Summary
Extracellular Hsp70 (eHsp70) exerts its biological actions via Toll‐like receptors 2 and 4, and is increased in sera of chronic obstructive pulmonary disease (COPD) patients. The aim of this study was to explore the pro‐inflammatory effects and cytotoxicity of eHsp70 alone and in combination with bacterial components lipoteichoic acid (LTA) and lipopolysaccharide (LPS) on NCI‐H292 airway epithelial cells. NCI‐H292 cells were treated with recombinant human Hsp70 protein (rhHsp70), LPS, LTA and their combinations for 4, 12, 24 and 48 hours. IL‐6, IL‐8 and TNF‐α levels were measured by an ELISA method. Cell viability was determined by the MTS method, and caspase‐3/7, caspase‐8 and caspase‐9 assays. rhHsp70 induced secretion of IL‐6 and IL‐8 in a concentration‐ and time‐dependent manner, with the highest secretion at 24 hours. rhHsp70 combined with LTA had antagonistic and with LPS synergistic effect on IL‐6 secretion, while the interactions between rhHsp70 and LPS or LTA on IL‐8 were synergistic. TNF‐α was not detected in the applied conditions. rhHsp70, LPS or LTA did not affect cell viability, and rhHsp70 even suppressed caspase‐3/7 activities. We suggest that pro‐inflammatory effects of eHsp70, together with other damaging molecules and/or COPD risk factors, might contribute to the aggravation of chronic inflammation in human bronchial epithelium.
Keywords: COPD, extracellular Hsp70, LPS, LTA, NCI‐H292 cells
1. INTRODUCTION
In the industrialized and developed countries, chronic obstructive pulmonary disease (COPD) is considered as one of the leading causes of morbidity and mortality.1 Beside well‐known risk factor tobacco smoking, other exogenous (air pollution, occupational exposure, etc) and endogenous (age, sex, genetic factors, etc) factors determine the susceptibility for development of the disease.1, 2 Furthermore, much evidence indicates the important role of the chronic inflammatory and immune responses in the development and progression of COPD.3
Chronic obstructive pulmonary disease is characterized by irreversible chronic airflow limitation that is the consequence of defective tissue repair and chronic inflammation, leading to parenchymal destruction and small airway fibrosis.4, 5 In addition to the different types of cellular reaction, many inflammatory mediators such as reactive oxygen and nitrogen species, interleukin (IL)‐8, tumour necrosis factor (TNF)‐α, IL‐6 and interferon (IFN)‐γ are involved in the process of inflammation in COPD.5 Danger signals, such as pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs), activate families of pattern recognition receptors (PRRs) that include the Toll‐like receptors (TLRs).6 PAMPs, represented by pathogen‐derived proteins, nucleic acid and lipids such as lipopolysaccharide (LPS), peptidoglycan and lipoteichoic acid (LTA), can trigger an immune response to an infectious insult.7 On the other hand, DAMPs are secreted by damaged cells and can trigger an immune response to tissue injury that may occur in trauma, ischaemia‐reperfusion injury and oxidative stress.7
Heat‐shock proteins (Hsps) are molecular chaperones that can be up‐regulated when cells are exposed to different kinds of stressors. It has been demonstrated that 72 kDa, the major inducible Hsp70 protein, possesses antagonistic actions, depending on its localization.5, 7, 8, 9 While intracellular Hsp70 exerts a powerful protective and anti‐inflammatory effect, extracellular Hsp70 (eHsp70) is considered as one of the DAMPs and therefore might be able to activate innate and inflammatory responses.8, 10, 11 eHsp70 can stimulate a pro‐inflammatory effect through binding to several receptors, most notably TLR2 and TLR4, followed by activation of mitogen‐activated protein kinases (MAPKs) and/or nuclear factor κB (NF‐κB) signal transduction pathways, resulting in cytokine production.8, 12, 13, 14, 15
Hsp70 has been implicated in the pathogenesis of COPD. A case‐control study reported that higher level of plasma Hsp70 might be associated with an increased risk of COPD among coal workers.16 In addition, Hacker et al17 measured increased concentrations of Hsp70 in sera of COPD patients compared with the healthy individuals. It is possible that eHsp70 may activate signal transduction cascades, leading to the activation of an immune response, by inducing production and release of various cytokines, which could cause continuous propagation of inflammation observed in COPD.
The aim of this study was to explore the pro‐inflammatory effects of extracellular Hsp70 alone and in combination with TLR2 and TLR4 agonists LTA and LPS, respectively, on NCI‐H292 cell line, used as a well‐established model of human tracheo‐bronchial airways in COPD,18 by measuring IL‐6, IL‐8 and TNF‐α concentration. As in vivo studies on COPD patients measured eHsp70 in peripheral blood mostly in nanogram per millilitre concentrations, we used recombinant human Hsp70 protein (rhHsp70) in the range of concentrations from nanogram to microgram per millilitre for the treatment of NCI‐H292 cells. Inflammation and cell death are crucial cellular processes that control organism's homoeostasis and viability. Therefore, the cytotoxicity of eHsp70, LTA and LPS was also assessed by measuring cellular metabolic activity and caspase‐3/7, caspase‐8 and caspase‐9 activities.
2. MATERIALS AND METHODS
2.1. Cell culture
The NCI‐H292 human bronchial epithelial cell line (American Type Culture Collection) was cultured in RPMI 1640 medium containing 10% foetal bovine serum, 2 mmol/L l‐glutamine, 1 mmol/L pyruvate and 1% penicillin and streptomycin, at 37°C in a humidified 5% CO2 atmosphere. Cells were grown in 75‐cm2 culture flasks and subcultured using 0.025% trypsin‐EDTA every 3 or 4 days.
2.2. Ethical approval
Appropriate ethical approval was obtained for this study and the study followed all ethical guidelines for cell lines culturing and treatments.
