This cohort study compares cardiovascular and safety outcomes of chlorthalidone and hydrochlorothiazide as first-line therapies for adults with hypertension.
Key Points
Question
What are the relative effectiveness and safety of chlorthalidone and hydrochlorothiazide?
Findings
In this comparative cohort study of 730 255 individuals from multiple large databases, no difference in association with effectiveness between the 2 drugs was found, but a significantly worse safety profile for chlorthalidone was observed.
Meaning
Recommendations to prefer chlorthalidone to hydrochlorothiazide are not supported by real-world evidence.
Abstract
Importance
Chlorthalidone is currently recommended as the preferred thiazide diuretic to treat hypertension, but no trials have directly compared risks and benefits.
Objective
To compare the effectiveness and safety of chlorthalidone and hydrochlorothiazide as first-line therapies for hypertension in real-world practice.
Design, Setting, and Participants
This is a Large-Scale Evidence Generation and Evaluation in a Network of Databases (LEGEND) observational comparative cohort study with large-scale propensity score stratification and negative-control and synthetic positive-control calibration on databases spanning January 2001 through December 2018. Outpatient and inpatient care episodes of first-time users of antihypertensive monotherapy in the United States based on 2 administrative claims databases and 1 collection of electronic health records were analyzed. Analysis began June 2018.
Exposures
Chlorthalidone and hydrochlorothiazide.
Main Outcomes and Measures
The primary outcomes were acute myocardial infarction, hospitalization for heart failure, ischemic or hemorrhagic stroke, and a composite cardiovascular disease outcome including the first 3 outcomes and sudden cardiac death. Fifty-one safety outcomes were measured.
Results
Of 730 225 individuals (mean [SD] age, 51.5 [13.3] years; 450 100 women [61.6%]), 36 918 were dispensed or prescribed chlorthalidone and had 149 composite outcome events, and 693 337 were dispensed or prescribed hydrochlorothiazide and had 3089 composite outcome events. No significant difference was found in the associated risk of myocardial infarction, hospitalized heart failure, or stroke, with a calibrated hazard ratio for the composite cardiovascular outcome of 1.00 for chlorthalidone compared with hydrochlorothiazide (95% CI, 0.85-1.17). Chlorthalidone was associated with a significantly higher risk of hypokalemia (hazard ratio [HR], 2.72; 95% CI, 2.38-3.12), hyponatremia (HR, 1.31; 95% CI, 1.16-1.47), acute renal failure (HR, 1.37; 95% CI, 1.15-1.63), chronic kidney disease (HR, 1.24; 95% CI, 1.09-1.42), and type 2 diabetes mellitus (HR, 1.21; 95% CI, 1.12-1.30). Chlorthalidone was associated with a significantly lower risk of diagnosed abnormal weight gain (HR, 0.73; 95% CI, 0.61-0.86).
Conclusions and Relevance
This study found that chlorthalidone use was not associated with significant cardiovascular benefits when compared with hydrochlorothiazide, while its use was associated with greater risk of renal and electrolyte abnormalities. These findings do not support current recommendations to prefer chlorthalidone vs hydrochlorothiazide for hypertension treatment in first-time users was found. We used advanced methods, sensitivity analyses, and diagnostics, but given the possibility of residual confounding and the limited length of observation periods, further study is warranted.
Introduction
The 2017 American College of Cardiology/American Heart Association hypertension guideline1 recommends thiazide and thiazidelike diuretics as one of the first-line treatment classes. Hydrochlorothiazide is the most commonly prescribed member of the class, but the guideline states that chlorthalidone is preferred on the basis of longer half-life and proven trial reduction of cardiovascular disease. However, to our knowledge there are no large, completed randomized clinical trials comparing these medications, although one is in progress.2 Indirect network meta-analyses showed superior effectiveness with chlorthalidone,3,4 but a large observational study showed approximately equal effectiveness.5 Short-term, small randomized clinical trials6,7 showed only nominal differences in safety issues such as hypokalemia, but the observational study5 showed a worse safety profile for chlorthalidone with higher rates of hypokalemia and hyponatremia.
Both indirect network meta-analyses and traditional observational studies are vulnerable to bias, but recent analytic methods are providing improved strategies to mitigate this risk. These strategies include the balancing of large numbers of baseline patient covariates to make comparison groups more equivalent,8 using many negative controls to detect and correct residual bias9,10 and testing of the consistency among heterogeneous data sources.11 We used these techniques to compare chlorthalidone and hydrochlorothiazide on 55 outcomes in 3 large observational databases of patients from the United States.
Methods
This multicenter controlled cohort study was part of the Observational Health Data Sciences and Informatics (OHDSI)12 Large-Scale Evidence Generation and Evaluation in a Network of Databases for Hypertension initiative.13 The research was approved by the Columbia University institutional review board as an OHDSI network study. The use of databases was reviewed by the New England Institution Review Board and was determined to be exempt from broad institutional review board approval because this research project did not involve human subjects research. Informed consent was also waived for this reason.
Data Sources
We included the 3 OHDSI databases that had at least 2500 patients with exposures to each drug who met the eligibility criteria enumerated below. The MarketScan Commercial Claims and Encounters database (CCAE) (IBM Watson Health; 2001 to 2018) database includes adjudicated health insurance claims and enrollment data from individuals enrolled in US employer-sponsored insurance health plans. The deidentified Clinformatics Data Mart Database (ie, Optum) (OptumInsight; 2001 to 2017) is an adjudicated US administrative health claims database with commercial and Medicare claims from inpatient and outpatient medical services, prescriptions as dispensed, and outpatient laboratory test results processed by participating large national laboratory vendors. The Optum deidentified Electronic Health Record Dataset (ie, PanTher) (Optum; 2007 to 2017) database comprises deidentified electronic health record data including prescriptions as prescribed and administered, laboratory results, vital signs, body measurements, diagnoses, procedures, and information derived from clinical notes using natural language processing. The databases were encoded in OHDSI’s Observational Medical Outcomes Partnership common data model version 5.12,14,15 The 3 databases were deidentified.
