Abstract
Aging-related cellular and molecular processes including low-grade inflammation are major players in the pathogenesis of cardiovascular disease (CVD) and Alzheimer’s disease (AD). Epidemiological studies report an independent interaction between the development of dementia and the incidence of CVD in several populations, suggesting the presence of overlapping molecular mechanisms. Accumulating experimental and clinical evidence suggests that amyloid-beta (Aβ) peptides may function as a link among aging, CVD, and AD. Aging-related vascular and cardiac deposition of Αβ induces tissue inflammation and organ dysfunction, both important components of the Alzheimer’s disease amyloid hypothesis. In this review, the authors describe the determinants of Aβ metabolism, summarize the effects of Aβ on atherothrombosis and cardiac dysfunction, discuss the clinical value of Αβ1-40 in CVD prognosis and patient risk stratification, and present the therapeutic interventions that may alter Aβ metabolism in humans.
Key Words: Alzheimer’s disease, amyloid-beta, amyloid precursor protein, atherosclerosis, cardiovascular disease, cardiovascular therapy, cerebral amyloid angiopathy, coronary artery disease, endothelial cells, leukocytes, platelets, prognosis, vascular dementia, vascular stiffness
Abbreviations and Acronyms: Aβ, amyloid-beta; ACS, acute coronary syndrome; AD, Alzheimer’s disease; ApoE−/−, apolipoprotein E-deficient; APP, amyloid precursor protein; BACE, beta amyloid cleaving enzymes; CAA, cerebral amyloid angiopathy; CAD, coronary artery disease; CVD, cardiovascular disease
Central Illustration
Highlights
-
•
Aging is the most important risk factor for the development of cardiovascular disease and dementia. Yet, the overlapping underlying mechanisms are not completely appreciated.
-
•
Aβ, an aging-induced peptide and the hallmark of the amyloid hypothesis of Alzheimer’s disease, constitutes an independent cardiovascular risk factor.
-
•
We review the determinants and the role of Aβ in cardiovascular system and disease.
-
•
We call for research gathering evidence on how to treat this newly recognized entity.
Several cardiovascular risk factors have long been associated with a greater risk for future cognitive decline in nondemented individuals (1). Control of vascular risk factors effectively reduces the incidence of dementia in both healthy and cognitively impaired individuals (2). The presence of intracerebral atherosclerotic vascular disease (3) exacerbates all types of dementia and has been independently associated with worse cognitive performance even in nondemented individuals (4). These observations indicate that the aging-related inflammatory nature of both atherosclerosis and dementia involves multiple common cellular and molecular mechanisms. Recent accumulating evidence points toward the existence of a possible nonexclusive shared systems biology process that may drive aging-associated diseases, atherosclerotic cardiovascular disease (CVD), and dementia (Figure 1).
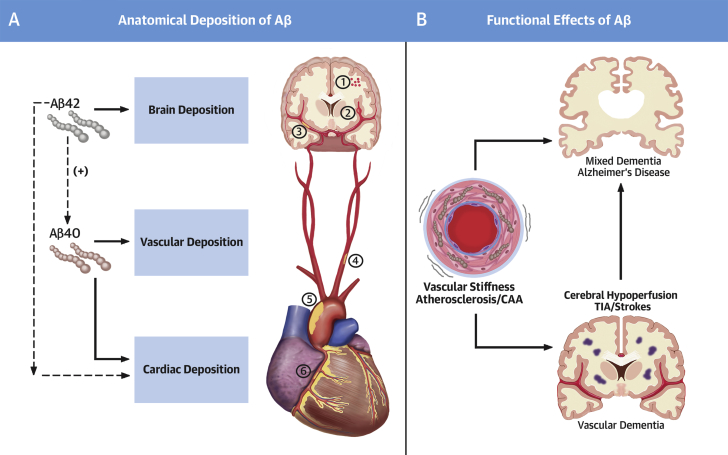
Figure 1.
The Continuum of Cardiovascular and Neurotoxic Effects of Αβ Peptides
(A) Amyloid-beta (Aβ) 1-42 peptides have been found in brain parenchymal and cardiac depositions and, to a lesser extent, in vessels. Depositions composed of Αβ1-40 peptides have been described mainly in the heart and vessels including several vascular beds ranging from: (1) leptomeningeal and cortical vessels in cerebral amyloid angiopathy (CAA); to (2) cerebral microvasculature; (3) intracerebral arteries/circle of Willis; (4) carotid arteries; (5) aorta; and (6) coronary/extracerebral arteries. (B) Brain Αβ deposits trigger a number of events involved in neuronal dysfunction clinically manifested as cognitive decline and progressive Alzheimer’s type dementia. Cardiac depositions are associated with cardiomyocyte dysfunction. Vascular Αβ deposition induces functional changes (vascular stiffening) and promotes vascular inflammation and atherosclerosis. Aging-associated Αβ-induced cardiovascular disease leads to cerebral hypoperfusion, which is a risk factor for vascular, Alzheimer’s, or mixed dementia.