2.3. Treatments of NCI‐H292 cells
Cells were treated with low endotoxin rhHsp70 (Enzo Life Sciences). Endotoxin concentration in rhHsp70 was 2.7 EU/mg, determined by Limulus amebocyte lysate (LAL) test by manufacturer. Equivalent amount of LPS was used as negative control in all experiments. Cells were also treated with LPS (positive control for TLR4 activation) and LTA (positive control for TLR2 activation). LPS was isolated from Escherichia coli O111:B4 (Sigma‐Aldrich). LTA was isolated from Staphylococcus aureus (Invivogen) and had endotoxin concentration of 10 EU/mg. Concentrations of rhHsp70 (0.1‐30 μg/mL), LPS (0.1 μg/mL) and LTA (1 μg/mL) were used in this study based on our preliminary results. Three independent experiments (n = 3) were performed for all analysis (cytokines and viability measurements).
2.4. Measurement of cytokine concentration
NCI‐H292 cells were seeded on 12‐well cell culture plates (1 × 106 cells per well) for 24 hours in 1 mL complete cell medium. Cells were treated with different concentrations of rhHsp70, 0.1 μg/mL LPS, 1 μg/mL LTA, and their combinations for 4, 12, 24 and 48 hours. Cell medium was harvested and frozen at −80°C until analysis.
Concentrations of IL‐6, IL‐8 and TNF‐α were determined in cell medium by DuoSet ELISA kits (Human IL‐6 DuoSet ELISA, Human CXCL8/IL‐8 DuoSet ELISA, Human TNF‐α DuoSet ELISA; R&D Systems).
Ninety‐six‐well plate was coated with 100 μL per well of cytokine‐specific capture antibody diluted in PBS and incubated overnight at room temperature. Each well was washed three times with 400 μL of washing buffer. Then the plate was blocked with 300 μL of reagent diluent and incubated at room temperature for a minimum of 1 hour, followed by washing. 100 μL of diluted samples or standards was added and incubated at room temperature for 2 hours, followed by washing. After that, 100 μL of the specific detection antibody was added, and plate was incubated for 2 hours at room temperature. After washing, 100 μL of streptavidin‐HRP was added, and plate was incubated for 20 minutes at room temperature in the dark, followed by washing. 100 μL of substrate solution (equal volume of hydrogen peroxide and tetramethyl benzidine) was added, and plate was once again incubated for 20 minutes at room temperature in the dark. The reaction was stopped by adding 50 μL of stop solution (2 N sulphuric acid). The optical density was determined at 450 nm, using a microplate reader (VICTOR3 1420 Multilabel counter; Perkin Elmer).
2.5. MTS assay
NCI‐H292 cells were seeded on 96‐well cell culture plates (5 × 103 cells per well) for 24 hours in 100 μL of complete cell medium. Cells were treated with rhHsp70, LPS, LTA and their combinations for 24 hours. Afterwards, 20 μL of MTS (3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium; Promega) reagent was added, and cells were incubated for 4 hours in a humidified 5% CO2 atmosphere. Absorbance was measured at 490 nm using a microplate reader (VICTOR3 1420 Multilabel counter; Perkin Elmer).
2.6. Caspases' activity (caspase‐3/7, caspase‐8, caspase‐9)
NCI‐H292 cells were seeded on white 96‐well cell culture plates (1 × 104 cells per well for caspases‐3/7, and 1.5 × 104 cells per well for caspase‐8 and caspase‐9) for 24 hours in 100 μL of cell medium. Cells were treated with rhHsp70 (0.3‐3 μg/mL), 0.1 μg/mL LPS and 1 μg/mL LTA for 2, 4, 6 and 8 hours. After incubation, activities of caspase‐3/7, caspase‐8 and caspase‐9 were detected according to the manufactures' instructions by using the luminescent assay kits Caspase‐Glo® 3/7 Assay, Caspase‐Glo® 8 Assay and Caspase‐Glo® 9 Assay (Promega). Briefly, 100 µl of reagent for each specific caspase was added to each well and the plate was gently mixed at 300‐500 rpm for 30 seconds. Then the plate was incubated at room temperature for 3 hours. Luminescence of samples was measured using a microplate reader (VICTOR3 1420 Multilabel counter; Perkin Elmer).
2.7. Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Unpaired t test or one‐way analysis of variance (ANOVA) followed by post hoc testing by Sidak method was used for statistical analysis. The level of P < .05 was considered statistically significant. Data were analysed using graphpad prism 6.01 software (GraphPad Software Inc).
When calculating combined effects of rhHsp70 with LPS and LTA, we compared our measured values with calculated (expected) values. The values were calculated as the mean value obtained after exposure to one substance alone (rhHsp70) plus the mean value obtained after exposure to the second substance (LPS or LTA)19:
The results were interpreted as follows: a synergistic effect was implied if the measured values were significantly above the expected values; an antagonistic effect was implied if the measured values were significantly below the expected values.
3. RESULTS
3.1. Influence of eHsp70, LPS and LTA on cell viability
We wanted to investigate the cytotoxicity of eHsp70. First we measured the metabolic activity of NCI‐H292 cells by MTS assay. Cells were treated with rhHsp70 (0.1, 0.3, 1, 3, 10 and 30 μg/mL), 0.1 μg/mL LPS and 1 μg/mL LTA for 24 hours. Cells were also treated for 24 hours with combinations of rhHsp70 concentrations (0.3, 1 or 3 μg/mL) and LPS or LTA. Results are presented as percentages of absorbance of non‐treated cells (expressed as 100%). Compared to the control cells, different rhHsp70 concentrations did not influence cell viability (P = .2245), nor did the treatment with LPS or LTA (P = .2574). In addition, when we compared combined rhHsp70 and LPS or LTA treatments with individual LPS and LTA treatments, we did not observe any significant differences in metabolic activity (P = .1877 and P = .9304 respectively; Figure 1).
Figure 1.