Study Design
This study follows a retrospective, observational, comparative cohort design.16 We included all patients initiating antihypertensive treatment with chlorthalidone or hydrochlorothiazide, and we defined the index time as the first observed exposure to either drug, including only patients with a prior or concurrent diagnosis of hypertension. We excluded patients having known prior exposure to any hypertension therapies (eAppendix in the Supplement) and those initiating another hypertension treatment within 7 days after starting chlorthalidone or hydrochlorothiazide; however, a patient remained in the cohort if they initiated another hypertension treatment after the 7 days. We required that patients have continuous observation in the database for at least 365 days before treatment initiation. We excluded patients with known prior outcome events and less than 1 day at risk. Full cohort details are provided in the eAppendix in the Supplement.
We used more than 60 000 patient features per database, including demographics (age, sex, index year, index month), all other drugs in the 365 days before the index date, all diagnoses in the 365 days before the index date, and the Charlson Comorbidity Index score,17 as baseline covariates for balancing cohorts. Table 1 shows a sample of covariates.
Table 1. Selected Baseline Characteristics for CCAEa.
| Characteristic | Before Stratification | After Stratification | ||||
|---|---|---|---|---|---|---|
| No. (%)b | Standard Difference | No. (%)b | Standard Difference | |||
| Chlorthalidone | Hydrochlorothiazide | Chlorthalidone | Hydrochlorothiazide | |||
| Age, mean (SD), y | 49.0 (10.4) | 48.2 (10.6) | 0.05 | 48.7 (10.4) | 48.2 (10.6) | 0.03 |
| Age, y | ||||||
| 15-19 | 60 (0.4) | 1700 (0.6) | −0.03 | 60 (0.4) | 1700 (0.6) | −0.03 |
| 20-24 | 230 (1.6) | 4600 (1.6) | 0 | 200 (1.4) | 4600 (1.6) | −0.02 |
| 25-29 | 420 (3.0) | 10 300 (3.6) | −0.03 | 480 (3.4) | 10 100 (3.5) | −0.01 |
| 30-34 | 850 (6.0) | 19 000 (6.6) | −0.02 | 850 (6.0) | 19 000 (6.6) | −0.02 |
| 35-39 | 1260 (8.9) | 28 500 (9.9) | −0.03 | 1340 (9.5) | 28 500 (9.9) | −0.01 |
| 40-44 | 1850 (13.1) | 38 500 (13.4) | −0.01 | 1860 (13.2) | 38 500 (13.4) | 0 |
| 45-49 | 2190 (15.5) | 47 100 (16.4) | −0.02 | 2260 (16.0) | 47 100 (16.4) | −0.01 |
| 50-54 | 2600 (18.4) | 50 600 (17.6) | 0.02 | 2550 (18.1) | 50 900 (17.7) | 0.01 |
| 55-59 | 2430 (17.2) | 46 000 (16.0) | 0.03 | 2400 (17.0) | 46 300 (16.1) | 0.02 |
| 60-64 | 2050 (14.5) | 37 600 (13.1) | 0.04 | 1950 (13.8) | 37 600 (13.1) | 0.02 |
| 65-69 | 180 (1.3) | 3200 (1.1) | 0.02 | 160 (1.1) | 3200 (1.1) | 0 |
| Female | 7310 (51.8) | 175 600 (61.1) | −0.19 | 8590 (60.9) | 174 200 (60.6) | 0 |
| Medical History | ||||||
| General | ||||||
| Acute respiratory disease | 3300 (23.4) | 75 600 (26.3) | −0.07 | 3640 (25.8) | 75 000 (26.1) | −0.01 |
| Attention-deficit/hyperactivity disorder | 210 (1.5) | 3200 (1.1) | 0.04 | 140 (1.0) | 3200 (1.1) | −0.01 |
| Chronic liver disease | 160 (1.1) | 3200 (1.1) | 0 | 140 (1.0) | 3200 (1.1) | −0.01 |
| Chronic obstructive lung disease | 170 (1.2) | 4000 (1.4) | −0.02 | 200 (1.4) | 4000 (1.4) | 0 |
| Dementia | 10 (0.1) | 300 (0.1) | −0.02 | 10 (0.1) | 300 (0.1) | −0.01 |
| Depressive disorder | 1170 (8.3) | 23 300 (8.1) | 0 | 1090 (7.7) | 23 300 (8.1) | −0.02 |
| Diabetes mellitus | 630 (4.5) | 13 200 (4.6) | 0 | 660 (4.7) | 12 900 (4.5) | 0.01 |
| Gastroesophageal reflux disease | 1140 (8.1) | 21 600 (7.5) | 0.02 | 1020 (7.2) | 21 600 (7.5) | −0.01 |
| Gastrointestinal hemorrhage | 210 (1.5) | 4600 (1.6) | −0.01 | 230 (1.6) | 4600 (1.6) | 0 |
| HIV infection | 40 (0.3) | 900 (0.3) | 0.01 | 30 (0.2) | 900 (0.3) | −0.02 |
| Hyperlipidemia | 3810 (27.0) | 72 700 (25.3) | 0.04 | 3740 (26.5) | 72 700 (25.3) | 0.03 |
| Lesion of liver | 30 (0.2) | 600 (0.2) | 0 | 10 (0.1) | 600 (0.2) | −0.01 |
