This multicenter cohort study assesses the association of maximal extent of resection of contrast-enhanced and non–contrast-enhanced tumor with survival among patients with newly diagnosed glioblastoma in different molecular subgroups to develop a new road map for cytoreductive surgery.
Key Points
Question
Is maximal extent of resection of non–contrast-enhanced and contrast-enhanced tumor associated with improved survival within molecularly defined subgroups of newly diagnosed glioblastoma?
Findings
In this cohort study of 761 patients with newly diagnosed glioblastoma, maximal resection of contrast-enhanced plus non–contrast-enhanced tumor was found to be associated with increased overall survival in younger patients, whereas maximal resection of contrast-enhanced tumor was associated with increased overall survival in older patients, regardless of molecular subgroup.
Meaning
These findings indicate that maximal extent of resection of the contrast-enhanced tumor in all patients and the contrast-enhanced plus non–contrast-enhanced tumor in younger patients is associated with increased overall survival regardless of molecular subgroup and suggest a need to reconsider surgical strategies for these patients in the molecular era.
Abstract
Importance
Per the World Health Organization 2016 integrative classification, newly diagnosed glioblastomas are separated into isocitrate dehydrogenase gene 1 or 2 (IDH)–wild-type and IDH-mutant subtypes, with median patient survival of 1.2 and 3.6 years, respectively. Although maximal resection of contrast-enhanced (CE) tumor is associated with longer survival, the prognostic importance of maximal resection within molecular subgroups and the potential importance of resection of non–contrast-enhanced (NCE) disease is poorly understood.
Objective
To assess the association of resection of CE and NCE tumors in conjunction with molecular and clinical information to develop a new road map for cytoreductive surgery.
Design, Setting, and Participants
This retrospective, multicenter cohort study included a development cohort from the University of California, San Francisco (761 patients diagnosed from January 1, 1997, through December 31, 2017, with 9.6 years of follow-up) and validation cohorts from the Mayo Clinic (107 patients diagnosed from January 1, 2004, through December 31, 2014, with 5.7 years of follow-up) and the Ohio Brain Tumor Study (99 patients with data collected from January 1, 2008, through December 31, 2011, with a median follow-up of 10.9 months). Image accessors were blinded to patient groupings. Eligible patients underwent surgical resection for newly diagnosed glioblastoma and had available survival, molecular, and clinical data and preoperative and postoperative magnetic resonance images. Data were analyzed from November 15, 2018, to March 15, 2019.
Main Outcomes and Measures
Overall survival.
Results
Among the 761 patients included in the development cohort (468 [61.5%] men; median age, 60 [interquartile range, 51.6-67.7] years), younger patients with IDH–wild-type tumors and aggressive resection of CE and NCE tumors had survival similar to that of patients with IDH-mutant tumors (median overall survival [OS], 37.3 [95% CI, 31.6-70.7] months). Younger patients with IDH–wild-type tumors and reduction of CE tumor but residual NCE tumors fared worse (median OS, 16.5 [95% CI, 14.7-18.3] months). Older patients with IDH–wild-type tumors benefited from reduction of CE tumor (median OS, 12.4 [95% CI, 11.4-14.0] months). The results were validated in the 2 external cohorts. The association between aggressive CE and NCE in patients with IDH–wild-type tumors was not attenuated by the methylation status of the promoter region of the DNA repair enzyme O6-methylguanine-DNA methyltransferase.
Conclusions and Relevance
This study confirms an association between maximal resection of CE tumor and OS in patients with glioblastoma across all subgroups. In addition, maximal resection of NCE tumor was associated with longer OS in younger patients, regardless of IDH status, and among patients with IDH–wild-type glioblastoma regardless of the methylation status of the promoter region of the DNA repair enzyme O6-methylguanine-DNA methyltransferase. These conclusions may help reassess surgical strategies for individual patients with newly diagnosed glioblastoma.
Introduction
In 2016, the World Health Organization (WHO) reclassified glioma by integrating molecular and histologic characteristics. The resulting molecular subclassification of glioblastoma, according to presence or absence of mutation in the isocitrate dehydrogenase 1 or 2 gene (IDH [OMIM 147700]), has prognostic significance.1 Overall, approximately 91% of glioblastomas have IDH–wild-type mutations with median overall patient survival of 1.2 years, whereas the remaining 9% of tumors are IDH mutant, with a median overall patient survival of 3.6 years.2 For both types of glioblastoma, the standard of care for patients with newly diagnosed disease is surgical resection followed by radiotherapy given in combination with the DNA-alkylating agent temozolomide.3 Maximum resection of contrast-enhanced (CE) tumor on T1-weighted magnetic resonance imaging has been consistently associated with longer survival.4,5,6,7 However, the association of maximal resection of the CE tumor with survival within glioblastoma subgroups and the potential importance of resection of non–contrast-enhanced (NCE) disease remain poorly understood.4,5,8,9,10,11,12 A clear understanding of the association of maximal extent of resection within molecular subgroups with survival is essential for counseling patients and medical decision-making.
