Abstract
Traumatic brain injury (TBI) impacts over 3.17 million Americans. Management of hemorrhage and coagulation caused by vascular disruption after TBI is critical for the recovery of patients. Cerebrovascular pathologies play an important role in the underlying mechanisms of TBI. The objective of this study is to evaluate a novel regenerative medicine for the injured tissue after brain injury. We utilized a recently described synthetic growth factor with angiogenic potential to facilitate vascular growth in situ at the injury site. Previous work has shown how this injectable self-assembling peptide-based hydrogel (SAPH) creates a regenerative microenvironment for neovascularization at the injury site. Supramolecular assembly allows for thixotropy; the injectable drug delivery system provides sustained in vivo efficacy. In this study, a moderate blunt injury model was used to cause physical vascular damage and hemorrhage. The angiogenic SAPH was then applied directly on the injured rat brain. At day 7 post-TBI, significantly more blood vessels were observed than the sham and injury control group, as well as activation of VEGF-receptor 2, demonstrating the robust angiogenic response elicited by the angiogenic SAPH. Vascular markers von-Willebrand factor (vWF) and α-smooth muscle actin (α-SMA) showed a concomitant increase with blood vessel density in response to the angiogenic SAPH. Moreover, blood brain barrier integrity and blood coagulation were also examined as the parameters to indicate wound recovery post TBI. Neuronal rescue examination by NeuN and myelin basic protein staining showed that the angiogenic SAPH may provide and neuroprotective benefit in the long-term recovery.
Keywords: Traumatic brain injury, Cerebrovascular injury, Regenerative medicine, Angiogenesis, Self-assembling peptide hydrogels
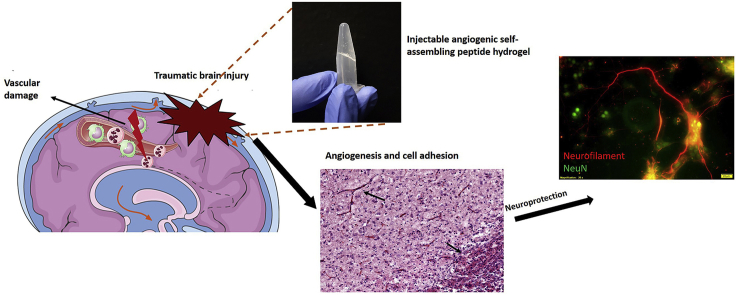
Graphical abstract
Highlights
-
•
Cerebral vascular damage plays a key role in traumatic brain injury and recovery.
-
•
Injectable self-assembling peptide-based hydrogels (SAPH) create a regenerative microenvironment for neovascularization.
-
•
VEGF-R2 was upregulated by angiogenic SAPH, promoting endothelial cell and smooth muscle based neovascularization.
-
•
Angiogenic SAPH provided potential neuroprotection in recovery post brain injury.
1. Introduction
Traumatic brain injury (TBI) is typically caused by a mechanical force or sudden acceleration-deceleration [1]. In 2013 alone, there were about 2.8 million ER visits for TBI in the US, where 3.2–5.3 million people live with permanent long-term disability [2]. TBI has gained increased public awareness in recent years with a number of sports-related concussive injuries and military-related blunt/diffuse blast injuries – altogether these are known to increase the risk of chronic traumatic encephalopathy and other cognitive disorders [3].
TBI is composed of primary and secondary mechanisms, primary injury is the physical damage to the tissue, including contusion, vascular penetration, or axon stretch [4,5]. Secondary injury happens within minutes and lasts years with consequences of neuroinflammation and neurodegeneration [6]. A major concern of TBI is the direct damage to the cerebral vasculature in the early stages following the insult, observed in TBI patients and recapitulated in animal models of TBI [[7], [8], [9]]. At a cellular level, the cerebral vasculature contributes several key components to the expanded neurovascular unit (eNVU), consisting of endothelial cells, smooth muscle cells, neurons, astrocytes, and pericytes [10]. Disruption of the vascular trees precedes ischemia, immune cell infiltration, altered delivery of metabolites, hypoxia and tissue death [11]. Previously, we have found that hemorrhage related coagulation leads to the acute necrotic cell death in a blunt TBI model [5], suggesting that agents which promote neoangiogenesis may allow enhanced recovery from TBI.
Clinical trials in TBI to date have not specifically targeted at angiogenesis and neurogenesis in situ [12]. Preclinical studies in cerebral ischemia are replete with strategies that deliver vascular endothelial growth factor, sildenafil, atorvastatin, carbamylated erythropoietin (EPO) and EPO monotherapy, to name a few [[13], [14], [15], [16], [17], [18]]. Treatments with growth factors also contribute to eNVU remodeling, improving outcomes after ischemic brain injury [18]. However, these systemic or local treatments are cleared by the body in a matter of minutes to hours and do not have durable localized effect. Further, few strategies report the generation of a supportive in situ microenvironment for wound healing [19].
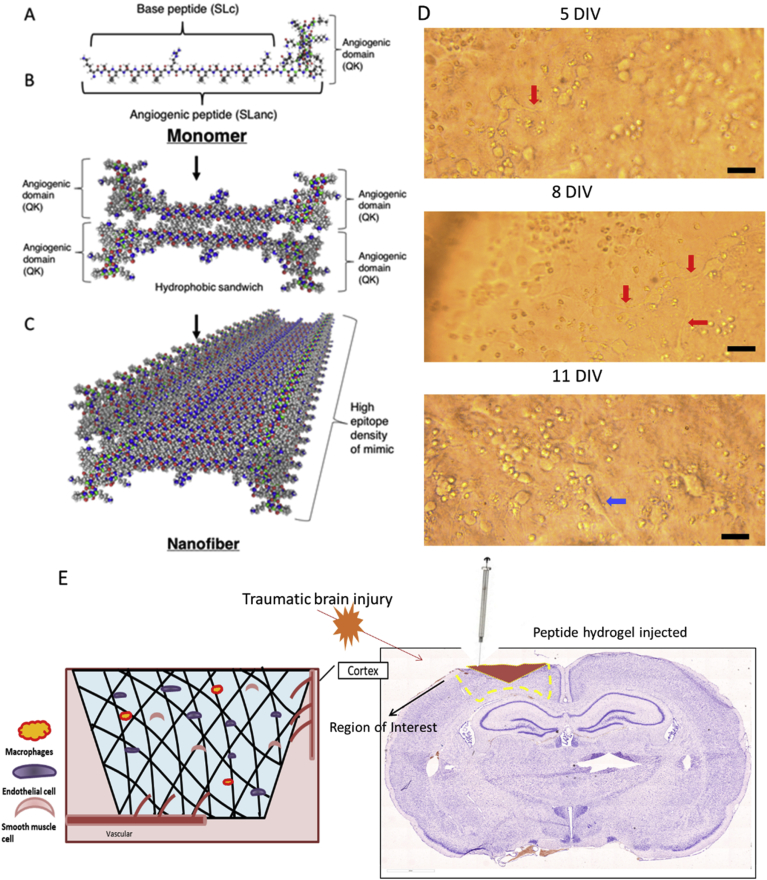
Self-assembling peptide hydrogels (SAPH) present a facile method to present potent biological signals in a localized tissue microenvironment. SAPH are composed of chemically synthesized oligo peptides (typically 5-50 amino acids long), with multiple domains [[20], [21], [22], [23]]. The principle self-assembling domain (that give SAPH their namesake) consists of polar amino acid residues flanking a midblock of alternating hydrophilic and hydrophobic amino acid residues [24]. SAPH can be engineered to contain degradation sequences, such as MMP-2 susceptible -LRG- domains in the midblock to hasten in vivo biodegradation [25]. Additionally, SAPH can promote cell specific signaling when the termini are functionalized with short peptide mimics of growth factors [26]. Noteworthy is a SAPH that has an appended mimic of vascular endothelial growth factor (VEGF-165), QK [25,27]. This angiogenic SAPH, termed SLanc, assembles into fibers displaying the QK epitope in very high density (Fig. 1A–C) [21,25,[27], [28], [29], [30], [31], [32]]. Owing to non-covalent interactions, these peptides rapidly assemble and dissemble upon needle shear.
