46) | 17.86% (n = 10) | 34.48% (n = 20) | 65.52% (n = 38) | ||
| Yes | 60% (n = 6) | 40% (n = 4) | 50% (n = 4) | 50% (n = 4) | ||
| Presentation with CI symptoms | 1(f) | .8523 | ||||
| No | 78.26% (n = 36) | 21.74% (n = 10) | 37.5% (n = 12) | 62.5% (n = 20) | ||
| Yes | 80% (n = 16) | 20% (n = 4) | 35.29% (n = 12) | 64.71% (n = 22) | ||
| Presentation with both HL and CI symptoms | .7415(f) | .1445 | ||||
| No | 77.08% (n = 37) | 22.92% (n = 11) | 28.95% (n = 11) | 71.05% (n = 27) | ||
| Yes | 83.33% (n = 15) | 16.67% (n = 3) | 46.43% (n = 13) | 53.57% (n = 15) | ||
| CI screen positive | .1825 | |||||
| No | NA | NA | 30% (n = 12) | 70% (n = 28) | ||
| Yes | NA | NA | 46.15% (n = 12) | 53.85% (n = 14) |
Notes: Fisher's exact and Cochran‐Mantel‐Haenszel used in place of chi‐square when appropriate. Values reported in the format % (n). Chi‐square by default; (f)‐Fishers.
Abbreviations: CI, cognitive impairment; HL, hearing loss.
Among providers who refer patients for CI evaluation, there was no consensus on which patient age group or degree of HL should prompt referral (Table 3). Similarly, there was no consensus regarding the role of age or degree of HL in CI referral among providers who do not regularly refer. Over half of all providers would not consider age (59.1%, n = 39) nor degree of HL (59.1%, n = 39) a factor for CI referral. A minority of providers (12.12%, n = 8) would refer a new patient with HL for CI evaluation; however, more than half (51.5%, n = 34) would refer a new patient presenting with neurologic symptom for CI evaluation. Interestingly, a fewer number of providers (42.42%, n = 28) would refer a new patient with both HL and a neurologic symptom for CI evaluation than a new patient with a neurologic symptom alone. Finally, only 42.42% (n = 28) of providers would refer a patient for CI evaluation if a CI screen were positive.
There was no variable found to be significantly associated with screening in the multivariable logistic regression model after adjustment for a priori variables; however, the odds of screening among providers who allot more than 1 hour for a new patient visit for HL is 5.875 [95% CI: 0.800, 43.12] times than providers who allot 30 minutes to 1 hour. As in univariable categorical analysis, profession was significantly associated with referral in the multivariable logistic regression model (P < .004). The odds of referral among audiologists and SLPs combined and neurotologists is 4.828 [95% CI: 1.054, 22.124] times and 18.933 [95% CI: 3.33, 107.638] times, respectively, as compared to general otolaryngologists.
Just under half of respondents (42%, n = 28) chose the Mini‐Mental Status Exam (MMSE) as their screening test of choice, but nearly a quarter (23%, n = 15) were not sure of what test should be used (Figure 1). There was no consensus on the type of specialists to which a referral for CI evaluation be made, however, more respondents would refer patients for CI evaluation to neurologists (35%, n = 23) and internists (23%, n = 15) than gerontologists, psychiatrists and neuropsychologists (Figure 2).
Figure 1.
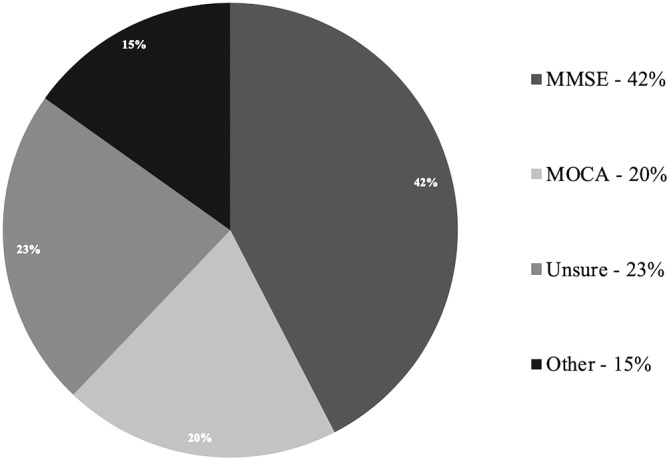
Distribution of screening test choices
Figure 2.