We hypothesized that maximal extent of resection for CE and NCE tumor would be associated with improved patient survival regardless of IDH mutation status. The Stupp protocol3 with its accompanying improved survival became the accepted standard of care for glioblastoma in 2005. For this reason, we first focused our analysis on patients newly diagnosed with glioblastoma since 2005. In the first such study to our knowledge, we analyzed whether extent of resection of CE and NCE tumor was associated with overall survival among patients with known IDH mutation status. We then verified the findings in an independent patient cohort from 2 different institutions. Last, we examined overall survival in association with extent of resection among patients with known methylation status of the promoter region of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT).13,14,15,16
Methods
In this retrospective, multicenter cohort study, overall survival risk models were first established in a development cohort and then tested in an external validation cohort, both of which are described below. Additional details on patient, tumor, imaging, and clinical data collection are given in the eMethods in the Supplement. The study was approved by the institutional review boards of the University of California, San Francisco (UCSF), Mayo Clinic, and the University Hospitals of Cleveland. Written informed consent was obtained from all participants in all studies. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Development Cohort
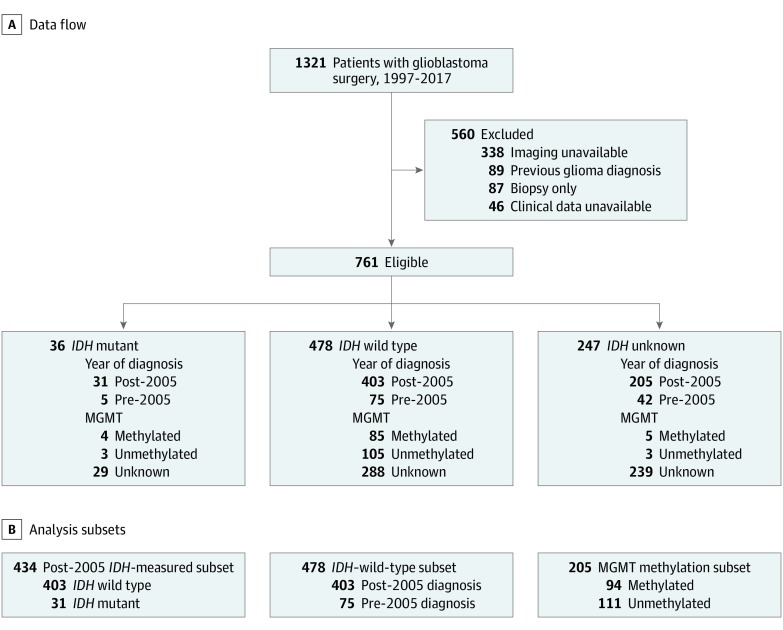
Clinical and imaging data were retrievable for 761 of 1321 consecutive patients (57.6%) who were newly diagnosed with glioblastoma after 18 years of age and had an initial surgical procedure at UCSF from January 1, 1997, through December 31, 2017 (Figure 1). The UCSF Cancer Registry was used to identify each patient’s vital status, and data collection ended on December 10, 2018.
Figure 1. Data Flow Diagram for the UCSF Development Cohort.
IDH indicates isocitrate dehydrogenase gene 1 or 2; MGMT, promoter region of the DNA repair enzyme O6-methylguanine-DNA methyltransferase.
External Validation Cohort
The external validation cohort consisted of 206 patients from the Mayo Clinic and the Ohio Brain Tumor Study (OBTS) (eTable 1 in the Supplement). The Mayo Clinic provided clinical, surgical, and imaging data for 107 consecutive patients with newly diagnosed glioblastoma from January 1, 2004, through December 31, 2014. The OBTS is an ongoing prospective study and provided clinical, surgical, and imaging data for 99 consecutive patients with newly diagnosed glioblastoma from January 1, 2008, through December 31, 2011.
Summary of Statistical Methods
Data were analyzed from November 15, 2018, through March 15, 2019. Details of analytic methods are in the eMethods in the Supplement. To summarize, the characteristics considered for each patient are presented in the Table. We used the unpaired 2-tailed t and χ2 tests to test for differences in these variables between cohorts. Overall survival was calculated from the date of first surgery until death or last follow-up. Cox proportional hazards regression models evaluated associations of variables with survival. The proportional hazards assumption was not met in all models, particularly for extent of resection; thus, we used recursive partitioning methods with all univariable significant variables except MGMT status (due to unstable imputed values [eMethods in the Supplement]). Recursive partitioning survival trees divided patients into different survival risk groups.17,18 Median overall survival, hazard ratios (HRs), and 95% CIs were computed for each risk group using the Kaplan-Meier method and Cox proportional hazards regression model with and without adjustment for MGMT status. Two-sided P < .05 indicated significance. All analyses were conducted using the statistical software R, version 3.5.1 (R Institute for Statistical Computing).
Table. Patient Characteristicsa.
