Summary
One of the most important causes of visual loss (blindness) is glaucoma, which occurs due to the degeneration of the ganglion cells in retina. It has been shown that hydrogen sulphide (H2S) acts an antioxidant, neuroprotective and neuromodulator and provides protection against oxidative stress and apoptosis. This study aims to examine through which apoptotic pathway H2S acts in experimental glaucoma model. Twenty‐two male wistar albino rats were used in this study. Group 1 (n = 6, control group): Intravitreal saline was given in the third week without inducing ocular hypertension (OHT) with laser photocoagulation. Group 2 (n = 8): After the induction of OHT with laser photocoagulation, intravitreal saline was given in the third week. Group 3 (n = 8): After the induction of OHT with laser photocoagulation, intravitreal H2S’s donor sodium hydrosulphide (NaSH) 100 nmol/L was given in the third week. At the end of the 6th week, the eyes of the rats were sacrified under anaesthesia and extracted and then routine tissue follow‐up was undertaken. Besides haematoxylin & eosin (H&E) staining, Bax, Bcl‐2, p53 and caspase‐3 activation were examined immunohistochemically in the retina and the cornea. This showed that ocular hypertension caused apoptosis through the intrinsic pathway, due to Bax and caspase‐3 activation, in both retina and cornea, and that this led to DNA damage due to p53 activation. Also, we found that H2S exposure in glaucoma distinctly suppressed Bax, caspase‐3 and p53 activations in retina but that it has a limited effect on the cornea. According to these results, glaucoma caused apoptosis in the retina through intrinsic pathway, and the damage to the retina could be compensated partially by H2S but would have limited on the cornea.
Keywords: apoptosis, cornea, glaucoma, hydrogen sulphide, rat, retina
1. INTRODUCTION
Glaucoma is a disease which appears as irreversible loss of vision and is characterized by the degeneration in retinal ganglion cells (RGCs) and optic nerve head (ONH) axons. It is observed in 70 million people all over the world.1, 2, 3, 4 One of the most important factors of glaucoma is increased intraocular pressure (IOP).5 Increased IOP can cause deformation, axonal damage and transport impairment especially in lamina cribrosa and the surrounding tissues due to mechanical stress.3 Although increased IOP is a major cause of glaucoma, the molecular relationship between the deaths of ONHs and RGCs respectively has not been resolved completely.4, 6 The mechanism of RGC degeneration is not clearly established but may implicate either single or multiple mechanisms. Those that have been invoked include axonal transport failure, activation of intrinsic and extrinsic apoptotic signals, mitochondrial dysfunction, excitotoxic damage and oxidative stress.2, 5, 6, 7, 8, 9 Apart from decreasing IOP, an increasing number of molecular studies have suggested the significance of neuroprotective applications in preventing RGC death in glaucoma.
Hydrogen sulphide is a gasotransmitter with growing importance which is synthesized from L‐cysteine by two enzymes [cystathionine beta synthase (CBS) and cystathionine gamma‐lyase (CSE)].10, 11 Experimental studies report that CBS enzyme is abundant in both the cornea and the retina of mammals and that H2S is produced endogenously in bovine ocular tissues.12, 13, 14, 15 H2S also regulates intracellular calcium concentration in the nervous system and can be neuroprotective against neurodegenerative disorders due to its anti‐oxidant, anti‐inflammatory and anti‐apoptotic effect. Recently, there have been questions whether any such effect of H2S could be applicable in glaucoma. Therefore it has been tested in some previous studies.16, 17, 18, 19, 20
Apoptosis is essential in normal development for the maintenance of tissue homeostasis.21, 22 The process of apoptosis induction mechanism is initiated by several factors, including decrease in growth factors and DNA damage. Apoptosis can be documented through two pathways ‐ intrinsic and extrinsic.23 The B‐cell lymphoma 2 (Bcl‐2) family regulates the intrinsic pathway of apoptosis (mitochondrial pathway). After an apoptotic stimulus is received with intracellular signals, the Bcl interacting domain [Bid (proapoptotic protein)] ensures the oligomerization of Bcl‐2 antagonist/ killer (Bak) and Bcl‐2–associated X (Bax) proteins and inactivates Bcl‐2. Subsequently, activated Bax changes the mitochondrial membrane potential and stimulates cytochrome c release from mitochondrial membrane pores. Cytochrome c interacts with apoptotic protease activating factor 1 (Apaf‐1), adenosine triphosphate (ATP) and procaspase‐9 to form a structure known as an apoptosome. After this caspase‐9 transforms procaspase‐3 into active caspase‐3. During the process following caspase‐3 activation, apoptosis takes place and is reflected in a series of identifiable changes such as cytoplasmic shrinkage and nuclear condensation.22, 23, 24