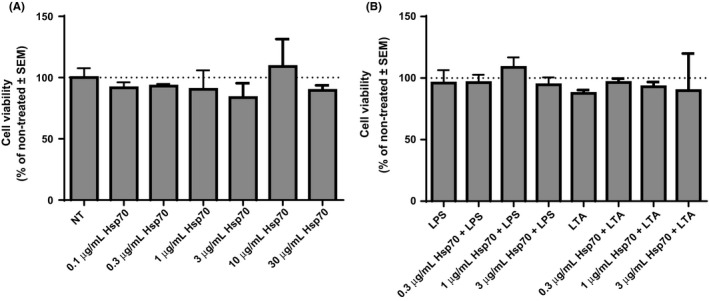
Cytotoxicity of rhHsp70 (A), LPS and LTA alone, and their combinations with rhHsp70 (B), assessed by MTS assay. Data are presented as mean ± SEM from three independent experiments (n = 3). Dotted line represents viability of non‐treated cells set as 100%. LPS, lipopolysaccharide; LTA, lipoteichoic acid
3.2. Caspase‐3/7, caspase‐8 and caspase‐9 activities after treatment with rhHsp70, LPS and LTA
In addition to MTS assay, eHsp70 cytotoxicity was also explored by luminometric determination of apoptotic caspase activities. Activities of caspase‐3/7, caspase‐8 and caspase‐9 were measured in NCI‐H292 cells after treatment with different concentration of rhHsp70 (0.3, 1 and 3 μg/mL), 0.1 μg/mL LPS and 1 μg/mL LTA for 2, 4, 6 and 8 hours. rhHsp70 did not activate pro‐apoptotic caspases. On the contrary, rhHsp70 even suppressed caspase‐3/7 activities at 2 and 8 hours, compared to non‐treated cells (Table 1). Cells treated with LPS or LTA had similar caspase‐3/7 activities to non‐treated cells (data not shown). In addition, caspase‐8 and caspase‐9 activities were similar in non‐treated cells, cells treated with rhHsp70 as well as in cells treated with LPS or LTA at all time points examined.
Table 1.
Caspase‐3/7 activities in NCI‐H292 cells treated with rhHsp70
| 0.3 µg/mL Hsp70 | 1 µg/mL Hsp70 | 3 µg/mL Hsp70 | |
|---|---|---|---|
| CASPASES‐3/7 | |||
| 2 h | 75.77 ± 0.70* | 77.79 ± 2.25* | 79.91 ± 1.35* |
| 4 h | 86.76 ± 7.56 | 86.02 ± 3.16 | 90.28 ± 5.68 |
| 6 h | 106.00 ± 11.74 | 106.00 ± 3.89 | 120.50 ± 2.82 |
| 8 h | 93.45 ± 3.72 | 34.01 ± 10.89* | 31.28 ± 11.06* |
Results are expressed as percentages of relative light units (RLU) of non‐treated cells (expressed as 100%) and are presented as mean ± SEM.
Statistically significant (P < .05) vs non‐treated cells.
3.3. Concentration‐ and time‐dependent effects of eHsp70 on cytokines' secretion
Concentration‐dependent effects of eHsp70 on secretion of IL‐6, IL‐8 and TNF‐α were determined after 24 hours treatment of NCI‐H292 cells with different concentration of rhHsp70 (0.1, 0.3, 1, 3, 10 and 30 μg/mL). Cytokine concentrations in cell culture media of non‐treated (control) cells are expressed as 100%, and those of treated cells as percentages of concentration of non‐treated cells. Concentration of IL‐6 was significantly higher compared to the control cells after treatment with 