Key Points
Question
What are the cumulative incidence and temporal dynamics of acute cortical microinfarcts?
Findings
In this cohort study of 54 participants with cerebral small vessel disease who were recruited to undergo 10 monthly 3-T magnetic resonance imaging (MRI) scans, including high-resolution diffusion-weighted imaging, 21 acute cortical microinfarcts were observed in 7 of 54 participants (13%). All acute cortical microinfarcts disappeared on follow-up MRI.
Meaning
We show that incident acute cortical microinfarcts never evolved into chronically MRI-detectable lesions and suggest that these acute microinfarcts underlie part of the submillimeter cortical microinfarcts visible only on neuropathology, thereby providing a source for the high microinfarct burden encountered on neuropathology.
Abstract
Importance
Neuropathology studies show a high prevalence of cortical microinfarcts (CMIs) in aging individuals, especially in patients with cerebrovascular disease and dementia. However, most, are invisible on T1- and T2-weighted magnetic resonance imaging (MRI), raising the question of how to explain this mismatch. Studies on small acute infarcts, detected on diffusion-weighted imaging (DWI), suggest that infarcts are largest in their acute phase and reduce in size thereafter. Therefore, we hypothesized that a subset of the CMI that are invisible on MRI can be detected on MRI in their acute phase. However, to our knowledge, a serial imaging study investigating the temporal dynamics of acute CMI (A-CMI) is lacking.
Objective
To determine the prevalence of chronic CMI (C-CMI) and the cumulative incidence and temporal dynamics of A-CMI in individuals with cerebral small vessel disease (SVD).
Design, Setting, Participants and Exposures
The RUN DMC—Intense study is a single-center hospital-based prospective cohort study on SVD performed between March 2016 and November 2017 and comprising 10 monthly 3-T MRI scans, including high-resolution DWI, 3-dimensional T1, 3-dimensional fluid-attenuated inversion recovery, and T2. One hundred six individuals from the previous longitudinal RUN DMC study were recruited based on the presence of progression of white matter hyperintensities on MRI between 2006 and 2015 and exclusion of causes of cerebral ischemia other than SVD. Fifty-four individuals (50.9%) participated. The median total follow-up duration was 39.5 weeks (interquartile range, 37.8-40.3). Statistical data analysis was performed between May and October 2019.
Main Outcomes and Measures
We determined the prevalence of C-CMI using the baseline T1, fluid-attenuated inversion recovery, and T2 scans. Monthly high-resolution DWI scans (n = 472) were screened to determine the cumulative incidence of A-CMI. The temporal dynamics of A-CMI were determined based on the MRI scans collected during the first follow-up visit after A-CMI onset and the last available follow-up visit.
Results
The median age of the cohort at baseline MRI was 69 years (interquartile range, 66-74 years) and 34 participants (63%) were men. The prevalence of C-CMI was 35% (95% CI, 0.24-0.49). Monthly DWI detected 21 A-CMI in 7 of 54 participants, resulting in a cumulative incidence of 13% (95% CI, 0.06-0.24). All A-CMI disappeared on follow-up MRI.
Conclusions and Relevance
Acute CMI never evolved into chronically MRI-detectable lesions. We suggest that these A-CMI underlie part of the submillimeter C-CMI encountered on neuropathological examination and thereby provide a source for the high CMI burden on neuropathology.
This cohort study examines the prevalence of chronic cortical microinfarcts and the cumulative incidence and temporal dynamics of acute cortical microinfarcts in Finnish individuals with cerebral small vessel disease.
Introduction
Cortical microinfarcts (CMIs) are ischemic lesions between 0.05 and 5 mm that are frequently encountered on neuropathological examination of brains of elderly people.1,2 Most of these lesions are invisible on magnetic resonance imaging (MRI), raising the question of how to explain this mismatch. In vivo studies on the causes and consequences of CMI are limited when most cannot be captured with MRI.
Recently, a few MRI studies demonstrated the disappearance of acute small (subcortical) infarcts, which were detected on diffusion-weighted imaging (DWI), on follow-up T1, and/or T2-weighted images.3,4,5,6 Small cortical infarcts in particular seem to disappear on follow-up MRI.5,6,7 Consequently, it is possible that a subset of CMI identified on neuropathology but invisible on in vivo MRI may only be visible in their acute phase on MRI.
