Key Points
Question
What is the short-term and long-term comparative efficacy among biologics and oral agents for plaque psoriasis?
Findings
In a network meta-analysis of 60 clinical trials for short-term efficacy, brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa had the highest Psoriasis Area and Severity Index response rates at 10 to 16 weeks from baseline. A meta-analysis of long-term efficacy suggested that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa had the highest response rates at 44 to 60 weeks.
Meaning
This study provides an assessment of both short-term and long-term comparative efficacy among treatments for moderate to severe plaque psoriasis which can help health care stakeholders optimize treatment regimens.
Abstract
Importance
The clinical benefits of novel treatments for moderate to severe psoriasis are well established, but wide variations exist in patient response across different therapies. In the absence of head-to-head randomized trials, meta-analyses synthesizing data from multiple studies are needed to assess comparative efficacy among psoriasis treatments.
Objective
To estimate the relative short-term and long-term efficacy of biologics and oral agents for the treatment of moderate to severe psoriasis.
Data Sources
A systematic literature review was conducted on December 4, 2017, and updated on September 17, 2018. The Embase, MEDLINE, and Cochrane Central Register databases were included.
Study Selection
Phase 2, 3, or 4 randomized clinical trials of treatments licensed by the US Food and Drug Administration and the European Medicines Agency for adults with moderate to severe psoriasis with data on Psoriasis Area and Severity Index assessment of 75%, 90%, and 100% reductions (PASI 75, 90, and 100) at 10 to 16 weeks (short-term efficacy) or 44 to 60 weeks (long-term efficacy) from baseline.
Data Extraction and Synthesis
Data were extracted based on the Preferred Reporting Items for Systematic Review and Meta-analysis guidelines. A bayesian network meta-analysis was conducted to estimate short-term PASI response rates; to account for variation across trials, an ordinal model that adjusted for reference arm response was implemented. The long-term PASI rates were estimated via a traditional meta-analysis.
Main Outcomes and Measures
PASI 75, 90, and 100 response rates at 10 to 16 weeks and 44 to 60 weeks from baseline.
Results
Sixty trials meeting all inclusion criteria were included. At weeks 10 to 16, the highest PASI 90 rates were seen with risankizumab-rzaa (71.6%; 95% credible interval [CrI], 67.5%-75.4%), brodalumab (70.8%; 95% CrI, 66.8%-74.6%), ixekizumab (70.6%; 95% CrI, 66.8%-74.6%), and guselkumab (67.3%; 62.5%-71.9%). At weeks 44 to 60, the treatments with the highest PASI 90 rates were risankizumab-rzaa (79.4%, 95% CI, 75.5%-82.9%), guselkumab (76.5%; 95% CI, 72.1%-80.5%), brodalumab (74.0%; 95% CI, 69.3%-78.1%), and ixekizumab (73.9%; 95% CI, 69.9%-77.5%). Findings were consistent for short-term and long-term PASI 75 and 100 responses.
Conclusions and Relevance
This study provides an assessment of the comparative efficacy among treatments for moderate to severe plaque psoriasis. The meta-analysis suggests that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were associated with the highest PASI response rates in both short-term and long-term therapy.
This meta-analysis examines the use of biologics and oral agents for treatment of moderate to severe psoriasis.
Introduction
The treatment options for patients with moderate to severe psoriasis have expanded greatly over the past decade.1,2,3,4 Among the treatments, biologics provide targeted inhibition of immune-mediated pathways involving specific cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-17, and IL-23.5,6 Biologics licensed by the US Food and Drug Administration and the European Medicines Agency for the treatment of moderate to severe psoriasis include the tumor necrosis factor inhibitors adalimumab, etanercept, infliximab, and certolizumab pegol; the IL-12/23 inhibitor ustekinumab; the IL-17 inhibitors secukinumab, ixekizumab, and brodalumab; and the IL-23 inhibitors tildrakizumab-asmn, guselkumab, and risankizumab-rzaa.7,8,9
Although the increased options of biologics and oral treatments for moderate to severe psoriasis have provided substantial benefit to patients, it can be challenging for clinicians to determine how the medications compare with one another. Variations exist across different therapies with regard to efficacy, safety, and dosing profiles.10,11
Although several head-to-head trials exist,12,13,14,15,16,17,18,19,20,21,22 they are not available for all possible comparisons. In the absence of head-to-head trials across the entire set of comparators, studies that combine and analyze data from multiple studies are needed to determine comparative efficacy. The overall objective in this study is to evaluate the comparative efficacy of systemic treatments for psoriasis, including newly developed biologics. Specifically, the short-term, relative rates of Psoriasis Area and Severity Index (PASI) response are estimated via a network meta-analysis (NMA), and the long-term PASI response rates following maintenance therapy are estimated via a traditional meta-analysis.
Methods
Search Strategy
A systematic literature review was performed on December 4, 2017, and updated on September 17, 2018, to identify randomized clinical trials of treatments licensed by the US Food and Drug Administration and the European Medicines Agency for adults with moderate to severe psoriasis. This systematic review was conducted based on the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines for Network Meta-Analysis (PRISMA-NMA) using Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials.23 Identified studies were independently assessed by 2 reviewers for inclusion at each stage of study selection. Any discrepancies between the inclusion/exclusion decisions were resolved by a third independent reviewer.
Inclusion Criteria
To be included in the analyses, studies were required to (1) be a phase 2, 3, or 4 randomized clinical trial on adults with moderate to severe psoriasis who were eligible for systemic therapies and phototherapy, (2) include European Medicines Agency–licensed treatments and dosages for moderate to severe psoriasis, and (3) report at least 1 of the efficacy outcomes of interest (PASI 75, 90, and 100, indicating 75%, 90%, or 100% reductions) at the end of the primary response period (10-16 weeks from baseline) or at the end of the maintenance period (44-60 weeks from baseline).