| Obesity | 1930 (13.7) | 28 500 (9.9) | 0.12 | 1380 (9.8) | 28 700 (10.0) | −0.01 |
| Osteoarthritis | 1590 (11.3) | 30 500 (10.6) | 0.02 | 1520 (10.8) | 30 500 (10.6) | 0 |
| Pneumonia | 200 (1.4) | 4000 (1.4) | −0.01 | 230 (1.6) | 4000 (1.4) | 0.02 |
| Psoriasis | 140 (1.0) | 2600 (0.9) | 0.02 | 100 (0.7) | 2600 (0.9) | −0.02 |
| Renal impairment | 140 (1.0) | 1400 (0.5) | 0.06 | 80 (0.6) | 1400 (0.5) | 0.02 |
| Rheumatoid arthritis | 130 (0.9) | 2300 (0.8) | 0.01 | 160 (1.1) | 2300 (0.8) | 0.03 |
| Ulcerative colitis | 40 (0.3) | 600 (0.2) | 0.01 | 30 (0.2) | 600 (0.2) | −0.01 |
| Urinary tract infectious disease | 750 (5.3) | 18 400 (6.4) | −0.04 | 890 (6.3) | 18 100 (6.3) | 0 |
| Viral hepatitis C | 40 (0.3) | 900 (0.3) | 0 | 40 (0.3) | 900 (0.3) | −0.01 |
| Visual system disorder | 2130 (15.1) | 42 800 (14.9) | 0 | 2060 (14.6) | 42 500 (14.8) | −0.01 |
| Cardiovascular disease | ||||||
| Atrial fibrillation | 40 (0.3) | 600 (0.2) | 0.02 | 30 (0.2) | 600 (0.2) | 0 |
| Cerebrovascular disease | 140 (1.0) | 2900 (1.0) | 0 | 110 (0.8) | 2300 (0.8) | 0.01 |
| Coronary arteriosclerosis | 160 (1.1) | 2900 (1.0) | 0.02 | 160 (1.1) | 2600 (0.9) | 0.02 |
| Heart disease | 1020 (7.2) | 18 400 (6.4) | 0.03 | 900 (6.4) | 17 500 (6.1) | 0.01 |
| Heart failure | 60 (0.4) | 900 (0.3) | 0.01 | 30 (0.2) | 600 (0.2) | −0.01 |
| Ischemic heart disease | 110 (0.8) | 2600 (0.9) | −0.01 | 110 (0.8) | 2300 (0.8) | 0 |
| Peripheral vascular disease | 550 (3.9) | 9200 (3.2) | 0.04 | 440 (3.1) | 8600 (3.0) | 0 |
| Pulmonary embolism | 30 (0.2) | 600 (0.2) | 0 | 10 (0.1) | 600 (0.2) | −0.01 |
| Neoplasms | ||||||
| Hematologic neoplasm | 70 (0.5) | 1100 (0.4) | 0.01 | 60 (0.4) | 1100 (0.4) | −0.01 |
| Malignant lymphoma | 30 (0.2) | 600 (0.2) | 0.02 | 40 (0.3) | 600 (0.2) | 0.02 |
| Malignant neoplastic disease | 550 (3.9) | 10 900 (3.8) | 0.01 | 560 (4.0) | 10 900 (3.8) | 0.01 |
| Malignant tumor of breast | 130 (0.9) | 2900 (1.0) | −0.01 | 170 (1.2) | 2900 (1.0) | 0.02 |
| Malignant tumor of colon | 10 (0.1) | 600 (0.2) | −0.01 | 10 (0.1) | 600 (0.2) | −0.02 |
| Malignant tumor of urinary bladder | 10 (0.1) | 300 (0.1) | 0 | 10 (0.1) | 300 (0.1) | 0.01 |
| Primary malignant neoplasm of prostate | 60 (0.4) | 900 (0.3) | 0 | 60 (0.4) | 900 (0.3) | 0 |
| Medication use | ||||||
| Antibacterials for systemic use | 6500 (46.1) | 146 300 (50.9) | −0.10 | 7180 (50.9) | 145 400 (50.6) | 0 |
| Antidepressants | 2500 (17.7) | 55 200 (19.2) | −0.04 | 2650 (18.8) | 54 900 (19.1) | −0.01 |
| Antiepileptics | 860 (6.1) | 17 200 (6.0) | 0 | 860 (6.1) | 17 000 (5.9) | 0.01 |
| Anti-inflammatory and antirheumatic products | 3470 (24.6) | 75 900 (26.4) | −0.04 | 3720 (26.4) | 75 300 (26.2) | 0 |
| Antineoplastic agents | 210 (1.5) | 4300 (1.5) | 0.01 | 240 (1.7) | 4300 (1.5) | 0.02 |
| Antipsoriatics | 60 (0.4) | 1100 (0.4) | 0 | 60 (0.4) | 1100 (0.4) | 0 |
| Antithrombotic agents | 380 (2.7) | 6300 (2.2) | 0.03 | 340 (2.4) | 5700 (2.0) | 0.02 |
| Drugs for acid-related disorders | 1890 (13.4) | 40 200 (14.0) | −0.02 | 1960 (13.9) | 39 900 (13.9) | 0 |
| Drugs for obstructive airway diseases | 2960 (21.0) | 58 300 (20.3) | 0.02 | 2910 (20.6) | 58 300 (20.3) | 0.01 |
| Drugs used in diabetes | 410 (2.9) | 8900 (3.1) | −0.01 | 450 (3.2) | 8600 (3.0) | 0.01 |
| Immunosuppressants | 240 (1.7) | 4300 (1.5) | 0.02 | 200 (1.4) | 4300 (1.5) | −0.01 |
| Lipid modifying agents | 2000 (14.2) | 38 800 (13.5) | 0.02 | 1990 (14.1) | 38 500 (13.4) | 0.02 |
| Opioids | 2200 (15.6) | 46 000 (16.0) | −0.01 | 2260 (16.0) | 45 400 (15.8) | 0 |
| Psycholeptics | 2580 (18.3) | 52 300 (18.2) | 0 | 2620 (18.6) | 52 000 (18.1) | 0.01 |
Values are rounded, with prestratification counts estimated from percentages and totals and poststratification counts showing estimated effective numbers.