In addition to a larger size, the detection of acute infarcts on MRI is facilitated by the hyperintense DWI signal due to cytotoxic edema. Because the DWI signal is elevated for approximately 4 weeks,8 an at least monthly serial MRI study, including DWI, is required to detect acute CMI (A-CMI) and examine their evolution. However, to our knowledge, such a study is lacking.
We aimed to determine the prevalence of chronic CMI (C-CMI) and the incidence and temporal dynamics of A-CMI in a high-frequent MRI study. Furthermore, we explored variables associated with CMI presence, including cardiovascular risk factors, MRI markers of cerebral small vessel disease (SVD), and cognition.
Methods
The RUN DMC—Intense study is a single-center observational study among 54 individuals with progressive SVD, defined as progression of white matter hyperintensities (WMH) between 2006 and 2015 on MRI scans collected in the RUN DMC study but no other causes of cerebral ischemia (median [interquartile range (IQR)] baseline age 69 years [66-74]; 34 men [63%]).9 Participants underwent a prescreening visit to assess cardiovascular risk factors and cognition and 10 monthly MRI scans.10 Data were collected between March 2016 and November 2017. The medical ethics committee of region Arnhem-Nijmegen approved the study. All participants gave written informed consent. Three-Tesla MRI scans, all collected on the same scanner (MAGNETOM Prisma; Siemens Healthineers), included 3-dimensional (3-D) T1 (0.85 mm isotropically), multiple spin-echo T2 with quantitative T2 mapping (reconstructed voxels 0.36 × 0.36 × 3.0 mm), 3-D fluid-attenuated inversion recovery (FLAIR) (0.85 mm isotropically), multishell DWI (10 × b = 0; 30 × b = 1000; 60 × b = 3000 seconds/mm2 diffusion weightings, 1.7 mm isotropically), 1 b equal to 0 image with reversed phase encoding, and 3-D multiecho fast low-angle shot images (0.8 × 0.8 × 2.0 mm) to generate susceptibility weighted images.
Chronic CMIs, defined according to previously reported criteria and without hyperintensity on DWI,11 were manually detected using the T1-, FLAIR, and T2-weighted images.11,12 Two trained assessors (A. t. T., B. S. B.) performed a sensitive rating of all baseline and last follow-up scans and were masked to clinical characteristics. In the case of disagreement, a final consensus was reached involving more raters (M.D.; S.J. van Veluw, PhD, Massachusetts General Hospital).
Acute CMIs were manually detected using all monthly b equal to 1000 and b equal to 3000 DWI trace images.2,7 The temporal dynamics of A-CMI were determined using the DWI, T1-, FLAIR, and T2-weighted scans of the first follow-up visit after A-CMI onset and the last follow-up visit. Neuroimaging markers of SVD, including WMH, lacunes, microbleeds, and mean diffusivity within main white matter tracts, cardiovascular risk factors, and cognitive domain scores were assessed as previously described.7,13
Statistical analyses were performed in R (R Foundation). We determined the prevalence and incidence of C-CMI and cumulative incidence of A-CMI. Differences in baseline characteristics and the progression of SVD imaging markers between participants with any CMI (chronic or acute) and those without were assessed using Mann-Whitney U tests and χ2 tests (or Fisher exact tests when appropriate). A 2-tailed α was set at .05. All variables with P < .05 were assessed in a multiple logistic regression model. For the progression of WMH and mean diffusivity, we calculated linear mixed models (packages “lme4” and “lmerTest”). Associations between CMI presence and baseline cognition were assessed using linear regression models.
Results
At baseline, 81 C-CMIs were observed in 19 of 54 participants, resulting in a prevalence of 35% (95% CI, 0.24-0.49) (Figure 1). The median number of C-CMIs was 3 (IQR, 1-7). Fifty-two of 54 participants (96%) had 1 or more follow-up MRI scans. After a median follow-up period of 39.5 weeks (IQR, 37.8-40.3), the incidence of C-CMI was 0% (95% CI, 0.00-0.07). In total, 21 A-CMIs were observed on 9 of 472 DWI scans (2.0%) in 7 of 54 participants, resulting in a cumulative incidence of 13% (95% CI, 0.06-0.24).
