This systematic review and meta-analysis examines the association of use of gastric acid suppressants with the risk of colonization with multidrug-resistant microorganisms (MDROs).
Key Points
Question
Is gastric acid suppression therapy associated with an increased risk of intestinal colonization with multidrug-resistant microorganisms?
Findings
This systematic review and meta-analysis, including 26 observational studies and 29 382 patients, found that the use of acid suppressants was associated with an increased risk of colonization of the intestinal tract with multidrug-resistant microorganisms of the Enterobacterales order (producing extended-spectrum β-lactamases, carbapenemases, or plasmid-mediated AmpC β-lactamases) and with vancomycin-resistant enterococci.
Meaning
This adverse effect of acid suppressant use adds to others recently described and, in view of the global increase in antimicrobial resistance, calls for a more prudent use of acid suppression therapy, which may help to reduce multidrug-resistant microorganism colonization rates.
Abstract
Importance
Acid suppressants inhibit gastric acid secretion and disrupt the intestinal microbiome. Whether acid suppression increases the risk of colonization with multidrug-resistant microorganisms (MDROs) is unclear.
Objectives
To systematically examine the association of use of acid suppressants with the risk of colonization with MDROs and to perform a meta-analysis of current evidence.
Data Sources
PubMed, Embase, the Web of Science Core Collection, and the Cochrane Central Register of Controlled Trials were searched from database inception through July 8, 2019.
Study Selection
Study selection was performed independently by 2 authors (R.P.J.W. and C.M.J.E.V.-G.) on the basis of predefined selection criteria; conflicts were resolved by consensus or by an adjudicator (K.v.D.). Human observational studies (case control, cohort, and cross-sectional) and clinical trial designs were selected if they quantified the risk of MDRO colonization in users of acid suppressants in comparison with nonusers.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) recommendations were followed. Data were extracted independently by the same 2 authors, and adjudication was conducted when necessary. Risk of bias was assessed according to a modified Newcastle-Ottawa Scale. Pooled odds ratios (ORs) were estimated using random-effects models; heterogeneity was evaluated using the I2 method.
Main Outcomes and Measures
The primary outcome measure was intestinal colonization with MDROs of the Enterobacterales order (producing extended-spectrum β-lactamases, carbapenemases, or plasmid-mediated AmpC β-lactamases), vancomycin-resistant enterococci, methicillin-resistant or vancomycin-resistant Staphylococcus aureus, or multidrug-resistant Pseudomonas or Acinetobacter species.
Results
A total of 26 observational studies including 29 382 patients (11 439 [38.9%] acid suppressant users) met the selection criteria. Primary meta-analysis of 12 studies including 22 305 patients that provided adjusted ORs showed that acid suppression increased the odds of intestinal carriage of MDROs of the Enterobacterales order and of vancomycin-resistant enterococci by roughly 75% (OR = 1.74; 95% CI, 1.40-2.16; I2 = 68%). The odds were concordant with the secondary pooled analysis of all 26 studies (OR = 1.70; 95% CI, 1.44-1.99; I2 = 54%). Heterogeneity was partially explained by variations in study setting and the type of acid suppression.
Conclusions and Relevance
Acid suppression is associated with increased odds of MDRO colonization. Notwithstanding the limitations of observational studies, the association is plausible and is strengthened by controlling for confounders. In view of the global increase in antimicrobial resistance, stewardship to reduce unnecessary use of acid suppressants may help to prevent MDRO colonization.
Introduction
Antibiotic resistance is an increasing threat to human health.1 Carriers of multidrug-resistant microorganisms (MDROs) are at increased risk for developing infections that are difficult to treat and may contribute to further spread of these strains.2,3,4,5 To our knowledge to date, several risk factors for colonization with MDROs have been described, including antibiotic use, age, underlying illness, and international travel.6,7,8 Recent evidence has pointed to the use of acid suppression therapy as a possible risk factor for colonization with MDROs.9,10
Acid suppressants inhibit stomach acid secretion and can change the composition of the intestinal microbiome11,12,13; stomach acid and a healthy intestinal microbiome protect the gastrointestinal tract against colonization by exogenous bacteria.14 Whether acid suppression facilitates colonization and infection with MDROs remains unclear. Current evidence from observational studies has been inconsistent, considering that some epidemiologic studies report an increased risk of MDRO colonization with acid suppression,10 whereas others do not demonstrate such an association.15
During the past couple of decades, acid suppressants have become widely prescribed and are freely available at drugstores.16 According to data from the National Health and Nutrition Examination Survey,17 nearly 8% of US adults used proton pump inhibitors (PPIs) in 2011 and 2012, a doubling compared with 1999 and 2000. This PPI use is highest in older adults—approximately 17% of those aged 60 to 79 years use PPIs.18 In addition, as much as 50% to 70% of PPI use seems to be inappropriate based on incorrect indications or failure to stop use when no longer needed.19,20,21,22 In view of the possible risks associated with use of these drugs, we performed a systematic review and meta-analysis to determine whether acid suppression therapy is associated with colonization by MDROs.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.23,24 The protocol was preregistered in PROSPERO (CRD42018092541). In the eMethods in the Supplement, we describe the MDROs eligible for inclusion, search strategies, data collection items, and quality assessment scale in detail.
Eligibility Criteria
Clinical and observational studies (cohort, case control, and cross-sectional) were selected when they reported the association of acid suppression with the risk of colonization with MDROs in human participants. Eligible studies investigated intestinal carriage with the target MDROs. We considered urinary tract infections (UTIs) to be a proxy of rectal carriage, since most UTIs are caused by bacterial species that colonize the intestinal tract.25,26 Therefore, studies of UTI were also included. We placed no restrictions on study setting, size, or location. The inclusion was limited to studies reporting enough data to calculate odds ratios (ORs) and their corresponding 95% CIs. Studies restricted to populations with Clostridium difficile were excluded because acid suppression is a well-known risk factor for infection with this microorganism.27
Search Strategy and Study Selection
PubMed, Embase, the Web of Science Core Collection (Clarivate Analytics), and the Cochrane Central Register of Controlled Trials (Wiley-Cochrane Library) were systematically searched from database inception through July 8, 2019 (R.P.J.W. and J.C.F.K.), without language restrictions. We used index terms or free-text words (including synonyms and closely related words) that were associated with MDROs and acid suppressants. Second, we performed a cross-reference check of relevant articles and reviews, supplemented by a search of the European Society of Clinical Microbiology and Infectious Diseases eLibrary. The most up-to-date versions of full-text publications were included.
Study selection was performed in 2 stages using a validated Web application.28 First, titles and abstracts were screened; then, selected full-text articles were included according to the eligibility criteria. Screening was performed independently by 2 authors (R.P.J.W. and C.M.J.E.V.-G.). Conflicts were handled by consensus, and an adjudicator (K.v.D.) was consulted when necessary.