The Commercial Claims and Encounters Database (CCAE) chlorthalidone group had 14 104 patients, and the hydrochlorothiazide group had 287 390.
The primary outcomes, which we prespecified, were hospitalization for acute myocardial infarction, heart failure, ischemic or hemorrhagic stroke, and a composite cardiovascular disease outcome including the first 3 outcomes and sudden cardiac death (eAppendix in the Supplement). International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline was followed.
The 51 safety outcomes, which we prespecified, included electrolyte disorders, such as hypokalemia and hyponatremia, acute and chronic kidney disease, and gout. They were assembled from safety concerns reported on hypertension drug product labels, and they are defined in the eAppendix in the Supplement. We began the outcome risk window 1 day after treatment initiation and used 2 design choices to define the window end. First, we ended the outcome time-at-risk window at first cessation of continuous drug exposure, analogous to an on-treatment design. Second, we ended the outcome time-at-risk window when the patient was no longer in the database or the outcome occurred, analogous to an intention-to-treat design. Continuous drug exposures were constructed from the available longitudinal data by considering sequential dispensing or prescriptions with gaps less than 30 days. We show on-treatment results in this article and intention-to-treat results in the eAppendix in the Supplement.
Statistical Analysis
Analysis began June 2018. We conducted our cohort study using the open-source OHDSI CohortMethod R package,18 with large-scale analytics from the Cyclops R package.19 We used propensity scores20 to balance the chlorthalidone and hydrochlorothiazide cohorts with respect to measured confounding variables, with a separate model developed for each database. Propensity scores estimated the treatment exposure probability conditional on 60 535 to 70 072 pretreatment baseline covariates in the 1 year prior to treatment initiation. We performed propensity score stratification and then estimated comparative chlorthalidone-vs-hydrochlorothiazide hazard ratios (HRs) using a Cox proportional hazards model, accounting for time on therapy and censoring; we used Kaplan-Meier survival plots to assess the assumption of proportionality. Detailed covariate and methods information is provided in the eAppendix in the Supplement. We used propensity score and covariate balance metrics to assess the success of measured confounding control, defined as all covariates having a standardized difference of the mean less than 0.1. We used preference score distributions to judge equipoise, defined as having most patients within 0.25 to 0.75 propensity score–based preference scores.21 The HRs and associated SEs from each of the 3 database analyses were then combined through a random-effect meta-analysis to produce a composite effect estimate.
We estimated residual bias using 76 negative control outcomes11 (eAppendix in the Supplement) (ie, outcomes believed to be caused by neither chlorthalidone nor hydrochlorothiazide, which therefore have an assumed HR of 1) identified through a data-rich algorithm,22 and we augmented the set by injecting events into the negative controls to create synthetic positive controls9 (ie, outcomes where the true HR is assumed known and greater than 1). We measured how often the true relative risks for controls were inside of their CIs (it should be 95% of the time for 95% CIs), and we calibrated all HR estimates, their 95% CIs, and their 2-sided P values so that approximate 95% coverage was achieved for the controls.
To address multiplicity concerns, we indicate which estimates remain statistically significant after a Bonferroni correction for 55 hypotheses. However, we report all differences.
Time at Risk, Baseline Blood Pressure, Dose, and Potassium
Outcomes may differ in timing: electrolyte imbalances may occur quickly while cardiovascular outcomes may take longer to occur, issues that were identified after the prespecified analyses. We therefore performed a post hoc analysis with risk period starting 91 days after the first drug exposure. This both ensured that all recorded days at risk had at least a 91-day exposure to the drug and shifted the median time at risk to longer time than for the primary analysis.
Only the electronic health record database, PanTher, had systolic and diastolic blood pressure readings recorded to assess baseline blood pressure. We repeated the analysis on that database with last systolic and last diastolic blood pressures in the year prior to index treatment included in the propensity score model using cubic splines.
PanTher also had appropriately timed blood potassium levels for some patients. We compared the last potassium level up to a year before first drug dose with the last potassium level 30 to 90 days after the first dose. In the largest database, CCAE, we addressed differences in dosing and potency by restricting the analysis to patients whose dose was 12.5 mg of chlorthalidone or 25 mg of hydrochlorothiazide for the entire on-treatment period.
Results
Balance Between Cohorts
For CCAE, there were 14 104 individuals receiving chlorthalidone and 287 390 individuals receiving hydrochlorothiazide; for Optum there were 7696 and 189 834, respectively; and for PanTher there were 15 118 and 216 113, respectively. Table 1 summarizes a selection of baseline characteristics before and after propensity score stratification for CCAE (eAppendix, eTables 3-8 in the Supplement), with substantial prestratification differences in sex, obesity, and several other variables but with all of the covariates having small (less than 0.1) standardized differences of the mean after propensity score stratification. Figure 1A and B shows the preference score distribution21 for CCAE, demonstrating sufficient between-group equipoise. Figure 1C shows the standardized differences of the means of all covariates before and after propensity score stratification. Before stratification, variables differed by up to almost 0.3, but after stratification all differed by substantially less than 0.1 and most less than 0.05, indicating excellent balance on all variables (eAppendix in the Supplement, section 2.4, reports the other databases, which had similar results; eFigure 4 in the Supplement).
Figure 1. Comparability of the Populations for Commercial Claims and Encounters Database (CCAE).