| Characteristic | UCSF Cohort (n = 761) | Post-2005/IDH Known Subset (n = 434) |
|---|---|---|
| Sex | ||
| Male | 468/761 (61.5) | 271/434 (62.4) |
| Female | 293/761 (38.5) | 163/434 (37.6) |
| Age at diagnosis, y | ||
| Mean (SD) | 59.5 (12.0) | 59.6 (11.5) |
| Median (IQR) | 60.0 (51.6-67.7) | 60.5 (52.2-67.4) |
| Range | 19.0-89.0 | 21.3-89.0 |
| Diagnosis year | ||
| Before 2005 | 122/761 (16.0) | 0/434 |
| 2005 and after | 639/761 (84.0) | 434/434 (100) |
| KPSb | ||
| <60 | 35/451 (7.8) | 19/241 (7.9) |
| 60 | 24/451 (5.3) | 10/241 (4.1) |
| 70 | 50/451 (11.1) | 34/241 (14.1) |
| 80 | 149/451 (33.0) | 72/241 (29.9) |
| 90 | 173/451 (38.4) | 92/241 (38.2) |
| 100 | 20/451 (4.4) | 14/241 (5.8) |
| Median KPS (IQR) | 80 (80-90) | 80 (70-90) |
| Tumor location by lobe | ||
| Brainstem, insular, basal ganglia, or thalamus | 14/704 (2.0) | 11/414 (2.7) |
| Cerebellum | 2/704 (0.3) | 1/414 (0.2) |
| Frontal | 268/704 (38.1) | 153/414 (37.0) |
| Occipital | 46/704 (6.5) | 29/414 (7.0) |
| Parietal | 137/704 (19.5) | 74/414 (17.9) |
| Temporal | 237/704 (33.7) | 146/414 (35.3) |
| Tumor location by hemisphere | ||
| Bilateral | 8/705 (1.1) | 4/414 (1.0) |
| Left | 357/705 (50.6) | 205/414 (49.5) |
| Right | 340/705 (48.2) | 205/414 (49.5) |
| IDH status | ||
| Wild type | 478/514 (93.0) | 403/434 (92.9) |
| Mutant | 36/514 (7.0) | 31/434 (7.1) |
| MGMT status | ||
| Methylated | 94/205 (45.9) | 89/197 (45.2) |
| Unmethylated | 111/205 (54.1) | 108/197 (54.8) |
| Postoperative adjuvant therapy | ||
| Postoperative radiotherapy | 677/741 (91.4) | 399/424 (94.1) |
| Postoperative temozolomide | 628/741 (84.8) | 386/424 (91.0) |
| Both | 619/741 (83.5) | 380/424 (89.6) |
| Neither | 64/741 (8.6) | 20/424 (4.7) |
| Preoperative volume, mL | ||
| CE tumors | ||
| Mean (SD) | 32.6 (28.2) | 31.3 (27.9) |
| Median (IQR) | 24.8 (10.7-46.9) | 22.9 (11.0-44.3) |
| Range | 0.1-173.8 | 0.1-172.1 |
| NCE tumors | ||
| Mean (SD) | 85.3 (55.7) | 82.6 (54.7) |
| Median (IQR) | 75.0 (40.3-121.2) | 73.3 (37.7-121.1) |
| Range | 1.2-274.8 | 1.2-266.3 |
| Postoperative volume, mL | ||
| CE tumors | ||
| Mean (SD) | 3.2 (6.9) | 3.1 (7.1) |
| Median (Q1-Q3) | 0.6 (0.0-3.1) | 0.5 (0.0-2.8) |
| Range | 0.0-57.6 | 0.0-57.6 |
| NCE tumors | ||
| Mean (SD) | 40.2 (33.4) | 36.7 (32.6) |
| Median (IQR) | 33.8 (13.5-56.8) | 29.8 (10.8-51.7) |
| Range | 0.0-200.3 | 0.0-200.3 |
| Extent of resection, % by volume | ||
| CE tumors | ||
| Mean (SD) | 89.6 (17.2) | 90.0 (16.9) |
| Median (Q1-Q3) | 97.3 (87.3-100) | 97.5 (88.4-100) |
| Range | 9.9-100.0 | 9.9-100.0 |
| NCE tumors | ||
| Mean (SD) | 53.7 (23.3) | 56.7 (23.3) |
| Median (Q1-Q3) | 54.0 (39.0-70.0) | 58.0 (43.0-73.0) |
| Range | 0.0-100 | 0.0-100 |
Abbreviations: CE, contrast enhanced; IDH, isocitrate dehydrogenase 1 or 2 gene; IQR, interquartile range; KPS, Karnofsky Performance Score; MGMT, promoter region of the DNA repair enzyme O6-methylguanine-DNA methyltransferase; NCE, non–contrast enhanced.
Unless otherwise indicated, data were expressed as number/total number (percentage) of patients. Percentages have been rounded and may not total 100.
Higher scores indicate a better ability to carry out daily activities.
Results
Of the 761 patients with newly diagnosed glioblastoma in the UCSF development cohort, 468 (61.5%) were men and 293 (38.5%) were women; median age at diagnosis was 60 (interquartile range [IQR], 51.6-67.7) years (Table). Of the 514 patients with IDH measured, 478 (93.0%) had IDH–wild-type tumors; of the 205 with tumor MGMT methylation measured, 94 (45.9%) had MGMT methylated tumors. Similar to the findings of other studies, MGMT promoter methylation rates were lower (approximately 45%) in patients with IDH–wild-type tumors and higher (approximately 60%) in patients with IDH-mutant tumors.2,19 Of the 741 patients with treatment recorded, 619 (83.5%) received combined adjuvant temozolomide and radiotherapy, because 639 of the 761 patients in the cohort (84.0%) were diagnosed since 2005.3 The median percentage of CE tumor resected was 97% (IQR, 87%-100%), and the median percentage of NCE tumor resected was 54% (IQR, 39%-70%). Of the 514 patients with IDH measured, the percentage of CE tumor resected was the same in patients with IDH–wild-type glioblastoma as it was in patients with IDH-mutant glioblastoma (89.9% vs 89.5%; P = .90). As of December 10, 2018, median follow-up was 9.6 (95% CI, 7.7-13.4) years, and median overall survival was 14.2 (95% CI, 13.3-15.2) months. As of final data collection, 50 patients (6.6%) were still alive or lost to follow-up. In univariable models, age at diagnosis (HR, 1.42; 95% CI, 1.33-1.52; P < .001), Karnofsky Performance Score (KPS) (HR for 90-100, 0.60; 95% CI, 0.47-0.76; P < .001), IDH status (HR for IDH-mutant status, 0.26; 95% CI, 0.17-0.41; P < .001), MGMT status (HR for unmethylated status, 1.55; 95% CI, 1.14-2.10; P = .005), adjuvant radiotherapy (HR, 3.13; 95% CI, 2.30-4.25; P < .001), adjuvant temozolomide treatment (HR, 1.36; 95% CI, 1.01-1.82; P = .04), location of tumor (HR for cerebellum, 4.29; 95% CI, 1.06-17.37; P = .04), postoperative CE tumor volume (HR, 1.04; 95% CI, 1.03-1.05; P < .001), NCE tumor volume (HR, 1.01; 95% CI, 1.0-1.01; P < .001), and percentage extent of resection of the CE (HR, 0.99; 95% CI, 0.98-0.99; P < .001) and NCE (HR, 0.99; 95% CI, 0.99-0.99; P < .001) tumors were significantly associated with overall survival (eTable 2 in the Supplement). In eFigure 1 in the Supplement, the univariable association of percentage of enhancing tumor resected with the relative death rate is shown. Using a previously determined cutoff ranging from 75% to 80%,5 a reduction in the relative death rate was noted for resections of greater than 80%, whereas an increase in the relative death rate was noted for resections of less than 40%, with a plateau in effect from 40% to 80%.