Data Collection
Data were collected independently by R.P.J.W. and C.M.J.E.V.-G. using a predesigned spreadsheet (Excel [Microsoft]) that was pilot-tested beforehand. Conflicts were settled by discussion or adjudication (K.v.D.).
Collected data items included authors, year of publication, study setting and design, participant characteristics, details of acid suppressant use, outcomes, and risk estimators. Acid suppression was categorized according to the Anatomical Therapeutic Chemical classification system.29 Most studies defined acid suppressant use as current use or any use within a specific time window before the index date. Corresponding authors were asked via email to clarify or provide additional information.
Outcomes
The outcome of interest was intestinal colonization with target MDROs. In addition, we included studies investigating the association of UTI with MDROs of the Enterobacterales order (MDR-E).
Risk of Bias Assessment
Along with data extraction, 2 authors (R.P.J.W. and C.M.J.E.V.-G.) independently judged study quality according to a modified Newcastle-Ottawa Scale30 without blinding to authors or journals. Conflicts were resolved either by consensus or by the adjudicator (K.v.D.).
Statistical Analysis
First, pooled ORs with 95% CIs were estimated using random-effects meta-analysis with the generic inverse-variance method for only studies that provided fully adjusted ORs.31 In a second analysis with this same method, we included all studies; in this analysis, fully adjusted ORs were used when available. Inconsistency across studies was measured with the I2 method. Cutoff values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively.32 We visualized the results with forest plots.
Second, to examine heterogeneity, we performed analyses of predefined subgroups based on study design and type of acid suppressant studied. Subsequent subgroup analyses were conducted by looking further into target MDROs and study setting. Next, to determine the influence of the surrogate outcome measure of UTI, all analyses were repeated with exclusion of the studies of UTI. Additionally, to address potential bias and verify our results, we performed various sensitivity analyses by (1) excluding low-quality studies, (2) restricting the analysis to high-quality studies that adjusted for classic confounders, (3) using a leave-one-out method, (4) Mantel-Haenszel weighting, and (5) calculating the summary estimate with the Knapp-Hartung modification.33
Finally, to investigate the risk of publication bias, we applied the Egger test and the test used by Peters et al31,34,35 and visually inspected the funnel plots.All analyses were carried out using Review Manager, version 5.3 (Nordic Cochrane Centre), complemented by STATA statistical software, version 14.1 (StataCorp).
Results
Study Selection
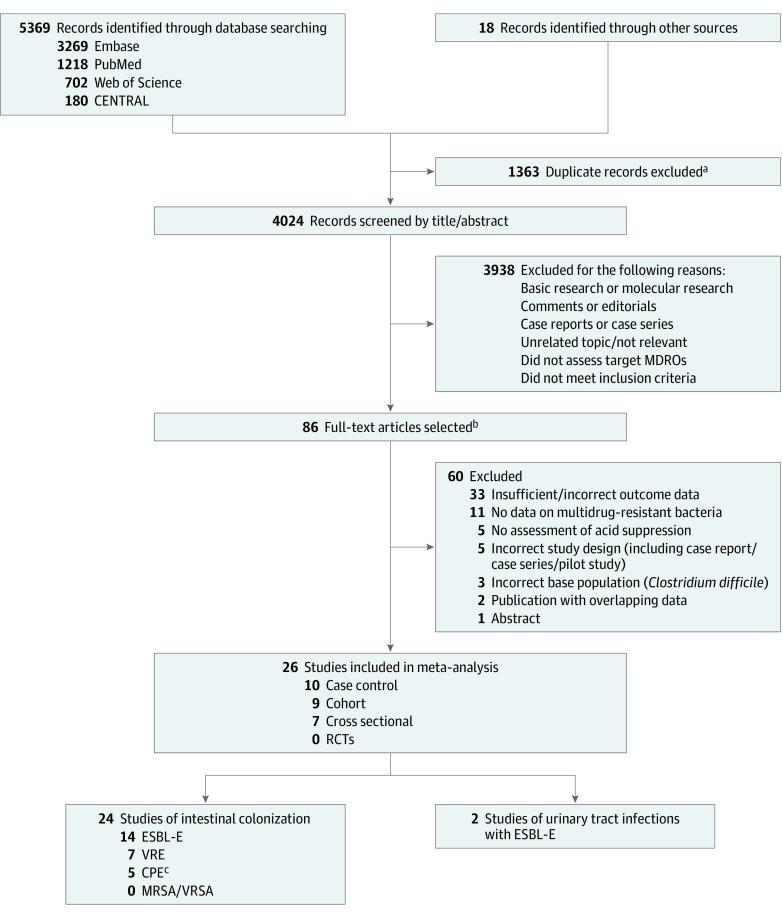
Study selection is presented in Figure 1.36 We retained 26 nonduplicate studies that met the purpose of the meta-analysis.8,9,10,15,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 Among these 26 studies, 2 clinical studies of interventions not related to the use of acid suppressants were included as cohort studies because no intervention effect was found and they included the analysis of exposure to acid suppressants as a covariate.50,53 A total of 24 studies measured intestinal carriage, 19 of MDR-E8,9,10,15,36,37,39,42,43,44,45,46,48,49,51,52,54,56,57 and 7 of vancomycin-resistant enterococci (VRE).38,40,41,47,50,53,55 Additionally, 2 studies had UTI as the outcome measure.46,54 We found no eligible randomized clinical trials and no eligible studies of intestinal colonization with methicillin-resistant Staphylococcus aureus or vancomycin-resistant S aureus. One study of carbapenemase-producing microorganisms included Pseudomonas and Acinetobacter species.36 We contacted 12 author groups.8,9,10,37,40,41,42,44,54,55,56,57 Authors from all but 1 study responded, and those from 5 of the studies provided additional data that we included in the analyses.8,9,41,55,56
Figure 1. PRISMA Diagram of Study Selection.
CENTRAL indicates Cochrane Central Register of Controlled Trials; CPE, carbapenemase-producing multidrug-resistant microorganisms of the Enterobacterales order; ESBL-E, extended-spectrum β-lactamase–producing multidrug-resistant microorganisms of the Enterobacterales order; MDROs, multidrug-resistant microorganisms; MRSA, methicillin-resistant Staphylococcus aureus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT, randomized clinical trial; VRE, vancomycin-resistant enterococci; VRSA, vancomycin-resistant S aureus.
aEndNote software (Clarivate Analytics) was used to remove duplicates.
bThe Cohen κ indicated strong agreement for the full-text stage (κ = 0.82).
cGoodman et al36 included carbapenemase-producing Acinetobacter and Pseudomonas species in addition to CPE.