Fig. 1.
Angiogenic peptides display in vitro compatibility and have been used to stimulate angiogenesis in the brain after injury. (A-C) Injectable hydrogels consist of self-assembled peptides – sequence and assembly schematic (adapted from Kumar et al. [25], copyright 2015 ACS ®). Of critical importance is cytocompatibility with primary rat cortex neurons cultures (D), arrows indicate neuronal growth; cytocompatibility with 1 w% SLanc is demonstrated over a 14 day period with primary neurons in culture that show minimal neuronal loss, with concomitant neurite growth, scale bar 30 μm. (E) Fluid percussion injury on the exposed dura (Right image of Nissl stained whole brain section). Hydrogels assemble into an ECM-mimetic niche for cellular infiltration and neo-angiogenesis (Left image).
From our previous studies, the current injectable SAPH has been examined in vitro assays for cytocompatibility, angiogenesis, recovery from hind limb ischemia, and management of a number of ischemic tissue diseases [21,27,28]. In the current study, we assess the angiogenic SAPH termed SLanc in the injured rat brain, for its angiogenic and neuroprotective effect in the central nervous system. A lateral fluid percussion injury model (FPI) was used to induce a moderate traumatic brain injury, with physical vascular disruption and tissue deformation [33]. SLanc peptide hydrogels were injected into the injury site immediately after FPI, and bio-distribution along with histological recovery (angiogenesis and neuronal survivability) were evaluated at day 7 and day 14 post injury.
2. Materials and methods
2.1. Synthesis and characterization of peptides
SLanc was prepared as previously described [25,27]. Briefly, SLanc was synthesized by standard Fmoc solid-phase peptide synthesis protocol and purified through dialysis against DI water for 2 days (see Table 1 for peptide sequences). The dialyzed peptide was frozen, lyophilized and stored in the powder form at −80 °C until formulation. The peptide hydrogel was prepared initially at a concentration of 20 mg/ml (2 w. %) in sterile 298 mM sucrose and pH adjusted to 7. To obtain the final concentration of 10 mg/ml, the same volume of sterile 1X HBSS as sucrose was added. This formulation was easily injectable with a 25G needle.
Table 1.
Primary sequences of peptides used in this study.
| Name | Sequence |
|---|---|
| SLanc | K(SL)3RG(SL)3K–G–KLTWQELYQLKYKGI |
| F-SLanc | Carboxyfluorescein-K(SL)3RG(SL)3K–G–KLTWQELYQLKYKGI |
2.2. Cytocompatibility of SLanc on primary neurons in vitro
1% (w/v) SLanc was placed into the wells of a 96-well plate (100 μL/well) and incubated at 37 °C for 2 h to gel. Complete neurobasal medium containing 2% Gibco B27 supplement with antioxidants (50X), 1% penicillin-streptomycin, and 0.2% glutamate was added to the well and incubated at 37 °C for 2 h to condition the SLanc. Primary neuronal cells were obtained by neuronal isolation. Briefly, cortices were extracted from the embryos of a Sprague Dawley timed pregnant rat (Charles River) at day 16 gestation. Cortices were then digested in trypsin-EDTA (0.25%) at 37 °C for 30 min and agitated with a pipette to release cells. Primary cortical neurons were separated by straining with 70 μm and 40 μm pore filters. Neurons were seeded at a density of 50,000 cells/cm2 on top of the SLanc and supplemented with complete neurobasal medium. Cell morphology and neurite growth was observed over time by microscopy.
2.3. Animal handling
Adult male and pregnant female Sprague-Dawley or Wistar rats were purchased from Charles River Laboratory (Wilmington, MA). The animals were housed with free access to food and water in a 12 h dark-light cycle at room temperature. The protocols were approved by the NJIT-Rutgers-Newark Institutional Animal Care and Use Committee, and followed the guidelines in the Guide for the Care and Use of Laboratory Animals.
2.4. Subcutaneous implantation
1% (w/v) SLanc was injected within the dorsal subcutaneous space of 200–225 g Wistar rats to evaluate normo-physiological response in vivo. 200 μL of SLanc was injected (n = 3), tissue was retrieved at day 7, and prepared for immunofluorescence staining.
2.5. Fluid percussion injury (FPI)
Sprague Dawley male rats (250-300 g; 8–11 weeks old) were randomly selected and subjected to lateral FPI for a moderate TBI [34]. Sham-injured cases served as control. 5 rats were used for each group (Sham, FPI+PBS, FPI+SLanc). All rats were anesthetized with mixture of ketamine (100 mg/kg), and xylazine (10 mg/kg) was administered via intraperitoneal injection and placed in a stereotaxic frame. Craniotomy (3.0 mm) was performed over the left parietal skull, 2.5 mm lateral from the midline and 3.0 mm caudal from bregma, with the dura intact. A Luer-lock hub was fitted to the craniotomy window and secured with cyanoacrylate gel on the skull. The hub was sealed by application of methyl-methacrylate (Henry Schein, Melville, NY, USA). Once the methyl methacrylate was hardened, the Luer-lock was filled with sterile saline. One day after surgery, animals were randomly assigned to receive either sham or FPI. Sham animals were subjected to all surgical procedures except the induction of the injury. For induction of the FPI, the pendulum hammer was released onto the piston of the fluid filled cylinder to induce the injury. The recorded apnea and righting reflex time after a moderate FPI (1.6–1.8 atm.) were 10–12 s and 6-8 min, respectively.