A, The preference score is a transformation of the propensity score that adjusts for differences in the sizes of the 2 treatment groups. A higher overlap indicates individuals in the 2 groups were more similar in terms of their predicted probability of receiving 1 treatment over the other. This plot shows sufficient equipoise (majority of both distributions being between 0.25 and 0.75) in CCAE that propensity score stratification should be able to create balance without discounting a large proportion of the population, but it shows sufficient difference (nonoverlap) that propensity score stratification is necessary. B, Same plot as panel A but showing essentially perfect overlap after adjustment (matching shown here). This illustrates the success of the adjustment in achieving balance. C, Each dot represents the standardized difference of the means for a single covariate before and after stratification on the propensity score. The panel shows poor balance before but excellent balance after stratification, with all 63 069 under 0.1 and most under 0.05. All measured variables were successfully balanced by the adjustment, and the 2 cohorts were in fact similar on all measured aspects.
Effectiveness
We found no statistically significant differences in risk of acute myocardial infarction, hospitalized heart failure, stroke, or the composite cardiovascular outcome between individuals receiving chlorthalidone and hydrochlorothiazide (Table 2; eTables 9-12 in the Supplement). The calibrated and uncalibrated HRs were very close, and this similarity indicated that the 76 negative controls and the synthetic positive controls revealed little evidence of residual confounding (in the form of false-positive or skewed results in the controls). The HR for the composite cardiovascular outcome for patients receiving chlorthalidone compared with patients receiving hydrochlorothiazide was 1.00 (95% CI, 0.85-1.17). Figure 2 shows the estimate to be consistent across databases (eFigure 5 in the Supplement).
Table 2. Effectiveness by Outcome (Propensity Score Stratification, On-Treatment).
| Outcome | Chlorthalidone, Total No. | Hydrochlorothiazide, No. (%) | Hazard Ratio (95% CI)a | |||
|---|---|---|---|---|---|---|
| Events | Patientsb | Events | Patientsb | Uncalibrated | Calibrated | |
| Acute myocardial infarction | 41 | 36 859 | 952 | 692 371 | 0.93 (0.63-1.36) | 0.92 (0.64-1.31) |
| Hospitalization for heart failure | 62 | 36 833 | 1248 | 691 409 | 1.07 (0.82-1.39) | 1.05 (0.82-1.34) |
| Stroke | 60 | 36 755 | 1141 | 689 698 | 1.13 (0.86-1.47) | 1.10 (0.86-1.41) |
| Composite cardiovascular diseasec | 149 | 36 628 | 3089 | 687 106 | 1.01 (0.86-1.20) | 1.00 (0.85-1.17) |
Hazard ratio for chlorthalidone vs hydrochlorothiazide (lower hazard ratio favors chlorthalidone).
Number of patients exposed varies by outcome owing to differences in whether database has hospitalization information and outcome-specific preexposure exclusions.
Composite cardiovascular disease includes the first 3 outcomes and sudden cardiac death.
Figure 2. Homogeneity on Effectiveness.
Hazard ratios (HRs) and forest plot of the 3 databases and the meta-analysis for chlorthalidone vs hydrochlorothiazide on the composite cardiovascular disease outcome. The 3 databases showed excellent agreement. CCAE indicates Commercial Claims and Encounters Database.
Safety
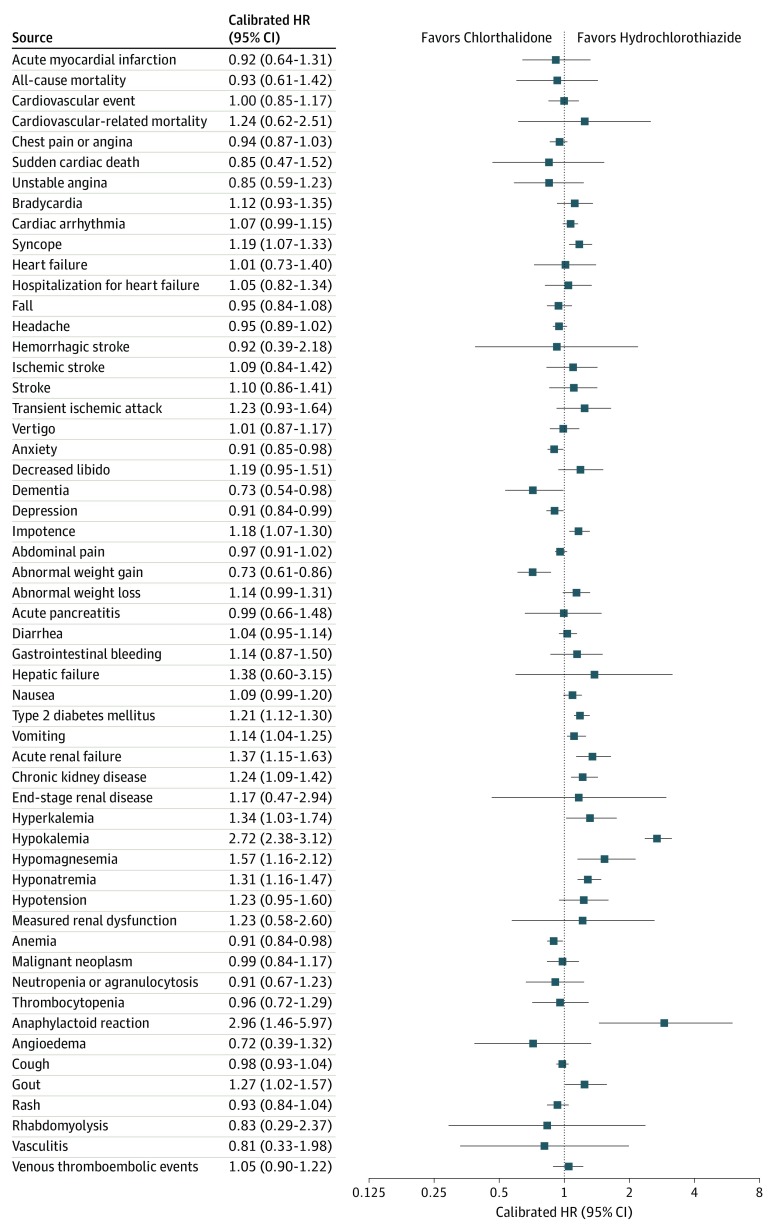
Figure 3 shows the comparative safety profile for the 2 drugs (eAppendix, eFigure 6, and eTables 9-12 in the Supplement). Chlorthalidone shows a different safety profile compared with hydrochlorothiazide, with the following outcomes different after correction for multiple hypotheses: chlorthalidone was associated with an increased risk for hypokalemia, hyponatremia, acute renal failure, chronic kidney disease, and type 2 diabetes mellitus. Chlorthalidone was associated with a decreased risk for diagnosed abnormal weight gain. Hypokalemia had an uncalibrated HR of 2.99 (95% CI, 2.58-3.46) and calibrated HR of 2.72 (95% CI, 2.38-3.12) (eFigure 5 in the Supplement). The uncalibrated and calibrated HR for hyponatremia was 1.36 (95% CI, 1.20-1.53) and 1.31 (95% CI, 1.16-1.47), respectively; acute renal failure, 1.42 (95% CI, 1.18-1.72) and 1.37 (95% CI, 1.15-1.63), respectively; and abnormal weight gain, 0.72 (95% CI, 0.60-0.87) and 0.73 (95% CI, 0.61-0.86), respectively. The following findings had CIs that excluded 1 but did not surpass the Bonferroni threshold: chlorthalidone was associated with an increased risk of hypomagnesemia, hyperkalemia, vomiting, syncope, gout, impotence, and anaphylactoid reaction and associated with a decreased risk of anemia, depression, dementia, and anxiety.