Production and accumulation of amyloid-beta (Aβ) peptides in the brain are considered the hallmark of Alzheimer’s disease (AD) amyloid hypothesis (5). The prototypic cerebrovascular disease associated with Αβ40 deposits is cerebral amyloid angiopathy (CAA) (6). CAA describes a group of aging-associated brain disorders with characteristic pathological findings of amyloid deposits predominantly in the arteriolar wall. Clinical and imaging features of CAA vary from asymptomatic microbleeds to severe hemorrhage, neurological deficits, cognitive impairment, dementia, and death. Defective perivascular drainage of neuronal-derived Aβ is probably the main mechanism of Αβ deposition. Among Aβ peptides, Αβ1-40 is the main peptide involved in the pathogenesis of CAA, whereas Αβ1-42 is mainly involved in development of AD. The vascular preference of Aβ1-40 has led to the hypothesis that this molecule may exert proinflammatory properties not only in cerebral but also in peripheral vasculature, mediating arterial disease as depicted in Figure 1, suggesting a continuum of Aβ1-40 deposits in the circulatory system ranging from leptomeningeal and cortical cerebral microvasculature (CAA) to intracerebral, carotid, aortic, or coronary vascular wall or heart. Interestingly, in contrast to studies examining associations between Aβ1-40 plasma levels and cardiovascular disease, studies assessing the association of plasma Αβ1-40 with cognitive function have not yielded consistent results (7). The detrimental properties of Αβ1-40 species on vascular brain pathology affecting memory and cognition secondarily to microvasculature damage rather than through direct neurotoxicity, may explain this discrepancy.