2.6. Therapeutic intervention and bio-distribution of F-SLanc
After FPI and anesthesia, the rats were placed in a stereotaxic frame to be treated with 5 μl SLanc peptide hydrogel, Fluorescent-tagged SLanc peptide, or PBS as injury control. The location of injection according to the stereotaxic coordinates was: AP = −3, L = −2.5 from Bregma, dorsoventral (DV) = 0.5 mm. Then the injury hub was removed, and the head was sutured. The rats recovered on a heating pad and then they were returned to their home cage.
2.7. Intracisterna CSF tracer infusion
Fluorescent FITC tracer (Dextran, Fluorescein, 2,000,000 MW, (Thermo Fisher) reconstituted in artificial CSF (5 mg/ml) was infused into anesthetized rats via intracisterna magna [35], 2 h before sacrifice. This established protocol was used to track the vascular damage as this large molecule tracer is distributed along the perivascular space [5]. Ten microliters of the FITC tracer was infused into anesthetized rats by a 30-gauge needle at a rate of 1 μl/min for 10 min with a syringe pump (Harvard Apparatus).
2.8. Tissue processing
3, 7, 14 days after FPI/FPI + treatment, the rats were euthanized via slow transcardial perfusion with ice cold phosphate buffered saline (PBS), followed by ice cold 4% paraformaldehyde in PBS. Brain were immersed in 4% paraformaldehyde overnight, followed by cryoprotection in 30% sucrose in PBS. 20 μm thick coronal sections were processed from each sample and stored at −80 °C for further analysis.
2.9. Immunofluorescence and microscopy
Brain tissue sections were washed with PBS and fixed in acetone-methanol (1:1 volumetric ratio) for 10 min at −20 °C for immunofluorescence staining. Fixed slides were then blocked with 3% bovine serum albumin at 25 °C for 1 h in the presence of 0.1% Triton X- 100. Slides were incubated with the primary antibodies shown in Table 2 overnight at 4 °C for probing the respective antigens. Sample slides were washed with PBS and then incubated with Alexa Fluor 488/594 conjugated with anti-mouse/goat/rabbit/sheep immunoglobulin-G (IgG) for 1 h. After washing with PBS, sample slides were mounted on immunomount containing DAPI (Thermo Fisher). Whole brain tissue sections images were scanned (20x magnification) by Leica Aperio Versa 200 digital pathology grade slide scanner, and detailed regional fluorescence images were captured by a fluorescence microscope (IX81 DSO; Olympus, Somerset, NJ).
Table 2.
Sources, catalogue numbers, and dilutions factors for antibodies used for immunofluorescence staining and western blotting analyses.
| Antibody | Marker for | Company | Catalogue # | Dilution for IF | Dilution for WB |
|---|---|---|---|---|---|
| Rabbit anti-MBP | Myelin basic protein | Abcam | Ab40390 | 1:150 | -- |
| Mouse anti-NeuN | Neurons | Abcam | Ab104224 | 1:200 | -- |
| Rabbit anti-Von Willebrand Factor | Endothelial cells | Abcam | Ab6994 | 1:200 | 1:1000 |
| Mouse anti-alpha smooth muscle actin | Smooth muscle cells | Abcam | Ab7817 | 1:200 | -- |
| Goat VEGF-Receptor 2 | VEGF-R2 | Abcam | Ab10972 | 1:200 | 1:1000 |
| Rabbit CD42d | Blood platelets | Abcam | Ab65017 | -- | 1:1000 |
| Rabbit ZO-1 | Tight junction protein | Abcam | Ab96587 | 1:200 | 1:1000 |
2.10. Western blot
Fresh brain tissue was homogenized with CellLytic-M (Sigma) lysis buffer (500 μL buffer for every 10 mg of tissue) on ice using sonication. After 30 min incubation on ice, this homogenization was centrifuged at 13,000 rpm for 20 min at 4 °C and supernatant was collected and assessed for protein concentration by the bicinchoninic acid (BCA) method (Thermo Fisher). Protein was loaded 20 μg/lane in 4–15% SDS-PAGE gradient gels (Thermo Fisher), transferred on the PVDF membranes, blocked with 5% milk, incubated overnight with primary antibodies at 4 °C, washed, and incubated with respective horse-radish peroxidase conjugated secondary antibodies (1:10000 dilution) for 1 h at room temperature. Immunoreactive bands were detected by West Pico chemiluminescence substrate (Thermo Fisher). Data was quantified as densitometry intensity using ImageJ software.
2.11. Statistical analysis
All results are expressed as the mean ± SEM. Statistical analysis of the data was performed using GraphPad Prism 7 (Sorrento Valley, CA, USA). Comparisons between samples were performed by one-way ANOVA with Tukey's multiple comparison tests. Differences were considered significant at p < 0.05.
3. Results
3.1. Angiogenic peptide hydrogel properties
As previously established, SLanc was synthesized via conventional Fmoc solid phase peptide synthesis and purified by dialysis and HPLC. Peptides, in aqueous buffer, undergo self-assembly into a nanofibrous hydrogel through simultaneous hydrogen bond formation and hydrophobic packing (Fig. 1A–C). We have assessed the self-assembly, cytocompatibility, and angiogenic potential of SLanc in vitro and in vivo (subcutaneous and rodent hind limb ischemia model) [25,27,29]. From our previous cytocompatibility assays in-vitro, SLanc increased cell adhesion of human mesenchymal stem cells and promoted wound healing in endothelial scratch assays [25]. In this study, we critically analyzed the efficacy of this facile strategy in wound recovery subsequent to traumatic brain injury (TBI). Germane to this work, cytocompatibility of SLanc with primary rat neurons showed neurite outgrowth was found in SLanc hydrogel, Fig. 1D. Subcutaneous implantation of SLanc was performed to investigate angiogenesis (Fig. S1), prior to evaluation in the rat TBI model, Fig. 1E. Immunostaining images for endothelial cells and smooth muscle cells showed a robust angiogenic effect in subcutaneous at day 7 and day 14 post treatment (Fig. S1. Importantly, cytotoxicity of the hydrogels was not noted in any of the cultures over the range of concentrations tested: 0.1w% solutions - 1w% hydrogels for a range of cells types in these and previous studies [25,27].