0.1 μg/mL (P = .0079), 0.3 μg/mL (P < .0001), 1 μg/mL (P < .0001), 3 μg/mL (P < .0001) and 30 μg/mL rhHsp70 (P = .0077; Figure 2A). In addition, concentration of IL‐8 significantly increased after treatment with 0.3 μg/mL (P = .0011), 1 μg/mL (P < .0001) and 3 μg/mL rhHsp70 (P < .0001; Figure 2B). However, TNF‐α was not detected either in control or in rhHsp70‐treated cells.
Figure 2.
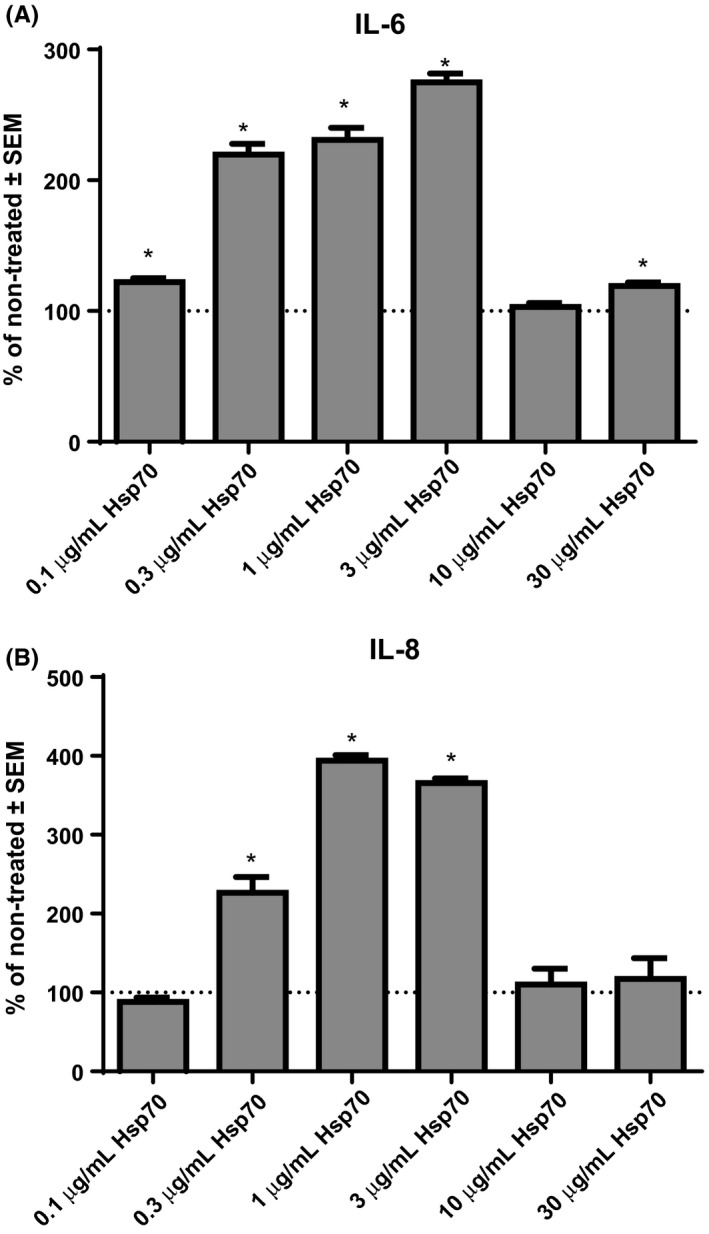
Concentration‐dependent effects of rhHsp70 on production of IL‐6 (A) and IL‐8 (B). Data are presented as mean ± SEM from three independent experiments (n = 3). Dotted line represents concentrations of cytokines in non‐treated cells set as 100%. *Statistically significant (P < .05) vs non‐treated cells
Next, we wanted to explore time‐dependent effects of eHsp70 on cytokine secretion. Based on the results obtained in this study, we have selected 1 μg/mL rhHsp70 concentration, and we treated NCI‐H292 cells for 4, 12, 24 and 48 hours. Compared to the non‐treated cells, cells treated with rhHsp70 produced significantly higher level of IL‐6 after 12 hours (P = .0004) and 24 hours (P = .0024), but after 48‐hour secretion of IL‐6 was reduced (P = .0432; Figure 3A). The level of IL‐8 was not detectable after 4‐hour treatment with 1 μg/mL rhHsp70. On the other hand, the level of IL‐8 was significantly increased after 12‐hour (P < .0001) and 24‐hour (P < .0001) treatment with rhHsp70, but concentration of IL‐8 decreased after 48 hours (P = .0003), compared to control cells (Figure 3B).
Figure 3.
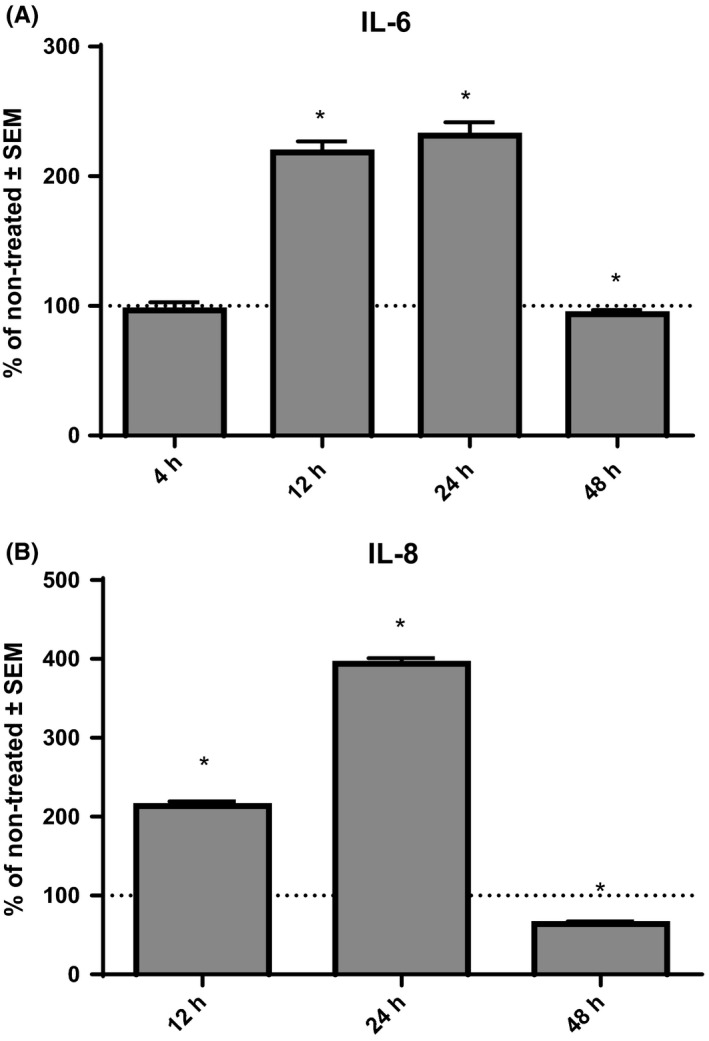
Time‐dependent effects of 1 μg/mL rhHsp70 on production of IL‐6 (A) and IL‐8 (B). Data are presented as mean ± SEM from three independent experiments (n = 3). Dotted line represents concentrations of cytokines in non‐treated cells set as 100%. *Statistically significant (P < .05) vs non‐treated cells
3.4. Inflammatory effects of eHsp70 in combination with LPS or LTA
LTA and LPS are constituents of Gram‐positive and Gram‐negative bacteria that might trigger TLR2 and TLR4 activation, respectively, and consequently cytokine production. Thus, we wanted to determine cytokine secretion by LPS and LTA alone as well as in combination with rhHsp70. Based on our previous preliminary results (data not shown), we have selected 0.1 μg/mL LPS and 1 μg/mL LTA concentrations, and we have treated NCI‐H292 cells for 4, 12, 24 and 48.