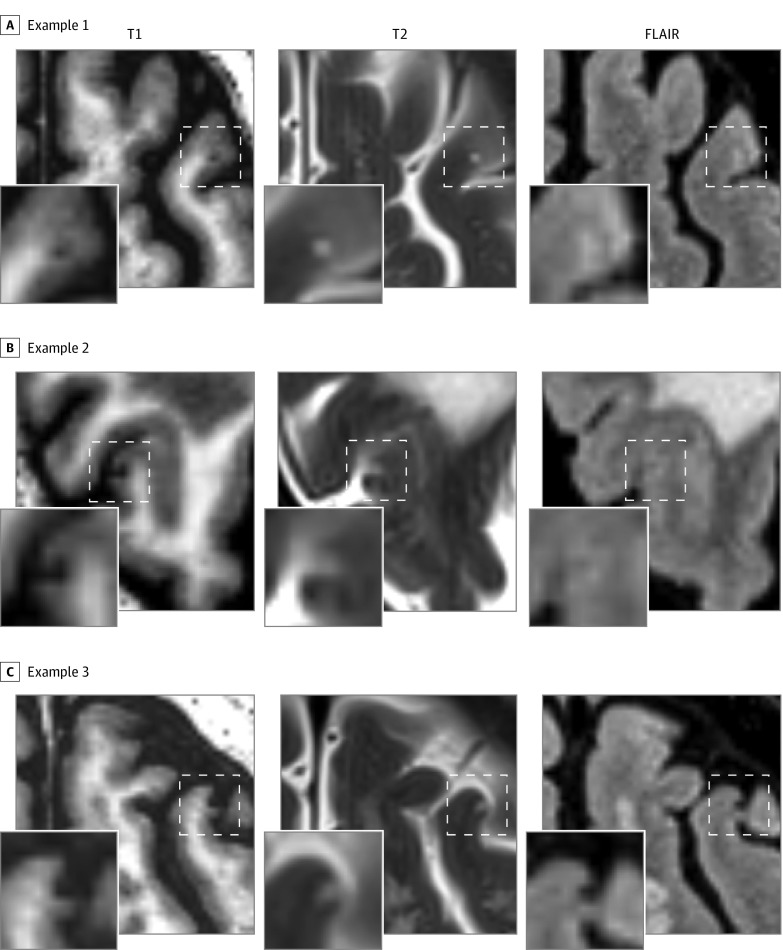
Figure 1. Examples of Chronic Cortical Microinfarcts on High-Resolution 3-T Imaging.
Chronic cortical microinfarcts were defined as hypointense lesions on T1-weighted imaging in combination with a hyperintense or isointense signal on T2 and a visible signal alteration on fluid-attenuated inversion recovery (FLAIR). A and B, The microinfarct is characterized on FLAIR by a hyperintense signal, whereas in panel C the signal is hypointense.
The incidence of A-CMI was not significantly higher in participants having also C-CMI compared with those without (21% vs 9%; P = .23). On visual inspection, C-CMI and A-CMI were similarly distributed throughout the brain (Figure 2).
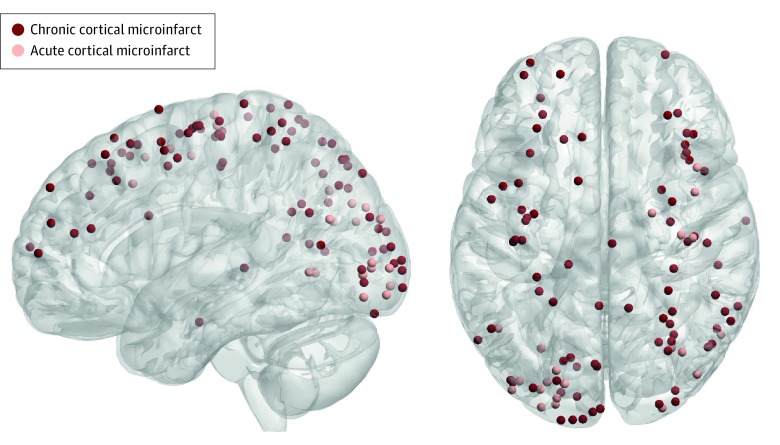
Figure 2. Topographical Distribution of Chronic and Acute Cortical Microinfarcts.
To determine whether chronic and acute cortical microinfarcts have a similar topographical distribution throughout the brain, which would be an indication of a common pathophysiological mechanism, we projected all lesion coordinates onto a 3-dimensional surface rendering of the Montreal Neurological Institute–152 1-mm template using BrainNet Viewer (https://www.nitrc.org/projects/bnv/). As illustrated, chronic and acute cortical microinfarcts were similarly distributed, predominantly within the occipital, parietal, and frontal cortices, and seemed to correspond partially to the watershed areas.
