Abstract
Over the past 30 years in the United States, increasing identification of small thyroid nodules has led to a dramatic rise in the detection of small thyroid cancers, many of which are unlikely to progress to overt clinical disease. Because autopsy studies reveal that up to 30% of people harbor clinically occult thyroid cancers, the growing use of diagnostic technologies has identified an increasing number of small, clinically low risk papillary thyroid cancers (PTCs). In recent years, clinical practice has evolved to de‐intensify the treatment for PTCs, with fewer total thyroidectomy and nodal dissection procedures being performed, in favor of more limited operations. In addition, vigilant observation of selected low risk cancers has demonstrated outcomes comparable to those patients who undergo immediate surgical intervention. Active surveillance has emerged as a new option within the treatment algorithm of PTCs. There is now robust data from cancer centers in Japan and Korea which have reported excellent oncologic outcomes among patients undergoing active surveillance for PTC, as well as more recent, similar data from the United States. American Thyroid Association guidelines now include the option of active surveillance for appropriately selected patients with low‐risk PTC. With active surveillance now one option within the standard of care for patients with certain thyroid cancers, surgeons have become critical to facilitating shared decision‐making for patients facing this diagnosis.
Keywords: active surveillance, de‐escalation, immediate surgery, low risk, papillary thyroid carcinoma
1. BACKGROUND
1.1. Rising incidence of thyroid cancer is primarily an epidemic of diagnosis, not disease
Thyroid cancer is the most common malignancy of the endocrine system. The incidence of thyroid cancer has increased in much of the world, especially in developed nations. Vaccarella and colleagues described the rising incidence of thyroid cancer within high‐income countries and noted dramatic increases in diagnosis in the United States, Italy, France, and other developed nations. By far the most dramatic increases in thyroid cancer incidence have been noted in South Korea,1 where the incidence among women skyrocketed from 30 to 120 cases per 100, 000 women between the years 2003 and 2007.
Recent literature, published over both the short and medium term, highlights the increasing incidence of thyroid cancer worldwide, and has linked this phenomenon to the wider use of diagnostic techniques such as ultrasound scans2, 3, 4, 5, 6, 7 coupled with fine‐needle aspiration of incidentally found small nodules. In the United States alone, new diagnoses of thyroid cancer have more than tripled in the last five decades.8 While hypotheses explaining these rising trends have included environmental and genetic etiologies, a growing body of evidence has confirmed that the chief contribution to this rising incidence has been improvements in, and wider usage of, diagnostic modalities such as ultrasound.3, 9 Indeed, a strong predictor of a region or country's incidence of thyroid cancer is the level of health care access and utilization of that region10 and the degree to which the health system is privately funded.11
The increasing incidence is nearly entirely attributable to papillary thyroid carcinoma (PTC), observed to have the least aggressive behavior of the thyroid malignancies, and as primarily being of tumors less than 2 cm in size, consistent with increasing diagnosis of otherwise clinically occult disease.2, 4, 8, 9 It has been estimated that 70%‐80% of cases in the United States, and 50%‐90% of cases in other developed nations are cases that would not have gone on to cause symptoms, harm, or death if never discovered—a phenomenon termed overdiagnosis.1
Despite the dramatic rise in incidence, the mortality rate (defined as the number of deaths from disease per 100,000 population) attributable to thyroid cancer has remained essentially static.8 Recently, analyses of national cancer registry data have uncovered a slight increase in thyroid cancer mortality—an increase in a subset of patients of 0.06 per 100,000 over two decades, in the context of a parallel increase in incidence of 8.2 per 100,00012 suggesting that nearly all of the additional cases now being diagnosed do not ultimately prove to be lethal cancers.