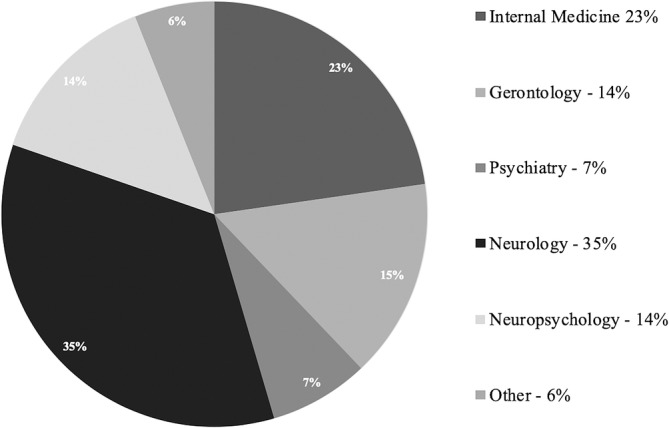
Distribution of specialty referrals
4. DISCUSSION
HL has been identified as a modifiable risk factor for CI, and audiologists and otolaryngologists who regularly see adults with ARHL should be aware of this relationship and its implications for practice. This group of practitioners are uniquely positioned to evaluate a high‐risk community of patients for CI and ensure that they are receiving appropriate care by way of referral. Unsurprisingly, however, the majority of respondents to this survey reported not screening for CI in their practices. We predicted that screening rates would be low, because of the relatively recently established relationship between CI and HL, the previously vague recommendations for CI screening in the community, and the time constraints of specialized practices. Though we anticipated that audiologists might screen more than neurotologists or general otolaryngologists because of the nature of their training and generally longer length appointment times, we found that SLPs working with patients who will receive cochlear implants screen the most. In the free response sections, these three providers, reported screening all patients who are undergoing evaluation for cochlear implantation. Generalizability of these results should be cautioned because of the small number of SLPs, however. Further inquiry should be made into the specific techniques and indications for CI screening among this group for guideline development.
Unexpectedly, we found that seeing fewer than 10 patients with ARHL weekly was associated with a higher rate of screening than seeing more than 10, although this association was not statistically significant, and the relationship became even weaker on multivariable logistic regression. Furthermore, we found that respondents with greater than 1‐hour time slots for a new patient visit for ARHL were more likely to screen than respondents with shorter time slots, though again this relationship was not statistically significant. Together, these trends may suggest that providers who have more time and see less patients may be more apt to screen; however, again, generalizability should be cautioned and these trends should be further investigated. Still, prior to the widespread dissemination of literature surrounding primary care identification of CI, barriers to diagnosis in the primary care setting included limited time, lack of routine, and physician attitudes.22
Despite the low rate of reported CI screening, it is reassuring that a larger proportion of respondents reported referring patients for CI evaluation. This calls into question the views of respondents on the role they play in caring for these patients; providers may feel that screening for CI is out of the scope of their practice or that they are ill‐equipped to perform screening, as previously suggested.22 The more specialized providers, neurotologists, SLPs and audiologists, referred at higher rates than general otolaryngologists. Though small sample sizes, this suggests that these providers may see more patients who warrant referral or highlights the relative ease of referral to CI specialists within an academic center, among several possible explanations. What is alarming, however, is that only half of respondents would refer a new patient presenting with a neurologic symptom, like memory loss, for CI evaluation, and under half would refer a new patient presenting with both a neurologic symptom, like memory loss, and HL. This suggests that although many providers recognize the benefit of specialized evaluation for CI, still many may not appreciate the value. Early detection of CI can lead patients and families to prepare for risks associated with CI, seek out education and support groups, enlist in research studies, begin medications to reduce symptoms, and obtain more appropriate medical care for comorbidities.3 Furthermore, literature from the primary care setting suggests that barriers to appropriate diagnosis (in this instance, referral for CI evaluation after a complaint of memory loss) may be related to lack of knowledge, concern for potential negative impact on families and patients, or low prioritization of CI recognition.23
In addition to the limited consensus on patient presentations that should prompt screening and referral for CI, there was no consensus on the best screening assessment to use. A plethora of research has been conducted on the relationship between CI and HL using a variety of CI screening and diagnostic tools, yet only one study to our knowledge has been conducted to attempt establishment of validation of an instrument designed specifically for patients with HL.21 Though the audiology literature has suggested that the MoCA may be the best CI screening tool to use for adults with ARHL, mounting evidence points toward the need for development of tools specifically validated for this population.17, 19, 24, 25, 26 The lack of a validated screening tool may also explain the low rates of screening, even for patients presenting with a complaint of memory loss.