Study Characteristics
The 26 studies included 29 382 participants (11 439 [38.9%] were acid suppressant users; 15 866 [54.0%] were female). Twelve studies provided risk estimates that were adjusted for confounding using multivariable analysis.8,9,10,37,39,40,43,45,47,54,55,57 Overall, the 12 studies included 22 305 participants (8491 [38.1%] were acid suppressant users; 12 714 [57.0%] were female). Of these, 7 studies were cross-sectional,9,10,37,43,45,55,57 3 were case control,39,40,54 and 2 were cohort studies.8,47
We summarized the study characteristics in the Table. Studies were published between 1996 and 2019; most were of adult populations (age ≥18 years). Three studies were designed to determine the risk associated with acid suppressants,9,39,54 whereas the remaining studies evaluated risk factors in general. Most studies were conducted in the World Health Organization European region (13 of 26 studies) and the region of the Americas (11 of 26 studies) (eTable 1 in the Supplement). Baseline values together with covariates adjusted for, as well as details of exposure and outcome ascertainment, are presented in eTables 2, 3, and 4 in the Supplement.
Table. Study Characteristics.
| Source; Country | Years of Study | Design | Study Setting | Outcome Measure | MDRO Subtype | Acid Suppression Therapy | Sampling Method |
|---|---|---|---|---|---|---|---|
| With Statistical Adjustment | |||||||
| Arcilla et al,8 2017; the Netherlands | 2012-2013 | Cohort, prospective, multicenter | Travel clinics | Colonization | ESBL-E | Acid suppression, unspecified | Stool |
| Ben-Ami et al,37 2006; Israel | 2002-2003 | Cross-sectionala | Tertiary care hospital | Colonization | ESBL-E | PPIs and H2RAs | Stool |
| Cheng et al,39 2016; China | 2011-2015 | Case control, prospective multicenterb | Hospitals (teaching hospital and multiple extended-care hospitals) | Colonization | CPE | PPIs | Stool or rectal swab |
| Falk et al,40 2000; United States | 1996-1997 | Case control, retrospective | University hospital burn ICU | Colonization | VRE | Antacids | Rectal swab |
| Hamprecht et al,43 2016; Germany | 2014 | Cross-sectional, multicentera | Tertiary care hospitals | Colonization | ESBL-Ec | Acid suppression, unspecified | Stool or rectal swab |
| Huizinga et al,9 2017; the Netherlands | 2014; 2015 | Cross-sectionala,b | Teaching hospital | Colonization | ESBL-E | PPIs and H2RAs | Rectal swab |
| Latour et al,45 2019 Belgium | 2015 | Cross-sectional, multicenter | Nursing homes | Colonization | ESBL-E | PPIs and H2RAs | Rectal swab |
| McNeil et al,47 2006; United States | 2000-2003 | Cohort, prospective | Tertiary care hospital liver transplant unit | Colonization | VRE | PPIs | Stool or rectal swab |
| Reuland et al,10 2016; the Netherlands | 2011 | Cross-sectional | Community | Colonization | ESBL-E | PPIs, H2RAs, and antacids | Stool or perirectal swab |
| Søgaard et al,54 2017; Denmark | 2007-2012 | Case control, retrospectiveb | Community | Urinary tract infection | ESBL-E | PPIs | Urine |
| Tan et al,55 2018; Singapore | 2014; 2015; 2016 | Cross-sectional, multicenter | Mixed (acute-care hospital and multiple intermediate-term and long-term care facilities) | Colonization | VRE | PPIs, H2RAs, and antacids | Stool or rectal swab |
| Wielders et al,57 2017; the Netherlands | 2014-2015 | Cross-sectional | Community | Colonization | ESBL-E; AmpC-E | PPIs | Stool |
| Without Statistical Adjustment | |||||||
| Chanderraj et al,38 2019; United States | 2013-2016 | Case control, retrospective | Tertiary care hospital (ICU, hemato-oncology unit, and bone marrow transplant unit) | Colonization | VRE | PPIs | Rectal swab |
| Ford et al,41 2015; United States | 2006-2012 | Cohort, retrospective | Tertiary care hospital (hematology and bone marrow transplant units) | Colonization | VRE | PPIs | Stool |
| Goodman et al,36 2019; United States | 2016-2017 | Cross-sectionala | Teaching hospital (medical ICU or solid-organ transplant unit) | Colonization | CPOd | PPIs and H2RAs | Perirectal swab |
| Hagel et al,42 2019; Germany | 2013-2015 | Cohort, prospective | University hospital | Colonization | ESBL-E | Acid suppression, unspecified | Rectal swab |
| Kuenzli et al,44 2014; Switzerland | 2012-2013 | Cohort, prospective, multicenter | Travel clinics | Colonization | ESBL-E | PPIs | Rectal swab |
| Lee et al,46 2018; Republic of Korea | 2015-2016 | Case control, retrospective | University hospital emergency department | Urinary tract infection | ESBL-E | PPIs | Urine |
| Okamoto et al,15 2017; United States | 2012-2013 | Case control, prospective, multicenter | Long-term acute-care hospitals | Colonization | KPC-E | PPIs and H2RAs | Rectal swab |
| Östholm-Balkhed et al,48 2013; Sweden | 2008-2009 | Cohort, prospective, multicenter | Vaccination clinics | Colonization | ESBL-E | Acid suppression, unspecified | Stool |
| Prasad et al,49 2016; United States | NA | Cross-sectional | Long-term care facility | Colonization | KPC-E | PPIs | Rectal swab |
| Puzniak et al,50 2001; United Statese | 1997-1998 | Cohort, prospective | Tertiary care hospital medical ICU | Colonization | VRE | Acid suppression, unspecified | Stool or rectal swab |
| Rodríguez-Baño et al,51 2008; Spain | 2005-2006 | Cross-sectional | Community | Colonization | ESBL-E | Acid suppression, unspecified | Stool |
| Seekatz et al,52 2018; United States | 2014-2016 | Case control, prospective | Long-term acute-care hospital | Colonization | KPC-E | PPIs | Stool or rectal swab |
| Slaughter et al,53 1996; United Statese | 1994-1995 | Cohort, prospective | Teaching hospital medical ICU | Colonization | VRE | PPIs, H2RAs, and antacids | Rectal swab |
| Vading et al,56 2016; Sweden | 2013-2015 | Cohort, prospective | Travel clinic | Colonization | ESBL-E | PPIs and antacids | Rectal swab |
Abbreviations: AmpC-E, plasmid-mediated AmpC β-lactamase–producing multidrug-resistant microorganisms of the Enterobacterales order; CPE, carbapenemase-producing multidrug-resistant microorganisms of the Enterobacterales order; CPO, carbapenemase-producing organisms; ESBL-E, extended-spectrum β-lactamase–producing multidrug-resistant microorganisms of the Enterobacterales order; H2RA, histamine2 receptor antagonist; ICU, intensive care unit; KPC-E, Klebsiella pneumoniae carbapenemase-producing multidrug-resistant microorganisms of the Enterobacterales order; MDROs, multidrug-resistant microorganisms; NA, not available; PPI, proton pump inhibitor; VRE, vancomycin-resistant enterococci.