1.2. Large subclinical reservoir of thyroid cancer in healthy persons
Thyroid cancer is usually asymptomatic and has been commonly identified by large autopsy studies in patients with no evidence of known thyroid disease. The prevalence of occult thyroid cancers has been found to be as high as 30% in a classic study from Finland.13, 14 Additional autopsy studies have placed the range of subclinical PTC in the range of 5%‐30%, suggesting that it might be considered an essentially normal finding. Another way for estimating the prevalence of occult papillary carcinoma, in younger patients, has been to examine thyroid glands removed for benign disease. In these studies, the prevalence of small microcarcinomas has been shown to be up to 10.5%, with Pelizzo et al showing no evidence of disease in this occult patient group at follow‐up greater than 5 years.15 Similarly, a large Japanese screening study detected thyroid cancer in 3.6% of otherwise healthy Japanese women of which 85% were PTC sized 15 mm or smaller.16 Again, this reported incidence was more than 1000 times higher than the reported prevalence of clinical thyroid carcinoma in Japanese women at the time. Taken together, these data indicate that the true incidence of subclinical thyroid carcinoma is much higher than the reported prevalence of clinical disease, suggesting that increasing use of diagnostic modalities would be likely to identify additional cases.
Occult carcinomas tend to be predominantly small PTCs17, 18 and more specifically, microcarcinomas, or microPTC (defined as <1 cm in maximal diameter). MicroPTCs tend to be indolent in course with low rates of locoregional and distant metastases and have excellent disease‐specific survival of nearly 100%.19, 20, 21 However, many incidentally detected thyroid cancers are larger in size. When coupled with the understanding that close to none of the cases represented by the increasing incidence of PTC appear to result in a meaningful increase in the mortality rate in the United States, these identified trends can be reasonably attributed to increased sensitivity in diagnosing occult disease and the majority of these microPTCs are termed low risk.8, 18 Thus, it is apparent that relatively small thyroid carcinomas are regularly present in otherwise healthy adults, and many will never adversely impact the well‐being and life of their host. Cancers that would never cause symptoms or death if undiscovered and untreated are termed “overdiagnosed,” and, by definition, treating overdiagnosed cancers carries no benefit, only potential for harm, to the patient.
Recent analyses by our group now demonstrate the degree to which early stage thyroid cancers are overdiagnosed and associated with health care activities. The 10‐year disease‐specific survival for stage I thyroid cancer in the United States is 99.7%—this number is high because of the indolent nature of disease. Because patients who are diagnosed with a small thyroid cancer are often healthy, health‐conscious persons in whom the subclinical tumor was incidentally discovered, thyroid cancer patients actually tend to be healthier on average than patients who do not end up having a small thyroid cancer incidentally discovered. As a result, the relative survival rates for patients with thyroid cancer are over 100% (approximately 102% at 10 years), indicating that patients diagnosed with early stage thyroid cancers actually live longer than patients of similar age, sex, and race in whom a thyroid cancer is not diagnosed.22
1.3. Low‐risk PTC
Low‐risk PTCs are described as small, intrathyroidal lesions without gross extra‐thyroidal extension, or clinically apparent lymph node metastases, or distant metastases. They do not exhibit invasion into surrounding structures such as the recurrent laryngeal nerve, esophagus, or airway. Additionally, many low‐risk PTCs have been shown to remain indolent over time without any overt disease. Ito et al performed a comparison of 340 patients with papillary thyroid microcarcinoma (PTMC) who underwent observation to 1055 patients also with PTMC who underwent immediate surgery.23 They found relatively low rates of tumor growth over time (enlargement by 3 mm or more among 6.4% and 15.9% of patients at 5 and 10 years, respectively) and low levels of nodal metastases (3.4% at 10 years—comparable to the probability of nodal recurrence after thyroidectomy) during the course of observation. Even among those patients requiring surgery after originally undergoing surveillance, no evidence of disease recurrence was noted and the patients were successfully surgically salvaged as indicated. Given the stable nature of this disease process, active surveillance (AS) of low‐risk PTC has been proposed as a treatment modality in an effort to avoid unnecessary and overly aggressive surgical intervention.
1.4. Management of PTC has de‐escalated
There is conflicting data within the literature regarding the need for low‐intensity vs high‐intensity treatment of papillary thyroid cancer. In the past, strong advocacy has existed for aggressive treatment of PTC in an attempt to improve survival outcomes for patients with the disease. Unfortunately, there are no prospective randomized controlled trials comparing aggressive initial treatment to lesser intensity management; thus, decisions regarding intervention are often guided by retrospective and population‐based registry data.