Finally, practitioners have a wide base of referral for CI evaluation, including internists, neurologists, psychologists, gerontologists and neuropsychologists, and there was no single group to which respondents of this survey were likeliest to refer patients. It is therefore important for this group of providers to recognize that they all may be referred patients with ARHL and should have tools specifically designed for them. Furthermore, audiology and otolaryngology communities may be benefit from improved collaboration and communication with these specialists in to establish appropriate and consistent referral patterns.
There are several limitations with this study. First, this survey was designed to determine practice patterns and perceptions, but the methodology likely results in best practice patterns, a proxy for what actually occurs in practice, as currently there is no objective tool (like the medical record system) that could be used to understand what providers are actually doing. Therefore, the results of this survey are estimates, and perhaps overestimates, of reality. Second, we estimate the response rate to be ~12%. The survey was sent by email LISTSERV to one national group (ANS/AOS) and two Georgia state groups (GAA and GSO), for which the membership to the email LISTSERV was known. However, the number of potential survey recipients of ASHA is unknown, because the survey was posted on a web forum, rather than sent by email. This is problematic in three ways: Georgia state practices may be over‐represented because of the imbalance between national and statewide recipients, audiologists may be under‐represented because of the lack of automatic receival of the survey, and the response rate may be a slight over‐estimation. Additionally, we hoped that by choosing representative groups from general otolaryngology (the GSO) and audiology (the GAA and two SIGs from ASHA) we would obtain generalizability results; however, caution must be used in generalizing to the broader community because of this response rate. Additionally, the distribution technique may have led to sampling bias, in which providers with a special interest in CI chose to respond to the survey at higher rates than those without an interest. Finally, the survey was not designed for respondents to justify their practices, therefore we did not collect data on why providers do not screen or refer. Given the unexpectedly low rates of reported referral, this should be a target for future investigation.
5. CONCLUSION
The practice of screening for CI and referring for CI evaluation for adults with ARHL among audiologists and otolaryngologists is not routine. There is no consensus on which age or degree of HL should prompt screening. Neither is there adequate screening and referral for patients who present with a neurologic complaint, such as memory loss. Audiologists and otolaryngologists are uniquely positioned to improve the care of an adult population at high risk for modifiable CI and dementia, and should therefore, work toward improved knowledge of the relationship between HL, age and CI. Future research should focus on the development of simple, time efficient CI screening tools and guidelines surrounding their use for this particular patient population.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Supporting Information A. Listing of the introduction and consent, as they would appear to respondents in email form, and survey questions, in order of appearance in Google Forms
ACKNOWLEDGMENTS
None
Raymond MJ, Lee AC, Schader LM, Moore RH, Raol NR, Vivas EX. Practices and perceptions of cognitive assessment for adults with age‐related hearing loss. Laryngoscope Investigative Otolaryngology. 2020;5:137–144. 10.1002/lio2.339
REFERENCES
- 1. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. World Alzheimer Report 2015. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. 2015. Accessed August 18, 2018.