Figure 3. Forest Plot of Safety and Effectiveness Outcomes.
Forest plot of hazard ratio estimates and calibrated 95% CIs for chlorthalidone vs hydrochlorothiazide for 55 safety and effectiveness outcomes. The safety signals predominantly favored hydrochlorothiazide.
Furthermore, the event rates for hypokalemia were substantial. In CCAE, the rates per patient were 6.3% and 1.9% for chlorthalidone and hydrochlorothiazide, respectively. The Kaplan-Meier curves (eAppendix and eFigure 7 in the Supplement) were consistent with our assumption of proportionality for our use of the Cox proportional hazards model.
Sensitivity to Time at Risk
When we shifted the time at risk to begin 91 days after first drug exposure in the largest database, CCAE, the median end of the at-risk period shifted from 92 days to 267 days after the first drug exposure, and the upper quartile shifted from 425 days to 689 days. The HR estimates for the composite cardiovascular outcome was 0.94 (95% CI, 0.60-1.38) for the delayed risk period, which was similar to that for the original period, which was 0.96 (95% CI, 0.70-1.29).
Sensitivity to Baseline Blood Pressure
We assessed balance on baseline blood pressure in the PanTher database. Before any stratification, the standardized difference of the mean for blood pressure was 0.200 for systolic and 0.168 for diastolic. After propensity score stratification, but without including blood pressure in the model, the differences were 0.126 and 0.094, respectively. Therefore, without knowing blood pressure, balancing on the other 60 535 PanTher covariates resulted in marked improvement in balance on blood pressure. When we included blood pressure in the propensity model, the differences improved to 0.046 and less than 0.001 for systolic and diastolic blood pressure, respectively, with good balance among all other covariates (eFigure 1 in the Supplement). Furthermore, there were no major shifts in any of the effectiveness or safety outcomes (eFigure 2 in the Supplement) between propensity models with and without blood pressure, pointing to low sensitivity to slight imbalance in baseline blood pressure.
Sensitivity to Dose
The subgroup receiving 12.5 mg of chlorthalidone vs 25 mg of hydrochlorothiazide had an uncalibrated HR for hypokalemia of 1.71 (95% CI, 1.37-2.11) and calibrated HR of 1.57 (95% CI, 1.25-2.01), passing the Bonferroni threshold (eTables 1-2 in the Supplement). No other outcomes passed the threshold.
Change in Measured Potassium
PanTher showed greater reduction in blood potassium level in the chlorthalidone group than in the hydrochlorothiazide group (chlorthalidone, 0.22 mEq/L; hydrochlorothiazide, 0.12 mEq/L; P = .03; 95% CI, 0.01-0.18). Visualization of the changes reveals a greater downward shift in chlorthalidone (eFigure 3 in the Supplement).
Discussion
Our observational analysis across 3 large and disparate databases showed no significant difference in the effectiveness of chlorthalidone compared with hydrochlorothiazide for a range of cardiovascular outcomes, but chlorthalidone had a worse safety profile, including an association with an increased risk of hypokalemia with an HR of 2.72 (95% CI, 2.38-3.12). Other electrolyte abnormalities were also more frequent.
Our study is the largest multisite analysis of real-world evidence to address this comparison, with 36 918 records of individuals prescribed chlorthalidone and 693 337 prescribed hydrochlorothiazide across the 3 databases. We found consistent results across our 3 databases, excellent balance on more than 60 000 covariates after stratification, little bias based on our controls, little sensitivity to changes in time at risk, to inclusion of baseline blood pressure or to initial dose, and confirmation of differences in potassium by laboratory measurement.
Chlorthalidone use was associated with a higher rate of electrolyte and renal disorders, with an increase in hypokalemia, hyponatremia, acute renal failure, and chronic kidney disease. Based on the electrolyte findings, chlorthalidone’s association with an increase in rate of type II diabetes may be associated with potassium depletion or to dehydration. Chlorthalidone’s lower rate of abnormal weight gain may be associated with more effective diuresis.