Comparators
Comparators for the base case analyses included anti-TNF agents (etanercept, 25 mg, twice weekly or 50 mg once weekly, or etanercept, 50 mg twice weekly until week 12, then once weekly; adalimumab, 80 mg, at week 0, then 40 mg every 2 weeks starting at week 1; certolizumab pegol, 400 mg, at weeks 0, 2, and 4, then 200 mg every 2 weeks; certolizumab pegol, 400 mg, every 2 weeks; infliximab, 5 mg/kg, at weeks 0, 2, and 6, then every 8 weeks), apremilast, 30 mg, twice daily after initial titration schedule; dimethyl fumarate, ustekinumab weight-based dosage (45 mg ≤100 kg, 90 mg >100 kg at weeks 0 and 4, then every 12 weeks), anti-IL-17 agents (secukinumab, 300 mg, at weeks 0, 1, 2, and 3, then every 4 weeks starting at week 4; ixekizumab, 160 mg, at week 0, then 80 mg every 2 weeks; brodalumab, 210 mg, at weeks 0, 1, and 2, then every 2 weeks), and anti-IL-23 agents (tildrakizumab-asmn, 100 mg, at weeks 0, 4, then every 12 weeks; tildrakizumab-asmn, 200 mg, at weeks 0, 4, then every 12 weeks; guselkumab, 100 mg, at weeks 0, 4, then every 8 weeks; risankizumab-rzaa, 150 mg, at weeks 0, 4, then every 12 weeks). Different dosing schedules of etanercept with 25 mg twice weekly and 50 mg once weekly were assumed to have the same clinical efficacy, and the 2 dosages were pooled into a single etanercept, 25 mg twice weekly/50 mg once weekly, treatment arm. For the outcomes at the end of the maintenance period, a dose of 50 mg twice weekly until week 12 then once weekly (approved by the US Food and Drug Administration and also as an alternative dosage licensed by the European Medicines Agency) was used. In addition, the following conventional systemic treatments were included in a sensitivity analysis for the short-term analyses: acitretin, 0.4 mg/kg daily, cyclosporine, 2.5-3 mg/kg/d (initial dose), fumaric acid esters (licensed in Germany only), low-dose methotrexate (initial dose of 5-7.5 mg once weekly, increased as needed and as tolerated up to a maximum of 15-25 mg once weekly), and high-dose methotrexate (initial dose of 15 mg once weekly, increased as needed up to a maximum of 20-22.5 mg once weekly).
Outcomes
In this analysis, the outcomes of interest were the proportion of patients that achieved 75%, 90%, and 100% reductions in PASI and the associated number needed to treat (NNT) at the end of the primary response period (10-16 weeks from baseline) and at the end of the maintenance period (44-60 weeks from baseline).
Statistical Analysis
Short-term Efficacy: NMA
An ordinal bayesian random effects NMA24 was conducted to compare the relative PASI 75, 90, and 100 responses at the end of the primary response period (10-16 weeks from baseline) across treatments. To account for heterogeneity across trials, a model that adjusted for reference-arm response was implemented.25,26 The base-case analysis included all biologics, apremilast, and dimethyl fumarate. The estimated PASI response rates were summarized using posterior medians and their associated 95% credible intervals (CrIs). The NNT for each treatment was calculated as the reciprocal of the treatment effect difference vs placebo. Pairwise comparisons of the estimated treatment effects relative to risankizumab-rzaa, ixekizumab, brodalumab, and guselkumab were summarized using odds ratios (ORs) and their associated 95% CrIs. All analyses were conducted using R, version 3.5.1 statistical software (R Foundation) and WinBUGS, version 1.4.3 with noninformative priors, 40 000 burn-in iterations, a thinning factor of 10, and 3 chains each with 100 000 posterior iterations. Three sensitivity analyses were conducted: 1 that included only global trials (country-specific trials were excluded to assess the associations of homogeneous populations with the study results), 1 that included only phase 3 trials, and 1 that focused on an expanded treatment space that also included conventional systemic treatments (acitretin, cyclosporine, fumaric acid esters, and methotrexate).
Long-term Efficacy: Meta-analysis
Most of the psoriasis trials feature crossover (typically, the placebo arm switching to the active treatment) after the primary short-term end point. In addition, some psoriasis trials rerandomize patients based on the level of PASI response achieved. Owing to the relative dearth of trials that did not feature crossover or rerandomization, a traditional random-effects meta-analysis27 was conducted to estimate the PASI 75, 90, and 100 response rates at the end of the maintenance period (44-60 weeks from baseline). Only data from trial treatment arms in which baseline treatment assignment was maintained during the maintenance period were used. Each intervention’s response rate and its associated 95% CI were summarized. Pairwise comparisons of the estimated response rates relative to brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were summarized using ORs and their associated 95% CIs. Three sensitivity analyses were conducted: an analysis of only global trials (country-specific trials were excluded to assess the association between the homogeneous populations and the study results), 1 that included only phase 3 trials, and 1 that included only trials that reported nonresponse imputed (NRI) data.
Results
Literature Search
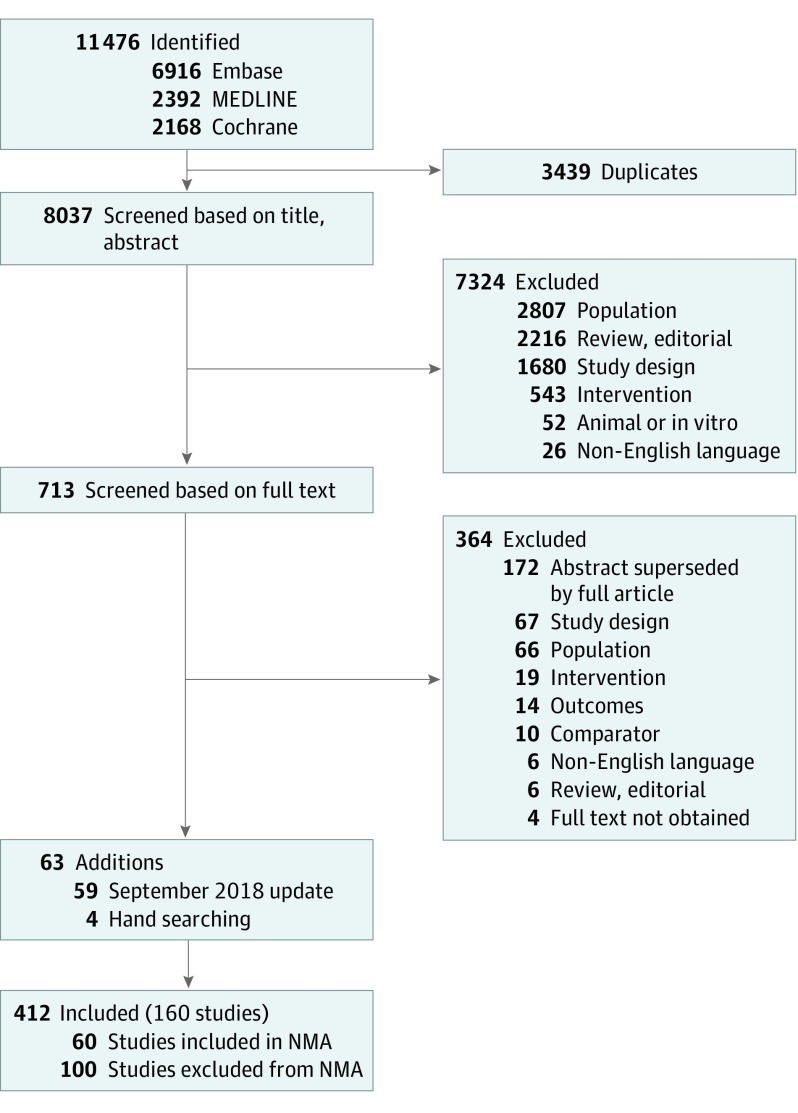
A total of 11 476 studies were systematically identified. Following elimination of duplicate studies, 8037 studies were screened based on title and abstract and 713 full-text studies were evaluated. An updated search on September 17, 2018, identified an additional 59 randomized clinical trials of treatments and dosages licensed by the European Medicines Agency for adults with moderate to severe psoriasis. A total of 412 publications covering 160 studies met the inclusion criteria (Figure 1). The full list of clinical trials13,15,16,17,18,19,20,21,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86 and their reported efficacy data that were analyzed are summarized in eTable 1 in the Supplement.