Certain trends in treatment and outcomes over the last few decades do favor conservative approaches to the treatment of PTC. As noted previously, the advent of sensitive diagnostic imaging has led to an increase in the incidence of PTC over the last few decades, particularly small PTCs, without a resultant increase in mortality.8 From this data, it can be inferred that early diagnosis of small PTCs treated with aggressive interventions has not improved survival outcomes. Subsequent studies have found no statistically significant survival advantage for total thyroidectomy (compared with lobectomy) among patients with tumors less than 4 cm in size.24, 25, 26, 27, 28, 29 This further supports the role of deescalating therapy, potentially including an AS component, in the long‐term management of PTC. Based on this new evidence, the ATA guidelines have been updated to allow for conservative treatment in carefully selected patients. Earlier recommendations of total thyroidectomy with postoperative radioactive iodine ablation (RAI) for all thyroid cancers have been replaced with a new risk stratified approach, which utilizes less aggressive surgery (lobectomy or hemithyroidectomy) without requiring routine use of postoperative RAI in low‐risk and intermediate‐risk PTCs.30, 31
1.5. Active Surveillance in Asia
Active surveillance for microPTC was first proposed by Akira Miyauchi in 1993 based on his observations of occult PTCs on autopsy studies and during ultrasound screenings.32 This approach was subsequently implemented at Kuma Hospital in Kobe, Japan, making Miyauchi the first physician to offer AS to patients as an alternative to immediate surgery. His colleague Yashuhiro Ito continued this practice in Kobe, as did Iwao Sugitani at the Cancer Institute Hospital in Tokyo, Japan. These two hospitals collectively have been the largest and longest experience in offering both AS and surgery to patients with microPTC.
To date, there have been no prospective randomized controlled trials within or outside of the United States evaluating AS compared to up‐front surgery for patients with PTC. However, as stated above, AS has been utilized overseas in large prospective and retrospective, non‐randomized patient series with robust data. The results of the largest prospective series of patients undergoing AS over a period of 22 years at Kuma Hospital were published by Ito and Miyauchi.33, 34 In the trial, which included 2153 patients, 1235 patients underwent AS as opposed to immediate surgery with similar oncologic outcomes between the two treatment groups. Among those patients who underwent AS, only 8% demonstrated increases in tumor size by 3 mm or more over 10 years. Similarly, only 3.8% of patients were noted to develop new lymph node metastases over the same time period. With close observation, the patients who required surgery for disease progression after initiating AS were successfully treated without complications or any evidence of poorer clinical outcome. No patients in either group died of disease.
A second prospective study similarly performed AS on 230 patients with asymptomatic microPTC over a time period of up to 17 years and found that the majority of patients had no appreciable change in the size of their lesions during follow‐up.35 More importantly, none of the observed patients developed evidence of extrathyroidal extension (ETE) or distant metastases. Twelve patients ultimately required surgery for lymph node metastases or increase in tumor size and had no noted recurrence during postoperative follow‐up. Lastly, a retrospective report from a Korean group also published its experience with AS of 192 patients with PTMC.36 The investigators noted relatively low rates of tumor growth with 24 patients undergoing delayed thyroid surgery after commencing observation. None of these patients had noted recurrence following surgery.
The results of the three trials revealed the overall safety of AS as a management option. Additionally, as noted by Miyauchi, patients who required surgical intervention after initiating AS for disease progression were able to avoid multiple operations and instead underwent one definitive procedure.33 Most importantly though was the finding that even those who demonstrated disease progression during monitoring were able to undergo appropriate surgery with excellent oncologic outcomes.