In individual LPS and LTA treatments, secretion of IL‐6 increased after 4‐hour treatment with both LPS (P < .0001) and LTA (P = .0104) as well as after 12‐hour treatment (P < .0001 and P = .0001, respectively), in comparison with control cells. However, only cells treated with LPS, but not with LTA, showed significantly higher concentration of IL‐6 compared to non‐treated cells after 24 hours (P = .0036) and 48 hours (P = .0306; Figure 4). As for IL‐8, NCI‐H292 cells treated with LPS or LTA secreted higher concentrations of IL‐8 after 12 hours (P < .0001 for LPS, and P < .0001 for LTA), and 48 hours (P < .0001 for LPS, and P < .0001 for LTA), when compared to controls (Figure 5). Treatment of cells with LPS also induced production of IL‐8 after 24 hours of treatment (P = .0085). After 4‐hour treatment, we could not measure detectable level of IL‐8 in cell supernatants. Once again, TNF‐α was not detected in LPS‐ or LTA‐treated cells.
Figure 4.
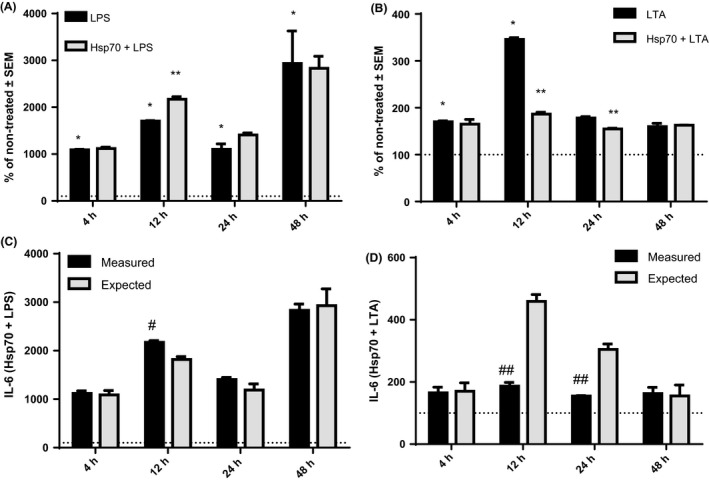
Production of IL‐6 after 4‐, 12‐, 24‐ and 48‐h treatment with LPS (A) and LTA (B) alone and in combination with rhHsp70. Data are presented as mean ± SEM from three independent experiments (n = 3). Dotted line represents concentrations of cytokines in non‐treated cells set as 100%. *Statistically significant (P < .05) LPS or LTA alone vs non‐treated cells. **Statistically significant (P < .05) combination of rhHsp70 with LPS or LTA vs LPS or LTA alone. rhHsp70 and LPS (C) or LTA (D) interactions on IL‐6 secretion. Dark bars represent the measured values and grey bars the expected (calculated) values. Data are presented as mean ± SEM from three independent experiments (n = 3). #Statistically significant (P < .05) synergistic effect. ##Statistically significant (P < .05) antagonistic effect. LPS, lipopolysaccharide; LTA, lipoteichoic acid
Figure 5.
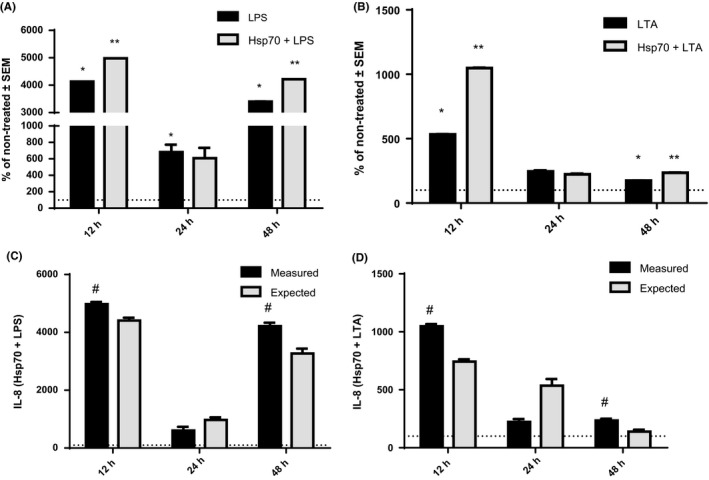
Production of IL‐8 after 12‐, 24‐ and 48‐h treatment with LPS (A) and LTA (B) alone and in combination with rhHsp70. Data are presented as mean ± SEM from three independent experiments (n = 3). Dotted line represents concentrations of cytokines in non‐treated cells set as 100%. *Statistically significant (P < .05) LPS or LTA alone vs non‐treated cells. **Statistically significant (P < .05) combination of rhHsp70 with LPS or LTA vs LPS or LTA alone. rhHsp70 and LPS (C) or LTA (D) interactions on IL‐8 secretion. Dark bars represent the measured values and grey bars the expected (calculated) values. Data are expressed as mean ± SEM from three independent experiments (n = 3). #Statistically significant (P < .05) synergistic effect. LPS, lipopolysaccharide; LTA, lipoteichoic acid
In our combined treatments, we compared the values obtained from cells treated with combination of rhHsp70 and LPS or LTA to those obtained from cells treated with LPS or LTA alone. IL‐6 secretion was higher in the cells treated together with rhHsp70 and LPS for 12 hours (P = .0145) compared to the cells treated with LPS alone (Figure 4A). In contrast, cells treated with combination of rhHsp70 and LTA for 12‐hour (P = .012) and 24‐hour (P = .0227) treatment produced significantly lower concentrations of IL‐6 compared to the cells treated with LTA alone (Figure 4B). IL‐8 secretion was higher in the cells treated with rhHsp70 and LPS for 12 hours (P < .0001) and 48 hours (P < .0001) compared to the cells treated with LPS alone (Figure 5A). Similar results were observed for combined treatment with rhHsp70 and LTA after 12 and 48 hours (P < .0001 for 12 hours, and P = .0002 for 48 hours; Figure 5B).