The tumour suppressor protein, p53, encodes a transcription factor which is activated as a response to stress signals. Monitoring genetic integrity, p53 blocks cell cycle in the G1 phase according to the degree of damage. DNA damage may occur and there is time for DNA repair, or it may suppress Bcl‐2 and Bcl‐xL by increasing Bax, Apaf‐1 and Fas formations thus contributing to apoptosis.25, 26
Although intravitreous or surgical applications are applied in clinical practices in order to decrease IOP in glaucoma treatment, neuroprotective factors are necessary in order to protect RGC since IOP increase is not the only contributory cause for glaucoma ‐ glaucoma can arise even when there is low IOP.6 Our previous study (Ozer et al17) demonstrated that H2S application decreased apoptosis and lowered IOP in an experimental glaucoma model. The current study aims to extend this by analysing immunohistochemically Bcl‐2, p53, Bax and caspase‐3 activities in order to reveal though which apoptotic pathway are affected by H2S.
2. MATERIAL AND METHOD
Twenty‐two male wistar albino rats (10‐12 weeks old; 200‐250 g) were used in this study. The animals were maintained under 12‐hour light and 12‐hour dark cycle at 20 ± 2°C and 55%‐60% humidity conditions. The rats were divided into three groups. In the control group (n = 6), 10 μL saline was injected intravitreously into the right eyes without laser coagulation. In the OHT group (n = 8), photocoagulation was applied with argon laser to the limbal plexus, dorsal vein and temporal episcleral vein of the right eyes. At the end of the third week, 10 μL saline was injected intravitreously into the right eyes. Finally, in the OHT+H2S group, photocoagulation was applied with argon laser to the limbal plexus, dorsal vein and temporal episcleral vein of the right eyes. At the end of the third week, 10 μL of 100 nmol/L NaSH (H2S’s donor) was injected intravitreously into the right eyes.
2.1. Induction of ocular hypertension
Initially the rats were anaesthetized before the procedure of IOP increase (50 mg/kg ketamine and 5 mg/kg intraperitoneal xylazine). After anaesthesia, general frontal and posterior segment examinations were made, and the IOPs of all the eyes were measured. One temporal episcleral vein, two dorsal episcleral veins and limbal plexus were targeted in the superior temporal areas of the eyes (the area between superior rectus and lateral rectus) in order to increase IOP. These areas were subjected to 50 shots of argon laser photocoagulation (ARC Argon Laser, Nuremberg, Germany) with 1000 mW power and 100 µm diameter for 0.2 msec, and thermal burns were avoided in this process.27
2.2. Ethical approval
This study has been carried out upon the approval of the Local Research Ethics Committee for Lab Animals of Giresun University (Ethics approval number: 2018/9)
2.3. IOP measurement
IOP was measured prior to and 10 minutes after photocoagulation. This process was repeated every week for six weeks. IOPs were measured two minutes after twilight anaesthesia in order to eliminate the possible decreases in IOP values. Before IOP measurement, 0.5% proparacaine HCI was dropped on to the eyes to prevent corneal reflex and pain. All the measurements were taken between 12:00 and 14:00 in order that circadian IOP changes did not affect the results. All the IOP measurements were evaluated under biomicroscope using Goldmann applanation tonometer.17
2.4. Intravitreous injection
All the subjects were given intravitreous injection on the nasal part of the eye under deep anaesthesia. In order to correctly adjust needle tip and fundus reflex prior to injection, rat pupils were dilated with tropicamide, and the injections were made from the nasal quadrant at 2 mm distance to limbus. Intactness of lens and retina was checked by examination. A total of 33 gauge syringes (Hamilton, Bonaduz AG, Bonaduz, Switzerland) were used for injection. At the end of the third week, H2S donor was applied intravitreously to NaSH and OHT group rats as 10 µL and 100 nmol/L NaSH (Sigma Chemical Co., Taufkirchen, Germany) and to control group rats as 10 µL and 0.9% NaCl.