Studies that used screening at admission to the hospital.
Studies specifically designed to assess the risk associated with acid suppression (all other studies evaluated acid suppression as 1 risk factor among many).
Study assessed third-generation cephalosporin-resistant MDR-E; ESBL was the predominant resistance mechanism detected in 90% of the isolates.
Study assessed carbapenemase-producing glucose-nonfermenting Gram-negative MDR-E in addition to CPE.
Intervention studies analyzed as cohort studies.
Risk of Bias and Primary Analysis
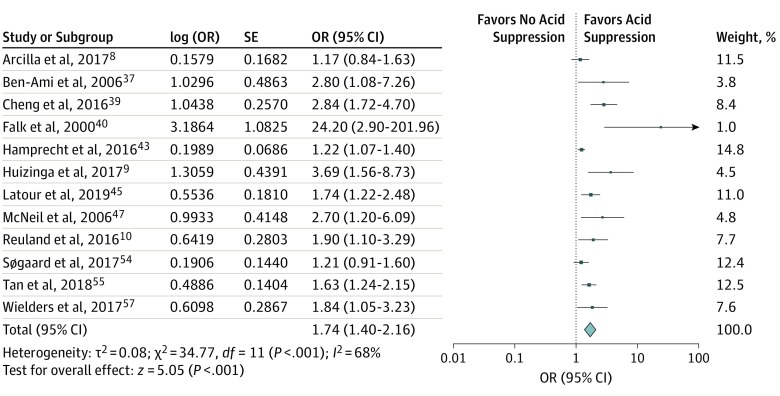
The median (range) Newcastle-Ottowa Scale30 score was 6 (3-9) (eTable 5 in the Supplement). In the primary analysis, we included the 12 studies that adjusted for confounding.8,9,10,37,39,40,43,45,47,54,55,57 This showed that acid suppression was associated with MDRO colonization (OR = 1.74; 95% CI, 1.40-2.16) (Figure 2). Among these studies, heterogeneity, as measured using the I2 method, was 68%. Restriction of the analysis to the 11 studies8,9,10,37,39,40,43,45,47,55,57 that directly evaluated intestinal carriage (not UTI) yielded a summary OR of 1.86 (95% CI, 1.46-2.37); heterogeneity remained the same (I2 = 70%).
Figure 2. Forest Plot for the Association of Multidrug-Resistant Microorganism Colonization With Acid Suppression.
Odds ratios (ORs) are presented as random effects with inverse variance (except for the log [OR] column). Among studies, acid suppression mainly included exposure to proton pump inhibitors and/or histamine2 receptor antagonists, with few studies including other antacids.
Secondary Analysis
A secondary analysis of all 26 studies revealed odds consistent with those found in the primary analysis and showed that acid suppression was associated with MDRO colonization (OR = 1.70; 95% CI, 1.44-1.99; I2 = 54%) (eFigure 1 in the Supplement). Analysis of the 24 studies8,9,10,15,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,55,56,57 that directly evaluated intestinal carriage yielded an OR of 1.77 (95% CI, 1.48-2.10; I2 = 56%).
Subgroup Analysis
By MDRO Subtype
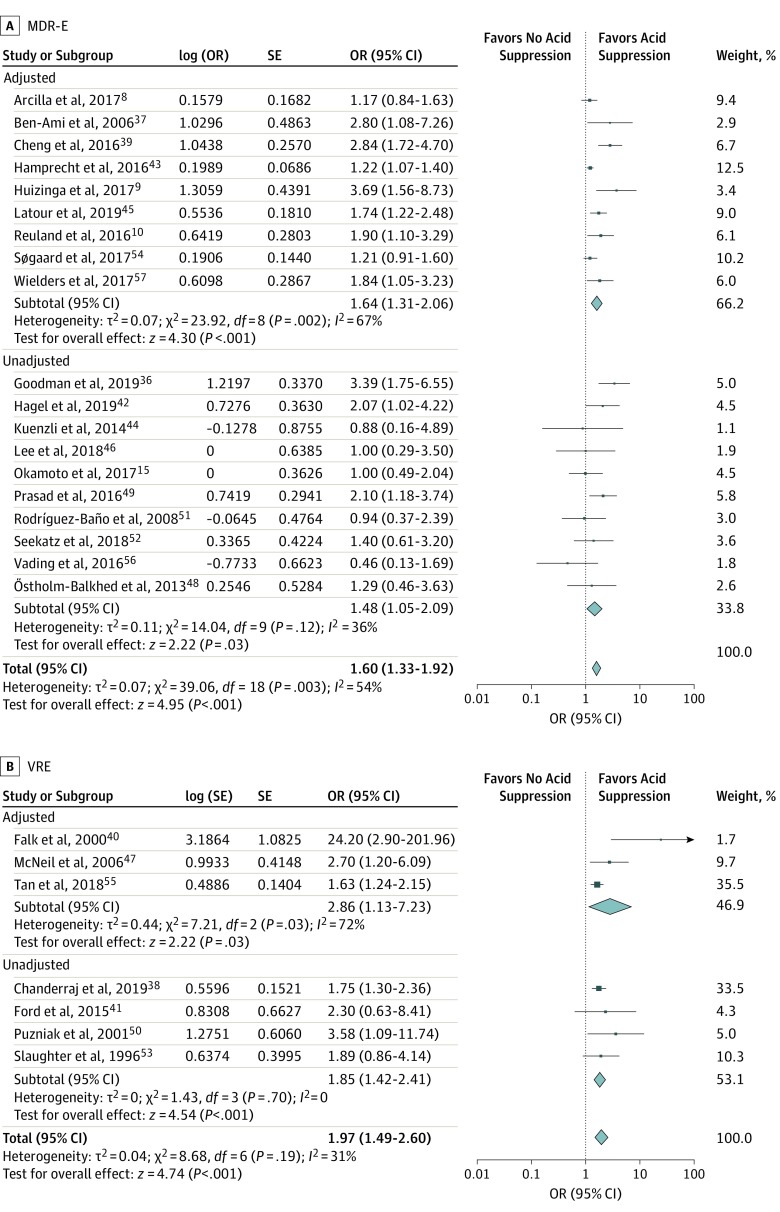
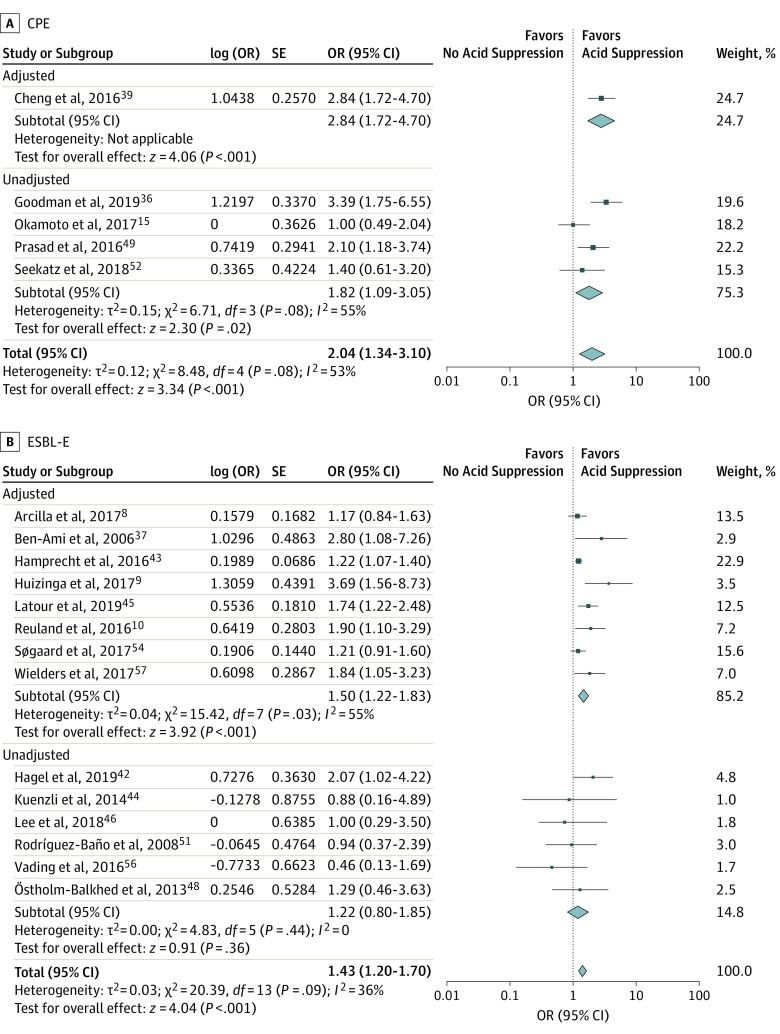
Acid suppression was associated with MDR-E carriage as well as VRE carriage (MDR-E: OR = 1.60; 95% CI, 1.33-1.92; I2 = 54%; VRE: OR = 1.97; 95% CI, 1.49-2.60; I2 = 31%). The association was larger for carbapenemase-producing MDR-E (CPE) than for extended-spectrum β-lactamase–producing MDR-E (ESBL-E), although the ORs had overlapping CIs (CPE: OR = 2.04; 95% CI, 1.34-3.10; I2 = 53%; ESBL-E: OR = 1.43; 95% CI, 1.20-1.70; I2 = 36%) (Figure 3 and Figure 4).
Figure 3. Subgroup Analysis by Multidrug-Resistant Microorganism Subtype.
A, Multidrug-resistant microorganisms of the Enterobacterales order (MDR-E). B, Vancomycin-resistant enterococci (VRE). Odds ratios (ORs) are presented as random effects with inverse variance (except for the log [OR] column).
Figure 4. Subgroup Analysis by Multidrug-Resistant Microorganism Subtype.