In addition to demonstrating the safety of AS, these experiences in Japan and Korea greatly enhanced current understanding of the natural progression of small low‐risk PTCs. In a retrospective review of 169 patients undergoing AS over a period of 10 years, the authors noted a wide spectrum of behavior of PTMC lesions. While the vast majority of cancers remained unchanged in size (97 patients, 57%), a small number underwent more pronounced growth (5 patients [3%] with rapid growth and 38 patients [22%] with slow growth), while some cancers were noted to decrease in size (29 patients, 17%).37 Further evaluation of these lesions among 1235 patients demonstrated low rates of growth and progression overall with respect to nodule size, appearance of new lymph node metastases, and progression to clinical disease.34 However, young age (<40 years) was a notable independent predictor for nodule growth over time whereas older patients had very rare growth.34, 38 Additional observations allowed for risk stratification of lesions based on location, relation to the tumor capsule, and histological appearance. Histological evaluation of confirmed PTC lesions revealed distinct growth patterns, specifically expansive growth and invasive growth.39 Lesions with expansive growth demonstrated sharp margins between the tumor and surrounding gland parenchyma with an intact capsule, whereas lesions with an invasive growth pattern exhibited irregular margins, lack of tumor capsule, and infiltration into the thyroid parenchyma and had a higher risk of recurrence.39 These data together allow for risk stratification of patients when considering AS.
1.6. Active Surveillance in the United States
In more recent years, AS has been practiced in the United States, with the largest experience at Memorial Sloan Kettering Cancer Center (MSKCC) in New York. In 2017, Tuttle and colleagues described the findings from an initial cohort of 291 patients who underwent close observation as an alternative to immediate surgery for PTC or probable PTC (Bethesda categories V or VI) based on fine‐needle aspiration and ultrasonography findings, with tumor size of up to 1.5 cm.40 In accordance with other studies, this investigation also found low rates of tumor growth and no distant or regional metastases during surveillance. Tumor growth (defined as an increase in tumor diameter >3 mm) was observed in only 3.8% of all study patients, whereas a decrease in tumor size was noted in 6.5% of patients. Younger age at diagnosis and tumor risk category were both independent risk factors for tumor growth. Measurement of tumor volume was a better predictor of further tumor growth compared to tumor size and allowed for early identification of tumors at risk for growth during the observation period and timing of therapy if needed. Importantly, these findings supported the results from Japanese and Korean populations with respect to the safety of AS, although it is important to note that the published MSKCC cohort had shorter duration of median follow‐up—25 months. As of 2019, more than 500 patients have undergone AS for PTCs of up to 2 cm in size at MSKCC, with median follow‐up now approximately 4 years, and rates of growth and nodal metastasis that remain comparable to those reported in Japan (Figure 1).
Figure 1.
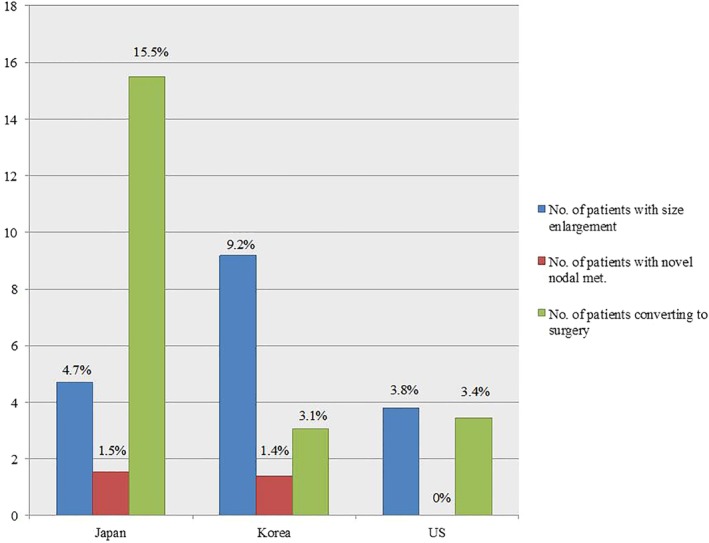
Proportion of patients who demonstrate progression during active surveillance.33, 34, 39 Proportion of patients who demonstrate nodule size enlargement (% total, defined as 3 mm size increase), novel nodal metastases, or convert to surgery during active surveillance. Japan, n = 1235; Korea, n = 360; United States, n = 291
1.7. Instituting Active Surveillance
Due to the results of these new data, namely the safety of AS, the low rates of recurrence, the success of delayed surgery, and finally the lack of mortality associated with surveillance, the ATA guidelines were updated in 2015 to incorporate AS as an option within the management paradigm of PTC.31 AS is therefore not considered an “experimental” treatment option, but rather one acceptable option within the standard of care for appropriately selected patients with low‐risk thyroid cancers (Table 1).