3.2. Angiogenic hydrogels increase blood vessel density in the injured brain
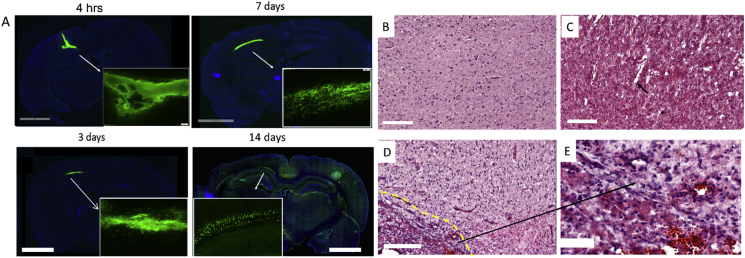
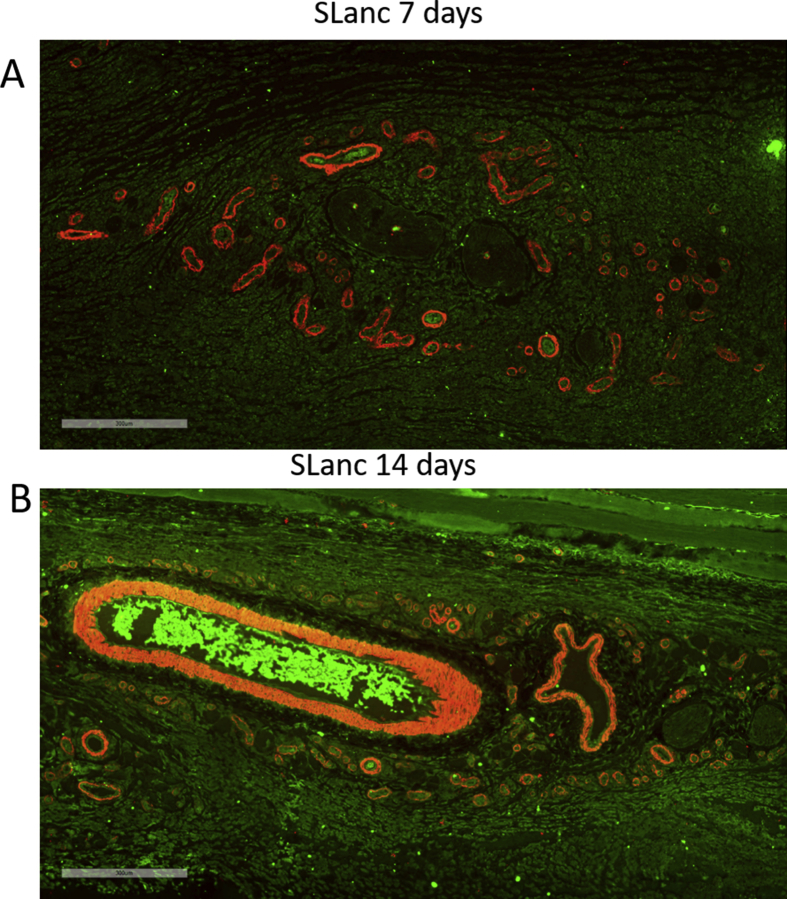
To examine the bio-distribution of the peptide hydrogel in situ at the impact site, fluorescently tagged SLanc was injected intracranially directly after FPI in the injury site. The peptide hydrogel exhibited thixotropic shear thinning and near instantaneous recovery into a hydrogel in situ after injection [25]. The bulk of the gel remained localized up to 1 week post injection (Fig. 2A). At day 14 post treatment, the implanted hydrogel showed modest biodegradation as expected, and with some distribution in the cortex, hippocampus, partially in the ventricles. The significance of using the peptide hydrogel is to create a depot for persistent drug-epitope presentation in the injury site for therapeutic angiogenesis, with subsequent degradation. SLanc persistence in CNS to 14 days is comparable to subcutaneous and intramuscular persistence previously reported [27].
Fig. 2.
Self-assembly of peptides and promotion of angiogenesis in the cranial tissue. (A) Fluorescently tagged SLanc (1:10 ratio with untagged SLanc) showed localization and as an injected bolus. Representative whole brain sections from different rats at 4hrs–14 days shows persistence of SLanc hydrogels in the brain, scale bar 3 mm. (B-E) Promotion of material driven angiogenesis in cranial tissue was first observed using H&E staining of whole rat brain sections at day 7 post TBI; sham (B) or injured control (C) did not show significant angiogenesis. Black arrow in C showed vasospasm in injured brain without treatment. The SLanc treated brain (D) showed a significant increase of new blood vessels (black arrows in D) in the proximity of hydrogel implants (dotted-yellow line indicates the implanted hydrogel interface extending to the bottom left), enlarged figure (E) clearly displayed the new blood vessels structure in (D). Scale bar for B-D is 200 m, for E is 50 m.
Post-TBI, Hematoxylin & Eosin (H&E) stained sections clearly showed cellular infiltration and angiogenesis (Fig. 2B–E), in SLanc treated injuries (Fig. 2D) vs. the injury-control brain (Fig. 2C) and sham (Fig. 2B). Vasospasm was observed in injury control with enlarged perivascular space in Fig. 2C. At 7 days post FPI, the enlarged figure captured in proximity of the hydrogel implant (Fig. 2E) showed clear angiogenesis and increased cell infiltration, indicating vascular regeneration by angiogenic SAPH.
3.3. Angiogenesis is promoted by SAPH treatment post TBI
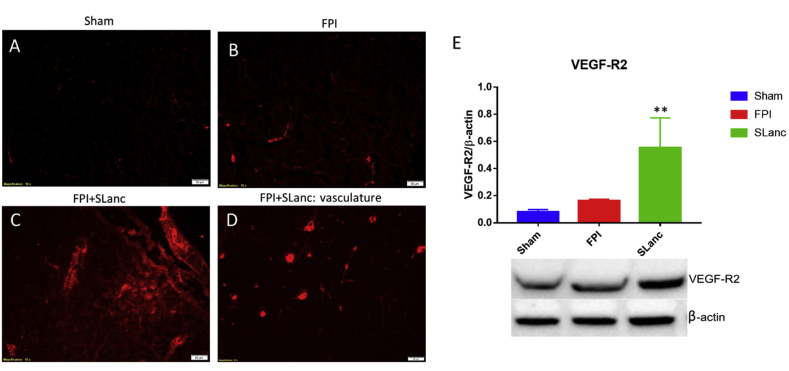
VEGF-receptor 2 is expected to be activated for self-recovery in the injured brain, and it plays an important role in angiogenesis and neuroprotection in brain injuries [[36], [37], [38], [39]]. Thus the mechanistic understanding of the angiogenic process, as well as the identification of new blood vessels were evaluated by VEGF-Receptor 2 expression (Fig. 3). It was found here that SLanc treatment resulted in significantly higher VEGFR2 expression at day 7 post injury (Fig. 3C), compared with injury control and sham brain (Fig. 3A–B). Corresponding to the increased number of blood vessels in histology staining (Fig. 2E), positive immunoactivity was specifically observed in the vascular structures (Fig. 3D). By western blot quantification, we also noted that VEGFR2 was upregulated in the FPI injured brain as a native response to repair the damaged vasculature, however our angiogenic peptide upregulated VEGFR2 significantly to promote the angiogenesis than the native wound healing process (Fig. 3E).