A, Carbapenemase-producing multidrug-resistant microorganisms of the Enterobacterales order (CPE). B, Extended-spectrum β-lactamase–producing multidrug-resistant microorganisms of the Enterobacterales order (ESBL-E). Odds ratios (ORs) are presented as random effects with inverse variance (except for the log [OR] column).
By Design
To evaluate the influence of research methods, we performed a subgroup analysis by study design. Overall, the association of acid suppression therapy with MDRO colonization was marginally moderated by study design (cohort: OR = 2.31; 95% CI, 1.56-3.43; I2 = 0%; case control: OR = 1.64; 95% CI, 1.13-2.38; I2 = 66%; cross-sectional: OR = 1.84; 95% CI, 1.47-2.30; I2 = 58%) (eFigure 2 in the Supplement).
By Type of Acid Suppressant
To evaluate whether the association depended on the type of acid suppressant used, we restricted the analysis to PPI users because PPIs exert more potent acid suppression than histamine2 receptor antagonists (H2RAs).58 Seventeen studies9,10,36,37,38,39,41,44,45,46,47,49,52,54,55,56,57 reported the risk of MDRO colonization in PPI users only; the meta-analysis yielded an OR of 1.81 (95% CI, 1.52-2.16; I2 = 33%). Four studies9,10,37,55 reported risk in H2RA users only. Use of these drugs did not seem to be associated with MDRO colonization (OR = 1.33; 95% CI, 0.86-2.08; I2 = 15%) (eFigure 3 in the Supplement); the large CI suggests that the lack of association may be due to the small number of studies.
By Setting
We divided the 15 hospital-based studies into 2 groups: 4 studies9,36,37,43 evaluated colonization at admission (screening within 48 hours of admission), and 11 studies15,38,39,40,41,42,46,47,50,52,53 evaluated colonization during hospital stay. The OR of colonization with MDROs at hospital admission was 2.39 (95% CI, 1.17-4.87; I2 = 82%). Meta-analysis of the studies that focused on colonization during hospital stay showed a similar association (OR = 1.98; 95% CI, 1.50-2.62; I2 = 33%); 4 community-based studies10,51,54,57showed similar results but with a slightly smaller association (OR = 1.41; 95% CI, 1.07-1.87; I2 = 21%). However, meta-analysis of 4 travel-based studies8,44,48,56 yielded an OR with a very broad CI (OR = 1.11; 95% CI, 0.82-1.50; I2 = 0%) (eFigure 4 in the Supplement). Three studies were conducted in residents of long-term care facilities and were therefore excluded from this subgroup analysis.45,49,55
Sensitivity Analysis
To ascertain the strength of our results, we performed additional sensitivity analyses (eTables 6, 7, and 8 and eFigures 5 and 6 in the Supplement). The results were consistent; the association remained significant in all analyses.
Both Mantel-Haenszel weighting and the Knapp-Hartung33 estimators yielded similar results. Using the leave-one-out method, we found no studies that influenced the results disproportionately (lowest value: OR = 1.64; 95% CI, 1.40-1.92; highest value: OR = 1.75; 95% CI, 1.49-2.07).