Table 1.
ATA 2015 guideline update on the management of papillary thyroid carcinoma30
| Active surveillance, in lieu of immediate surgery, can be used in: |
OR
OR
OR
|
Abbreviations: PTMC, papillary thyroid microcarcinoma; RLN, recurrent laryngeal nerve.
aOnly one of the criteria listed above needs to be met to consider an active surveillance approach.
Risk stratification scheme presented in Table 2.
It is important to note that only one of the criteria outlined in Table 1 must be met when considering a patient for AS and surgery can be offered at any point in the surveillance period. Additionally, the guidelines do not limit candidates to lesions smaller than 1 cm, but merely state that the lesion should be clinically low risk. As stated above, low‐risk lesions encompass a fairly wide range of tumors to include microcarcinomas (<1 cm) or completely intrathyroidal PTCs up to 1.5 or 2 cm in size without ETE or clinical nodes. While the majority of available data has evaluated lesions up to 1.5 cm in size, AS of larger lesions (up to 2 cm) may be appropriate, provided the tumor is intrathyroidal.
Based on the 2015 guidelines update Brito et al proposed a framework for risk stratification of patients being considered for observation of PTC.41 The approach encompasses evaluation of characteristics of the tumor, the patient, and the medical team (Table 2). These criteria are then used to stratify patients into “ideal,” “appropriate,” and “inappropriate” categories for AS. Ideal patients are defined as older patients with a single probable or proven tumor within the thyroid parenchyma, away from the thyroid capsule with well‐defined margins. Appropriate patients include those who are younger, with multifocal disease at noncritical locations near the thyroid capsule, or who may have concurrent conditions making US evaluation more difficult. Finally, inappropriate candidates are defined as those with tumors in critical locations (adjacent to the recurrent laryngeal nerve, esophagus, trachea), aggressive cytological features, have radiographic evidence of ETE, or evidence of disease growth and progression on serial imaging.
Table 2.
Brito categories for risk stratification of patients with papillary thyroid carcinoma when considering active surveillance41
| Ideal candidate | Appropriate candidate | Inappropriate candidate |
|---|---|---|
|
|
|
|
|
|
Abbreviations: AS, active surveillance; ETE, extrathyroidal extension; LNs, lymph nodes; PTMC, papillary thyroid microcarcinoma; RLN, recurrent laryngeal nerve.
Currently, there are no data to indicate that any molecular findings, such as BRAF mutation, impact the suitability of a small PTC for AS. The extensive Japanese, Korean, and more recent North American experiences have not used any molecular markers as inclusion or exclusion criteria, and it would be anticipated that a substantial portion of PTCs undergoing AS in each of these series would have harbored BRAF mutations. A recent mutational analysis by Tuttle and colleagues revealed that there are no clear mutational differences between indolent papillary microcarcinomas and those that develop nodal metastases.42 However, as experiences mature and more cases are available for molecular analysis, better molecular discriminators of clinical behavior may be identified in the future.
At MSKCC, patients with Bethesda V or VI nodules enrolling on an AS treatment approach undergo a baseline ultrasound scan for measurements of axial dimensions and calculation of tumor volume, followed by serial ultrasound scans every 6 months for 2‐3 years, then annually. Currently, surgery is recommended when the cancer exhibits an increase in size as defined by (a) a 3 mm increase in axial dimension or (b) a >50% increase in tumor volume with a short doubling time or in tumors located immediately adjacent to the thyroid capsule. The 3 mm increase in axial dimension corresponds to a 100% increase in tumor volume, so the 50% increase in tumor volume cutoff is an early indicator of an increase in tumor size that is usually clinically apparent before an increase of 3 mm in any single axial dimension can be documented. Furthermore, evidence of invasion into or through the thyroid capsule or cervical lymph node metastases are also indications for surgical intervention.