2.5. Histopathology
At the end of the 6th week, the animals were sacrificed under anaesthesia and the eyes were enucleated after. The extracted ocular tissues were fixed in 10% neutral formalin for 72 hours. Then, the tissues processed for histology using an Automatic Tissue Processor (Leica TP1050) and embedded in paraffin blocks. 5 µm coronal cross‐sections were taken from the optic discs on to Poly‐L‐Lysine coated slides. In addition to H&E staining, immunohistochemistry with anti‐Bax, anti‐Bcl‐2, anti‐caspase‐3 and anti‐p53 antibodies (signalling pathways of cell death) was performed.
2.5.1. Immunohistochemistry
The slides first deparaffined and then dehydrated, and antigen retrieval was performed. For antigen retrieval, the slides were boiled in microwave in 100 W through 20 minutes. Then, the slides were cooled at room temperature for 20 minutes. After two washes with PBS tissue peroxidase activity was blocked using 3% hydrogen peroxide in methanol. Then, the slides were washed again with PBS and the tissues encircled with PAPPEN. Background staining was blocked with protein block in secondary antibody for 5 minutes. After washing with TBS, the primary antibodies, anti‐Bax (1:50, sc20067 mouse monoclonal antibody, Santa Cruz Biotechnology Inc, USA), anti‐Bcl‐2 (1:50, ab196495 Rabbit polyclonal, Abcam, UK), anti‐caspase‐3 (1:50 PA1 29 157, polyclonal, Invitrogen) and anti‐p53 (1:50, ab131442 Rabbit polyclonal, Abcam, UK), were placed on the slides and they were kept at +4°C overnight. After that, the slides were placed at room temperature for 10 minutes. From this point, all the processes were carried out at room temperature. Biotinylated Goat Anti‐Polyvalent solution in horseradish peroxidase (HRP) secondary kit was put on to the tissues and the slide were incubated for 10 minutes. After washing with TBS, Streptavidin Peroxidase solution in the same secondary kit was put on to the slides and the tissues were incubated it for 20 minutes. After washing with PBS, the slides were stained with AEC chromogen (Thermo Fisher Scientific) for seven minutes, and then, Mayers Hematoxylin (ThermoFisher Scientific) was used for counterstaining for one minute. At last, all the slides were mounted with water‐based mounting medium (Thermo Fisher Scientific).
2.5.2. Image analysis
The immunostained slides were evaluated using a light microscope (Nikon, E600). During evaluation, the immunopositive cells were counted one by one under 40x objective magnification in four different randomly selected areas on each slide. As known, rats’ eyes are small in structure, and so, their corneas are only approximately 5‐6 mm in length. Also, the cornea is a thin structure which has only 2‐4 epithelial cell layers and an almost acellular matrix under the epithelium. Because of this limitation, during immunopositive cell counting, we could not count enough cells for statistical analysis. We counted the immunopositive cells in 24 areas for each group but the number of immunopositive cells was still very small. The number of immunopositive cells in the retina was also very low, although higher than in the cornea. Therefore we used classical semiquantitative evaluation methods for tissue evaluation, instead of HSCORE scoring system which we had aimed to use in the preparation of study.
According to the semiquantitative evaluation method, the same parameters were used for both cornea and retina evaluation. We scored the slides according to the mean number of Bax, Bcl‐2, caspase‐3 and p53 expression in each tissue. For this the scoring method described below was used.
If no immunopositive cells were present it was scored as 0.
If the total number of immunopositive cells were between 1% and 10%, it was scored as 1.
If the total number of immunopositive cells were between 11% and 30%, it was scored as 2.
If the total number of immunopositive cells were between 31% and 50%, it was scored as 3.
If the total number of immunopositive cells were higher than 51%, it was scored as 4.
This scale was used for each protein. We also combined all these data to get better and safer results reflecting cell death in tissues. The scoring method was used for four parameters calculated as a total score.