Restriction of the analyses to high-quality studies of intestinal carriage did not substantially change the summary estimate (OR = 1.74; 95% CI, 1.42-2.14; I2 = 64%).8,9,10,15,36,38,39,43,50,52,53,55,57 Four of these studies adjusted for at least age, sex, and antibiotic use and had a maximum Newcastle-Ottawa Scale30 score for ascertainment of the exposure; their summary estimate (OR = 2.15; 95% CI, 1.52-3.04; I2 = 49%)9,10,39,55 was similar to that of the primary meta-analysis.
Publication Bias
We observed no evidence of publication bias with inspection of the funnel plot or with the Egger test or the test used by Peters et al.31,34,35 Excluding both studies of UTI did not affect publication bias estimators (eFigure 7 in the Supplement).
Discussion
This systematic review and meta-analysis showed that the use of acid suppressants (mainly PPIs or H2RAs) is associated with a 75% increase in the odds of intestinal MDRO colonization, both in the community and in the health care setting. This association was found in a primary analysis of the 12 studies8,9,10,37,39,40,43,45,47,54,55,57 covering more than 22 000 patients, which provided adjusted risk estimates, as well as in the secondary analysis of all studies8,9,10,15,37,38 (>29 000 patients). The risk was similar for colonization with Gram-negative MDR-E and Gram-positive enterococci. The results from our sensitivity analyses, in which we address the risk of bias and confounding, buttress these findings.
Acid suppressants may promote colonization with MDROs through 3 different mechanisms. First, and most important, acid suppressants reduce gastric acid secretion; this is associated with bacterial survival and in turn the amount of viable exogenous bacteria that pass through the stomach to reach the intestine.59 Second, such agents have been shown to directly alter the composition of intestinal microbiota, leading to a decrease in mean species diversity.11,12,13 This may influence microbiota-mediated colonization resistance. For bacterial species such as VRE and MDR-E, resistance to colonization can be induced by microbiota-driven immune responses or by targeted depletion of nutrients or toxic substances.14 Third, a 2019 study of ESBL-producing Escherichia coli sequence type 13160 showed that these strains contain several protein amino acid substitutions that confer resistance to gastric acid. Therefore, MDROs might be better able to pass the gastric acid barrier. This characteristic may present an additional advantage, even in a gastric environment where this barrier is less effective than normal as a consequence of acid suppressant use.
Acid suppression conferred the largest risk for colonization with VRE and CPE (nearly 2-fold higher odds), whereas for ESBL-E, the OR was approximately 1.4. However, these differences should be interpreted with caution because the CIs of the ORs overlap.
We explored the association according to type of study design and setting. These did not influence the estimates substantially; the odds of MDRO colonization with acid suppression therapy remained nearly 2-fold higher. An exception was found for PPI use among travelers; in this group, there was not an association. However, it is conceivable that the small proportion of acid suppressant users in the traveler cohorts (between 3% and 12% of the total cohort) precluded the identification of an association. In addition, the influence of individual risk factors on the acquisition of intestinal carriage may be overshadowed by the large risk posed by travel to endemic regions.44 Up to 75% of travelers to southern Asia return with ESBL-E in their stool.8
Since the acid suppression induced by PPIs is more profound than that caused by H2RAs, we expected the association of PPI use with MDRO colonization to be larger than that of H2RA with MDRO colonization.58 The risk associated with PPI use was larger than the risk associated with H2RAs. However, the number of studies of H2RAs was small (n = 4),9,10,37,55 and the CI of the estimate was large. Therefore, to clearly define a difference in the associations of PPIs and H2RAs with MDRO colonization, more studies of H2RAs are needed.
Unfortunately, only 2 of the studies reported dose or duration of acid suppression therapy. These 2 studies, both of VRE colonization, did find an association of duration of acid suppressant exposure with increased risk of VRE colonization.38,40
Strengths
To the best of our knowledge, this is the first systematic review and meta-analysis to date of the association of gastric acid suppression with MDRO colonization. We were able to include 12 studies8,9,10,37,39,40,43,45,47,54,55,57 with adjusted ORs, comprising more than 22 000 patients; this large sample yielded an accurate estimate of the effect size. Inclusion of the studies that did not provide adjusted ORs in the analysis yielded the same results. We incorporated several sensitivity analyses to test whether our findings were robust. A major strength is that we strictly adhered to the PRISMA and MOOSE guidelines, following a focused hypothesis.23,24 We applied stringent criteria and restricted our review to studies that analyzed the presence of MDROs in the gastrointestinal tract, the site of action of acid suppression therapy, and the main route of acquisition of the MDROs (ie, MDR-E or enterococci).
Limitations
Our study has some limitations. The studies included in the analysis were heterogeneous, partly owing to differences in exposure and study setting. Nevertheless, we believe the effect of heterogeneity to be small given the steady summary estimates across the subgroup and sensitivity analyses.
This meta-analysis is based on observational studies, which are potentially limited by confounding factors such as age, sex, comorbidity, and especially antibiotic use. Users of PPIs may differ in lifestyle and severity of disease (possibly causing confounding by disease severity). However, analysis of the studies that adjusted for potential confounders showed that the odds of colonization with MDROs were consistently increased by use of acid suppressants.8,9,10,37,39,40,43,45,47,54,55,57 Furthermore, the adjusted group estimates were higher overall than the unadjusted group estimates across all analyses performed.
We included only 2 studies46,54 that investigated the surrogate outcome measure of UTI. Therefore, we cannot draw conclusions about whether the use of acid suppressants also increases the risk of infection with MDROs, irrespective of the association with intestinal carriage. However, the current literature underpins the concept of the intestinal reservoir; intestinal colonization appears to be an important intermediary step toward infection.2,3,4,5
Conclusions
In conclusion, our systematic review showed that acid suppression is associated with an increased risk of colonization with MDROs. This association is biologically plausible but should be interpreted with caution, since evidence from observational studies cannot prove causation. However, this adverse effect adds to many others that were described recently, such as the increased risks of Clostridium difficile colitis, bacterial gastroenteritis, and renal diseases.27,61,62,63,64 We advocate that acid suppressants should be used when necessary but that unnecessary use should be avoided.
Because up to 70% of PPI prescriptions appear to be based on indications without clear benefit20,21 and in view of the ever-growing problem of antimicrobial resistance, we see the possibility of a favorable interaction between infection control, antibiotic stewardship, and the promotion of rational use of PPIs. This rational use could be called PPI stewardship. Future intervention programs may provide further insight about whether the risks of MDRO colonization and infection are reduced after discontinuation of inappropriate acid suppression therapy.
eMethods. Supplemental Methods.
eTable 1. Study Distribution According to World Health Organization Region.
eTable 2. Baseline Characteristics.
eTable 3. Exposure and Outcome Ascertainment.
eTable 4. Adjusted Study Results.
eTable 5. Quality Assessment According to the Modified Newcastle-Ottawa Scale.
eTable 6. Sensitivity Analyses After Exclusion of Studies on Urinary Tract Infections.
eTable 7. Sensitivity Analysis by the Leaving-One-Out Method.
eTable 8. Sensitivity Analysis by Knapp-Hartung Modification.
eFigure 1. Secondary Analysis.
eFigure 2. Subgroup Analysis by Design.
eFigure 3. Subgroup Analysis by Type of Acid Suppressant.
eFigure 4. Subgroup Analysis by Setting.
eFigure 5. Sensitivity Analysis of Studies With High Quality.
eFigure 6. Sensitivity Analyses by Mantel-Haenszel Weighting.
eFigure 7. Funnel Plot.
eReferences.