Figure 1. Study Screening and Selection Flow.
NMA indicates network meta-analysis.
Short-term Efficacy
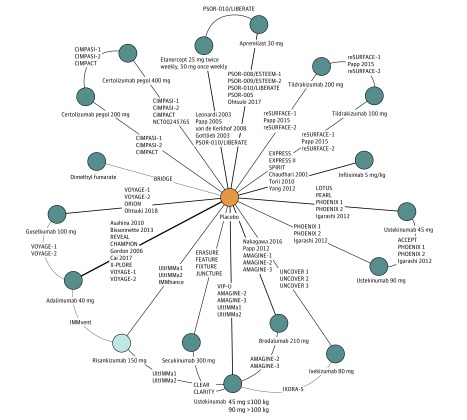
The evidence network for the base-case NMA of short-term efficacy 10 to 16 weeks from baseline is illustrated in Figure 2.13,15,16,17,18,19,20,21,30,31,32,33,34,35,37,38,39,40,41,42,43,44,45,46,47,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,71,72,73,74,83,84 A total of 60 trials meeting all inclusion criteria were included in the base-case NMA. The median posterior probability of each intervention achieving at least the given PASI response (PASI 75, 90, and 100) is presented in the Table. The estimated PASI 90 response rates at 10 to 16 weeks from baseline were 71.6% (95% CrI, 67.5%-75.4%) for risankizumab-rzaa; 70.8% (95% CrI, 66.8%-74.6%) for brodalumab; 70.6% (95% CrI, 66.8%-74.6%) for ixekizumab; 67.3% (95% CrI, 62.5%-71.9%) for guselkumab; 61.4% (95% CrI, 57.2%-65.6%) for secukinumab; 57.4% (95% CrI, 52.2%-62.8%) for infliximab; 45.6% (39.3%-52.2%) for certolizumab pegol, 400 mg; 43.9% (40.2%-47.9%) for ustekinumab; 43.7% (95% CrI, 40.0%-47.4%) for adalimumab; 40.2% (95% CrI, 33.5%-47.2%) for certolizumab pegol, 200 mg; 38.8% (95% CrI, 33.3%-44.7%) for tildrakizumab-asmn, 200 mg; 36.8% (31.4%-42.5%) for tildrakizumab-asmn, 100 mg; 17.9% (CrI, 14.9%-21.4%) for etanercept; 12.1% (95% CrI, 9.9%-14.7%) for apremilast; 11.4% (95% CrI, 7.5%-16.7%) for dimethyl fumarate; and 1.1% (95% CrI, 1.0%-1.3%) for placebo. In addition, the ORs of achieving the given PASI response (PASI 75, 90, and 100) for each intervention vs brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa are illustrated in eFigure 1 in the Supplement.
Figure 2. Evidence Network for Network Meta-analysis (NMA) of Short-term Psoriasis Area and Severity Index (PASI) (Base Case).
The reference citations for the NMA are as follows: PSOR-010/LIBERATE: reference 20; CIMPASI-1: reference 65; CIMPASI-2: reference 65; CIMPACT: reference 66; reSURFACE-1: reference 68; Papp 2005: reference 38; reSURFACE-2: reference 68; PSOR-008/ESTEEM-1: reference 71; PSOR-009/ESTEEM-2: reference 72; PSOR-005: reference 73; Ohtsuki 2017: reference 74; NCT00245765: reference 67; Papp 2015: reference 69; Leonardi 2003: reference 37; van de Kerkhof 2008: reference 39; Gottlieb 2003: reference 40; EXPRESS: reference 41; EXPRESS II: reference 42; SPIRIT: reference 43; Chaudhari 2001: reference 44; Torii 2010: reference 45; Yang 2012: reference 46; BRIDGE: reference 31; VOYAGE-1: reference 17; VOYAGE-2: reference 18; ORION: reference 60; Ohtsuki 2018: reference 61; LOTUS: reference 54; PEARL: reference 55; PHOENIX 1: reference 56; PHOENIX 2: reference 57; Igarashi 2012: reference 58; Asahina 2010: reference 30; Bissonnette 2013: reference 31; REVEAL: reference 32; CHAMPION: reference 33; Gordon 2006: reference 34; Cai 2017: reference 35; X-PLORE: reference 58; ACCEPT: reference 53; Nakagawa 2016: reference 62; Papp 2012: reference 63; AMAGINE-1: reference 64; AMAGINE-2: reference 19; AMAGINE-3: reference 19; ERASURE: reference 50; FEATURE: reference 51; FIXTURE: reference 50; JUNCTURE: reference 52; UltIMMa-1: reference 15; UltIMMa-2: reference 15; IMMhance: reference 83; UNCOVER 1: reference 47; UNCOVER 2: reference 48; UNCOVER 3: reference 48; VIP-U: reference 59; IMMvent: reference 21; CLEAR: reference 13; CLARITY: reference 26; and IXORA-S: reference 16.
Table. Estimated Response Rates From the NMA of Short-term PASI (Base Case).
| Treatment | Posterior Median, % (95% CrI) | ||
|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | |
| Risankizumab-rzaa, 150 mg | 89.2 (86.9-91.3) | 71.6 (67.5-75.4) | 40.4 (35.9-45.0) |
| Ixekizumab, 80 mg | 88.8 (86.5-90.9) | 70.8 (66.8-74.6) | 39.5 (35.2-44.0) |
| Brodalumab, 210 mg | 88.7 (86.5-90.8) | 70.6 (66.8-74.6) | 39.2 (35.2-43.9) |
| Guselkumab, 100 mg | 86.8 (83.8-89.4) | 67.3 (62.5-71.9) | 35.7 (30.9-40.7) |
| Secukinumab, 300 mg | 83.1 (80.2-85.7) | 61.4 (57.2-65.6) | 29.9 (26.3-33.9) |
| Infliximab, 5 mg/kg | 80.4 (76.5-84.0) | 57.4 (52.2-62.8) | 26.5 (22.3-31.4) |
| Certolizumab pegol, 400 mg | 71.1 (65.4-76.5) | 45.6 (39.3-52.2) | 17.7 (13.8-22.3) |
| Ustekinumab, 45 mg ≤100 kg, 90 mg >100 kg | 69.7 (66.3-73.1) | 43.9 (40.2-47.9) | 16.7 (14.4-19.3) |
| Adalimumab, 40 mg | 69.5 (66.0-72.6) | 43.7 (40.0-47.4) | 16.5 (14.2-19.0) |
| Certolizumab pegol, 200 mg | 66.2 (59.6-72.4) | 40.2 (33.5-47.2) | 14.4 (10.7-18.8) |
| Tildrakizumab-asmn, 200 mg | 64.9 (59.4-70.3) | 38.8 (33.3-44.7) | 13.6 (10.6-17.1) |
| Tildrakizumab-asmn, 100 mg | 62.9 (57.3-68.4) | 36.8 (31.4-42.5) | 12.5 (9.7-15.8) |
| Etanercept, 25 mg twice weekly/50 mg once weekly | 40.1 (35.4-45.1) | 17.9 (14.9-21.4) | 4.2 (3.1-5.4) |
| Apremilast, 30 mg | 30.8 (26.8-35.0) | 12.1 (9.9-14.7) | 2.4 (1.8-3.1) |
| Dimethyl fumarate | 29.6 (22.0-38.3) | 11.4 (7.5-16.7) | 2.2 (1.2-3.8) |
| Placebo | 5.3 (4.8-5.9) | 1.1 (1.0-1.3) | 0.1 (0.1-0.1) |
Abbreviations: CrI, credible interval; NMA, network meta-analysis; PASI, Psoriasis Area and Severity Index; PASI 75, 90, 100, a 75%, 90% or 100% decrease from baseline PASI.