In this review, we present contemporary evidence that links Αβ peptides with vascular inflammation and a wide range of associated extracerebral atherosclerotic manifestations and myocardial dysfunction, as well as adverse CVD outcomes and mortality (Central Illustration). Based on this evidence, we discuss the potential clinical utility of Αβ1-40 as a biomarker for risk stratification for mortality and present therapeutic interventions that may alter Αβ accumulation.
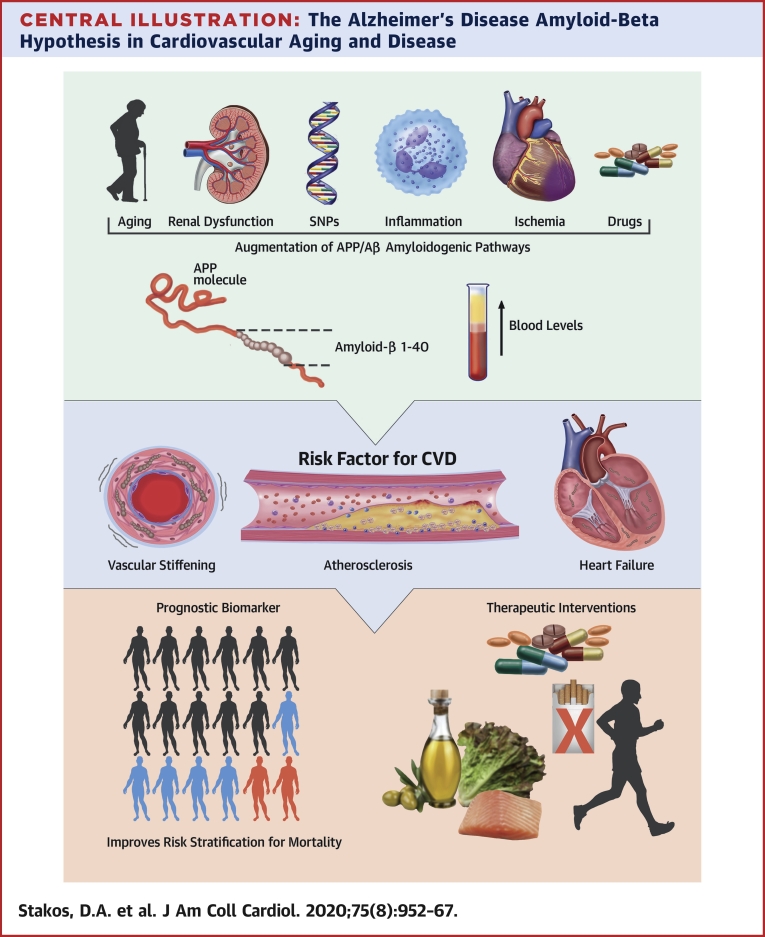
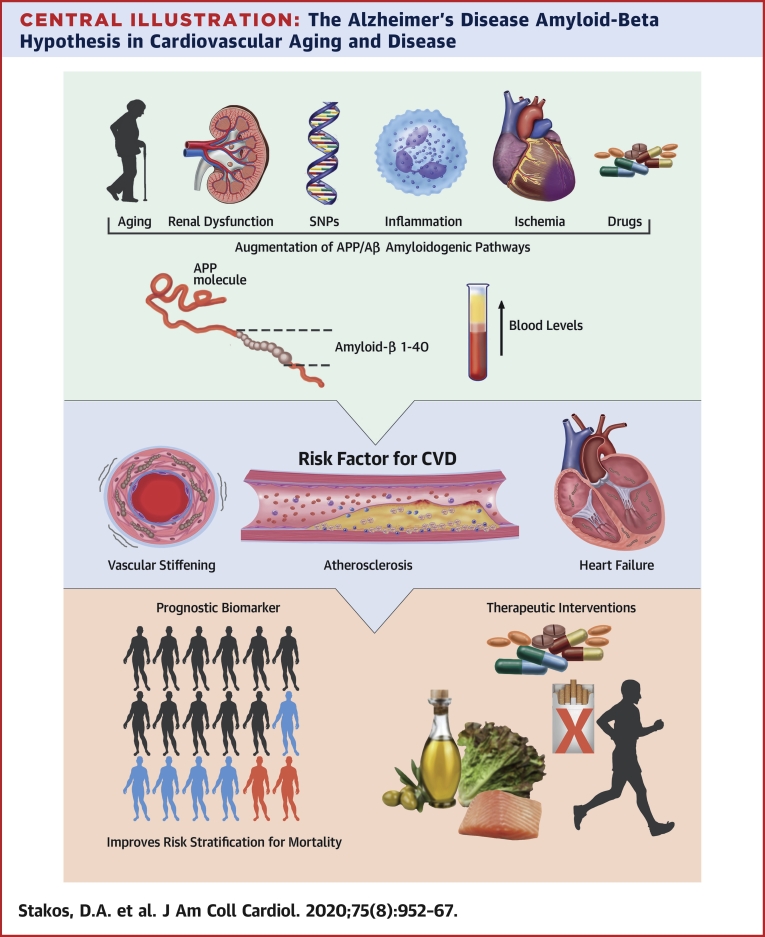
Central Illustration.
The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease
Several factors alter APP/Aβ metabolism by promoting amyloidogenic pathways leading to increased Αβ1-40 blood levels. Subsequent deposition of Αβ1-40 in heart and vessels induces cell damage, accelerating arterial stiffening, atherosclerosis, and cardiac dysfunction, which are manifestations of cardiovascular aging and disease. Epidemiological evidence supports the clinical relevance of these effects. Αβ1-40 blood levels fulfill several criteria as a cardiovascular prognostic biomarker for risk stratification. Lifestyle and medical interventions interfere with Αβ1-40 levels. Aβ = amyloid-beta; APP = amyloid precursor protein; CVD = cardiovascular disease; SNP = single-nucleotide polymorphism.
Amyloid Precursor Protein and Aβ Metabolism
Aβ peptides are proteolytic fragments of amyloid precursor protein (APP), an integral membrane protein (8,9). The APP gene produces 3 major splice variants (10), APP695, APP751, and APP770, produced in neurons, endothelial cells, and platelets, respectively. The exact physiological function of this well-conserved, site-specific APP/Αβ pathway is not fully elucidated, but it is associated with natural antimicrobial defense (11) and coagulation cascade proteolytic events (12). The latter is mediated by a Kunitz-type serine protease inhibitor domain contained in APP751 and APP770 molecules.