References
- 1.World Health Organization Antimicrobial resistance: global report on surveillance. https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf. Accessed June 1, 2019.
- 2.Carlet J. The gut is the epicentre of antibiotic resistance. Antimicrob Resist Infect Control. 2012;1(1):39. doi: 10.1186/2047-2994-1-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis. 2012;54(5):707-713. doi: 10.1093/cid/cir899 [DOI] [PubMed] [Google Scholar]
- 4.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39(2):219-226. doi: 10.1086/422002 [DOI] [PubMed] [Google Scholar]
- 5.Martin RM, Cao J, Brisse S, et al. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae [published online October 19, 2016] . mSphere. doi: 10.1128/msphere.00261-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834-844. doi: 10.7326/0003-4819-136-11-200206040-00013 [DOI] [PubMed] [Google Scholar]
- 7.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase–producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63(3):310-318. doi: 10.1093/cid/ciw283 [DOI] [PubMed] [Google Scholar]
- 8.Arcilla MS, van Hattem JM, Haverkate MR, et al. Import and spread of extended-spectrum β-lactamase–producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78-85. doi: 10.1016/S1473-3099(16)30319-X [DOI] [PubMed] [Google Scholar]
- 9.Huizinga P, van den Bergh MK, van Rijen M, Willemsen I, van ’t Veer N, Kluytmans J. Proton pump inhibitor use is associated with extended-spectrum β-lactamase–producing Enterobacteriaceae rectal carriage at hospital admission: a cross-sectional study. Clin Infect Dis. 2017;64(3):361-363. doi: 10.1093/cid/ciw743 [DOI] [PubMed] [Google Scholar]
- 10.Reuland EA, Al Naiemi N, Kaiser AM, et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother. 2016;71(4):1076-1082. doi: 10.1093/jac/dkv441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740-748. doi: 10.1136/gutjnl-2015-310376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749-756. doi: 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi: 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790-801. doi: 10.1038/nri3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto K, Lin MY, Haverkate M, et al. Modifiable risk factors for the spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae among long-term acute-care hospital patients. Infect Control Hosp Epidemiol. 2017;38(6):670-677. doi: 10.1017/ice.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482. doi: 10.1001/jamainternmed.2015.8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. doi: 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hales CM, Servais J, Martin CB, Kohen D. Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada. NCHS Data Brief, No. 347. Hyattsville, MD: National Center for Health Statistics; 2019. [PubMed] [Google Scholar]
- 19.Hanauer SB. Addicted to acid suppression. Nat Rev Gastroenterol Hepatol. 2009;6(9):497. doi: 10.1038/nrgastro.2009.146 [DOI] [PubMed] [Google Scholar]
- 20.Katz MH. Failing the acid test: benefits of proton pump inhibitors may not justify the risks for many users. Arch Intern Med. 2010;170(9):747-748. doi: 10.1001/archinternmed.2010.64 [DOI] [PubMed] [Google Scholar]
- 21.Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol. 2016;111(8):1085-1086. doi: 10.1038/ajg.2016.166 [DOI] [PubMed] [Google Scholar]
- 22.Linsky A, Simon SR. Reversing gears: discontinuing medication therapy to prevent adverse events. JAMA Intern Med. 2013;173(7):524-525. doi: 10.1001/jamainternmed.2013.4068 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) group . Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-284. doi: 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruppé E, Lixandru B, Cojocaru R, et al. Relative fecal abundance of extended-spectrum-β-lactamase–producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrob Agents Chemother. 2013;57(9):4512-4517. doi: 10.1128/AAC.00238-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. FDA drug safety communication: Clostridium difficile–associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-clostridium-difficile-associated-diarrhea-can-be-associated-stomach. Accessed November 14, 2017.
- 28.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Collaborating Centre for Drug Statistics Methodology Web site. Anatomical Therapeutic Chemical classification system. http://www.whocc.no/atc_ddd_index/. Accessed December 20, 2017.
- 30.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 12, 2017.
- 31.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. Chichester, England: Wiley-Blackwell; 2011. [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. doi: 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 34.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676-680. doi: 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman KE, Simner PJ, Klein EY, et al. ; CDC Prevention Epicenters Program . Predicting probability of perirectal colonization with carbapenem-resistant Enterobacteriaceae (CRE) and other carbapenem-resistant organisms (CROs) at hospital unit admission. Infect Control Hosp Epidemiol. 2019;40(5):541-550. doi: 10.1017/ice.2019.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Ami R, Schwaber MJ, Navon-Venezia S, et al. Influx of extended-spectrum beta-lactamase–producing Enterobacteriaceae into the hospital. Clin Infect Dis. 2006;42(7):925-934. doi: 10.1086/500936 [DOI] [PubMed] [Google Scholar]
- 38.Chanderraj R, Millar JA, Patel TS, et al. Vancomycin-resistant Enterococcus acquisition in a tertiary care hospital: testing the roles of antibiotic use, proton pump inhibitor use, and colonization pressure. Open Forum Infect Dis. 2019;6(4):ofz139. doi: 10.1093/ofid/ofz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng VC, Chen JH, So SY, et al. A novel risk factor associated with colonization by carbapenemase-producing Enterobacteriaceae: use of proton pump inhibitors in addition to antimicrobial treatment. Infect Control Hosp Epidemiol. 2016;37(12):1418-1425. doi: 10.1017/ice.2016.202 [DOI] [PubMed] [Google Scholar]
- 40.Falk PS, Winnike J, Woodmansee C, Desai M, Mayhall CG. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol. 2000;21(9):575-582. doi: 10.1086/501806 [DOI] [PubMed] [Google Scholar]
- 41.Ford CD, Lopansri BK, Haydoura S, et al. Frequency, risk factors, and outcomes of vancomycin-resistant Enterococcus colonization and infection in patients with newly diagnosed acute leukemia: different patterns in patients with acute myelogenous and acute lymphoblastic leukemia. Infect Control Hosp Epidemiol. 2015;36(1):47-53. doi: 10.1017/ice.2014.3 [DOI] [PubMed] [Google Scholar]