To our knowledge, there have been no completed large head-to-head randomized clinical trials comparing chlorthalidone and hydrochlorothiazide on cardiovascular effectiveness. An indirect meta-analysis by Thomopoulos et al23 looked at cardiovascular outcomes vs placebo for low-dose diuretics; there were nominal differences, but the 2018 European Society of Cardiology/European Society of Hypertension guideline24 interpreted the results as roughly equivalent for the 2 drugs. The indirect meta-analysis by Roush et al4 showed an improved relative risk for composite cardiovascular events for chlorthalidone compared with hydrochlorothiazide of 0.79 (95% CI, 0.72-0.88). The real-world evidence study by Dhalla et al5 estimated an HR for chlorthalidone vs hydrochlorothiazide of 0.93 (95% CI, 0.81-1.06), although 1 dose subgroup did reach statistical significance without adjustment for multiple hypotheses. An observational study of the MRFIT cohort by Dorsch et al25 showed a relative HR for chlorthalidone vs hydrochlorothiazide of 0.79 (95% CI, 0.68-0.92), but the doses for both drugs were high. A randomized clinical trial comparing hydrochlorothiazide with chlorthalidone currently in progress2 may provide more definitive information to inform drug choice.
Several factors may be contributing to the discordance between our effectiveness results and those of previous indirect network analyses. First, because we focus on first-time use of antihypertension drugs, we likely have higher proportion of patients with milder disease with less baseline cardiovascular disease risk than the randomized clinical trials included in the network analyses. Second, our time-at-risk periods may be shorter, but our sensitivity analysis showed no difference as we increased time at risk, and our previous study of hypertension drug classes,13 which used the same methods and databases and had the same follow-up times, was able to discern differences in effectiveness. Third, indirect network analysis is subject to bias26 if the underlying trials differ in populations of patients, in physician behavior, or in study design, and, similarly, our observational study may be subject to residual bias. A fourth factor, failure to account for differences in baseline blood pressure between the 2 drugs, does not appear to be a source of the disagreement based on the PanTher database results. Fifth, our 95% CI for the composite cardiovascular disease HR is 0.85 to 1.17, which does not rule out some superiority in either direction, although it does exclude the previous indirect network analysis results.
A simple difference in effective dose, with chlorthalidone known to have a greater-per-milligram potency for lowering blood pressure levels than hydrochlorothiazide,6,7,27,28 could underlie some of the observed differences in toxicities, but our dose-sensitivity analysis still revealed higher hypokalemia for chlorthalidone at a 1:2 chlorthalidone:hydrochlorothiazide dose ratio. Furthermore, if clinicians treat to similar blood pressure levels, then they may titrate the doses to levels with similar blood pressure reduction, explaining our lack of differences in effectiveness but not our differences in safety. Ernst et al6 found better chlorthalidone nighttime blood pressure control at a 1:2 dose ratio, which could explain some increased safety signals.
The literature inconsistently points to a difference in hypokalemia between the 2 drugs. At a 1:2 dose ratio, Ernst et al6 found little difference in potassium. At a 1:1 dose ratio, Bakris et al7 found no statistically significant difference in the hypokalemia rate although with only 5 events, the study lacked power. The network meta-analysis by Ernst et al29 found a small difference in potassium reduction. Two analyses of the MRFIT cohort25,30 showed increased reduction of potassium for chlorthalidone when both drugs were used at high doses (50 mg-100 mg). A study of real-world evidence by Dhalla et al5 showed odds ratios around 3 for hypokalemia, matching our results well, even in the 2 strata in which chlorthalidone was half the dose of hydrochlorothiazide; it also found an HR of 1.68 for hyponatremia.
Limitations
Our main limitation is the possibility of residual confounding including confounding by indication, differences in physician characteristics that may be associated with drug choice, concomitant use of other drugs started after the index date, differences in blood pressure measurement error, and informative censoring at the end of the on-treatment periods. To minimize this risk, we used newer methods to account for bias and to detect residual bias through our negative and positive controls.
Conclusions
Our findings based on currently available data and the most recent advances in observational research do not support the use of chlorthalidone over hydrochlorothiazide. This study found that chlorthalidone use was not associated with significant cardiovascular benefits when compared with hydrochlorothiazide, while its use was associated with greater risk of renal and electrolyte abnormalities. We acknowledge the possibility of residual confounding despite our analytic approach and diagnostics and look forward to the results of the ongoing randomized clinical trial.
eAppendix.