Among all of the comparators evaluated, brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa had the highest PASI 75, 90, and 100 rates at the end of the primary response period, and there were no statistically significant differences among these treatments. Brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa had significantly higher PASI 75, 90, and 100 rates (with 95% probability) compared with adalimumab, apremilast, certolizumab pegol, dimethyl fumarate, etanercept, tildrakizumab-asmn, and ustekinumab. Brodalumab, ixekizumab, and risankizumab-rzaa also had significantly higher rates compared with infliximab and secukinumab across all PASI outcomes (with 95% probability).
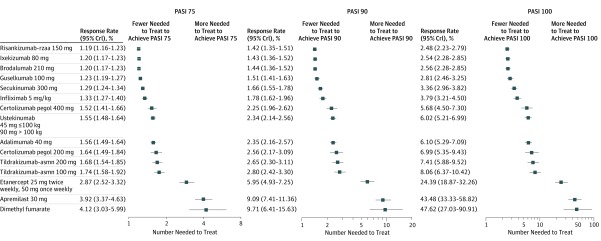
The NNT to achieve PASI 75, 90, or 100 for each treatment relative to placebo is presented in Figure 3. To achieve a PASI 75 response, the NNT was 1.19 (95% CrI, 1.16-1.23) with risankizumab-rzaa, 1.20 (95% CrI, 1.17-1.23) with ixekizumab and brodalumab, and 1.23 (95% CrI, 1.19-1.27) with guselkumab. The NNT to achieve a PASI 90 response was 1.42 (95% CrI, 1.35-1.51) with risankizumab-rzaa, 1.43 (95% CrI, 1.36-1.52) with ixekizumab, 1.44 (95% CrI, 1.36-1.52) with brodalumab, and 1.51 (95% CrI, 1.41-1.63) with guselkumab. The NNT to achieve a PASI 100 response was 2.48 (95% CrI, 2.23-2.79) with risankizumab-rzaa, 2.54 (95% CrI, 2.28-2.85) with ixekizumab, 2.56 (95% CrI, 2.28-2.85) with brodalumab, and 2.81 (95% CrI, 2.46-3.25) with guselkumab.
Figure 3. Estimated Number Needed to Treat (95% Credible Interval) Relative to Placebo for Short-term Psoriasis Area and Severity Index (PASI) (Base Case).
PASI 75, 90, 100, indicates 75%, 90%, or 100% decrease from baseline.
Sensitivity Analyses: Short-term Efficacy
Among the 60 trials included in the base-case, 10 are country-specific trials and were excluded from this sensitivity analysis. The median posterior probability of PASI 75, 90, and 100 rates for each intervention are presented in eTable 2 in the Supplement. Overall, the results from the sensitivity analysis including only global trials were consistent with the base-case analysis.
Among the 60 trials included in the base case, 47 were phase 3 trials that were included in the following sensitivity analysis. The median posterior probability of PASI 75, 90, and 100 rates for each intervention are presented in eTable 2 in the Supplement. The results from the sensitivity analysis based on phase 3 trials were consistent with those from the base-case analysis.
For the sensitivity analysis of the expanded treatment space, a total of 70 trials were included. The median posterior probability of PASI 75, 90, and 100 rates for each intervention are presented in eTable 2 in the Supplement. The results from the sensitivity analysis of the expanded treatment space were also consistent with those from the base-case analysis.
Long-term Efficacy
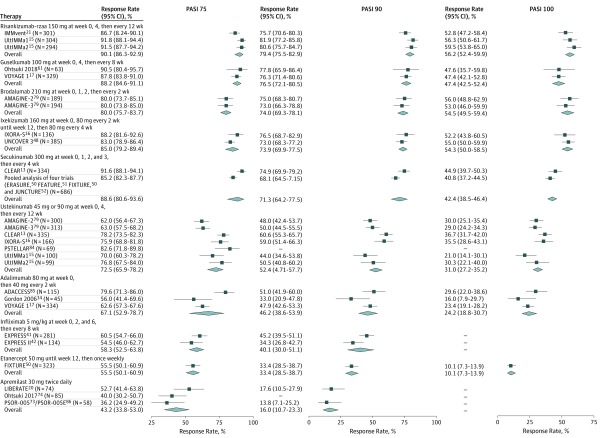
A total of 22 trials on 10 treatments meeting all inclusion criteria were included in the meta-analysis. The estimated response rates (PASI 75, 90, and 100) of each intervention are presented in Figure 4.13,15,16,17,18,20,21,34,41,42,47,50,51,52,73,74,84,85,86 The estimated PASI 90 response rates at 44 to 60 weeks from baseline were 79.4% (95% CI, 75.5%-82.9%) for risankizumab-rzaa, 76.5% (95% CI, 72.1%-80.5%) for guselkumab, 74.0% (95% CI, 69.3%-78.1%) for brodalumab, 73.9% (95% CI, 69.9%-77.5%) for ixekizumab, 71.3% (95% CI, 64.2%-77.5%) for secukinumab, 52.4% (95% CI, 47.1%-57.7%) for ustekinumab, 46.2% (95% CI, 38.6%-53.9%) for adalimumab, 40.1% (95% CI, 30.0%-51.1%) for infliximab, 33.4% (95% CI, 28.5%-38.7%) for etanercept, and 16.0% (95% CI, 10.7%-23.3%) for apremilast. In addition, the ORs of achieving PASI 75, 90, and 100 for each intervention vs brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa are presented in eFigure 2 in the Supplement. Among the comparators, the highest estimated PASI 75, 90, and 100 response rates at 44 to 60 weeks from baseline occurred following treatment with brodalumab, guselkumab, ixekizumab, risankizumab-rzaa, and secukinumab, which were significantly higher than those corresponding to adalimumab, apremilast, etanercept, infliximab, and ustekinumab. In addition, risankizumab-rzaa had significantly higher PASI 90 rates than ixekizumab and secukinumab; risankizumab-rzaa, brodalumab, and ixekizumab had significantly higher PASI 100 rates than secukinumab, and risankizumab-rzaa and ixekizumab also had significantly higher PASI 100 rates than guselkumab (eFigure 2 in the Supplement).