3. RESULTS
Bax, Bcl‐2, caspase‐3 and p53 cell distriburtions and apoptosis scorings resulting from the immunohistochemical staining in the cornea and retina are given in the following tables (Tables 1 and 2):
Table 1.
Anti‐Bax, anti‐Bcl‐2, anti‐caspase‐3 and anti‐p53 positive cell numbers in cornea
| Bax | Bcl‐2 | Caspase‐3 | P53 | Total | |
|---|---|---|---|---|---|
| Control | 0 | ‐ | ‐ | ‐ | 0 |
| OHT+H2S | 3 | ‐ | ‐ | 1 | 4 |
| OHT | 2 | ‐ | 2 | 1 | 5 |
Table 2.
Anti‐Bax, anti‐Bcl‐2, anti‐caspase‐3 and anti‐p 53 positive cell numbers in retina
| Bax | Bcl‐2 | Caspase‐3 | P53 | Total | |
|---|---|---|---|---|---|
| Control | ‐ | ‐ | ‐ | ‐ | 0 |
| OHT+H2S | 2 | ‐ | 1 | ‐ | 3 |
| OHT | 3 | ‐ | 2 | 1 | 6 |
In the general tissue evaluation, in the OHT group, the cornea was very destroyed as a histological structure. On the other hand, interestingly Bax expression was denser especially in the retina in the same group. In these cases, H2S application increases Bax expression in the cornea. On the other hand, H2S application decreases cell destruction in the retina with glaucoma. Bax‐mediated apoptotic cell death can be distinguished during the cell death process by caspase‐3 activation. Furthermore, p53 expression can also be distinctly seen in both corneal and retinal cells of the glaucoma cases. This indicates that OHT produces a negative effect on the DNA structure of these cells. These results show that glaucoma leads to apoptosis through intrinsic pathway and that H2S is also effective in the retina through the same pathway (Figures 1, 2 and 3).
Figure 1.
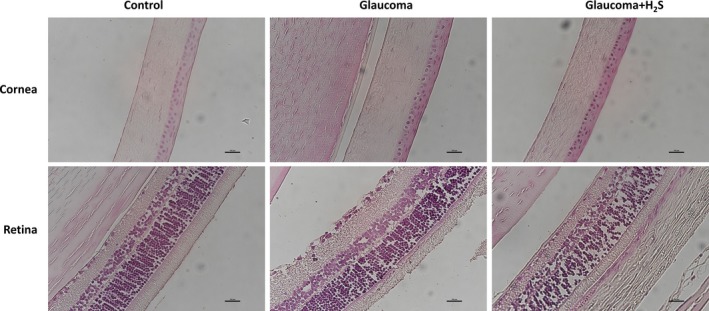
H&E stained retina cornea and retina cross‐section of control, glaucoma and glaucoma + H2S groups, ×40
Figure 2.
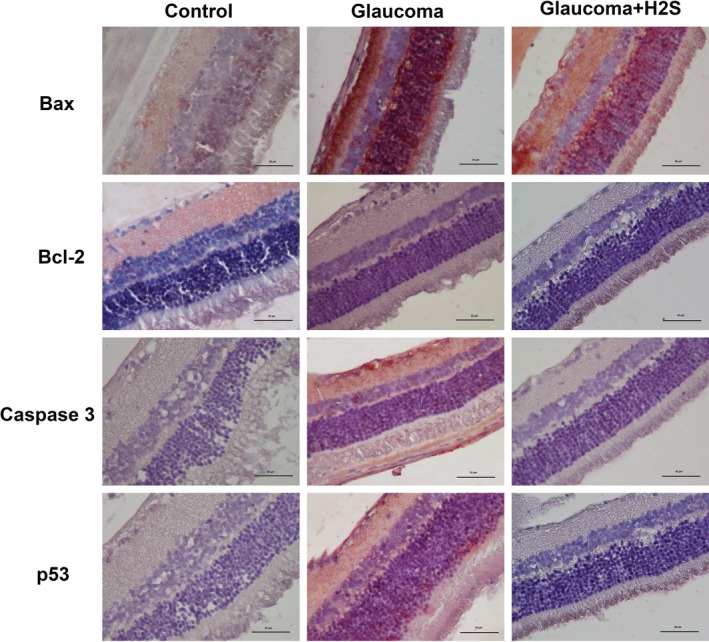
Immunohistochemically stained retina cross‐sections of control, glaucoma and glaucoma + H2S groups, ×40
Figure 3.