In order to explore the type of interactions between eHsp70 and LPS or LTA, we calculated the effects on cytokine secretion in those combined treatments. For IL‐6, combination of 1 μg/mL rhHsp70 and 0.1 μg/mL LPS showed significant synergistic effect after 12 hours and 24 hours (P = .0249 and P = .0001 respectively; Figure 4C), while combination of 1 μg/mL rhHsp70 and 1 μg/mL LTA showed significant antagonistic effect after 12 hours (P = .0004; Figure 4D). On the other hand, both combinations of 1 μg/mL rhHsp70 with 0.1 μg/mL LPS or 1 μg/mL LTA showed significant synergistic effect on IL‐8 secretion after 12 hours (P = .0002 and P < .0001, respectively) and 48 hours (P < .0001 and P = .0001 respectively; Figure 5C,D).
4. DISCUSSION
In this study, we explored the pro‐inflammatory and cytotoxic effects of extracellular Hsp70 on a COPD cellular model, human bronchial epithelial cell line NCI‐H292, that expresses both TLR2 and TLR4 receptors,20, 21 by using recombinant human Hsp70 protein. Furthermore, we also investigated those effects triggered by two well‐known PAMP molecules, LPS and LTA, alone and in combination with rhHsp70.
We have demonstrated that rhHsp70, LPS or LTA alone and in combinations did not influence metabolic activity of NCI‐H292 cells. In agreement with our results, it was reported that eHsp70, LPS and LTA do not exert toxic effects on various cell types.22, 23, 24, 25 For instance, Hsp70 treatment did not alter cell viability of A549 human lung cancer cells,14 HL‐60 promyelocytic leukaemia cells22 and neutrophils.25 In addition, treatment of human bronchial epithelial cell line BEAS‐2B with LPS did not affect cell viability24 as well as treatment of human lung carcinoma type II epithelial cells A549 with LTA.23
Apoptosis or programmed cell death is a vital process for normal cell turnover or proper development and function of the immune system, and inappropriate apoptosis is associated with different disorders and diseases.26 Caspases are the central players of the apoptotic machinery. Activation of intrinsic or mitochondrial apoptotic pathway results in activation of initiator caspase‐9. On the other hand, activation of extrinsic or death receptor apoptotic pathway results in activation of initiator caspase‐2, caspase‐8 and/or caspase‐10. Both pathways converge on downstream executioner caspase‐3, caspase‐6 and/or caspase‐7; when activated, those caspases cleave numerous substrates, leading to apoptotic cell death.27 In our study, activities of caspase‐8 and caspase‐9 were similar in non‐treated cells and cells treated with different concentrations of rhHsp70, LTA or LPS. Interestingly, we observed significantly lower activity of caspases‐3/7 in NCI‐H292 cells treated with 0.3, 1 or 3 µg/mL rhHsp70. This is in agreement with Franco et al28 who reported that extracellular exposure to Hsp70 reduced apoptotic cell death induced by hydrogen peroxide, and that eHsp70 suppressed caspase‐3 activation. Anti‐apoptotic function of Hsp70 was also confirmed in Schwann cells, and observed results indicated that Hsp70 function as a negative regulator of cytochrome c‐dependent activation of caspase‐3 and caspase‐9.29
In our study, treatment of NCI‐H292 cell with rhHsp70 showed concentration‐dependent pro‐inflammatory effects on IL‐6 and IL‐8 secretion. As for time‐dependence, significantly higher concentrations of IL‐6 and IL‐8 after 12‐ and 24‐hour treatment with rhHsp70 were produced. Interestingly, we measured significantly lower concentrations of both cytokines after 48‐hour treatment. Pro‐inflammatory effects of extracellular Hsp70 seem to be impaired after prolonged period, and we could only speculate that this might be due to the insensitivity of receptors to eHsp70 that might develop in time or due to inhibition of eHsp70 with some molecules secreted from the cells or already present in extracellular milieu. Indeed, some authors suggested that eHsp70 may also down‐regulate the innate immune response. In the absence of infection, repetitive binding of eHsp70 to TLR2/4 receptors may lead to unresponsiveness of the receptors to more dangerous pro‐inflammatory TLR activators.30
The potential immune regulatory role of extracellular Hsp70 has been challenged due to the possibility that the observed pro‐inflammatory effects might actually be reflecting bacterial LPS contamination of rhHsp70 preparations.31 Therefore, we used highly purified rhHsp70 with low levels of LPS for the treatments of NCI‐H292 cells (2.7 EU/mg, ie, 0.27 ng/mL LPS per mg of purified Hsp70 was detected by LAL test). We performed preliminary experiments using polymyxin B, boiling of rhHsp70, specific antibodies against Hsp70, TLR2 and TLR4, and we excluded the possibility that the effects on cytokine secretion and cell viability might be from endotoxin or some other contaminants rather than from rhHsp70 itself. In addition, we included in all our experiments 0.27 pg/mL LPS treatment, which represent the amount of endotoxin in 1 µg/mL rhHsp70. However, the results obtained with 0.27 pg/mL LPS did not significantly differ from non‐treated cells in any of the analysis performed (data not shown), meaning that eHsp70 alone is responsible for the observed pro‐inflammatory and anti‐apoptotic effects, and that this could not be the consequence of LPS contamination. Thus, our results are in accordance with previously published papers that showed the pro‐inflammatory effects of extracellular Hsp70 on different types of cells, including human bronchial epithelial cells.7, 22, 32, 33, 34