Initially, we examined the association of extent of resection adjusted for other prognostic variables separated by IDH status in Cox proportional hazards regression models (eTable 3 in the Supplement). In patients with IDH-mutant tumors, the percentages of CE (HR, 0.95; 95% CI, 0.91-0.99; P = .02) and NCE (HR, 0.96; 95% CI, 0.93-0.99; P = .02) resected tumor were significantly associated with better survival, whereas other possible prognostic variables (ie, age, temozolomide treatment, and KPS) were not significantly associated with survival. The association of MGMT status with survival among these patients with IDH-mutant glioblastoma could not be assessed owing to the small number of tumors with MGMT methylation measured (7 of 36). In the IDH–wild-type subset, the percentage resected of CE (HR, 0.98; 95% CI, 0.97-0.99; P < .001) and NCE (HR, 0.99; 95% CI, 0.98-1.00; P = .02) tumors were each statistically significantly associated with better survival after adjusting for additional prognostic variables, including MGMT methylation status. The interaction of extent of resection (CE or NCE) and MGMT status was not statistically significant. Furthermore, because the proportional hazards assumptions were not met in either model for temozolomide treatment or percentage resected, we used recursive partitioning survival models for risk stratification as described in more detail below.
Post-2005 IDH Subset
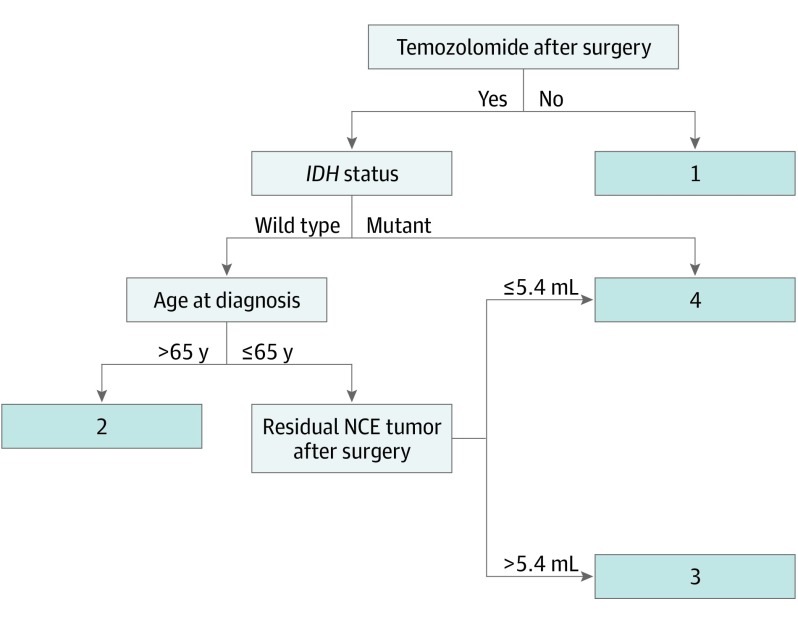
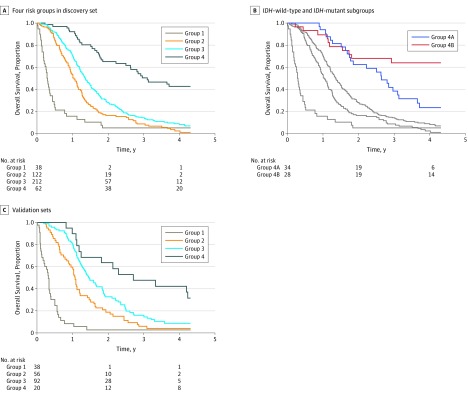
Given the differences in chemotherapeutic administration before 2005, we performed a specific subgroup analysis of the 434 patients whose glioblastoma was diagnosed since 2005 and had known tumor IDH status (post-2005 IDH measured subset) (Figure 1B). Clinical and surgical characteristics were similar to those of the entire cohort (Table). Four distinct survival risk groups were identified via recursive partitioning (Figure 2 and Figure 3A). Group 1 patients (n = 38) were those who did not receive temozolomide and had the poorest overall survival (median, 3.6 [95% CI, 2.6-5.4] months). Group 2 patients (n = 122) had better overall survival than group 1 and included patients who had IDH–wild-type tumor, were treated with temozolomide, and were older than 65 years at diagnosis (median, 12.4 [95% CI, 11.4-14.0] months). Group 3 patients (n = 212) had better overall survival than patients in group 2 and included patients with IDH–wild-type tumors who received temozolomide, were younger than 65 years of age, and had more than 5.4 mL of residual NCE tumor after resection (median, 16.5 [95% CI, 14.7-18.3] months). Group 4 patients had the best overall survival and included 2 subgroups of temozolomide-treated patients: those with IDH-mutated tumors (n = 28) or those with IDH–wild-type tumors who were younger than 65 years with a median of 100% of CE tumor resected and a median of 90% resection of NCE tumor resulting in no more than 5.4 mL of residual NCE tumor (n = 34) (median, 37.3 [95% CI, 31.6-70.7] months). The younger patients with complete resection with an IDH–wild-type tumor (Group 4A, Figure 3B) had similar survival to patients with IDH-mutant tumors treated with temozolomide (Group 4B, Figure 3B) during the first 3 years of treatment. After 3 years, patients with IDH–wild-type tumors declined at a faster rate than did those with IDH-mutant tumors. Clinical characteristics and HRs (with and without adjustment for MGMT status and KPS) are shown in the caption for Figure 3A and eTables 4-6 in the Supplement. The risks remained significant after adjusting for MGMT status and KPS. The model is substantiated by the external validation cohort (Figure 3C), in which the HRs were significant and the median survivals were almost identical to the development set median survivals (caption of Figure 3C and eTable 8 in the Supplement). Clinical characteristics are shown in eTable 7 in the Supplement.
Figure 2. Recursive Partitioning Analysis (RPA) for Post-2005/IDH-Known Subset.