Though appropriate patient selection is invariably critical to performing AS, other aspects must be taken into consideration when instituting this treatment modality. The experience and comfort of the clinical team is paramount and should ideally include members with multidisciplinary care experience, both surgeons and endocrinologists. Availability and use of high‐quality neck ultrasonography for baseline and follow‐up assessments and understanding of tumor kinetics is invaluable for making treatment decisions. Finally, establishing processes for tracking enrolled patients and ensuring adequate follow‐up is necessary. The ATA guidelines do accept AS as a treatment approach that falls within the standard of care, and this treatment modality is not regarded as experimental. Nevertheless, AS is a novel approach in many centers, and experiences may vary across diverse patient populations and practice settings. There is often value to formalizing the manner in which AS patients are followed. Examples of this include recurring multidisciplinary meetings to review patients on AS, and/or IRB‐approved protocols to prospectively track patient clinical and radiographic data.
1.8. Contribution of the thyroid surgeon to shared decision‐making
Undeniably, a key part of instituting AS protocols is the discussion that must take place between physician and patient. As implied by the terminology “active surveillance,” this is a treatment approach that requires a patient who is engaged as an active part of the surveillance program. The decision to pursue AS as opposed to immediate surgery is one that must be arrived upon after a process of shared decision‐making, frank and honest discussion of options, and mutual trust, involving both the patient and physician. It is imperative that patients understand the evidence and reasoning behind performing AS—namely avoiding unnecessary surgery and adjuvant treatments that are unlikely to significantly impact survival and disease‐specific outcomes, and circumventing the potential morbidity associated with those treatments. Given the nature of AS, patients must be fully committed to the process and feel confident in their choices of management options.
It is at this juncture that the thyroid surgeon plays a critical role in helping patients understand their choices. The surgeon is often best suited among the multidisciplinary team to help patients understand the potential risks and benefits of each treatment option, especially those of immediate surgery. It is well documented that thyroid surgery is not without risk even in the most experienced surgeons' hands, and these risks include temporary or permanent hypoparathyroidism, transient or permanent vocal cord paralysis (VCP), and need for life‐long thyroid hormone replacement. In the study evaluating 2135 patients over 20 years, Miyauchi reported significantly higher rates of temporary VCP, temporary hypothyroidism, and permanent hypothyroidism (4.1% vs 0.6%, 16.7% vs 2.8%, and 1.6% vs 0.08%, respectively) among patients who underwent immediate surgery compared to AS.33 Griffin et al performed a retrospective review of surgical patients eligible for AS to determine the number of surgical complications that could be avoided. In this study of 56 patients who met criteria for AS and all of whom underwent immediate surgery, 6 patients developed temporary hypoparathyroidism, 1 developed permanent hypoparathyroidism, 1 had temporary VCP, and 1 patient had permanent VCP.43 In both series, the reported rates of complications were low, but in the context of patients who do not require surgery (both in the short and potentially long term) these complication rates could be considered unacceptably high, as they could be avoided altogether.
In addition to avoiding surgical risks specifically associated with thyroidectomy, another consideration is the avoidance of multiple surgical procedures. In their review of AS candidates, Griffin et al identified 5 patients out of 56 (8.9%) who required revision surgery for local recurrence following upfront surgery for low‐risk PTC.40 Similarly, Oda et al described the surgical outcomes of 2153 patients who underwent either immediate surgery or AS for low‐risk PTC.44 Of this cohort, 974 patients chose immediate surgical resection for PTC and long‐term follow‐up identified 5 (0.051%) patients requiring reoperation for local recurrence (specifically nodal recurrence). In the AS group, 94 patients out of 1179 converted to surgery for various reasons after initiating AS. Of this group, only one patient (0.08%) required a second procedure for local recurrence. The finding highlighted by Oda et al was that risk of developing nodal metastases for patients who undergo AS is similar to those treated with immediate surgery.
Many patients may conclude that the risks of surgery, although low, outweigh the benefits, particularly given the evidence that equally curative operations can be performed at a later date should the thyroid cancer show signs of growth or progression. Other patients may come to the opposite conclusion, and decide that the risks of surgery are acceptable, and indeed preferable, to undergoing a process of AS. These conclusions are both reasonable. A thyroid surgeon is well suited to counsel a patient on the pros and cons, risks and sequelae, of either approach.