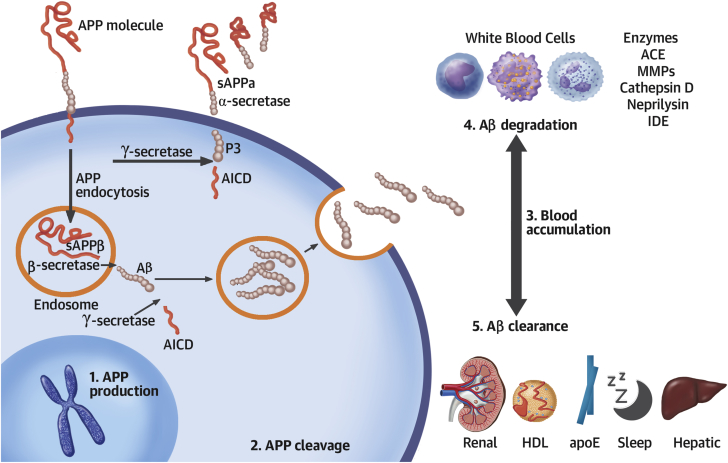
APP can be initially cleaved by α-secretases generating nonamyloidogenic products depending on its location on plasma membrane, the site of processing (membrane or endosomes), and environmental pH (13), or by β-secretases, also known as beta amyloid cleaving enzymes (BACE) (Figure 2). The β-secretase–mediated cleavage of APP retains the integrity of Αβ fragments within the remaining C99 peptide, while C99 subsequent cleavage by γ-secretases releases Aβ peptides (14). C99 cutting site by γ-secretases depends on the location of processing (endosomes or Golgi network) and generates amino acid peptides of length 40 (Αβ1-40 mostly found in vascular lesions) and 42 (Αβ1-42, mainly found in AD-associated brain lesions), as well as the intracellular domain of APP (Figure 2). Several factors, including aging, inflammation, renal dysfunction, ischemia, polymorphisms, and drugs, increase circulating levels and subsequent tissue deposition of Αβ by augmenting APP production and processing or by decreasing Αβ clearance and degradation (Figure 2, Online Tables 1 to 3). Under normal conditions an equilibrium exists between Aβ production and removal in various compartments inside or outside of the central nervous system (15). Deregulation of this equilibrium may lead to accumulation of Αβ1-40 in blood, vascular wall, and heart tissues, which has been associated with CVD.
Figure 2.
APP and Αβ Metabolism
Following (1) amyloid precursor protein (APP) gene transcription, (2) APP is cleaved in the nonamyloidogenic pathway (plasma membrane) by α- and γ- secretases or in the amyloidogenic pathway (endosomes) by β- and γ- secretases. The later pathway generates amyloid beta (Αβ) peptides that are released extracellularly. (3) Αβ accumulation in blood or tissues may result from enhanced production/cleavage or by (4) impaired degradation and/or (5) clearance. ACE = angiotensin converting enzyme; AICD = amyloid precursor protein intracellular domain; apoE = apolipoprotein E; HDL = high-density lipoprotein; IDE = insulin degrading enzyme; sAPP = soluble amyloid precursor protein.
Systemic Accumulation of Aβ and CVD
Peripheral vascular Aβ abundance
Although APP processing in different cell types gives rise preferentially to Αβ1-40 or -42 (16), it is not known what drives this differential final processing of the amyloidogenic pathway of APP. In cases of CAA, neuronal-derived Αβ (either Αβ1-40 or -42) fails to drain away from the leptomeningeal vessels, capillaries, and brain parenchyma (17). This defective depletion leads to its accumulation in brain arterioles. Αβ deposits are observed in the tunica media in close proximity as well as inside of the smooth muscle cells and in the adventitia, avoiding endothelial cells even at higher degrees of CAA (18,19). Because impairment of adventitial lymphatic capillaries in peripheral vessels also aggravates atherosclerosis, the role of lymphatic drainage in Aβ-related cardiovascular disease should be further explored. In peripheral atherosclerotic lesions, Αβ deposits consist almost exclusively from the Αβ1-40 species (20). Using mass spectrometry, Aβ1-40 peptide was on average 100 times more abundant than Aβ1-42 in human aortic atherosclerotic plaques (21). The 2-peptide-amino-acid-longer species Αβ1-42, being more hydrophobic and fibrillogenic, is the main amyloid peptide found in parenchymal lesions of AD; however, its “vascular” involvement is limited to deposits in pericapillary spaces and glia limitans, parenchymal brain vessels, and leptomeningeal vessels. Yet, overexpression of Αβ1-42 promotes Αβ1-40 vascular depositions in the brain (22), and factors that alter the Αβ1-40/-42 ratio, such as human apolipoprotein E4 (23), favor amyloid deposits in the form of CAA compared with parenchymal plaques. This differential tissue preference of Aβ species may be explained by the following observations: 1) using 3D models of cerebrovascular vessels, researchers have recently demonstrated that HDL and apolipoprotein E (ApoE) synergistically promote vascular clearance of Aβ1-42 more than that of Aβ1-40 (24); 2) Αβ1-40 is produced in significant amounts from platelets, plaque invading macrophages (25), endothelial cells (26), and vascular smooth muscle cells (27); and 3) different ApoE isoforms, which are proteins with an impact in cholesterol transport system, seem to differentially regulate Aβ production, aggregation, and clearance (28). More specifically, ApoE4 may inhibit Aβ clearance by competitively binding to the low-density lipoprotein receptor-related protein 1, and its presence has been associated with brain Αβ accumulation and increased AD risk. Interestingly, ApoE seems to affect also Αβ kinetics in blood (29).