In the acute phase, 16 of 21 A-CMIs (76.2%) were hypointense on T1 and hyperintense on FLAIR, 1 of 21 (4.8%) was only hypointense on T1, 3 of 21 (14.3%) were only hyperintense on FLAIR, and 1 of 21 (4.8%) was isointense on T1 and FLAIR. For 20 of 21 A-CMIs (95.2%), 1 or more follow-up MRI scans were available. Importantly, all A-CMIs were visible only on the MRI of the visit in which they first appeared. Already on the subsequent follow-up scan, they were not visible anymore on any modality, including DWI, T1-, and T2-weighted/FLAIR, also not with prior knowledge of the DWI-positive lesion (Figure 3).
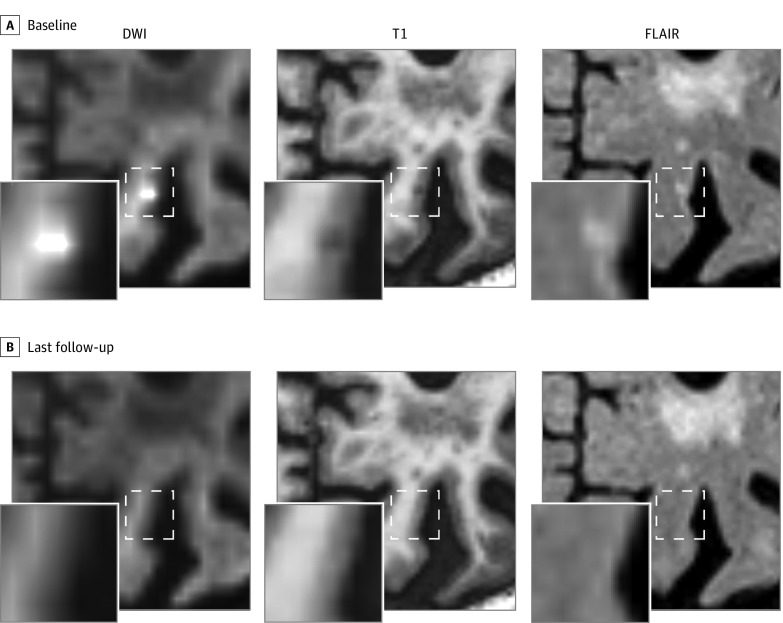
Figure 3. Disappearance of Acute Cortical Microinfarct.
An acute cortical microinfarct was detected on the diffusion-weighted imaging (DWI) trace image (shown in the figure; b = 3000), which was in the acute phase accompanied by a hypointense signal on T1 and a hyperintense signal on fluid-attenuated inversion recovery (FLAIR) (A). On the first follow-up magnetic resonance imaging (MRI) scan after acute microinfarct onset (not shown) and on the last available follow-up MRI scan (27 weeks later, shown in the figure), no visible signal alteration was observed on T1 and FLAIR (B).
The median age was significantly higher in individuals with any CMI (22 [40.7%]) compared with those without (32 [59.3%]; 73 [IQR, 67-80] vs 68 [IQR, 65-70] years; P = .01; eTable 1 in the Supplement). Furthermore, CMI presence was associated with increased median systolic blood pressure (145 [IQR, 136-157] vs 136 [IQR, 127-147] mm Hg; P = .04), although it was not independent of age (β = 0.01; odds ratio, 1.01; 95% CI, 0.98-1.05; P = .47). None of the remaining variables were significantly associated with CMI presence. Cortical microinfarct presence was not associated with the progression of SVD imaging markers.
Cortical microinfarct presence was significantly associated with lower scores on information processing speed (b = −0.35; 95% CI, −0.67 to −0.03; P = .03), memory (b = −0.55; 95% CI, −0.95 to −0.15; P = .01), and language (b = −0.63; 95% CI, −1.16 to −0.10; P = .02; eTable 2 in the Supplement). After correcting for age and baseline WMH volume, none of the associations remained significant.
Discussion
Limited evidence suggests that DWI-positive lesions do not correspond to the infarct core but contain salvageable tissue (ie, the penumbra).14 This may underlie their disappearance on MRI over time,4 even when using state-of-the-art high resolution at 3 T to detect C-CMI. We suggest that by applying serial imaging, part of the CMI detected on neuropathological examination but invisible on in vivo MRI can be detected in their acute phase on MRI. Thereby, we provide a source for the high CMI burden encountered on neuropathology. However, because we did not have histological verification of disappearing cortical DWI-positive lesions, combined in vivo MRI-histopathology studies are required to determine whether these A-CMIs contribute to the total CMI burden observed ex vivo or if their disappearance indicates tissue salvation.