In addition to guiding the patient through this discussion, the physician must take into account the patient experience and must be aware of intrinsic biases and assumptions that may lead the physician to strongly favor one option over another. Despite the avoidance of surgical risk and lack of significant survival benefit, the potential psychological impact of living with cancer upon the patient may preclude the physician from viewing AS as a viable option. A study performed by Davies et al sought specifically to assess the experience of patients undergoing AS. Through a combination of patient surveys and interviews, they revealed that patients overall worry about cancer recurrence, metastases, and need for surgical intervention to a similar degree as patients who undergo surgery.45 The proportion of patients who did not worry about their cancer outcomes steadily increased over the course of the surveillance period, and a large percentage of patients (83%) agreed that AS was the best management option for them. Additionally, and importantly, a large proportion of patients (77%) were not aware of AS as a treatment modality before it was offered to them. This study highlighted that patient experience with AS does not have a detrimental impact on psychological well‐being and is in fact comparable to those patients who undergo surgical intervention. The worry that a patient may experience due to living with an active cancer, while understandable and not unreasonable, need not prohibit consideration of AS as a reasonable management option. Oftentimes, the explanation that surgery can be performed at a later date with equal effectiveness is useful in providing patients with the space to consider their options carefully.
Ongoing research seeks to further define the patient experience during the AS process and identify roadblocks to the implementation of this treatment modality. Furthermore, research is needed to identify clinical, radiographic, and molecular biomarkers to better risk‐stratify tumors in terms of appropriateness for AS. Lastly, more prospective data are required to better define the optimal protocols for scans, clinical examination, and surgical intervention if needed.
2. CONCLUSION
Active surveillance is a safe alternative to immediate surgery for many patients with low‐risk papillary thyroid cancer. Patients and physicians weighing these options—observation or surgery—should understand that there is more than one “right answer” within the current standard of care. In this respect, thyroid surgeons are indispensable members of the multidisciplinary thyroid cancer team and best suited to guide patients through shared decision‐making. Patients facing a new diagnosis of thyroid cancer will benefit from consultation with a surgeon, to discuss these options.
CONFLICT OF INTEREST
The authors report no conflicts of interest. L.G.T.M. reports laboratory research funding from Illumina, Inc. and AstraZeneca, unrelated to this work.
Lohia S, Hanson M, Tuttle RM, Morris LGT. Active surveillance for patients with very low‐risk thyroid cancer. Laryngoscope Investigative Otolaryngology. 2020;5:175–182. 10.1002/lio2.356
BIBLIOGRAPHY
- 1. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid‐cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 2. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26(11):1541–1552. [DOI] [PubMed] [Google Scholar]
- 3. Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine‐needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154(6):1420–1426. discussion 1426‐1427. [DOI] [PubMed] [Google Scholar]
- 4. Rego‐Iraeta A, Perez‐Mendez LF, Mantinan B, Garcia‐Mayor RV. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid. 2009;19(4):333–340. [DOI] [PubMed] [Google Scholar]
- 5. La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–2195. [DOI] [PubMed] [Google Scholar]
- 6. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albores‐Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18(1):1–7. [DOI] [PubMed] [Google Scholar]
- 8. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. [DOI] [PubMed] [Google Scholar]
- 9. Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine‐needle aspirate cytology. Thyroid. 2006;16(1):47–53. [DOI] [PubMed] [Google Scholar]
- 10. Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(7):709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee TJ, Kim S, Cho HJ, Lee JH. The incidence of thyroid cancer is affected by the characteristics of a healthcare system. J Korean Med Sci. 2012;27(12):1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974‐2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer. 1985;56(3):531–538. [DOI] [PubMed] [Google Scholar]