Fig. 3.
Upregulation of angiogenic receptors. A canonical marker of upregulated angiogenesis was studied by VEGF-receptor 2 expression at day 7 post FPI/treatment. We noted a significantly higher expression of VEGFR2 in the SLanc treated FPI brain. Immunostaining of VEGFR2 showed a small increase in FPI without treatment (B), significant expression after SLanc treatment (C), and expressed in vascular structures in the vicinity of the implant (D), compared with sham (A), scale bar 50 m. (E) Western blot quantified VEGFR2 expression in the FPI+SLanc brain was significantly higher compared to FPI injured control and sham control. One-way ANOVA with Tukey's multiple analysis was used. Values are mean ± SEM (n = 5), **p < 0.01. T = 7 days post FPI/treatment.
Critical to the formation of vasculature is their nascency/maturity, permeability and ability to therapeutically revascularize ischemic tissue. This was observed by staining for nascent capillary based endothelial cells using von-Willebrand factor (vWF), and mature arteriole/venules marker smooth muscle cell marker (α-smooth muscle actin), Fig. 4. An increased number of endothelial cells were observed in and around SLanc hydrogel implants in the FPI injured brain at day 7 post TBI (Fig. 4A–B). Western blots of vWF showed significantly higher recruitment of endothelial cells in FPI+SLanc brain, compared to injury control and sham (Fig. 4C). Compared to sham, FPI+PBS and FPI+SLanc in Fig. 4D, we observed significantly more disruption of the vascular structure in injury control. SLanc treatment resulted in significantly more endothelial cell infiltration with smooth muscle cell linings, suggesting the development of mature stabilized vessels.
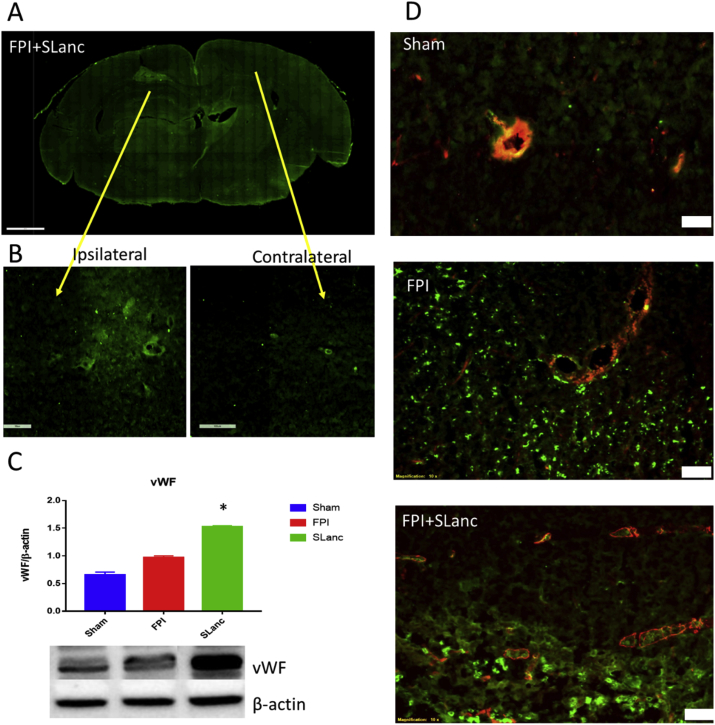
Fig. 4.
Higher expression of vascular markers in the SLanc treated injury brain sections. We noted increased endothelial cells adhesion and blood vessels regeneration in SLanc treated FPI brains. (A) Representative immunofluorescence staining of von-Willebrand factor in the whole brain section post FPI and SLanc treatment. Endothelial cells adhesion was observed in the hydrogel implant (B), comparing with contralateral site. (C) Western blot shows significant increased vWF in SLanc treated FPI brain at 7 days post FPI. One-way ANOVA with Tukey's multiple analysis was used, values are mean ± SEM (n = 5), *p < 0.05. (D) Co-localization of vWF and α-smooth muscle actin further indicates angiogenesis in FPI+SLanc brain section, compared with sham and FPI brain. Scale bar 50 m.
3.4. Angiogenic SAPH is potentially neuroprotective and reduces axonal damage
It was hypothesized that therapeutic angiogenesis promoted by SLanc treatment may translate to improved neuronal survivability in the injured brain. From previous studies, it was found that coagulation is significantly increased and accumulated along vasculature after FPI, and leads to thrombotic necrosis [5]. Here, a large molecule FITC-dextran tracer (2000 kDa) was injected into cerebrospinal fluid (CSF) (distributed along vasculature) to track the vascular damage as a parameter for injury recovery. Co-localization of the FITC tracer with tight junction protein Zonula occludens (ZO-1) further indicated blood brain barrier (BBB) recovery as a parameter of functional recovery. The bio-distribution of the FITC tracer was blocked along the impaired BBB in FPI injured brain at day 7 post injury (Fig. 5B), compared with the intact BBB and well-distributed tracer in sham animals. Fig. 5C showed that SLanc repaired the blood brain barrier (BBB) and recovered the distribution of tracer in the CSF pathway. Moreover, coagulation in the injured brain was found to be significantly reduced by the angiogenic peptide SLanc after 7 days post FPI, in western blot quantification (Fig. 5D).
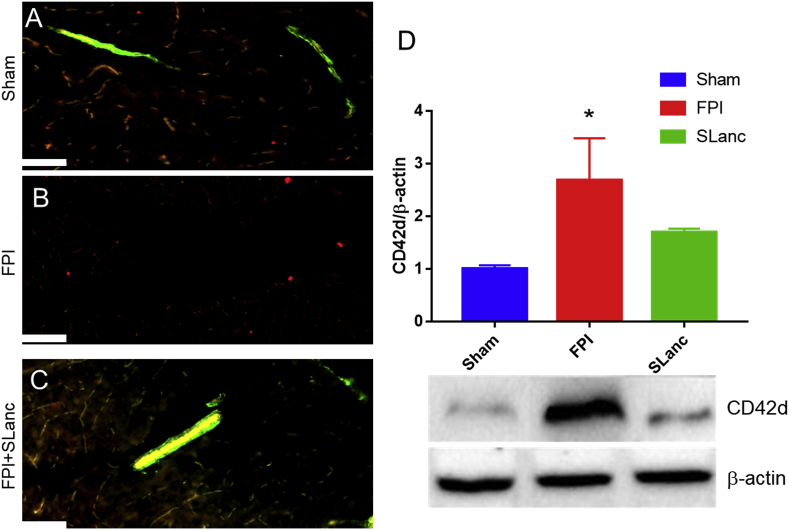
Fig. 5.