Aβ and subclinical vascular disease
Aβ1-40 is critically involved in vascular aging. SIRT1, a class III histone deacetylase, plays a pivotal protective role in vascular aging (30) as it up-regulates α-secretase activity shifting Aβ metabolism towards the non-amyloidogenic pathway (Figure 2). However, activation of the amyloidogenic pathway results in impairment of the vasodilating properties of small arterioles by enhancement of endothelin-1 expression (31), reduction of eNOS activity and endothelium-dependent vasodilation, enhancement of oxidative stress (32), and increased responsiveness to vasoconstrictors (33) (Table 1, Figure 3). Further, Aβ oligomers may inhibit telomerase activity leading to telomere shortening (34), which actively promotes vascular aging. This experimental evidence generates the hypothesis that increased Aβ systemic concentrations may be associated with measurable, accelerated arterial aging and deteriorated vascular function and structure in humans. Arterial pulse wave velocity is a well-established, noninvasive marker of arterial stiffness and vascular aging (35). Interestingly, the severity of cerebral β-amyloid deposition measured by positron emission tomography scan and its change over 2-year follow-up was associated with higher pulse wave velocity in nondemented elderly adults (36,37). To assess whether Aβ1-40 is involved in early processes of arterial disease and aging, we prospectively examined changes in pulse wave velocity and plasma Aβ1-40 in 107 young to middle-aged healthy adults (mean age 46.2 years), clinically followed for 5 years (38). We found that the 5-year change of plasma Aβ1-40 levels was an independent determinant of the 5-year change in aortic stiffness. Because Aβ1-40 deposits have been found in carotid human atherosclerotic plaques (25,39) and aortas (21), we examined whether plasma Aβ1-40 levels are associated with subclinical atherosclerosis in a population of 394 individuals with a wide range of CVD risk profiles. After adjustment for age, traditional CVD risk factors, and renal function, increased Αβ1-40 was independently associated with higher carotid intima-media thickness, lower ankle-brachial index, and the severity and extent of arterial damage assessed in the carotid and femoral arteries, aorta, and coronary circulation (38). Plasma Aβ1-40 was also associated with the severity of coronary artery calcium score in a sample of 3,266 adults from the Dallas Heart Study without clinically overt CVD (40).
Table 1.
Role of APP and Aβ in Cardiovascular Biology and Disease
| Molecule | Study Design | Tissue or Cell-Specific Effects | Ref. # |
|---|---|---|---|
| Endothelial Cells | |||
| APP | Murine and human cell line | Increased protein levels of proinflammatory mediators (COX-2, VCAM-1) and increased secretion of IL-1β and Aβ1-40 through Src kinase signaling pathway | (69) |
| Aβ1–40 | Human cell line | Increased expression of inflammatory genes (MCP-1, GRO, ΙL-1β, and IL-6) through JNK-AP1 signaling pathway | (48,70) |
| Aβ1–40 | Rat cell line | Increase of endoplasmic reticulum stress through unfolded protein response | (71) |
| Aβ1–40 | Human, mouse, rat, and bovine cell line | Inhibition of the KCa2+ channel opening and reduced Ca2+ efflux | (71,72) |
| Aβ1–40 | Human and rat cell line | Activation of caspase-dependent and -independent apoptosis through caspase 12 and cytochrome c | (48,71) |
| Aβ1–40 Aβ1–42 Aβ25–35 |
Human, mouse, bovine, and porcine cell line, rat arteries | Inhibition of NO signaling in a concentration-manner through interaction with CD36 | (72,73) |