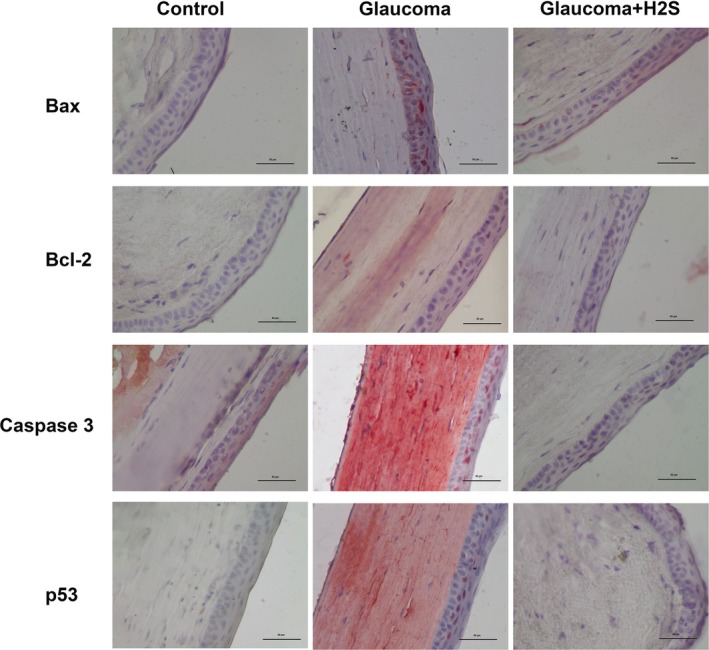
Immunohistochemically stained cornea cross‐sections of control, glaucoma and glaucoma + H2S groups, ×40
4. DISCUSSION
Glaucoma is a common neurodegenerative disease which results in blindness.6 It causes optic nerve degeneration in association with the death of retinal ganglion cells.28 Although it is important to decrease IOP in glaucoma treatment with topical and surgical interventions, the presence of degeneration in patients with low IOP highlights the significance of neuroprotection.28, 29, 30
Hydrogen sulphide (H2S) is a gasotransmitter.31 It is suggested that H2S, which is reported to be endogenously produced in ocular tissue, regulates intracellular calcium concentration in the nervous system and can be neuroprotective due to anti‐apoptotic effects against neurodegenerative disorders.12, 13, 14, 15, 32, 33
In our previous study, we revealed that intravitreous H2S application lowered IOP and decreased apoptosis in an experimental glaucoma model.17 In the current study which we carried out to determine through which apoptotic pathway H2S has it's effect, we found that it was associated with distinctive cell death activation both in the cornea and in the retina due to p53, Bax and caspase‐3 positivity through intrinsic pathway. Also H2S application caused distinctive apoptosis in the cornea on associated with Bax and p53 even though it prominently compensated apoptosis in the retina.
p53, a tumour suppressor protein, plays a significant role in the apoptosis in RGC.34, 35 According to some studies, the action of p53 upregulates the expression of the pro‐apoptotic gene Bax.34 Thus, it results in cytochrome c release by changing mitochondrial membrane permeability and in apoptosis by cytochrome c activating caspases.34 In the current study, there was an increase in p53 expression in the retinal and corneal cells of the OHT group. It H2S application decreased p53 expression, and there was also a decrease in the expression of Bax and caspase‐3.
The significant role of Bax which is a pro‐apoptotic protein for glaucoma formation was reported in previous studies.7, 9, 36 Libby et al9 carried out a study on mice with DBA/2J Bax ablation and revealed that Bax was a significant factor in RGC death, that Bax ablation prevented apoptosis in RGC, and that Bax ablation decelerated axonal death even though there is no Bax‐associated apoptosis.6, 9 In our study apoptosis increases both in retina and in cornea through Bax, a pro‐apoptotic protein, in glaucoma. This indicates that the treatment mechanisms using the Bax pathway could be important for the treatment of glaucoma. The research carried out by Libby et al9 demonstrated that Bax ablation prevented RGC death. Our study also suggests that H2S application, used as a neuroprotective agent, prevents Bax‐associated apoptosis especially in retina. However, it H2S application against glaucoma increases Bax‐associated apoptosis in the cornea.