Angiogenic peptide hydrogel repairs blood brain barrier and reduces coagulation. (A) Co-localization of FITC tracer along vasculature (green) with tight junction protein (ZO-1, red) in sham, FPI and FPI+SLanc brain sections. Scale bar is 40 μm, T = 7days post FPI. (B) Western blot quantification results show that coagulation persisted after 7 days post-FPI, with angiogenic treatment SLanc; blood platelets (CD42d) was significantly reduced compared with FPI group. One-way ANOVA with Tukey's multiple analysis was used, values are mean ± SEM (n = 5), *p < 0.05.
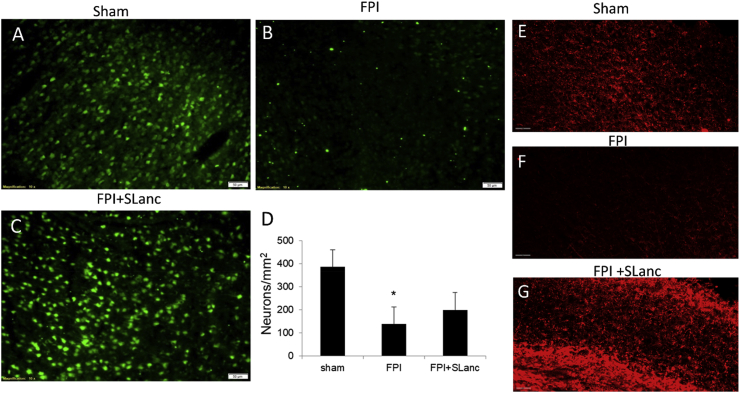
Neuronal survivability was expected to be improved based on the reduced coagulation and repaired BBB. Thus after 14 days post FPI/treatment, anti-NeuN (a neuronal marker) was used for the neuronal rescue examination. Representative immunofluorescence staining and cell counting showed significant neuronal loss in the FPI injured brain (Fig. 6B) compared with sham (Fig. 6A). SLanc treatments seemed to ameliorate neuronal loss at the injury site (Fig. 6C). The positive NeuN cell counting validated the neuronal rescue quantitatively (Fig. 6D). Myelin basic protein (MBP) was also used to label myelinated axons at day 14 post FPI/treatment. The reduced immunodensity of MBP in FPI brain validated the axonal impairment correlating with neuronal loss, but SLanc treatment ameliorated the axonal unmyelination (Fig. 6E–G). These results revealed that the SLanc peptide hydrogel is neuroprotective for neurons in grey matter and axonal branches in the white matter.
Fig. 6.
Angiogenic peptide is potentially neuroprotective. NeuN and Myelin basic protein (MBP) were used to investigate neuronal survival and axons at 14 days post FPI. (A-C) Immunostaining and Neuron cell counting (D) show SLanc is neuroprotective. Scale bar 50 m (E-G) MBP staining indicates the Angiogenic peptide could also promote axonal branches in the injured brain. One-way ANOVA with Tukey's multiple analysis was used, values are mean ± SEM (n = 6), *p < 0.05. Scale bar 60 m.
4. Discussion
SAPH have been utilized in a number of preclinical applications of the past decade [[21], [22], [23],[27], [28], [29], [30], [31],[40], [41], [42]]. Angiogenic SAPH have previously demonstrated angiogenic potential on human endothelial cells, promote rapid vascularization of subcutaneous bolus implants, and rapid recovery in a hind limb ischemia model [21,25,27]. The injured brain presents added complexity from eNVU disruption due to both vasculature damage and neuronal loss. Further driving the urgency of therapeutic developments is the lack of clinical modalities that have succeeded [43]. An often understudied aspect of TBI treatment is cerebrovascular repair and angiogenesis; critical milestones have focused on whole growth factor therapy, vasodilators, statins and promotors of red blood cell production [38,[44], [45], [46]]. In current study, we used a self-assembling peptide based injectable hydrogel (SLanc) containing a VEGF-165 mimic that can promote angiogenesis. The hydrogel can be injected into injury site post FPI and provide a consistent healing microenvironment for regeneration of the eNVU. Our results suggest that persistent presentation of a VEGF mimic through the use of SAPH in a hydrogel implant can guide axonal projections and angiogenesis after injury of the native brain ECM. Histologic staining and immunostaining of endothelium and smooth muscle indicate a robust angiogenic response induced by the angiogenic SAPH. Further, the underlying mechanism was found to be the activation and upregulation of VEGF-receptor 2. This was observed concomitant to a significant increase of endothelial cells, smooth muscle cells and VEGF-R2. Importantly, it has been established that VEGF-R2 drives direct activation of neuronal intracellular signaling pathway for neuroprotective actions, according to PI3-K/Akt pathway, as well as MAPK ERK kinase/extracellular signal-regulated kinase pathway [47]. Moreover, in an ischemia animal model, overexpression of VEGF was shown to reduce infarct volume by promoting neurogenesis and neuromigration [48]. These supporting studies provides a potential explanation for angiogenic SAPH to have neuroprotective efficacy.
Fluid percussion injury (FPI) is a well-established TBI injury model, with a mixed injury type including focal and diffuse injury from the impact site. Axonal injury was commonly reported in clinical TBI reports and experimental models [49]. It was demonstrated that targeting angiogenesis can also induce neurogenesis and thus improve cognitive impairment [37,45,46]. Here, we found that angiogenesis was activated by SLanc SAPH at 7 days post injury and treatment, followed with the clearance of coagulation, repair of blood brain barrier and CSF pathway recovery. These all lead to the amelioration of neuronal loss based on the previously established injury mechanisms [5]. We further examined the neuronal survivability and myelinated axonal recovery at day 14 post injury and treatment. We noted that along with repair of neurovascular unit, more neurons survived compared to injury control. Further, neurite outgrowth was observed surrounding the angiogenic SAPH, indicating a neuroprotective effect by SLanc in long-term.
Here, we argue that persistent and prolonged localized presentation of angiogenic moieties can help preserve neural function, and potentiate healing. While a number of angiogenic drugs have been used similarly (such as sildenafil, atorvastatin and EPO with a short half-life in vivo), their transient effect does not lead to functional/durable benefit. Conversely, too much, or persistent angiogenesis after the wound has healed may lead to cerebral edema or potential hemorrhage. While the vessels that SLanc promotes are lined by endothelial cells and smooth muscle cells (suggesting their maturity and stability), controlled and tailorable angiogenesis (on-demand) would be the preferred modus. To this end, future studies will investigate varying concentrations in CNS (and potentially the peptide sequence) to understand the temporal and functional benefit in restoring cerebral vasculature, and how this may relate to behavioral outcomes post TBI.