| Aβ1–40 Aβ1–42 |
Human cell line | Signature transcriptomic of essential endothelial function affected | (48) |
| Smooth Muscle Cells | |||
| Aβ1–42 | Human and porcine cell line | Decrease in sGC activity and cGMP production | (73) |
| Cardiomyocytes | |||
| Aβ1–40 Aβ1–42 |
Murine and human cell line | Decrease of cell viability | (48) |
| Monocytes | |||
| APP | Murine and human cell line | Recruitment of tyrosine kinases Lyn and Syk to APP during β1 integrin-mediated adhesion of monocyte through tyrosine kinase mechanism | (69,74,75) |
| Aβ1–42 | Human monocytes | Differentiation of monocytes into macrophages | (76) |
| Aβ1–40 Aβ1–42 Aβ25–35 |
Human monocytes Human cell line |
Hypersecretion of inflammatory cytokines (TNF-α and IL-1β) and chemokines (MCP-1, IL-8, MIP-1 α, and CCR5) through activation of ERK-1/-2 | (43,76, 77, 78, 79) |
| Aβ1–40 Aβ1–42 Aβ25–35 |
Human and murine cell line | Secretion of ROS | (79) |
| Aβ1-40 | Human cell line | Migration of monocyte through ERK-1/-2 and RAGE receptor | (74,80) |
| Aβ1-40 Aβ1-42 |
Human cell | Opsonization of lipoproteins enhances their uptake by human monocytes, resulting in cholesterol accumulation | (81) |
| Macrophages | |||
| Aβ1–40 | Murine cell line | Enhanced nitrite production in the presence of IFN-γ macrophage activation | (25) |
| Aβ1-40 Aβ1-42 |
Human cell | Opsonization of lipoproteins enhances their uptake by macrophages, resulting in cholesterol accumulation Accelerated formation of foam cells |
(81) |
| Aβ1–42 | Macrophages from CD36−/− mice | Production of ROS and proinflammatory cytokines IL-1β and TNF-α through CD36 signaling | (82,83) |
| Platelets | |||
| sAPP695α sAPP751α sAPP770α |
Human platelet | Inhibition of platelet aggregation and secretion | (84) |
| Aβ1–40 | Amyloid properties induced in unrelated proteins to stimulate human and murine platelets | Platelet aggregation through either a CD36-p38MAPK-TXA2 or a glycoprotein Ibα pathway | (85) |
| Aβ1–40 Aβ25–35 |
Human platelet | Platelet aggregation with Ca2+ mobilization and PLC γ 2-PKC pathway activation | (86) |
| Aβ25–35 | Human and murine platelet | Platelet activation through RhoA-dependent modulation of actomyosin Increase in intracellular Ca2+, leading to dense granule release and ADP secretion |
(87,88) |
| Aβ1–40 Aβ1–42 Aβ25–35 |
Human and murine platelet | Platelet adhesion and spreading through the elongation of filopodia and lamellipodia | (89,90) |
| Aβ1-42 | Human plasma | Thrombin generation in an FXII-dependent FXI activation | (91) |
| Aβ1–40 | Human and murine platelet | ROS generation and cell shrinkage | (89) |
| APP | Overexpression of human APP isoform 770 in mice platelets | Marked inhibition of thrombosis in vivo | (85) |
| APP | Overexpression of human APP isoform 751 in mice | Prothrombotic phenotype in vivo | (61) |
APP = amyloid precursor protein; Aβ = amyloid beta; CCR5 = chemokine receptor type 5; cGMP = cyclic guanosine monophosphate; COX = cyclooxygenase; ERK = extracellular signal–regulated kinase; FX = coagulation factor.; GRO = growth-related oncogene; IL = interleukin; IFN = interferon; JNK-AP = c-Jun N-terminal kinase–activator protein; MCP = monocyte chemo-attractant protein; MIP = macrophage inflammatory protein; NO = nitric oxide; PKC = protein kinase C; PLC = phospholipase C; RAGE = receptor advanced glycation end products; ROS = reactive oxygen species; sGC = soluble guanylyl cyclase; TNF = tumor necrosis factor; TXA2 = thromboxane A2; VCAM = vascular cell adhesion molecule.
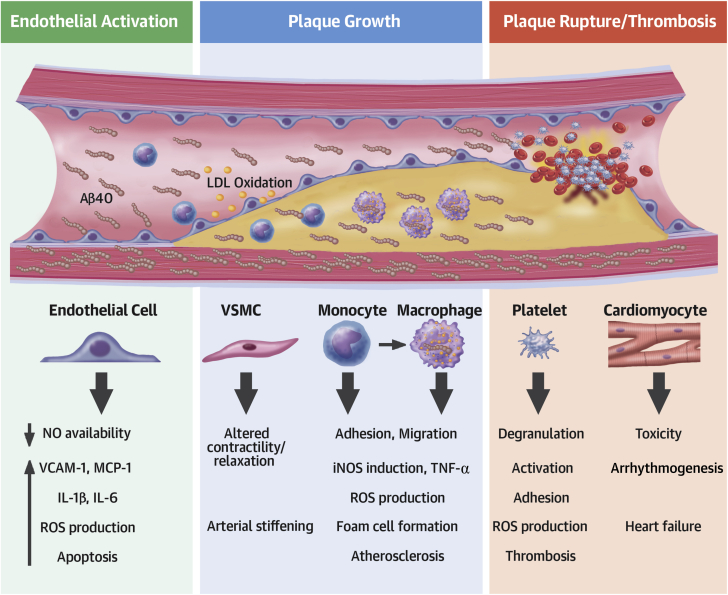
Figure 3.