Includes 434 patients. Four risk groups were determined by RPA based on adjuvant temozolomide treatment after surgery, isocitrate dehydrogenase gene 1 or 2 (IDH) status, age at diagnosis, and residual non–contrast-enhancing (NCE) tumor after surgery. Groups are denoted by numbers 1 through 4. Group 4 is the combination of 2 subgroups: temozolomide-treated patients with IDH-mutant tumors and temozolomide-treated patients aged 65 years or younger with IDH–wild-type tumors with no greater than 5.4 mL of NCE residual tumor.
Figure 3. Kaplan-Meier Curves for Overall Survival for 4 Risk Groups.
Groups are described in Figure 2. A, Includes patients in the post-2005 isocitrate dehydrogenase gene 1 or 2 status (IDH)-known (n = 434). For group 1, median overall survival was 3.6 (95% CI, 2.6-5.4) months (univariable hazard ratio [HR], 3.31 [95% CI, 2.31-4.74]; P < .001); group 2, 12.4 (95% CI, 11.4-14.0) months (univariable HR, 1.45 [95% CI, 1.15-1.83]; P = .001); group 3, 16.5 (95% CI, 14.7-18.3) months (univariable HR, 1 [reference]); and group 4, 37.3 (95% CI, 31.6-70.7) months (univariable HR, 0.36 [95% CI, 0.25-0.51]; P < .001). B, Includes groups 1 to 3 (gray) and the 2 subgroups in group 4. Group 4A represents the temozolomide-treated patients with IDH–wild-type tumors who were younger than 65 years and with no more than 5.4 mL of non–contrast-enhancing residual tumor (median overall survival, 31.7 [95% CI, 22.2-43.9] months); group 4B, the temozolomide-treated patients with IDH-mutant tumors (median overall survival, 78.4 [95% CI, 35.1-not applicable] months). C, Includes Mayo Clinic (n = 107) and Ohio Brain Tumor Study (n = 99) patients with glioblastoma. Median overall survival for group 1 was 3.8 (95% CI, 2.4-4.6) months (univariable HR, 6.17 [95% CI, 4.08-9.33]; P < .001); group 2, 12.8 (95% CI, 10.9-14.6) months (univariable HR, 1.58 [95% CI, 1.11-2.25]; P = .01); group 3, 17.5 (95% CI, 15.3-22.5) months (univariable HR, 1 [reference]); and group 4, 32.4 (95% CI, 21.7-not applicable) months (univariable HR, 0.54 [95% CI, 0.31-0.94]; P = .03).
IDH–Wild-type Subset
Given the known superior prognosis of patients with IDH-mutant disease2,15,20 and the similarity observed in survival with those with IDH–wild-type tumors who had extensive NCE tumor resection (Figure 2), we set out to determine the association of extent of resection with survival among patients in whom IDH was wild type regardless of diagnosis year (n = 478, Figure 1B). Four significant risk groups were identified (eFigure 3 in the Supplement). Group 1 patients (n = 25) were those who did not receive temozolomide and had more than 73.8 mL of NCE tumor preoperatively (median overall survival, 4.2 [95% CI, 3.3-4.9] months). Group 2 patients (n = 200) had better survival than group 1 patients and included those who did not receive temozolomide with less than or equal to 73.8 mL of NCE tumor preoperatively; those older than 65 years who did receive temozolomide; and those younger than 65 years who received temozolomide but had less than 77% of CE tumor resected (median overall survival, 11.6 [95% CI, 10.6-13.2] months). Group 3 patients (n = 217) had better survival than group 2 patients and were treated with temozolomide, were younger than 65 years of age, and had more than 77% of CE tumor resected with more than 5.4 mL residual NCE tumor (median overall survival, 17.9 [95% CI, 16.4-19.7] months). Group 4 patients (n = 36) had better survival than group 3 patients and were treated with temozolomide, were younger than 65 years, and had more than 77% of CE tumor resected and less than 5.4 mL of residual NCE tumor (median overall survival, 31.7 [95% CI, 22.2-56.2] months). Similar to the data above, the patients who had the best survival (group 4) were young with the most complete CE and NCE resections (ie, a median of 100% of the CE tumor resected and 92% of the NCE tumor resected) (eTable 9 in the Supplement). Clinical characteristics and HRs (with and without adjustment for MGMT and KPS) are shown in eFigure 3B and eTables 9 and 10 in the Supplement. The risk groups remain significant after adjusting for MGMT status and KPS. Validation included repeated imputation of IDH status for the 247 UCSF patients missing IDH status (eMethods 2 in the Supplement) in addition to the Mayo Clinic and OBTS cohorts (eFigure 3C in the Supplement).