Figure 4. Estimated Response Rate From the Meta-analysis of Long-term Placebo for Short-term Psoriasis Area and Severity Index (PASI) (Base Case).
Sensitivity Analyses: Long-term Efficacy
The estimated long-term response rates for PASI 75, 90, and 100 of each intervention in the sensitivity analyses are presented in eTable 3 in the Supplement. Among the 22 trials included in the base-case analysis, the sensitivity analysis including only global trials excluded 2 country-specific trials and produced results consistent with the base-case analysis. A total of 14 trials that reported NRI data at the end of the maintenance period (44-60 weeks from baseline) were included in the sensitivity analysis including only NRI data. The results of this sensitivity analysis were mostly consistent with the base-case analysis with the exception of secukinumab, which slightly increased because only 1 of the 5 trials that were included in the base case reported NRI values, and this trial had the highest PASI responses of the trials considered.13 Three phase 2 trials were excluded from the sensitivity analysis including only phase 3 trials; the results of this sensitivity analyses were consistent with the base-case analysis.
To test whether the method of meta-analysis would affect the results, we conducted an NMA using a fixed-effects bayesian multinomial likelihood model with a probit link for the long-term PASI outcomes using identified randomized clinical trials that maintained randomization through 52 weeks. Only 7 randomized clinical trials (AMAGINE 2,87 AMAGINE 3,88 CLEAR,13 FIXTURE,89 IXORA-S,90 UltIMMa1,15 and UltIMMa215) on 6 treatments (brodalumab, etanercept, ixekizumab, risankizumab-rzaa, secukinumab, and ustekinumab) were able to be included into this NMA because of the crossover in the studies evaluating long-term PASI outcomes (eFigure 3 in the Supplement). In the NMA, risankizumab-rzaa (81.3%; 95% CrI, 75.7%-86.1%) had the highest estimated PASI 90 rates, followed by brodalumab (76.6%; 95% CrI, 71.3%-81.4%), ixekizumab (69.8%; 95% CrI, 60.1%-78.3%), secukinumab (65.2%; 95% CrI, 58.1%-71.6%), ustekinumab (52.5%; 95% CrI, 49.3%-55.7%), and etanercept (35.9%; 95% CrI, 27.1%-45.6%). As demonstrated by this NMA, risankizumab-rzaa had significantly higher PASI response rates compared with ixekizumab (PASI 90 OR, 1.89; 95% CrI, 1.12-1.99), secukinumab (OR, 2.33; 95% CrI, 1.54-3.56), ustekinumab (OR, 3.95; 95% CrI, 2.90-5.45), and etanercept (OR, 7.80; 95% CrI, 4.75-12.94), and brodalumab had significantly higher PASI response rates compared with secukinumab (OR, 1.76; 95% CrI, 1.22-2.54), ustekinumab (OR, 2.97; 95% CrI, 2.32-3.83), and etanercept (OR, 5.86; 95% CrI, 3.72-9.36) (eTable 4 and eTable 5 in the Supplement).
Discussion
With increased therapeutic options for psoriasis, it has become increasingly challenging for clinicians to compare across the available treatments for clinical decision making. Meta-analytic approaches have important roles in integrating evidence from multiple studies to determine the relative efficacy of treatments and inform clinical decision making.91 This study provides a comprehensive assessment of the short-term and long-term comparative efficacy of biologics and oral therapies approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of moderate to severe plaque psoriasis.
Results from this study suggest that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa had significantly higher PASI response rates than adalimumab, apremilast, certolizumab pegol, dimethyl fumarate, etanercept, tildrakizumab-asmn, and ustekinumab at the end of the primary response period. There were no statistically significant differences in short-term efficacy among brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa. Over the long-term, brodalumab, guselkumab, ixekizumab, risankizumab-rzaa, and secukinumab had significantly higher PASI response rates than those corresponding to adalimumab, apremilast, infliximab, etanercept, and ustekinumab.
The short-term results from this study generally align with published NMAs that compared the efficacy of other biologic therapies for treatment of moderate to severe plaque psoriasis at the end of the primary response period.28,29,92,93,94,95,96,97,98 For example, a recent NMA study by Sawyer et al95 used similar methods and a similar list of trials to suggest that brodalumab, ixekizumab, risankizumab-rzaa, and guselkumab have the highest short-term PASI response rates.
The Sawyer et al95 NMA also pointed out the lack of a comprehensive systematic evaluation of long-term efficacy of systemic treatments in patients with moderate to severe psoriasis. Work by Nast et al99 found infliximab, secukinumab, and ustekinumab to be the most effective long-term treatment options. However, owing to clinical and methodologic differences among available studies and a lack of sufficiently long-term, head-to-head trials, the strength of the ranking of treatment efficacy was limited beyond 24 to 28 weeks.99 A 2017 NMA of four 52-week randomized clinical trials found that brodalumab achieved significantly higher PASI 100 response rates compared with secukinumab, ustekinumab, and etanercept.100 Nevertheless, only 4 trials were able to be included in the primary indirect comparison owing to study design heterogeneity and lack of long-term, active-comparator trial data. The present study overcame these limitations by using traditional meta-analysis methods.
Despite the differences in methods, the results from the long-term traditional meta-analysis are consistent with this NMA, suggesting the robustness of the meta-analysis results. However, the meta-analysis directly synthesized the PASI response rates for patients receiving each active treatment during both the induction and maintenance periods, making it possible to include additional treatments beyond the connected network.