The effect of extracellular Hsp70 is mediated through cell surface receptors such as c‐type lectin receptors, scavenger receptors and pattern recognition receptors for advanced glycation end products (RAGE) as well as TLR2 and TLR4. It was demonstrated that eHsp70 can stimulate the production of pro‐inflammatory cytokines through activation of NF‐κB and MAPKs pathways when acting as TLR2/4 receptors agonist.13, 14, 22, 30, 35, 36 Furthermore, signalling cascades triggered by various TLRs are known to interfere with each other, and cross‐talk between different signalling pathways activated by DAMPs or PAMPs may represent a regulatory mechanism that can control a potentially harmful immune response.30 In order to simulate an in vivo situation where different damage‐ and/or pathogen‐associated molecular patterns might be present at the same time, such as for instance during COPD exacerbations, we combined rhHsp70 treatments with LPS or LTA representing Gram‐negative and Gram‐positive bacterial infection respectively. When used alone, LPS treatment induced production of IL‐6 and IL‐8 at all measured time points. On the other hand, LTA triggered secretion of IL‐6 after 4 and 12 hours, and IL‐8 after 12‐ and 48‐hour treatment. In addition, in our experimental setting LPS has emerged as a stronger agonist of TLR that induced higher levels of IL‐6 and IL‐8 than LTA. Our results are in accordance with several published papers. Hutchison et al37 have shown that LPS from different Gram‐negative bacteria releases pro‐inflammatory cytokines, such as IL‐6 and IL‐8. Other groups of authors also reported significantly increased production of IL‐8 and IL‐6 by NCI‐H292 cells treated with LPS.38 LTA was also showed to induce, among other cytokines, expression of IL‐6 and IL‐8 in bronchial epithelial cells BEAS‐2B.39
In our study, combination of LPS and rhHsp70 induced significantly increased production of IL‐6 after 12 hours, and LPS or LTA applied together with rhHsp70 provoked significantly higher secretion of IL‐8 after 12 and 48 hours, compared to the cells treated with LPS or LTA alone. Those interactions between eHsp70 and LPS or LTA were shown to have a significant synergistic effect. In contrast, cells treated with combination of rhHsp70 and LTA for 12 and 24 hours produced significantly lower concentrations of IL‐6 compared to the cells treated with LTA alone, and calculated interaction was shown to be antagonistic. We also conducted similar study on another type of cells, THP‐1 monocytic cell line, and in comparison between those two studies, it is interesting to note that slightly lower concentrations of rhHsp70 were needed to induce cytokine secretion from NCI‐H292 cells. Cytokine secretion in THP‐1 cells seems to reach maximum at earlier time points than in NCI‐H292 cells. In the THP‐1 cell line, antagonistic type of interactions between rhHsp70 and LTA is more pronounced on all cytokine secretion.19
Our results confirmed that the interactions of eHsp70 with TLR2/4 receptors could be influenced by other extracellular molecules, in our case PAMPs LTA and LPS, and this might alter the sensitivity of the affected cells and provoke reduced or enhanced biological response through eHsp70 binding on its other receptors, but further investigation is needed to clarify the underlying mechanisms of pro‐inflammatory effects caused by extracellular Hsp70 alone as well as by interaction of eHsp70 with other DAMPs and/or PAMPs.
In conclusion, we demonstrated that extracellular Hsp70 induced concentration‐ and time‐dependent production of IL‐6 and IL‐8 in human bronchial epithelial cell line NCI‐H292. Moreover, combined treatment with LPS or LTA has led to a significantly increased secretion of IL‐8. However, while interaction of eHsp70 with LPS was synergistic for IL‐6, the one with LTA was antagonistic. It seems that elevated cytokines' levels were not the outcome of cells dying by apoptosis or its destruction by necrosis either primary or secondary, (ie, the ultimate fate of cells dying by apoptosis when cultured in vitro), as we have established that eHsp70 did not influence cell viability and did not increase caspase activities, nor did so LPS or LTA. It has been reported that concentration of eHsp70 is elevated in sera of COPD patients.16, 17 We suggest that pro‐inflammatory effects of extracellular Hsp70, together with other risk factors and/or damaging molecules, might contribute to the aggravation of chronic inflammation in human bronchial epithelium and might produce unwanted consequences in COPD patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work has been fully supported by the Croatian Science Foundation under the project number IP‐2014‐09‐1247.
Hulina‐Tomašković A, Grdić Rajković M, Jelić D, et al. Pro‐inflammatory effects of extracellular Hsp70 on NCI‐H292 human bronchial epithelial cell line. Int J Exp Path. 2020;100:320–329. 10.1111/iep.12335
Andrea Hulina‐Tomašković and Marija Grdić Rajković have contributed equally to this work.
REFERENCES
- 1. Artyukhov IP, Arshukova IL, Dobretsova EA, Dugina TA, Shulmin AV, Demko IV. Epidemiology of chronic obstructive pulmonary disease: a population‐based study in Krasnoyarsk region, Russia. Int J Chron Obstruct Pulmon Dis. 2015;10:1781‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faner R, Gonzalez N, Cruz T, Kalko SG, Agustí A. Systemic inflammatory response to smoking in chronic obstructive pulmonary disease: evidence of a gender effect. PLoS One. 2014;9(5):e97491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013;2013:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osei ET, Noordhoek JA, Hackett TL, et al. Interleukin‐1α drives the dysfunctional cross‐talk of the airway epithelium and lung fibroblasts in COPD. Eur Respir J. 2016;48(2):359‐369. [DOI] [PubMed] [Google Scholar]
- 5. Dong J, Guo L, Liao Z, et al. Increased expression of heat shock protein 70 in chronic obstructive pulmonary disease. Int Immunopharmacol. 2013;17(3):885‐893. [DOI] [PubMed] [Google Scholar]
- 6. Hansel TT, Barnes PJ. New drugs for exacerbations of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):744‐755. [DOI] [PubMed] [Google Scholar]