Apart from age, CMI presence was not associated with other variables. Cortical microinfarcts overlapped partially with the vascular border zones, suggesting a role for brain hypoperfusion in the etiology of CMI, although we did not have individual vascular territory maps.15
Limitations
Because of the small sample size, the study was probably underpowered to find associations with small-medium effect sizes between CMI and risk factors. As such, our risk factor analysis remains explorative. Future larger prospective studies, ideally combined with histopathological examination to capture part of the submillimeter microinfarcts, are needed to determine the causes of CMI. Likewise, larger prospective studies are required to evaluate the contribution of CMI to cognitive decline.
Conclusions
We demonstrated that A-CMI never evolved into chronically MRI-detectable lesions. We suggest that these A-CMI underlie part of the submillimeter C-CMI frequently encountered on neuropathological examination.
eTable 1. Group characteristics
eTable 2. Associations between presence of chronic or acute cortical microinfarcts and baseline cognitive performance
References
- 1.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32(3):425-436. doi: 10.1038/jcbfm.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Veluw SJ, Shih AY, Smith EE, et al. . Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017;16(9):730-740. doi: 10.1016/S1474-4422(17)30196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auriel E, Edlow BL, Reijmer YD, et al. . Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis. Neurology. 2014;83(2):182-188. doi: 10.1212/WNL.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duering M, Adam R, Wollenweber FA, et al. . Within-lesion heterogeneity of subcortical DWI lesion evolution, and stroke outcome: a voxel-based analysis [published online July 25, 2019]. J Cereb Blood Flow Metab. doi: 10.1177/0271678X19865916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havsteen I, Ovesen C, Willer L, et al. . Small cortical grey matter lesions show no persistent infarction in transient ischaemic attack? a prospective cohort study. BMJ Open. 2018;8(1):e018160. doi: 10.1136/bmjopen-2017-018160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Summers PM, Hartmann DA, Hui ES, et al. . Functional deficits induced by cortical microinfarcts. J Cereb Blood Flow Metab. 2017;37(11):3599-3614. doi: 10.1177/0271678X16685573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ter Telgte A, Wiegertjes K, Gesierich B, et al. . Contribution of acute infarcts to cerebral small vessel disease progression. Ann Neurol. 2019;86(4):582-592. doi: 10.1002/ana.25556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz UG, Flossmann E, Francis JM, Redgrave JN, Rothwell PM. Evolution of the diffusion-weighted signal and the apparent diffusion coefficient in the late phase after minor stroke: a follow-up study. J Neurol. 2007;254(3):375-383. doi: 10.1007/s00415-006-0381-y [DOI] [PubMed] [Google Scholar]
- 9.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 10.ter Telgte A, Wiegertjes K, Tuladhar AM, et al. . Investigating the origin and evolution of cerebral small vessel disease: the RUN DMC—InTENse study. Eur Stroke J. 2018;3(4):369-378. doi: 10.1177/2396987318776088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Veluw SJ, Hilal S, Kuijf HJ, et al. . Cortical microinfarcts on 3T MRI: clinical correlates in memory-clinic patients. Alzheimers Dement. 2015;11(12):1500-1509. doi: 10.1016/j.jalz.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 12.van Veluw SJ, Biessels GJ, Luijten PR, Zwanenburg JJ. Assessing cortical cerebral microinfarcts on high resolution MR images. J Vis Exp. 2015;(105). doi: 10.3791/53125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duering M, Finsterwalder S, Baykara E, et al. . Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. 2018;14(6):764-774. doi: 10.1016/j.jalz.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guadagno JV, Warburton EA, Aigbirhio FI, et al. . Does the acute diffusion-weighted imaging lesion represent penumbra as well as core? a combined quantitative PET/MRI voxel-based study. J Cereb Blood Flow Metab. 2004;24(11):1249-1254. doi: 10.1097/01.WCB.0000141557.32867.6B [DOI] [PubMed] [Google Scholar]
- 15.Ferro DA, van Veluw SJ, Koek HL, Exalto LG, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) study group . Cortical cerebral microinfarcts on 3 Tesla MRI in patients with vascular cognitive impairment. J Alzheimers Dis. 2017;60(4):1443-1450. doi: 10.3233/JAD-170481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Group characteristics
eTable 2. Associations between presence of chronic or acute cortical microinfarcts and baseline cognitive performance