- 42.Hagel S, Makarewicz O, Hartung A, et al. ESBL colonization and acquisition in a hospital population: the molecular epidemiology and transmission of resistance genes. PLoS One. 2019;14(1):e0208505. doi: 10.1371/journal.pone.0208505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamprecht A, Rohde AM, Behnke M, et al. ; DZIF-ATHOS Study Group . Colonization with third-generation cephalosporin-resistant Enterobacteriaceae on hospital admission: prevalence and risk factors. J Antimicrob Chemother. 2016;71(10):2957-2963. doi: 10.1093/jac/dkw216 [DOI] [PubMed] [Google Scholar]
- 44.Kuenzli E, Jaeger VK, Frei R, et al. High colonization rates of extended-spectrum β-lactamase (ESBL)–producing Escherichia coli in Swiss travellers to South Asia: a prospective observational multicentre cohort study looking at epidemiology, microbiology and risk factors. BMC Infect Dis. 2014;14:528. doi: 10.1186/1471-2334-14-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latour K, Huang TD, Jans B, et al. Prevalence of multidrug-resistant organisms in nursing homes in Belgium in 2015. PLoS One. 2019;14(3):e0214327. doi: 10.1371/journal.pone.0214327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H, Han SB, Kim JH, Kang S, Durey A. Risk factors of urinary tract infection caused by extended spectrum β-lactamase–producing Escherichia coli in emergency department. Am J Emerg Med. 2018;36(9):1608-1612. doi: 10.1016/j.ajem.2018.01.046 [DOI] [PubMed] [Google Scholar]
- 47.McNeil SA, Malani PN, Chenoweth CE, et al. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis. 2006;42(2):195-203. doi: 10.1086/498903 [DOI] [PubMed] [Google Scholar]
- 48.Östholm-Balkhed A, Tärnberg M, Nilsson M, Nilsson LE, Hanberger H, Hällgren A; Travel Study Group of Southeast Sweden . Travel-associated faecal colonization with ESBL-producing Enterobacteriaceae: incidence and risk factors. J Antimicrob Chemother. 2013;68(9):2144-2153. doi: 10.1093/jac/dkt167 [DOI] [PubMed] [Google Scholar]
- 49.Prasad N, Labaze G, Kopacz J, et al. Asymptomatic rectal colonization with carbapenem-resistant Enterobacteriaceae and Clostridium difficile among residents of a long-term care facility in New York City. Am J Infect Control. 2016;44(5):525-532. doi: 10.1016/j.ajic.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 50.Puzniak LA, Mayfield J, Leet T, Kollef M, Mundy LM. Acquisition of vancomycin-resistant enterococci during scheduled antimicrobial rotation in an intensive care unit. Clin Infect Dis. 2001;33(2):151-157. doi: 10.1086/321807 [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Baño J, López-Cerero L, Navarro MD, Díaz de Alba P, Pascual A. Faecal carriage of extended-spectrum beta-lactamase–producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother. 2008;62(5):1142-1149. doi: 10.1093/jac/dkn293 [DOI] [PubMed] [Google Scholar]
- 52.Seekatz AM, Bassis CM, Fogg L, et al. ; Centers for Disease Control and Prevention Epicenters Program . Gut microbiota and clinical features distinguish colonization with Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae at the time of admission to a long-term acute care hospital. Open Forum Infect Dis. 2018;5(8):ofy190. doi: 10.1093/ofid/ofy190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med. 1996;125(6):448-456. doi: 10.7326/0003-4819-125-6-199609150-00004 [DOI] [PubMed] [Google Scholar]
- 54.Søgaard M, Heide-Jørgensen U, Vandenbroucke JP, Schønheyder HC, Vandenbroucke-Grauls CMJE. Risk factors for extended-spectrum β-lactamase–producing Escherichia coli urinary tract infection in the community in Denmark: a case-control study. Clin Microbiol Infect. 2017;23(12):952-960. doi: 10.1016/j.cmi.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 55.Tan D, Htun HL, Koh J, et al. Comparative epidemiology of vancomycin-resistant enterococci colonization in an acute-care hospital and its affiliated intermediate- and long-term care facilities in Singapore. Antimicrob Agents Chemother. 2018;62(12):e01507-18. doi: 10.1128/AAC.01507-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vading M, Kabir MH, Kalin M, et al. Frequent acquisition of low-virulence strains of ESBL-producing Escherichia coli in travellers. J Antimicrob Chemother. 2016;71(12):3548-3555. doi: 10.1093/jac/dkw335 [DOI] [PubMed] [Google Scholar]
- 57.Wielders CCH, van Hoek AHAM, Hengeveld PD, et al. Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin Microbiol Infect. 2017;23(2):120.e1-120.e8. doi: 10.1016/j.cmi.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 58.Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2(2):132-139. doi: 10.1038/nrd1010 [DOI] [PubMed] [Google Scholar]
- 59.Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13(4):251-256. doi: 10.1136/gut.13.4.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura A, Komatsu M, Ohno Y, Noguchi N, Kondo A, Hatano N. Identification of specific protein amino acid substitutions of extended-spectrum β-lactamase (ESBL)–producing Escherichia coli ST131: a proteomics approach using mass spectrometry. Sci Rep. 2019;9(1):8555. doi: 10.1038/s41598-019-45051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14(12):697-710. doi: 10.1038/nrgastro.2017.117 [DOI] [PubMed] [Google Scholar]
- 62.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790. doi: 10.1001/archinternmed.2010.89 [DOI] [PubMed] [Google Scholar]
- 63.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102(9):2047-2056. doi: 10.1111/j.1572-0241.2007.01275.x [DOI] [PubMed] [Google Scholar]
- 64.Wei L, Ratnayake L, Phillips G, et al. Acid-suppression medications and bacterial gastroenteritis: a population-based cohort study. Br J Clin Pharmacol. 2017;83(6):1298-1308. doi: 10.1111/bcp.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods.
eTable 1. Study Distribution According to World Health Organization Region.
eTable 2. Baseline Characteristics.
eTable 3. Exposure and Outcome Ascertainment.
eTable 4. Adjusted Study Results.
eTable 5. Quality Assessment According to the Modified Newcastle-Ottawa Scale.
eTable 6. Sensitivity Analyses After Exclusion of Studies on Urinary Tract Infections.
eTable 7. Sensitivity Analysis by the Leaving-One-Out Method.
eTable 8. Sensitivity Analysis by Knapp-Hartung Modification.
eFigure 1. Secondary Analysis.
eFigure 2. Subgroup Analysis by Design.
eFigure 3. Subgroup Analysis by Type of Acid Suppressant.
eFigure 4. Subgroup Analysis by Setting.
eFigure 5. Sensitivity Analysis of Studies With High Quality.
eFigure 6. Sensitivity Analyses by Mantel-Haenszel Weighting.
eFigure 7. Funnel Plot.
eReferences.