- 7. Giuliano JS, Lahni PM, Wong HR, Wheeler DS. Pediatric sepsis ‐ part V: extracellular heat shock proteins: alarmins for the host immune system. Open Inflamm J. 2011;4:49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krause M, Heck TG, Bittencourt A, et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation‐driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015;2015:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qu B, Jia Y, Liu Y, Wang H, Ren G, Wang H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: a literature review. Cell Stress Chaperones. 2015;20(6):885‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim WK, Kanelakis KC, Neubig RR. Regulation of G protein signaling by the 70 kDa heat shock protein. Cell Signal. 2013;25(2):389‐396. [DOI] [PubMed] [Google Scholar]
- 11. Li C‐J, Ning W, Matthay MA, Feghali‐Bostwick CA, Choi AMK. MAPK pathway mediates EGR‐1‐HSP70‐dependent cigarette smoke‐induced chemokine production. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1297‐L1303. [DOI] [PubMed] [Google Scholar]
- 12. Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll‐like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028‐15034. [DOI] [PubMed] [Google Scholar]
- 13. Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll‐like receptors. Curr Top Microbiol Immunol. 2002;270:169‐184. [DOI] [PubMed] [Google Scholar]
- 14. Somensi N, Brum PO, de Miranda RV, et al. Extracellular HSP70 activates ERK1/2, NF‐kB and pro‐inflammatory gene transcription through binding with RAGE in A549 human lung cancer cells. Cell Physiol Biochem. 2017;42(6):2507‐2522. [DOI] [PubMed] [Google Scholar]
- 15. Joly A‐L, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2(3):238‐247. [DOI] [PubMed] [Google Scholar]
- 16. Cui X, Xing J, Liu Y, et al. COPD and levels of Hsp70 (HSPA1A) and Hsp27 (HSPB1) in plasma and lymphocytes among coal workers: a case‐control study. Cell Stress Chaperones. 2015;20(3):473‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hacker S, Lambers C, Hoetzenecker K, et al. Elevated HSP27, HSP70 and HSP90α in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55(1‐2):31‐40. [PubMed] [Google Scholar]
- 18. Adamson J, Haswell LE. In vitro models of chronic obstructive pulmonary disease (COPD) In: MartÃn‐Loeches I, eds. Bronchitis. In Tech; 2011. Available from: http://www.intechopen.com/books/bronchitis/in-vitro-models-ofchronicobstructivepulmonary-disease-copd [Google Scholar]
- 19. Hulina A, Grdić Rajković M, Jakšić Despot D, et al. Extracellular Hsp70 induces inflammation and modulates LPS/LTA‐stimulated inflammatory response in THP‐1 cells. Cell Stress Chaperones. 2018;23(3):373‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hulina‐Tomašković A, Rajković MG, Somborac‐Bačura A, Čeri A, Dabelić S, Rumora L. Extracellular Hsp70 modulates the inflammatory response of cigarette smoke extract in NCI‐H292 cells. Exp Physiol. 2018;103(12):1704‐1716. [DOI] [PubMed] [Google Scholar]
- 21. Gon Y, Asai Y, Hashimoto S, et al. A20 inhibits toll‐like receptor 2‐ and 4‐mediated interleukin‐8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31(3):330‐336. [DOI] [PubMed] [Google Scholar]
- 22. Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll‐like receptor (TLR)‐4. Respir Res. 2009;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu F‐L, Chuang C‐Y, Tai Y‐T, et al. Lipoteichoic acid induces surfactant protein‐A biosynthesis in human alveolar type II epithelial cells through activating the MEK1/2‐ERK1/2‐NF‐κB pathway. Respir Res. 2012;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verspohl EJ. LPS‐induced proliferation and chemokine secretion from BEAS‐2B cells. Pharmacol Pharm. 2012;3(April):166‐177. [Google Scholar]
- 25. Vinokurov M, Ostrov V, Yurinskaya M, et al. Recombinant human Hsp70 protects against lipoteichoic acid‐induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones. 2012;17(1):89‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duprez L, Wirawan E, Berghe TV, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11(13):1050‐1062. [DOI] [PubMed] [Google Scholar]
- 28. Franco L, Terrinca J, Rodríguez AB, Espino J, Pariente JA. Extracellular heat shock proteins protect U937 cells from H2O2‐induced apoptotic cell death. Mol Cell Biochem. 2016;412(1‐2):19‐26. [DOI] [PubMed] [Google Scholar]
- 29. Luo X, Tao L, Lin P, Mo X, Chen H. Extracellular heat shock protein 72 protects schwann cells from hydrogen peroxide‐induced apoptosis. J Neurosci Res. 2012;90(6):1261‐1269. [DOI] [PubMed] [Google Scholar]
- 30. Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175(5):2777‐2782. [DOI] [PubMed] [Google Scholar]
- 31. De Maio A, Vazquez D. Extracellular heat shock proteins: a new location, a new function. Shock. 2013;40(4):239‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4‐ and NF‐kappaB‐dependent mechanism. J Immunol. 2007;179(9):6318‐6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J. 2011;75(10):2445‐2452. [DOI] [PubMed] [Google Scholar]
- 34. Hulina‐Tomašković A, Heijink IH, Jonker MR, Somborac‐Bačura A, Grdić Rajković M, Rumora L. Pro‐inflammatory effects of extracellular Hsp70 and cigarette smoke in primary airway epithelial cells from COPD patients. Biochimie. 2019;156:47‐58. [DOI] [PubMed] [Google Scholar]
- 35. Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70. J Biol Chem. 2002;277:15028‐15034. [DOI] [PubMed] [Google Scholar]
- 36. Murshid A, Theriault J, Gong J, Calderwood SK. Investigating receptors for extracellular heat shock proteins. Methods Mol Biol. 2011;787:289‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hutchison ML, Bonell EC, Poxton IR, Govan J. Endotoxic activity of lipopolysaccharides isolated from emergent potential cystic fibrosis pathogens. FEMS Immunol Med Microbiol. 2000;27(1):73‐77. [DOI] [PubMed] [Google Scholar]
- 38. Fan XY, Chen B, Lu ZS, Jiang ZF, Zhang SQ. Poly‐l‐arginine acts synergistically with lps to promote the release of IL‐6 and IL‐8 via p38/ERK signaling pathways in NCI‐H292 cells. Inflammation. 2016;39(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 39. Jang J, Kim W, Kim K, et al. Lipoteichoic acid upregulates NF‐κB and proinfiammatory cytokines by modulating β‐catenin in bronchial epithelial cells. Mol Med Rep. 2015;12(3):4720‐4726. [DOI] [PubMed] [Google Scholar]