Strengths and Limitations
The present work analyzes the short-term and long-term efficacy that included all licensed medications to date (eg, brodalumab, dimethyl fumarate, guselkumab, risankizumab-rzaa, and tildrakizumab-asmn). This study also has several other strengths. First, the analytic methods used in the study are well-established, rigorous, treatment comparison techniques. Meta-analyses are a statistical tool used to synthesize direct evidence from multiple studies to compare 2 interventions at a time. Network meta-analyses extend traditional meta-analyses to allow for simultaneous comparison of several different treatments that are regularly used in clinical practice but have not necessarily been compared head-to-head in a randomized trial. These methods provide useful evidence about the comparative effectiveness of competing interventions—which would otherwise be lacking—in a cost-effective and timely manner. The NMA approach used in the analysis of short-term efficacy enabled an estimation of comparative effects among treatments that have not yet been investigated in head-to-head randomized clinical trials for moderate to severe plaque psoriasis. Adjusting for the reference arm response can reduce the outcomes associated with cross-trial heterogeneity, as the reference arm response rate integrates the results of other observed and unobserved trial-level factors likely to affect treatment arm outcomes. The traditional meta-analysis used to assess the long-term outcomes of treatments allowed for the amalgamation of scientific evidence and offered an objective appraisal of the available evidence. By combining data from multiple independent trials of the same treatment, the meta-analysis increased the sample size and improved statistical precision for the estimation of long-term PASI responses. In addition, the results from the sensitivity analyses were consistent with the base-case analyses for both short-term and long-term treatment, which supports the robustness of the primary findings. The comparative analyses of recently available biologic treatments in the present study fills an important gap in the current body of literature. Additional analyses comparing the short-term and long-term safety outcomes are warranted to provide a complete benefit-risk assessment of novel psoriasis therapies for health care stakeholders.101,102
The results of this analysis should be interpreted within the context of certain limitations. First, as with all meta-analyses, the presence of cross-trial differences and patient characteristics that may modify the treatment effect can introduce bias in the comparisons. Although adjusting for the reference arm response in this NMA can reduce the effects of cross-trial heterogeneity, there is no guarantee that adjustments will eliminate these effects. In addition, subgroup analyses (eg, by relevant patient characteristics) are generally not feasible because most published trials do not report detailed PASI outcomes for subgroups. Second, the findings of this study are based on clinical trial data, which may not be generalizable to real-world settings. Future research may study the comparative efficacy among treatments in real-world settings. Moreover, a holistic comparison of psoriasis treatments should include other key aspects, such as safety profile, effect on quality of life (eg, the Dermatology Life Quality Index), as well as administration and dosing frequency. Third, the short-term efficacy analysis only included efficacy data up to week 16, and any agent’s full efficacy may not have been achieved at this time point; however, the long-term analyses serve as a complement to this short-term assessment. Fourth, the long-term meta-analysis focused on conducting random-effects meta-analysis within treatments because an anchor-based approach (eg, NMA) would be limited for all treatments owing to crossover and rerandomization. Accordingly, the current meta-analysis using the DerSimonian and Laird27 method can only account for heterogeneity among trials of the same treatment but not for different treatments. Hence, the long-term meta-analysis results should be interpreted with caution beyond the relationships examined in the long-term NMA.
Conclusions
In the absence of head-to-head randomized clinical trials of treatments for moderate to severe plaque psoriasis, this study provides what we believe to be a comprehensive assessment of the comparative short-term and long-term efficacy among several novel treatments. Over the primary response period and long-term maintenance period, brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were associated with the highest estimated PASI 75, 90, and 100 response rates.
eTable 1. List of Clinical Trials
eFigure 1. Estimated Odds Ratios From the NMA of Short-term PASI (Base-Case)
eTable 2. Estimated Response Rates From the NMA of Short-term PASI (Sensitivity Analyses Including Global Trials Only, Including Phase III Trials Only, and in an Expanded Treatment Space)
eFigure 2. Estimated Odds Ratios From the Meta-analysis of Long-term PASI (Base-Case)
eTable 3. Estimated Response Rates From the Meta-analysis of Long-term PASI (Sensitivity Analyses: Including Global Trials Only, Including Trials Reporting NRI Data, and Including Phase III Trials Only)
eFigure 3. Evidence Network for NMA of Long-term PASI
eTable 4. Estimated Response Rates From the NMA of Long-term PASI
eTable 5. Estimated Odds Ratios for PASI 90 from the NMA of Long-term PASI
References
- 1.Young M, Roebuck HL. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor: a novel treatment option for nurse practitioners treating patients with psoriatic disease. J Am Assoc Nurse Pract. 2016;28(12):683-695. doi: 10.1002/2327-6924.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. 2015;28(6):390-403. doi: 10.1111/dth.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278-285. [PMC free article] [PubMed] [Google Scholar]
- 4.Levine D, Gottlieb A. Evaluation and management of psoriasis: an internist’s guide. Med Clin North Am. 2009;93(6):1291-1303. doi: 10.1016/j.mcna.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18(11):pii:E2297. doi: 10.3390/ijms18112297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46(1):1-23. doi: 10.1067/mjd.2002.120568 [DOI] [PubMed] [Google Scholar]
- 7.Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19(1):1-13. doi: 10.1007/s40257-017-0328-3 [DOI] [PubMed] [Google Scholar]
- 8.Haugh IM, Preston AK, Kivelevitch DN, Menter AM. Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis. Drug Des Dev Ther. 2018;12:3879-3883. doi: 10.2147/DDDT.S167149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AbbVie. European Commission approves SKYRIZI (risankizumab) for the treatment of moderate to severe plaque psoriasis. https://news.abbvie.com/news/press-releases/european-commission-approves-skyrizi-risankizumab-for-treatment-moderate-to-severe-plaque-psoriasis.htm. Published April 30, 2019. Accessed June 11, 2019.
- 10.Piaserico S, Cazzaniga S, Chimenti S, et al. ; Psocare Study Group . Efficacy of switching between tumor necrosis factor-alfa inhibitors in psoriasis: results from the Italian Psocare registry. J Am Acad Dermatol. 2014;70(2):257-62.e3. doi: 10.1016/j.jaad.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 11.Lecluse LL, Driessen RJ, Spuls PI, et al. Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol. 2010;146(2):127-132. doi: 10.1001/archdermatol.2009.347 [DOI] [PubMed] [Google Scholar]
- 12.Wan MT, Alvarez J, Shin DB, Dommasch ED, Wu JJ, Gelfand JM. Head-to-head trials of systemic psoriasis therapies: a systematic review of study design and maximum acceptable treatment differences. J Eur Acad Dermatol Venereol. 2019;33(1):42-55. doi: 10.1111/jdv.15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400-409. doi: 10.1016/j.jaad.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831-839. doi: 10.1016/S0140-6736(19)31773-8 [DOI] [PubMed] [Google Scholar]
- 15.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650-661. doi: 10.1016/S0140-6736(18)31713-6 [DOI] [PubMed] [Google Scholar]
- 16.Reich K, Pinter A, Lacour JP, et al. ; IXORA-S investigators . Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014-1023. doi: 10.1111/bjd.15666 [DOI] [PubMed] [Google Scholar]
- 17.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. doi: 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- 18.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431. doi: 10.1016/j.jaad.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 19.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318-1328. doi: 10.1056/NEJMoa1503824 [DOI] [PubMed] [Google Scholar]
- 20.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507-517. doi: 10.1111/jdv.14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576-586. doi: 10.1016/S0140-6736(19)30952-3 [DOI] [PubMed] [Google Scholar]
- 22.Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results). Dermatol Ther (Heidelb). 2018;8(4):571-579. doi: 10.1007/s13555-018-0265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 24.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607-617. doi: 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity—subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618-640. doi: 10.1177/0272989X13485157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorovitch JE, Betts KA, Yan YS, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol. 2015;172(2):504-512. doi: 10.1111/bjd.13437 [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 28.Sawyer L, Fotheringham I, Wright E, Yasmeen N, Gibbons C, Holmen Møller A. The comparative efficacy of brodalumab in patients with moderate-to-severe psoriasis: a systematic literature review and network meta-analysis. J Dermatolog Treat. 2018;29(6):557-568. doi: 10.1080/09546634.2018.1427205 [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence (NICE) Ixekizumab for treating moderate to severe plaque psoriasis NICE Technology appraisal guidance. https://www.nice.org.uk/guidance/ta442/resources/ixekizumab-for-treating-moderate-to-severe-plaque-psoriasis-pdf-82604781265093. Published April 26, 2017. Accessed November 25, 2019.