Huang et al20 reported that H2S application against glaucoma decreases RGC apoptosis. The research pointed out that Bax and caspase‐3 expressions increased in RGC in glaucoma and that H2S application decreased the activity of the intrinsic apoptotic pathway. Similar to the foregoing, our study found that the expression of Bax and caspase‐3 increased in glaucoma while they were decreased in retinal cells with H2S application. However, in contradistinction to the above research, our study examined p53 and Bcl‐2 expressions both in retina and in cornea in addition to Bax and caspase‐3. We did not observe any activation on Bcl‐2. Nevertheless, there have been doubts that H2S may cause Bax‐mediated apoptosis in cornea.
Considering that retinal neurons have common characteristics with CNS neurons, Gao et al37 showed in their study on the neuroprotective role of H2S on Alzheimer's disease that 200 µmol/I sodium hydrosulphide (NaHS) blocked the upregulation of caspase‐3 and the downregulation of Bcl‐2 expression. They further revealed that H2S had anti‐oxidant and anti‐apoptotic effects that suppressed reactive oxidative stress. Even though we inferred that H2S did not cause any change in Bcl‐2, it was seen that it decreased the upregulation of caspase‐3 in support of the aforementioned study.
5. CONCLUSION
In the experimental glaucoma model, it was concluded that H2S application distinctly decreased apoptosis in the retina and that this was mediated through the intrinsic pathway but that it was not effective as regard to the apoptotic compensation in cornea.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
ACKNOWLEDGEMENT
None.
Erisgin Z, Ozer MA, Tosun M, Ozen S, Takir S. The effects of intravitreal H2S application on apoptosis in the retina and cornea in experimental glaucoma model. Int. J. Exp. Path. 2020;100:330–336. 10.1111/iep.12334
REFERENCES
- 1. Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;390(10108):2183‐2193. [DOI] [PubMed] [Google Scholar]
- 2. Kim KY, Perkins GA, Shim MS, et al. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6:e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nickells R, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153‐179. [DOI] [PubMed] [Google Scholar]
- 5. Tamm ER, Ethier CR, Lasker IIoA . Biological aspects of axonal damage in glaucoma: A brief review. Exp Eye Res. 2017;157:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howell GR, Soto I, Libby RT, et al. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2013;246:54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152‐181. [DOI] [PubMed] [Google Scholar]
- 8. Dai Y, Weinreb RN, Kim KY, et al. Inducible nitric oxide synthase‐mediated alteration of mitochondrial OPA1 expression in ocular hypertensive rats. Invest Ophthalmol Vis Sci. 2011;52(5):2468‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1(1):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowicka E, Beltowski J. Hydrogen sulfide (H2S) ‐ the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59(1):4‐24. [PubMed] [Google Scholar]
- 12. Bankhele P, Salvi A, Jamil J, et al. Comparative effects of hydrogen sulfide‐releasing compounds on [(3)H]D‐aspartate release from bovine isolated retinae. Neurochem Res. 2018;43(3):692‐701. [DOI] [PubMed] [Google Scholar]
- 13. Kulkarni M, Njie‐Mbye YF, Okpobiri I, et al. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem Res. 2011;36(8):1540‐1545. [DOI] [PubMed] [Google Scholar]
- 14. Pong WW, Stouracova R, Frank N, et al. Comparative localization of cystathionine beta‐synthase and cystathionine gamma‐lyase in retina: differences between amphibians and mammals. J Comp Neurol. 2007;505(2):158‐165. [DOI] [PubMed] [Google Scholar]
- 15. Persa C, Osmotherly K, Chao‐Wei Chen K, et al. The distribution of cystathionine beta‐synthase (CBS) in the eye: implication of the presence of a trans‐sulfuration pathway for oxidative stress defense. Exp Eye Res. 2006;83(4):817‐823. [DOI] [PubMed] [Google Scholar]