- 14. Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol. 1988;90(1):72–76. [DOI] [PubMed] [Google Scholar]
- 15. Pelizzo MR, Piotto A, Rubello D, Casara D, Fassina A, Busnardo B. High prevalence of occult papillary thyroid carcinoma in a surgical series for benign thyroid disease. Tumori. 1990;76(3):255–257. [DOI] [PubMed] [Google Scholar]
- 16. Takebe K, Arai T. Problems of thyroid ultrasound screening–based on the results of thyroid screening conducted at our facility 25 years ago in Japan. Ultrasound Med Biol. 2017;43:S114. [Google Scholar]
- 17. Ahn HS, Kim HJ, Welch HG. Korea's thyroid‐cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–1767. [DOI] [PubMed] [Google Scholar]
- 18. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 19. Jeon MJ, Kim WG, Choi YM, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid. 2016;26(1):161–168. [DOI] [PubMed] [Google Scholar]
- 20. Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016;26(6):807–815. [DOI] [PubMed] [Google Scholar]
- 21. Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(4):653–660. [DOI] [PubMed] [Google Scholar]
- 22. Marcadis AR, Marti JL, Ehdaie B, Hakimi AA, Davies L, Morris LGT. Characterizing relative and disease‐specific survival in early‐stage cancers. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34(1):28–35. [DOI] [PubMed] [Google Scholar]
- 24. Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg 2014;260(4):601–605; discussion 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barney BM, Hitchcock YJ, Sharma P, Shrieve DC, Tward JD. Overall and cause‐specific survival for patients undergoing lobectomy, near‐total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011;33(5):645–649. [DOI] [PubMed] [Google Scholar]
- 26. Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low‐ or high‐risk papillary thyroid cancer. Ann Surg Oncol. 2005;12(1):81–89. [DOI] [PubMed] [Google Scholar]
- 27. Matsuzu K, Sugino K, Masudo K, et al. Thyroid lobectomy for papillary thyroid cancer: long‐term follow‐up study of 1,088 cases. World J Surg. 2014;38(1):68–79. [DOI] [PubMed] [Google Scholar]
- 28. Mendelsohn AH, Elashoff DA, Abemayor E, St John MA. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136(11):1055–1061. [DOI] [PubMed] [Google Scholar]
- 29. Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151(4):571–579. [DOI] [PubMed] [Google Scholar]
- 30. American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer , Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 31. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito Y, Miyauchi A. Active surveillance as first‐line management of papillary microcarcinoma. Annu Rev Med. 2019;70:369–379. [DOI] [PubMed] [Google Scholar]
- 33. Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40(3):516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222–1231. [DOI] [PubMed] [Google Scholar]
- 36. Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center's experience in Korea. J Clin Endocrinol Metab. 2017;102(6):1917–1925. [DOI] [PubMed] [Google Scholar]
- 37. Miyauchi A, Kudo T, Ito Y, et al. Natural history of papillary thyroid microcarcinoma: kinetic analyses on tumor volume during active surveillance and before presentation. Surgery. 2019;165(1):25–30. [DOI] [PubMed] [Google Scholar]
- 38. Ito Y, Miyauchi A, Kobayashi K, Miya A. Prognosis and growth activity depend on patient age in clinical and subclinical papillary thyroid carcinoma. Endocr J. 2014;61(3):205–213. [DOI] [PubMed] [Google Scholar]
- 39. Kakudo K, Tang W, Ito Y, Mori I, Nakamura Y, Miyauchi A. Papillary carcinoma of the thyroid in Japan: subclassification of common type and identification of low risk group. J Clin Pathol. 2004;57(10):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perera D, Ghossein R, Camacho N, et al. Genomic and transcriptomic characterization of papillary microcarcinomas with lateral neck lymph node metastases. J Clin Endocrinol Metab. 2019;104(10):4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffin A, Brito JP, Bahl M, Hoang JK. Applying criteria of active surveillance to low‐risk papillary thyroid cancer over a decade: how many surgeries and complications can be avoided? Thyroid. 2017;27(4):518–523. [DOI] [PubMed] [Google Scholar]
- 44. Oda H, Miyauchi A, Ito Y, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low‐risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64(1):59–64. [DOI] [PubMed] [Google Scholar]
- 45. Davies L, Roman BR, Fukushima M, Ito Y, Miyauchi A. Patient experience of thyroid cancer active surveillance in Japan. JAMA Otolaryngol Head Neck Surg. 2019;145(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]