Discussion
In 2019, more than 12 000 glioblastomas were diagnosed, accounting for more than 70% of all new gliomas.2,21 The WHO 2016 classification for brain and central nervous system tumors separates glioblastoma tumors into 2 groups, IDH mutant and wild type. To date, being younger, a higher KPS, treatment with temozolomide and radiotherapy, MGMT methylation, smaller CE tumor at presentation, and greater extent of resection of the CE tumor have consistently been associated with longer survival.2,9,12,15 The interplay between factors such as molecular classification and extent of resection has been a topic of intense interest. In addition, recent studies have attempted to determine whether there is benefit in resection of surrounding tumor that is NCE but hyperintense on T2-weighted or fluid-attenuated inversion recovery imaging.11,12,22 Herein we present the first study, to our knowledge, to examine the role of maximal resection of CE and NCE disease across glioblastoma subgroups for subsets of cases classified according to WHO 2016 classifications (IDH mutation status) and by MGMT methylation status (eMethods in the Supplement), and to offer guidance for clinical decision-making by subgroup.
As a result of the seminal clinical trial published by Stupp et al3 in 2005, most patients with glioblastoma are treated with temozolomide and radiotherapy after surgery. For the purposes of a contemporary comparison, we restricted our UCSF cohort of 761 patients to those diagnosed since 2005 with IDH status measured (post-2005 IDH subset [n = 434]). The recursive partitioning analysis indicates that temozolomide-treated patients with IDH-mutant tumor and those patients younger than 65 years with IDH–wild-type tumors have similar survival after maximal resection of NCE tumor and complete resection of CE tumor (Figure 2). In fact, these patients experience a similar survival to 3 years.
This study is the first, to our knowledge, in the molecular era to show that maximal resection of the NCE tumor in addition to that of the CE tumor outweighed the negative prognostic implication of IDH–wild-type status in younger patients. Prior published reports have suggested that patients with IDH-mutant gliomas are more likely to have complete tumor resection, potentially contributing to the survival benefit seen from aggressive resection.23 However, in the subset of patients in our large cohort whose tumors were tested for IDH, extent of resection was the same in patients with IDH–wild-type glioblastoma as it was in patients with IDH-mutant glioblastoma (89.9% vs 89.5%; P = .90 [n = 514]). It therefore appears unlikely that extent of resection of CE disease is simply a surrogate for IDH status, in contrast with previously published results.23
Most newly diagnosed glioblastomas are IDH wild type. We therefore performed a specific analysis of IDH–wild-type glioblastoma, noting important differences after the patients with favorable IDH-mutant tumors were removed. Again, the recursive partitioning analysis based on this subset indicates that for temozolomide-treated patients younger than 65 years, maximal resection of CE and NCE tumor is associated with improved overall survival (median, 31.7 vs 11.6 months) (eFigure 3 and eTables 10 and 11 in the Supplement). This finding does not support a previous report23 suggesting that only patients with IDH-mutant tumors benefit from maximal resection of the CE and NCE disease, whereas those with IDH–wild-type tumors benefit solely from resection of the enhancing disease. The previous study focused on resection and IDH status as main effects in a smaller cohort of patients; thus, the interaction among IDH status, age, and resection of CE and NCE disease was likely missed. For those older than 65 years in the present study, resection of the CE tumor was associated with improved survival (with adjustment for MGMT status), whereas resection of NCE tumor was not (eTable 11 in the Supplement).
Given that MGMT methylation improves prediction and prognosis, we looked at the association of MGMT status with the risk groups (eTables 6 and 10 in the Supplement). The association of the risk groups remained significant when adjusted for MGMT status, signifying the risk groups as independently associated with survival; and the interactions were insignificant, signifying that the association of the risk groups does not differ by MGMT status (see discussion in eTable 6D in the Supplement). We also performed an analysis on those patients with MGMT methylation measured separated by methylation status (n = 205) (Figure 1B and eMethods in the Supplement). In the 2 subsets, the patients treated with temozolomide (for the MGMT-methylated tumors) or younger than 65 years (for the MGMT-unmethylated tumors) who had maximum resection of the CE (median, 100%) and NCE (median, 63%-64%) tumor had the best and most similar survival (eFigures 4-7 and eTables 12-15 in the Supplement).