Detrimental Cellular and Molecular Effects of Aβ1-40 in the Cardiovascular System
Excess in blood Αβ1-40 levels exerts detrimental effects in vascular and blood cells promoting endothelial activation, atherosclerosis, and atherothrombosis. IL = interleukin; iNOS = inducible isoform of nitric oxide synthases; LDL = low-density lipoprotein; MCP = monocyte chemoattractant protein; NO = nitric oxide; ROS = reactive oxygen species; TNF = tumor necrosis factor; VCAM = vascular cell adhesion molecule; VSMC = vascular smooth muscle cells.
Overall, these findings are indicative of direct and indirect roles of Aβ1-40 in accelerated arterial aging, atherosclerosis at various stages, and vascular beds, taking place long before the establishment of clinically overt CVD.
Αβ1-40 in coronary artery disease
Circulating Aβ1-40 levels were independently associated with the presence of angiographically documented stable coronary artery disease (CAD) in 2 independent cohorts consisting of 514 and 396 patients (38). This association was confirmed in subsequent studies, including adults with normal cognitive function or patients with AD (41,42).
Experimental evidence indicates that Aβ peptides may be actively involved in downstream pathways leading to plaque rupture, thrombosis, and subsequent clinical manifestations of the acute coronary syndrome (ACS) (Figure 3). Αβ1-40 stimulates platelet activation and adhesion in humans and mice (Table 1) and induces release of matrix metalloproteinases by human monocytes to increase plaque vulnerability (43). Interestingly, in a myocardial infarction rat model, early surges in plasma sAPP770 concentrations preceded the release of cardiac injury enzymes (26), while plasma sAPP was also increased in patients with ACS (26), suggesting that enhanced APP/Aβ processing and subsequent release of sAPP770 and Αβ1-40 may trigger plaque rupture or its sequalae in ACS. In support of this hypothesis (Figure 3), we recently reported that in 2 independent cohorts of patients with non-ST-segment elevation ACS, higher blood Aβ1-40 levels were associated with worse risk profile, including a higher GRACE (Global Registry of Acute Coronary Events) score high sensitivity cardiac troponin T and lower systolic blood pressure and estimated glomerular filtration rate (44), implying a concentration-dependent relation of Aβ with the severity of ACS. Overall, the results of these studies provide conceptual proof that Aβ metabolism is enhanced in CAD and Aβ1-40 levels in blood are increased and associated with its clinical presentation.
Aβ1-40, Mortality, and Risk Stratification
General population
High plasma Aβ1-40 concentrations were independently associated with increased risk of mortality in 1,254 elderly subjects after adjustment for CVD risk factors and frailty (45). However, significance was lost after adjustment for cystatin C, suggesting that this association may be mediated by differences in renal function and/or inflammatory status. The prognostic value of circulating Aβ1-40 in nonelderly subjects from the general population as well as its reclassification potential remain unknown.
Coronary artery disease
We have recently shown that circulating Aβ1-40 blood levels measured in 2 independent populations of patients with stable CAD were predictive of a 3-fold increased risk of cardiovascular death for highest versus lowest quartile (38). Importantly, adding Aβ1-40 improved risk stratification over the best predictive model by reclassifying 22% of the population to correct risk categories for cardiovascular mortality.
In-hospital and midterm mortality in patients with ACS vary considerably from <1% to >8% according to risk score calculators (46,47). However, no indexes of vascular inflammation are currently included in risk estimation scores such as the widely recommended GRACE score assessing mortality (46,47). To this end, we have demonstrated that measuring Aβ1-40 in patients with non–ST-segment elevation ACS improves prognostic assessment and provides incremental reclassification value over the GRACE score (44). A single measurement of circulating Aβ1-40 at presentation was independently associated with mortality in both cohorts (44). Importantly, Αβ1-40 substantially improved risk stratification of patients with non–ST-segment elevation ACS into correct risk categories over the GRACE score (net reclassification index 33.4% to 47.1%).
Collectively, these findings suggest that Αβ1-40 may be a clinically useful risk biomarker in stable CAD and particularly in non–ST-segment elevation ACS where Aβ1-40’s performance was complementary to that of the GRACE score, a commonly used risk score in clinical practice. However, clinical application of this peptide as a biomarker needs further research to set reference values and thus allow its investigation as part of novel prognostic algorithms in CAD.