- 2. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 3.The Gerontological Society of America Workgroup on Cognitive Impairment Detection and Earlier Diagnosis; 2015. https://www.geron.org/images/gsa/documents/gsaciworkgroup2015report.pdf. Accessed October 1, 2018.
- 4. Lin JS, O'Connor E, Rossom RC, et al. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2013. http://www.ncbi.nlm.nih.gov/books/NBK174643/. [PubMed] [Google Scholar]
- 5. Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2013;159(9):601‐612. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . Deafness and hearing loss. http://www.who.int/mediacentrefactsheets/fs300/en/. Accessed August 18, 2018.
- 7. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population‐based study. Otol Neurotol. 2014;35(5):775‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age‐related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115‐126. 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442‐450. 10.1001/jamaoto.2015.129. [DOI] [PubMed] [Google Scholar]
- 11. Völter C, Götze L, Falkenstein M, Dazert S, Thomas JP. Application of a computer‐based neurocognitive assessment battery in the elderly with and without hearing loss. Clin Interv Aging. 2017;12:1681‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jayakody DMP, Friedland PL, Nel E, Martins RN, Atlas MD, Sohrabi HR. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otol Neurotol. 2017;38(8):e289‐e295. [DOI] [PubMed] [Google Scholar]
- 13. Sonnet MH, Montaut‐Verient B, Niemier JY, Hoen M, Ribeyre L, Parietti‐Winkler C. Cognitive abilities and quality of life after cochlear implantation in the elderly. Otol Neurotol. 2017;38(8):e296‐e301. [DOI] [PubMed] [Google Scholar]
- 14. Völter C, Götze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosetti MK, Pinkston JB, Flores JM, et al. Neurocognitive testing and cochlear implantation: insight into performance in older adults. Clin Interv Aging. 2016;11:603‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosnier I, Bebear JP, Mathieu M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otololaryngol Head Neck Surg. 2015;141(5):442‐450. [DOI] [PubMed] [Google Scholar]
- 17. Pye A, Charalambous AP, Leroi I, Thodi C, Dawes P. Screening tools for the identification of dementia for adults with age‐related acquired hearing or vision impairment: a scoping review. Int Psychogeriatr. 2017;29(11):1771‐1784. [DOI] [PubMed] [Google Scholar]
- 18. Lim MYL, Loo JHY. Screening an elderly hearing impaired population for mild cognitive impairment using Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Int J Geriatr Psychiatry. 2018;33(7):972‐979. [DOI] [PubMed] [Google Scholar]
- 19. Shen J, Anderson MC, Arehart KH, Souza PE. Using cognitive screening tests in audiology. Am J Audiol. 2016;25(4):319‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cullen B, O'Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin VY, Chung J, Callahan BL, et al. Development of cognitive screening test for the severely hearing impaired: hearing‐impaired MoCA. Laryngoscope. 2017;127(suppl 1):S4‐S11. [DOI] [PubMed] [Google Scholar]
- 22. Boise L, Camicioli R, Morgan DL, et al. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999;39:457‐464. [DOI] [PubMed] [Google Scholar]
- 23. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerreiro MJS, Van Gerven PWM. Disregarding hearing loss leads to overestimation of age‐related cognitive decline. Neurobiol Aging. 2017;56:180‐189. [DOI] [PubMed] [Google Scholar]
- 25. Dupuis K, Pichora‐Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22(4):413‐437. [DOI] [PubMed] [Google Scholar]
- 26. Jorgensen LE, Palmer CV, Pratt S, Erickson KI, Moncrieff D. The effect of decreased audibility on MMSE performance: a measure commonly used for diagnosing dementia. J Am Acad Audiol. 2016;27(4):311‐323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information A. Listing of the introduction and consent, as they would appear to respondents in email form, and survey questions, in order of appearance in Google Forms