5. Conclusions and future directions
In this study, we investigated an angiogenic SAPH in the injured brain tissue. The injectable self-assembling peptide hydrogel (SAPH) with a vascular endothelial growth factor mimic provides persistent angiogenic signal in the injury site, while providing a matrix for tissue infiltration and regeneration. Angiogenesis was further validated by immunohistochemistry and biomarkers for vasculature and VEGF-receptor 2. Angiogenic SAPH was shown to activate angiogenesis by inducing endothelial cells adhesion and upregulation of VEGF-R2. Neuroprotection and axonal outgrowth were observed in and surrounding angiogenic SAPH, indicating repair of neurovascular unit is associated with enhanced neuronal survivability. This study establishes the preclinical potential of SAPH for use in the injured brain. Longer-term studies that track functional recovery and behavioral outcomes in larger animal models will definitely demonstrate efficacy prior to human translation. Overall, angiogenic SAPH may create regenerative environments for neuroprotection, potential neuronal regeneration and a variety of other (neuro) ischemic tissue diseases.
Ethical approval and consent to participate
All procedures performed in studies involving animals followed the National Institutes of Health guidelines for the ethical care of laboratory animals, and the Institutional Animal Care and Use Committee (IACUC) at the animal facility of Rutgers-Newark University.
CRediT authorship contribution statement
Xiaotang Ma: Conceptualization, Data curation, Formal analysis. Agnieszka Agas: Formal analysis, Data curation. Zain Siddiqui: Data curation, Methodology. KaKyung Kim: Data curation, Methodology. Patricia Iglesias-Montoro: Validation. Jagathi Kalluru: Validation. Vivek Kumar: Funding acquisition, Supervision, Investigation, Writing - review & editing. James Haorah: Funding acquisition, Supervision, Investigation, Writing - review & editing.
Declaration of competing interest
V.A.K. has equity interests in start-up companies attempting to translate peptides from peptide-based technological platform. Other authors do not have conflict of interest.
Acknowledgements
This work was supported by grant 1R21AA022734-01A1 (to JH) from National Institutes of Health, and NIH R15 EY029504, NSF IIP 1903617, the NJIT Undergraduate Research and Innovation (URI) Program and NJIT Startup funds (to V.A.K.).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2020.01.005.
Contributor Information
Vivek Kumar, Email: vak@njit.edu.
James Haorah, Email: jhaorah@njit.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Faul M., Coronado V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:3–13. doi: 10.1016/B978-0-444-52892-6.00001-5. [DOI] [PubMed] [Google Scholar]
- 2.Taylor C.A., Bell J.M., Breiding M.J., Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveillance Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iverson K.M., Dardis C.M., Pogoda T.K. Traumatic brain injury and PTSD symptoms as a consequence of intimate partner violence. Compr. Psychiatr. 2017;74:80–87. doi: 10.1016/j.comppsych.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Ma X., Cheng Y., Garcia R., Haorah J. Hemorrhage associated mechanisms of neuroinflammation in experimental traumatic brain injury. J. Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09882-x. the official journal of the Society on NeuroImmune Pharmacology. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J. Intensive Care. 2016;4:29. doi: 10.1186/s40560-016-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maegele M., Schochl H., Menovsky T., Marechal H., Marklund N., Buki A., Stanworth S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16(8):630–647. doi: 10.1016/S1474-4422(17)30197-7. [DOI] [PubMed] [Google Scholar]
- 8.Hochstadter E., Stewart T.C., Alharfi I.M., Ranger A., Fraser D.D. Subarachnoid hemorrhage prevalence and its association with short-term outcome in pediatric severe traumatic brain injury. Neurocritical Care. 2014;21(3):505–513. doi: 10.1007/s12028-014-9986-7. [DOI] [PubMed] [Google Scholar]
- 9.Perel P., Roberts I., Bouamra O., Woodford M., Mooney J., Lecky F. Intracranial bleeding in patients with traumatic brain injury: a prognostic study. BMC Emerg. Med. 2009;9:15. doi: 10.1186/1471-227X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhowmick S., D'Mello V., Caruso D., Wallerstein A., Abdul-Muneer P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019;317:260–270. doi: 10.1016/j.expneurol.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Stein S.C., Graham D.I., Chen X.H., Smith D.H. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery. 2004;54(3):687–691. doi: 10.1227/01.neu.0000108641.98845.88. discussion 691. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y., Mahmood A., Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs. 2010;11(3):298–308. [PMC free article] [PubMed] [Google Scholar]
- 13.Beck H., Plate K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117(5):481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 14.Wu H., Jiang H., Lu D., Qu C., Xiong Y., Zhou D., Chopp M., Mahmood A. Induction of angiogenesis and modulation of vascular endothelial growth factor receptor-2 by simvastatin after traumatic brain injury. Neurosurgery. 2011;68(5):1363–1371. doi: 10.1227/NEU.0b013e31820c06b9. discussion 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y., Mahmood A., Meng Y., Zhang Y., Qu C., Schallert T., Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 2010;113(3):598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y., Mahmood A., Zhang Y., Meng Y., Zhang Z.G., Qu C., Sager T.N., Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J. Neurosurg. 2011;114(2):549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Sun L., Tian Y., Li Z., Wei H., Wang D., Yang Z., Chen J., Zhang J., Jiang R. Effects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J. Neurol. Sci. 2012;319(1–2):117–123. doi: 10.1016/j.jns.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Thau-Zuchman O., Shohami E., Alexandrovich A.G., Leker R.R. Subacute treatment with vascular endothelial growth factor after traumatic brain injury increases angiogenesis and gliogenesis. Neuroscience. 2012;202:334–341. doi: 10.1016/j.neuroscience.2011.11.071. [DOI] [PubMed] [Google Scholar]