- 30.Asahina A, Nakagawa H, Etoh T, Ohtsuki M; Adalimumab M04-688 Study Group . Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37(4):299-310. doi: 10.1111/j.1346-8138.2009.00748.x [DOI] [PubMed] [Google Scholar]
- 31.Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6(1):83-90. doi: 10.1161/CIRCIMAGING.112.975730 [DOI] [PubMed] [Google Scholar]
- 32.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106-115. doi: 10.1016/j.jaad.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 33.Saurat JH, Stingl G, Dubertret L, et al. ; CHAMPION Study Investigators . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558-566. doi: 10.1111/j.1365-2133.2007.08315.x [DOI] [PubMed] [Google Scholar]
- 34.Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598-606. doi: 10.1016/j.jaad.2006.05.027 [DOI] [PubMed] [Google Scholar]
- 35.Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89-95. doi: 10.1111/jdv.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldminz AM, Suárez-Fariñas M, Wang AC, Dumont N, Krueger JG, Gottlieb AB. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol. 2015;151(8):837-846. doi: 10.1001/jamadermatol.2015.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardi CL, Powers JL, Matheson RT, et al. ; Etanercept Psoriasis Study Group . Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014-2022. doi: 10.1056/NEJMoa030409 [DOI] [PubMed] [Google Scholar]
- 38.Papp KA, Tyring S, Lahfa M, et al. ; Etanercept Psoriasis Study Group . A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304-1312. doi: 10.1111/j.1365-2133.2005.06688.x [DOI] [PubMed] [Google Scholar]
- 39.van de Kerkhof PC, Segaert S, Lahfa M, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177-1185. doi: 10.1111/j.1365-2133.2008.08771.x [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627-1632. doi: 10.1001/archderm.139.12.1627 [DOI] [PubMed] [Google Scholar]
- 41.Reich K, Nestle FO, Papp K, et al. ; EXPRESS study investigators . Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367-1374. doi: 10.1016/S0140-6736(05)67566-6 [DOI] [PubMed] [Google Scholar]
- 42.Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1-31.e15. doi: 10.1016/j.jaad.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534-542. doi: 10.1016/j.jaad.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 44.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842-1847. doi: 10.1016/S0140-6736(00)04954-0 [DOI] [PubMed] [Google Scholar]
- 45.Torii H, Nakagawa H; Japanese Infliximab Study investigators . Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59(1):40-49. doi: 10.1016/j.jdermsci.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 46.Yang HZ, Wang K, Jin HZ, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl). 2012;125(11):1845-1851. [PubMed] [Google Scholar]
- 47.Gordon K, Blauvelt A, Langley R Ixekizumab for treatment of moderate‐to‐severe plaque psoriasis: 60‐week results from a double‐blind phase 3 induction and randomized withdrawal study (UNCOVER‐1). In 73rd Annual Meeting of the American Academy of Dermatology. 2015;F010. [Google Scholar]
- 48.Griffiths CE, Reich K, Lebwohl M, et al. ; UNCOVER-2 and UNCOVER-3 investigators . Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541-551. doi: 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 49.Reich K, Pinter A, Leutz A, et al. A randomized, open-label comparison of ixekizumab vs. methotrexate in patients with moderate-to-severe plaque-type psoriasis naive to systemic therapy: interim analysis of week 12 findings. Br J Dermatol. 2017;177:61-61. [Google Scholar]
- 50.Langley RG, Elewski BE, Lebwohl M, et al. ; ERASURE Study Group; FIXTURE Study Group . Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326-338. doi: 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 51.Blauvelt A, Prinz JC, Gottlieb AB, et al. ; FEATURE Study Group . Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484-493. doi: 10.1111/bjd.13348 [DOI] [PubMed] [Google Scholar]
- 52.Paul C, Lacour JP, Tedremets L, et al. ; JUNCTURE study group . Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082-1090. doi: 10.1111/jdv.12751 [DOI] [PubMed] [Google Scholar]
- 53.Griffiths CE, Strober BE, van de Kerkhof P, et al. ; ACCEPT Study Group . Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118-128. doi: 10.1056/NEJMoa0810652 [DOI] [PubMed] [Google Scholar]
- 54.Zhu X, Zheng M, Song M, et al. ; LOTUS Investigators . Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12(2):166-174. [PubMed] [Google Scholar]
- 55.Tsai TF, Ho JC, Song M, et al. ; PEARL Investigators . Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63(3):154-163. doi: 10.1016/j.jdermsci.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 56.Leonardi CL, Kimball AB, Papp KA, et al. ; PHOENIX 1 study investigators . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665-1674. doi: 10.1016/S0140-6736(08)60725-4 [DOI] [PubMed] [Google Scholar]
- 57.Papp KA, Langley RG, Lebwohl M, et al. ; PHOENIX 2 study investigators . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675-1684. doi: 10.1016/S0140-6736(08)60726-6 [DOI] [PubMed] [Google Scholar]
- 58.Igarashi A, Kato T, Kato M, Song M, Nakagawa H; Japanese Ustekinumab Study Group . Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242-252. doi: 10.1111/j.1346-8138.2011.01347.x [DOI] [PubMed] [Google Scholar]
- 59.Gelfand JM, Shin DB, Alavi A, et al. A Phase IV, Randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). J Invest Dermatol. 2020;140(1):85-93.e2. doi: 10.1016/j.jid.2019.07.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferris L, Ott E, Jiang G, Hong H, Baran W. Efficacy and safety of guselkumab administered with a novel self-Injection device for the treatment of moderate to severe psoriasis: results from the phase III ORION self-dose study through week 16. Acta Derm Venereol. 2018;29:P063. [Google Scholar]
- 61.Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053-1062. doi: 10.1111/1346-8138.14504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakagawa H, Niiro H, Ootaki K; Japanese brodalumab study group . Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44-52. doi: 10.1016/j.jdermsci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 63.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181-1189. doi: 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
- 64.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273-286. doi: 10.1111/bjd.14493 [DOI] [PubMed] [Google Scholar]
- 65.Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302-314.e6. doi: 10.1016/j.jaad.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 66.Lebwohl M, Blauvelt A, Paul C, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol. 2018;79(2):266-276.e5. doi: 10.1016/j.jaad.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 67.Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167(1):180-190. doi: 10.1111/j.1365-2133.2012.10941.x [DOI] [PubMed] [Google Scholar]
- 68.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276-288. doi: 10.1016/S0140-6736(17)31279-5 [DOI] [PubMed] [Google Scholar]