eFigure 1. Covariate balance after inclusion of baseline blood pressure in the PanTher database
eFigure 2. Sensitivity to balancing on baseline blood pressure in the PanTher database
eFigure 3. Changes in blood potassium level in the PanTher database
eFigure 4. Comparability of the populations for the three databases
eFigure 5. Homogeneity on effectiveness and hypokalemia across databases and analytic approaches
eFigure 6. Forest plot of safety and effectiveness outcomes across analytic approaches
eFigure 7. Kaplan-Meier plots for effectiveness and hypokalemia outcomes across databases and analytic approaches
eTable 1. Effectiveness and safety for CCAE for stratified on-treatment for limited dosing: 12.5mg chlorthalidone versus 25mg hydrochlorothiazide
eTable 2. Effectiveness and safety for CCAE for matching on-treatment for limited dosing: 12.5mg chlorthalidone versus 25mg hydrochlorothiazide
eTable 3. Baseline characteristics for CCAE with stratification
eTable 4. Baseline characteristics for Optum with stratification
eTable 5. Baseline characteristics for PanTher with stratification
eTable 6. Baseline characteristics for CCAE with matching
eTable 7. Baseline characteristics for Optum with matching
eTable 8. Baseline characteristics for PanTher with matching
eTable 9. Effectiveness and safety for meta-analysis for stratified on-treatment (these are the primary results reported in the main paper)
eTable 10. Effectiveness and safety for meta-analysis for stratified intent-to-treat
eTable 11. Effectiveness and safety for meta-analysis for matching on-treatment
eTable 12. Effectiveness and safety for meta-analysis for matching intent-to-treat
eReferences
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task force on clinical practice guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Cushman WC, Ferguson RE, Brophy MT, Fiore Md LD. Chlorthalidone Versus hydrochlorothiazide: a new kind of Veterans Affairs cooperative study. Ann Intern Med. 2016;165(9):663-664. doi: 10.7326/M16-1208 [DOI] [PubMed] [Google Scholar]
- 3.Olde Engberink RH, Frenkel WJ, van den Bogaard B, Brewster LM, Vogt L, van den Born BJ. Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality: systematic review and meta-analysis. Hypertension. 2015;65(5):1033-1040. doi: 10.1161/HYPERTENSIONAHA.114.05122 [DOI] [PubMed] [Google Scholar]
- 4.Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi: 10.1161/HYPERTENSIONAHA.112.191106 [DOI] [PubMed] [Google Scholar]
- 5.Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158(6):447-455. doi: 10.7326/0003-4819-158-6-201303190-00004 [DOI] [PubMed] [Google Scholar]
- 6.Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47(3):352-358. doi: 10.1161/01.HYP.0000203309.07140.d3 [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med. 2012;125(12):1229.e1-1229.e10. doi: 10.1016/j.amjmed.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 8.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005-2014. doi: 10.1093/ije/dyy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci USA. 2018;115(11):2571-2577. doi: 10.1073/pnas.1708282114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsitch M, Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuemie MJ, Ryan PB, Hripcsak G, Madigan D, Suchard MA. Improving reproducibility by using high-throughput observational studies with empirical calibration. Philos Trans A Math Phys Eng Sci. 2018;376(2128):20170356. doi: 10.1098/rsta.2017.0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574-578. [PMC free article] [PubMed] [Google Scholar]
- 13.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394(10211):1816-1826. doi: 10.1016/S0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan PB, Schuemie MJ, Gruber S, Zorych I, Madigan D. Empirical performance of a new user cohort method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36(suppl 1):S59-S72. doi: 10.1007/s40264-013-0099-6 [DOI] [PubMed] [Google Scholar]
- 15.Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19(1):54-60. doi: 10.1136/amiajnl-2011-000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 18.GitHub. OHDSI/CohortMethod: New-user cohort method with large scale propensity and outcome models. https://github.com/OHDSI/CohortMethod. Accessed January 13, 2020.
- 19.Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23(1):10. doi: 10.1145/2414416.2414791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 21.Walker AM, Patrick AR, Lauer MS, et al. A tool for assessing the feasibility of comparative effectiveness research. Comp Effectiveness Res. 2013;3:11-20. doi: 10.2147/CER.S40357 [DOI] [Google Scholar]
- 22.Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72-81. doi: 10.1016/j.jbi.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4: effects of various classes of antihypertensive drugs–overview and meta-analyses. J Hypertens. 2015;33(2):195-211. doi: 10.1097/HJH.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 24.Williams B, Mancia G, Spiering W, et al. ; Task Force Members . 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953-2041. doi: 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 25.Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi: 10.1161/HYPERTENSIONAHA.110.161505 [DOI] [PubMed] [Google Scholar]
- 26.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417-428. doi: 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi: 10.1161/01.HYP.0000103632.19915.0E [DOI] [PubMed] [Google Scholar]
- 28.Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension. 2012;59(6):1104-1109. doi: 10.1161/HYPERTENSIONAHA.111.190637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23(4):440-446. doi: 10.1038/ajh.2010.1 [DOI] [PubMed] [Google Scholar]
- 30.Ernst ME, Neaton JD, Grimm RH Jr, et al. ; Multiple Risk Factor Intervention Trial Research Group . Long-term effects of chlorthalidone versus hydrochlorothiazide on electrocardiographic left ventricular hypertrophy in the multiple risk factor intervention trial. Hypertension. 2011;58(6):1001-1007. doi: 10.1161/HYPERTENSIONAHA.111.181248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.
eFigure 1. Covariate balance after inclusion of baseline blood pressure in the PanTher database
eFigure 2. Sensitivity to balancing on baseline blood pressure in the PanTher database
eFigure 3. Changes in blood potassium level in the PanTher database
eFigure 4. Comparability of the populations for the three databases
eFigure 5. Homogeneity on effectiveness and hypokalemia across databases and analytic approaches
eFigure 6. Forest plot of safety and effectiveness outcomes across analytic approaches
eFigure 7. Kaplan-Meier plots for effectiveness and hypokalemia outcomes across databases and analytic approaches
eTable 1. Effectiveness and safety for CCAE for stratified on-treatment for limited dosing: 12.5mg chlorthalidone versus 25mg hydrochlorothiazide
eTable 2. Effectiveness and safety for CCAE for matching on-treatment for limited dosing: 12.5mg chlorthalidone versus 25mg hydrochlorothiazide
eTable 3. Baseline characteristics for CCAE with stratification
eTable 4. Baseline characteristics for Optum with stratification
eTable 5. Baseline characteristics for PanTher with stratification
eTable 6. Baseline characteristics for CCAE with matching
eTable 7. Baseline characteristics for Optum with matching
eTable 8. Baseline characteristics for PanTher with matching
eTable 9. Effectiveness and safety for meta-analysis for stratified on-treatment (these are the primary results reported in the main paper)
eTable 10. Effectiveness and safety for meta-analysis for stratified intent-to-treat
eTable 11. Effectiveness and safety for meta-analysis for matching on-treatment
eTable 12. Effectiveness and safety for meta-analysis for matching intent-to-treat
eReferences