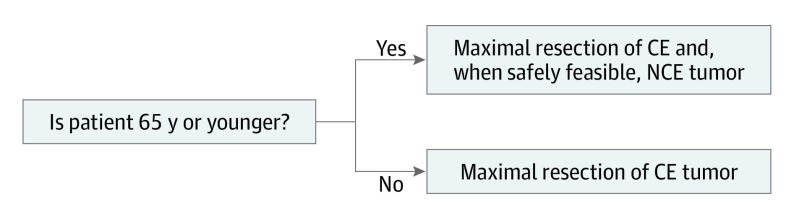
In summary, we found that reduction of CE tumor was significant regardless of IDH status and MGMT methylation status. Reduction of NCE tumor was significant in younger (<65 years) patients with IDH–wild-type tumors, regardless of MGMT status, and in all patients with IDH-mutant tumors. Thus, our proposed surgical strategy for newly diagnosed glioblastoma is to perform maximal resection of the CE tumor for all patients with the additional maximum resection of the NCE tumor in patients younger than 65 years, when safely feasible (Figure 4). Given the younger ages of patients with IDH-mutant tumors (median age, 38 years2), this guideline incorporates them in the younger group.
Figure 4. Proposed Surgical Strategy for Newly Diagnosed Glioblastoma.
Strategy consists of maximal resection of the contrast-enhanced (CE) tumors for all patients with the additional maximum resection of the non–contrast-enhanced (NCE) tumors for patients younger than 65 years, when safely feasible.
Limitations
This study has several limitations. This retrospective cohort involves patients from 3 large tertiary referral centers rather than a randomized clinical trial. As a surgical series, the distribution of volume resected is skewed toward surgically resectable glioblastoma, not tumors for which a neurosurgeon might recommend biopsy alone. Although we believe greater extent of resection, particularly of NCE disease, does not result in greater neurological compromise, we cannot comment on this topic in our data; in support, however, a large study (n = 643) comparing complete CE tumor resection with at least 53% vs less than 53% NCE resection found a significantly higher overall complication rate in the patients with less than 53% resection and a comparable rate of neurological complications between the 2 groups.12 In most cases, decisions about extent of resection are made without prior knowledge of molecular subclassification. Treating newly diagnosed presumed glioblastomas with biopsy before definitive resection is costly and would delay postresection chemoradiotherapy. Radiomic approaches and serum biomarkers have demonstrated the ability to diagnose glioblastoma based on imaging24 or serum samples only,25 but none of these innovations are currently available for clinical use. In light of these data, clinicians can make inferences about molecular subclassification based on previously published large-scale genomic analyses.
Conclusions
This study is the first, to our knowledge, to combine resection of CE and NCE tumors in conjunction with molecular and clinical information with validation in an external test set and paves the way for rethinking surgical strategies for individual patients with newly diagnosed glioblastoma. This study supports maximal extent of resection for the CE tumor, and in younger patients, the additional maximal resection of the NCE tumor, regardless of IDH and MGMT status. To maximize CE and NCE resection, advanced intraoperative imaging methods and fluorescence-based tumor biomarkers can be used, whereas stimulation mapping26 will help to decrease perioperative morbidity.
eMethods 1. Details and Cohorts
eMethods 2. Statistical Details
eResults. UCSF Subset With Tumor Methylation Status Known (Any Year of Diagnosis)
eTable 1. Demographic Table for UCSF Cohort and Mayo and OBTS Validation Cohorts
eTable 2. Univariable Survival Analysis for UCSF Cohort
eTable 3. Multivariate Cox Regression Analyses for UCSF Cohort
eTable 4. Demographic Table for 4 Risk Groups for UCSF Subset Newly Diagnosed After 2005 and Known Tumor IDH Status
eTable 5. Temozolomide-Treated Tumors for UCSF Subset Newly Diagnosed After 2005 by Known Tumor IDH Status
eTable 6. Hazard Ratios for 4 Risk Groups for UCSF Subset Newly Diagnosed After 2005 and Known Tumor IDH Status
eTable 7. Demographic Table for the 4 Risk Groups for External Validation
eTable 8. Hazard Ratios for Risk Groups for External Validation
eTable 9. Demographic Table for 4 Risk Groups for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 10. Hazard Ratios for 4 Risk Groups for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 11. Analysis by Age for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 12. Demographic Table for 3 Risk Groups for UCSF Subset With Tumor Methylation Status–Methylated
eTable 13. Hazard Ratios for 3 Risk Groups for UCSF Subset With Tumor Methylation Status Methylated
eTable 14. Demographic Table for 2 Risk Groups for UCSF Subset With Tumor Methylation Status–Unmethylated
eTable 15. Hazard Ratios for MGMT-Unmethylated Risk Groups
eFigure 1. Univariate Survival Analysis for Contrast-Enhancing Volumetric Resection via Splines
eFigure 2. Survival Curves for External Validation of the Risk Groups
eFigure 3. Recursive Partitioning Analysis and Survival Curves for IDH-Wildtype for Risk Groups
eFigure 4. Recursive Partitioning Analysis for MGMT-Methylated for Risk Groups
eFigure 5. Survival Curves for MGMT-Methylated Risk Groups
eFigure 6. Recursive Partitioning Analysis for MGMT-Unmethylated for Risk Groups
eFigure 7. Survival Curves for MGMT-Unmethylated Risk Groups
eReferences.