- 69.Papp K, Thaçi D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930-939. doi: 10.1111/bjd.13932 [DOI] [PubMed] [Google Scholar]
- 70.Altmeyer PJ, Matthes U, Pawlak F, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol. 1994;30(6):977-981. doi: 10.1016/S0190-9622(94)70121-0 [DOI] [PubMed] [Google Scholar]
- 71.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37-49. doi: 10.1016/j.jaad.2015.03.049 [DOI] [PubMed] [Google Scholar]
- 72.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387-1399. doi: 10.1111/bjd.14164 [DOI] [PubMed] [Google Scholar]
- 73.Papp K, Cather JC, Rosoph L, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738-746. doi: 10.1016/S0140-6736(12)60642-4 [DOI] [PubMed] [Google Scholar]
- 74.Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873-884. doi: 10.1111/1346-8138.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158(6):1345-1349. doi: 10.1111/j.1365-2133.2008.08564.x [DOI] [PubMed] [Google Scholar]
- 76.Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Derm Venereol. 1997;77(2):137-141. [DOI] [PubMed] [Google Scholar]
- 77.Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165(5):1109-1117. doi: 10.1111/j.1365-2133.2011.10615.x [DOI] [PubMed] [Google Scholar]
- 78.Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349(7):658-665. doi: 10.1056/NEJMoa021359 [DOI] [PubMed] [Google Scholar]
- 79.Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. Br J Dermatol. 2011;164(4):855-861. doi: 10.1111/j.1365-2133.2010.10195.x [DOI] [PubMed] [Google Scholar]
- 80.Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158(1):116-121. [DOI] [PubMed] [Google Scholar]
- 81.Blauvelt A, Ferris LK, Yamauchi PS, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). Br J Dermatol. 2017;177(6):1552-1561. doi: 10.1111/bjd.15722 [DOI] [PubMed] [Google Scholar]
- 82.Blauvelt A, Lacour JP, Fowler JF Jr, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623-631. doi: 10.1111/bjd.16890 [DOI] [PubMed] [Google Scholar]
- 83.Suleiman AA, Khatri A, Oberoi RK, Othman AA. Exposure-response relationships for the efficacy and safety of risankizumab in Japanese subjects with psoriasis [oublished online October 31, 2019]. Clin Pharmacokinet. 2019. doi: 10.1007/s40262-019-00829-2 [DOI] [PubMed] [Google Scholar]
- 84.US National Library of Medicine A Study of Ustekinumab to Evaluate a "Subject-tailored" Maintenance Dosing Approach in Subjects With Moderate-to-Severe Plaque Psoriasis (PSTELLAR). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01550744. Accessed August 2018.
- 85.US National Library of Medicine Study to Demonstrate Equivalent Efficacy and to Compare Safety of Biosimilar Adalimumab (GP2017) and Humira (ADACCESS). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT02016105. Accessed August 2018.
- 86.US National Library of Medicine Efficacy and Safety Study of Apremilast (CC-10004) in Subjects With Moderate-to-Severe Plaque-Type Psoriasis (Core Study). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT00773734. Accessed August 2018.
- 87.ClinicalTrials.gov Study of Efficacy and Safety of Brodalumab Compared With Placebo and Ustekinumab in Moderate to Severe Plaque Psoriasis Subjects (AMAGINE-2). https://clinicaltrials.gov/ct2/show/NCT01708603. Accessed December 9, 2019.
- 88.ClinicalTrials.gov Study of Efficacy and Safety of Brodalumab Compared With Placebo and Ustekinumab in Moderate to Severe Plaque Psoriasis Subjects (AMAGINE-3). https://clinicaltrials.gov/ct2/show/NCT01708629. Accessed December 9, 2019.
- 89.ClinicalTrials.gov Safety and Efficacy of Secukinumab Compared to Etanercept in Subjects With Moderate to Severe, Chronic Plaque-Type Psoriasis (FIXTURE). https://clinicaltrials.gov/ct2/show/NCT01358578. Accessed December 9, 2019.
- 90.Clinicaltrials.gov A 52-Week Multicenter, Randomized, Blinded, Parallel-Group Study Comparing the Efficacy and Safety of Ixekizumab to Ustekinumab in Patients With Moderate-to-Severe Plaque Psoriasis (IXORA-S). https://clinicaltrials.gov/ct2/show/NCT02561806. Accessed December 9, 2019.
- 91.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(suppl 1):29-37. [PMC free article] [PubMed] [Google Scholar]
- 92.Xu G, Xia M, Jiang C, et al. Comparative efficacy and safety of thirteen biologic therapies for patients with moderate or severe psoriasis: a network meta-analysis. J Pharmacol Sci. 2019;139(4):289-303. doi: 10.1016/j.jphs.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 93.Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170(2):274-303. doi: 10.1111/bjd.12663 [DOI] [PubMed] [Google Scholar]
- 94.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535. doi: 10.1002/14651858.CD011535.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14(8):e0220868. doi: 10.1371/journal.pone.0220868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wade R, Grosso A, South E, et al. Brodalumab for the treatment of moderate-to-severe plaque psoriasis: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(2):131-139. doi: 10.1007/s40273-018-0698-2 [DOI] [PubMed] [Google Scholar]
- 97.Cui L, Chen R, Subedi S, et al. Efficacy and safety of biologics targeting IL-17 and IL-23 in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;62:46-58. doi: 10.1016/j.intimp.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 98.Armstrong AW, Betts KA, Signorovitch JE, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018;34(7):1325-1333. doi: 10.1080/03007995.2018.1457516 [DOI] [PubMed] [Google Scholar]
- 99.Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2015;135(11):2641-2648. doi: 10.1038/jid.2015.206 [DOI] [PubMed] [Google Scholar]
- 100.Sawyer LM, Cornic L, Levin LA, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33(2):355-366. doi: 10.1111/jdv.15277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shear N, Joshi A, Zhao J, Betts K, Sinvhal R, Gisondi P Comparison of safety outcomes for treatments of moderate to severe plaque psoriasis through a network meta-analysis. Presented at: 24th World Congress of Dermatology; June 11, 2019; Milan, Italy. [Google Scholar]
- 102.Warren R, Joshi A, Betts K, Li J, Zhao J, Williams D Comparison of dermatology quality of life index for novel treatments of moderate-to-severe plaque psoriasis: a network meta-analysis. Presented at: 28th European Academy of Dermatology and Venereology Congress; October 12, 2019; Madrid, Spain. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of Clinical Trials
eFigure 1. Estimated Odds Ratios From the NMA of Short-term PASI (Base-Case)
eTable 2. Estimated Response Rates From the NMA of Short-term PASI (Sensitivity Analyses Including Global Trials Only, Including Phase III Trials Only, and in an Expanded Treatment Space)
eFigure 2. Estimated Odds Ratios From the Meta-analysis of Long-term PASI (Base-Case)
eTable 3. Estimated Response Rates From the Meta-analysis of Long-term PASI (Sensitivity Analyses: Including Global Trials Only, Including Trials Reporting NRI Data, and Including Phase III Trials Only)
eFigure 3. Evidence Network for NMA of Long-term PASI
eTable 4. Estimated Response Rates From the NMA of Long-term PASI
eTable 5. Estimated Odds Ratios for PASI 90 from the NMA of Long-term PASI