- 16. Huang S, Huang P, Lin Z, et al. Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp Eye Res. 2018;168:33‐48. [DOI] [PubMed] [Google Scholar]
- 17. Ozer MA, Erisgin Z, Ozen S, et al. Effects of intravitreous sodium hydrosulfide on intraocular pressure and retinopathy in ocular hypertensive rats. Biotech Histochem. 2018;93(1):8‐14. [DOI] [PubMed] [Google Scholar]
- 18. Ohia SE, Robinson J, Mitchell L, et al. Regulation of aqueous humor dynamics by hydrogen sulfide: potential role in glaucoma pharmacotherapy. J Ocul Pharmacol Ther. 2018;34(1–2):61‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Anders F, Thanos S, et al. Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci. 2017;58(12):5129‐5141. [DOI] [PubMed] [Google Scholar]
- 20. Huang S, Huang P, Liu X, et al. Relevant variations and neuroprotecive effect of hydrogen sulfide in a rat glaucoma model. Neuroscience. 2017;341:27‐41. [DOI] [PubMed] [Google Scholar]
- 21. Galvao J, Davis BM, Cordeiro MF. In vivo imaging of retinal ganglion cell apoptosis. Curr Opin Pharmacol. 2013;13(1):123‐127. [DOI] [PubMed] [Google Scholar]
- 22. Chipuk JE, Moldoveanu T, Llambi F, et al. The BCL‐2 family reunion. Mol Cell. 2010;37(3):299‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blankenberg FG, Norfray JF. Multimodality molecular imaging of apoptosis in oncology. AJR Am J Roentgenol. 2011;197(2):308‐317. [DOI] [PubMed] [Google Scholar]
- 24. Levkovitch‐Verbin H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog Brain Res. 2015;220:37‐57. [DOI] [PubMed] [Google Scholar]
- 25. Knillova J, Kolar Z. The significance of key regulators of apoptosis in the development and prognosis of prostate carcinoma. I. Proteins of the Bcl‐2 family and protein p53. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147(1):3‐10. [PubMed] [Google Scholar]
- 26. Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2(8):594‐604. [DOI] [PubMed] [Google Scholar]
- 27. Zhou X, Xia XB. Retinal stem cells transplantation combined with copolymer‐1 immunization reduces interferon‐gamma levels in an experimental model of glaucoma. Int J Ophthalmol. 2011;4(6):594‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuehn MH, Fingert JH, Kwon YH. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am. 2005;18(3):383‐395, vi. [DOI] [PubMed] [Google Scholar]
- 29. Mozaffarieh M, Flammer J. Is there more to glaucoma treatment than lowering IOP? Surv Ophthalmol. 2007;52(Suppl 2):S174‐179. [DOI] [PubMed] [Google Scholar]
- 30. Lambuk L, Iezhitsa I, Agarwal R, et al. Antiapoptotic effect of taurine against NMDA‐induced retinal excitotoxicity in rats. Neurotoxicology. 2019;70:62‐71. [DOI] [PubMed] [Google Scholar]
- 31. Lynch MJ, Crane BR. Design, validation, and application of an enzyme‐coupled hydrogen Sulfide detection assay. Biochemistry. 2019;58(6):474‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei HJ, Li X, Tang XQ. Therapeutic benefits of H(2)S in Alzheimer's disease. J Clin Neurosci. 2014;21(10):1665‐1669. [DOI] [PubMed] [Google Scholar]
- 33. Hu LF, Lu M, Hon Wong PT, et al. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15(2):405‐419. [DOI] [PubMed] [Google Scholar]
- 34. Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43(Suppl 1):S151‐161. [DOI] [PubMed] [Google Scholar]
- 35. Daugherty CL, Curtis H, Realini T, et al. Primary open angle glaucoma in a Caucasian population is associated with the p53 codon 72 polymorphism. Mol Vis. 2009;15:1939‐1944. [PMC free article] [PubMed] [Google Scholar]
- 36. Pietrucha‐Dutczak M, Amadio M, Govoni S, et al. The role of endogenous neuroprotective mechanisms in the prevention of retinal ganglion cells degeneration. Front Neurosci. 2018;12:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao C, Chang P, Yang L, et al. Neuroprotective effects of hydrogen sulfide on sodium azide‐induced oxidative stress in PC12 cells. Int J Mol Med. 2018;41(1):242‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]