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2.Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405-417. doi: 10.1038/s41582-019-0220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, van den Bent MJ, Hegi ME. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5(3):198-206. doi: 10.1007/s11910-005-0047-7 [DOI] [PubMed] [Google Scholar]
- 4.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156-162. doi: 10.3171/2008.4.17536 [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3-8. doi: 10.3171/2011.2.JNS10998 [DOI] [PubMed] [Google Scholar]
- 6.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ; ALA-Glioma Study Group . Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. doi: 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011;153(6):1211-1218. doi: 10.1007/s00701-011-1001-x [DOI] [PubMed] [Google Scholar]
- 8.Stummer W, Reulen HJ, Meinel T, et al. ; ALA-Glioma Study Group . Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576. doi: 10.1227/01.neu.0000317304.31579.17 [DOI] [PubMed] [Google Scholar]
- 9.Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774-782. doi: 10.1200/JCO.2013.51.8886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. doi: 10.3171/jns.2001.95.2.0190 [DOI] [PubMed] [Google Scholar]
- 11.Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? a single institution retrospective experience. J Neurooncol. 2017;135(1):129-139. doi: 10.1007/s11060-017-2559-9 [DOI] [PubMed] [Google Scholar]
- 12.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977-988. doi: 10.3171/2015.5.JNS142087 [DOI] [PubMed] [Google Scholar]
- 13.Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372-385. doi: 10.1038/nrneurol.2014.100 [DOI] [PubMed] [Google Scholar]
- 14.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 15.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743-5750. doi: 10.1200/JCO.2009.23.0805 [DOI] [PubMed] [Google Scholar]
- 16.Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926. doi: 10.1016/S1470-2045(12)70265-6 [DOI] [PubMed] [Google Scholar]
- 17.Molinaro AM, Lostritto K, van der Laan M. partDSA: deletion/substitution/addition algorithm for partitioning the covariate space in prediction. Bioinformatics. 2010;26(10):1357-1363. doi: 10.1093/bioinformatics/btq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lostritto K, Strawderman RL, Molinaro AM. A partitioning deletion/substitution/addition algorithm for creating survival risk groups. Biometrics. 2012;68(4):1146-1156. doi: 10.1111/j.1541-0420.2012.01756.x [DOI] [PubMed] [Google Scholar]
- 19.Bell EH, Zhang P, Fisher BJ, et al. Association of MGMT promoter methylation status with survival outcomes in patients with high-risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG Oncology/RTOG 0424 trial. JAMA Oncol. 2018;4(10):1405-1409. doi: 10.1001/jamaoncol.2018.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499-2508. doi: 10.1056/NEJMoa1407279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1-iv86. doi: 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lasocki A, Gaillard F. Non–contrast-enhancing tumor: a new frontier in glioblastoma research. AJNR Am J Neuroradiol. 2019;40(5):758-765. doi: 10.3174/ajnr.A6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81-91. doi: 10.1093/neuonc/not159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbari H, Macyszyn L, Da X, et al. Imaging surrogates of infiltration obtained via multiparametric imaging pattern analysis predict subsequent location of recurrence of glioblastoma. Neurosurgery. 2016;78(4):572-580. doi: 10.1227/NEU.0000000000001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler DC, Young AB, Cooper KD, et al. The ratio of HLA-DR and VNN2+ expression on CD14+ myeloid derived suppressor cells can distinguish glioblastoma from radiation necrosis patients. J Neurooncol. 2017;134(1):189-196. doi: 10.1007/s11060-017-2508-7 [DOI] [PubMed] [Google Scholar]
- 26.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18-27. doi: 10.1056/NEJMoa067819 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Details and Cohorts
eMethods 2. Statistical Details
eResults. UCSF Subset With Tumor Methylation Status Known (Any Year of Diagnosis)
eTable 1. Demographic Table for UCSF Cohort and Mayo and OBTS Validation Cohorts
eTable 2. Univariable Survival Analysis for UCSF Cohort
eTable 3. Multivariate Cox Regression Analyses for UCSF Cohort
eTable 4. Demographic Table for 4 Risk Groups for UCSF Subset Newly Diagnosed After 2005 and Known Tumor IDH Status
eTable 5. Temozolomide-Treated Tumors for UCSF Subset Newly Diagnosed After 2005 by Known Tumor IDH Status
eTable 6. Hazard Ratios for 4 Risk Groups for UCSF Subset Newly Diagnosed After 2005 and Known Tumor IDH Status
eTable 7. Demographic Table for the 4 Risk Groups for External Validation
eTable 8. Hazard Ratios for Risk Groups for External Validation
eTable 9. Demographic Table for 4 Risk Groups for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 10. Hazard Ratios for 4 Risk Groups for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 11. Analysis by Age for UCSF Subset With Tumor IDH Wildtype (Any Year of Diagnosis)
eTable 12. Demographic Table for 3 Risk Groups for UCSF Subset With Tumor Methylation Status–Methylated
eTable 13. Hazard Ratios for 3 Risk Groups for UCSF Subset With Tumor Methylation Status Methylated
eTable 14. Demographic Table for 2 Risk Groups for UCSF Subset With Tumor Methylation Status–Unmethylated
eTable 15. Hazard Ratios for MGMT-Unmethylated Risk Groups
eFigure 1. Univariate Survival Analysis for Contrast-Enhancing Volumetric Resection via Splines
eFigure 2. Survival Curves for External Validation of the Risk Groups
eFigure 3. Recursive Partitioning Analysis and Survival Curves for IDH-Wildtype for Risk Groups
eFigure 4. Recursive Partitioning Analysis for MGMT-Methylated for Risk Groups
eFigure 5. Survival Curves for MGMT-Methylated Risk Groups
eFigure 6. Recursive Partitioning Analysis for MGMT-Unmethylated for Risk Groups
eFigure 7. Survival Curves for MGMT-Unmethylated Risk Groups
eReferences.