- 19.Wang T.W., Chang K.C., Chen L.H., Liao S.Y., Yeh C.W., Chuang Y.J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale. 2017;9(42):16281–16292. doi: 10.1039/c7nr06528k. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen P.K., Sarkar B., Siddiqui Z., McGowan M., Iglesias-Montoro P., Rachapudi S., Kim S., Gao W., Lee E., Kumar V.A. Self-assembly of an anti-angiogenic nanofibrous peptide hydrogel. ACS Appl. Bio Mater. 2018;1(3):865–870. doi: 10.1021/acsabm.8b00283. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar B., Nguyen P.K., Gao W., Dondapati A., Siddiqui Z., Kumar V.A. Angiogenic self-assembling peptide scaffolds for functional tissue regeneration. Biomacromolecules. 2018;19(9):3597–3611. doi: 10.1021/acs.biomac.8b01137. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen P.K., Gao W., Patel S.D., Siddiqui Z., Weiner S., Shimizu E., Sarkar B., Kumar V.A. Self-assembly of a dentinogenic peptide hydrogel. ACS Omega. 2018;3(6):5980–5987. doi: 10.1021/acsomega.8b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitscherich P., Nguyen P.K., Kannan A., Chirayath A., Anur S., Sarkar B., Lee E.J., Kumar V.A. Injectable self-assembling peptide hydrogels for tissue writing and embryonic stem cell culture. J. Biomed. Nanotechnol. 2018;14(4):802–807. doi: 10.1166/jbn.2018.2583. [DOI] [PubMed] [Google Scholar]
- 24.Moore A.N., Hartgerink J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Accounts Chem. Res. 2017;50(4):714–722. doi: 10.1021/acs.accounts.6b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V.A., Taylor N.L., Shi S., Wang B.K., Jalan A.A., Kang M.K., Wickremasinghe N.C., Hartgerink J.D. Highly angiogenic peptide nanofibers. ACS Nano. 2015;9(1):860–868. doi: 10.1021/nn506544b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar B., Nguyen P.K., Gao W., Dondapati A., Siddiqui Z., Kumar V.A. Angiogenic self-assembling peptide scaffolds for functional tissue regeneration. Biomacromolecules. 2018;19(9):3597–3611. doi: 10.1021/acs.biomac.8b01137. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V.A., Liu Q., Wickremasinghe N.C., Shi S., Cornwright T.T., Deng Y., Azares A., Moore A.N., Acevedo-Jake A.M., Agudo N.R., Pan S., Woodside D.G., Vanderslice P., Willerson J.T., Dixon R.A., Hartgerink J.D. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials. 2016;98:113–119. doi: 10.1016/j.biomaterials.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrak K., Vissapragada R., Shi S., Siddiqui Z., Kim K.K., Sarkar B., Kumar V.A. Challenges in translating from bench to bed-side: pro-angiogenic peptides for ischemia treatment. Molecules. 2019;24(7) doi: 10.3390/molecules24071219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V.A., Wang B.K., Kanahara S.M. Rational design of fiber forming supramolecular structures. Exp. Biol. Med. 2016;241(9):899–908. doi: 10.1177/1535370216640941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V.A., Wickremasinghe N.C., Shi S., Hartgerink J.D. Nanofibrous snake venom hemostat. ACS Biomater. Sci. Eng. 2015;1(12):1300–1305. doi: 10.1021/acsbiomaterials.5b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar V.A., Shi S., Wang B.K., Li I.C., Jalan A.A., Sarkar B., Wickremasinghe N.C., Hartgerink J.D. Drug-triggered and cross-linked self-assembling nanofibrous hydrogels. J. Am. Chem. Soc. 2015;137(14):4823–4830. doi: 10.1021/jacs.5b01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar V.A., Taylor N.L., Shi S., Wickremasinghe N.C., D'Souza R.N., Hartgerink J.D. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–78. doi: 10.1016/j.biomaterials.2015.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyeth B.G. Historical review of the fluid-percussion TBI model. Front. Neurol. 2016;7:217. doi: 10.3389/fneur.2016.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X., Aravind A., Pfister B.J., Chandra N., Haorah J. Animal models of traumatic brain injury and assessment of injury severity. Mol. Neurobiol. 2019 doi: 10.1007/s12035-018-1454-5. [DOI] [PubMed] [Google Scholar]
- 35.Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33(46):18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. the official journal of the Society for Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafuente J.V., Argandona E.G., Mitre B. VEGFR-2 expression in brain injury: its distribution related to brain-blood barrier markers. J. Neural Transm. 2006;113(4):487–496. doi: 10.1007/s00702-005-0407-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu K.T., Sun C.L., Wo P.Y., Yen H.H., Tang T.H., Ng M.C., Huang M.L., Yang Y.L. Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J. Neurotrauma. 2011;28(3):441–450. doi: 10.1089/neu.2010.1473. [DOI] [PubMed] [Google Scholar]
- 38.Guo H., Zhou H., Lu J., Qu Y., Yu D., Tong Y. Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury. Neural Regen. Res. 2016;11(1):174–179. doi: 10.4103/1673-5374.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krum J.M., Mani N., Rosenstein J.M. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp. Neurol. 2008;212(1):108–117. doi: 10.1016/j.expneurol.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar B., Siddiqui Z., Nguyen P.K., Dube N., Fu W., Park S., Jaisinghani S., Paul R., Kozuch S.D., Deng D., Iglesias-Montoro P., Li M., Sabatino D., Perlin D.S., Zhang W., Mondal J., Kumar V.A. Membrane-disrupting nanofibrous peptide hydrogels. ACS Biomater. Sci. Eng. 2019;5(9):4657–4670. doi: 10.1021/acsbiomaterials.9b00967. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen P.K., Sarkar B., Siddiqui Z., McGowan M., Iglesias-Montoro P., Rachapudi S., Kim S., Gao W., Lee E.J., Kumar V.A. Self-assembly of an antiangiogenic nanofibrous peptide hydrogel. ACS Appl. Bio Mater. 2018;1(3):865–870. doi: 10.1021/acsabm.8b00283. [DOI] [PubMed] [Google Scholar]
- 42.Shi S., Nguyen P.K., Cabral H.J., Diez-Barroso R., Derry P.J., Kanahara S.M., Kumar V.A. Development of peptide inhibitors of HIV transmission. Bioact. Mater. 2016;1(2):109–121. doi: 10.1016/j.bioactmat.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopp S., Albert-Weissenberger C., Mencl S., Bieber M., Schuhmann M.K., Stetter C., Nieswandt B., Schmidt P.M., Monoranu C.M., Alafuzoff I., Marklund N., Nolte M.W., Siren A.L., Kleinschnitz C. Targeting coagulation factor XII as a novel therapeutic option in brain trauma. Ann. Neurol. 2016;79(6):970–982. doi: 10.1002/ana.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H., Lu D., Jiang H., Xiong Y., Qu C., Li B., Mahmood A., Zhou D., Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma. 2008;25(2):130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 45.Lee C., Agoston D.V. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J. Neurotrauma. 2010;27(3):541–553. doi: 10.1089/neu.2009.0905. [DOI] [PubMed] [Google Scholar]
- 46.Ortuzar N., Rico-Barrio I., Bengoetxea H., Argandona E.G., Lafuente J.V. VEGF reverts the cognitive impairment induced by a focal traumatic brain injury during the development of rats raised under environmental enrichment. Behav. Brain Res. 2013;246:36–46. doi: 10.1016/j.bbr.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Tovar-y-Romo L.B., Penagos-Puig A., Ramirez-Jarquin J.O. Endogenous recovery after brain damage: molecular mechanisms that balance neuronal life/death fate. J. Neurochem. 2016;136(1):13–27. doi: 10.1111/jnc.13362. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Jin K., Mao X.O., Xie L., Banwait S., Marti H.H., Greenberg D.A. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J. Neurosci. Res. 2007;85(4):740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 49.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.