Αβ1-40 and cardiac function
A deregulation of the BACE1/Αβ1-40 axis was identified in the hearts of nondemented individuals with ischemic heart failure (48), whereas histology confirmed Aβ1-40 and -42 aggregates in the heart of patients with AD (49), suggesting a novel form of aging-related cardiac amyloidosis that merits further investigation. Mechanistically, both peptides exert toxic effects on cardiomyocytes resulting in poorer cell viability and apoptosis (48,49). Treatment of zebrafish embryos with Aβ1-40 peptides induces impaired vascular development and angiogenesis (50), possibly by interfering with VEGF pathway (51). Because ischemia promotes both APP up-regulation and cleavage (52), and Aβ1-40 may induce vasoconstriction and reduced endothelium-dependent vasodilatation (53), the pathogenic consequences of short- or long-term myocardial ischemia on heart failure via enhanced cardiac amyloidogenesis should be explored.
Many aspects of Aβ-related cardiac amyloidosis are supported by clinical findings. Plasma Αβ1-40 has been associated with markers of cardiac dysfunction in several clinical conditions with variable degrees of myocardial functional impairment. We have recently demonstrated that in 3,266 individuals without clinically overt CVD from the Dallas Heart Study who underwent cardiac magnetic resonance imaging, plasma Αβ1-40 was associated with increased circulating N-terminal pro–B-type natriuretic peptide and high sensitivity cardiac troponin T, indicative of involvement of this peptide in early subclinical myocardial stretch and injury (40). Interestingly, we also found an association of Aβ1-40 with lower left atrial emptying fraction after adjustment for CVD risk factors. In contrast, although stroke volume index was lower at higher levels of Aβ1-40 by univariate analysis, we observed no independent associations with more advanced cardiac abnormalities such as left ventricular systolic dysfunction or remodeling, possibly because the population under study was free of established heart disease and such late changes were not discernible. Indeed, increased plasma Αβ1-40 was found in patients with established CAD and lower left ventricular ejection fraction (38). Given that Aβ1-40 is associated with lower cardiorespiratory fitness (VO2 max) independently of daily activity (40) and with left atrial dysfunction, further studies are needed to assess whether lower VO2 max is of cardiac origin possibly related to Αβ1-40–mediated subclinical myocardial damage. Accordingly, the presence of Aβ1-40 in the heart has been associated with echocardiographic findings of early diastolic dysfunction (49). Furthermore, in a prospective study of 939 patients with heart failure showing reduced or preserved ejection fraction, plasma Αβ1-40 levels were associated with symptoms of heart failure as described in New York Heart Association’s functional classification system (54). Because diastolic dysfunction and heart failure with preserved ejection fraction are considered prominent manifestations of myocardial aging (55), blood concentrations of Αβ1-40 may reflect the extent of its vascular and myocardial involvement in CVD. The clinical relevance of this concept is supported by recent findings showing that circulating Aβ1-40 predicts adverse clinical outcomes and mortality and improves risk stratification in patients with heart failure (54).
Experimental evidence of the link between Aβ and CVD
A dementia-CVD continuum hypothesis is further demonstrated through the vascular involvement of dementia-prone transgenic APP mice. The Tg2576 mouse model expresses 5 times the levels of endogenous murine APP (56) and shows progressive impairment of cognitive function together with Αβ1-40–dependent (57) and ROS-mediated (53,58) endothelial dysfunction, impaired vascular reactivity, and 30% attenuation in cerebral blood flow (59). B6Tg2576 mice develop more extensive aortic lesions than control mice when fed the same atherogenic or normal diet under similar lipid profiles (60). APP23 mice, which overexpress APP and Αβ1-40, show enhanced platelet integrin activation and degranulation as well as accelerated thrombus formation (61). Dementia-prone APP23 mice crossed with atherosclerosis-prone apolipoprotein E–deficient (ApoE−/−) mice develop larger and more inflammatory aortic atherosclerotic lesions compared with ApoE−/− mice (62). Conversely, ApoE−/− mice crossed with animals lacking APP (APP−/−) have significantly reduced atherosclerotic plaque size in thoracic and abdominal aorta (90% and 75% reduction, respectively) compared with ApoE−/− mice despite comparable cholesterol levels (63). More importantly, atherosclerotic plaques in APP−/−/ApoE−/− mice have reduced macrophage content, increased amount of collagen, and a thicker fibrous cap indicating a more stable plaque morphology. Mechanistically, a series of experimental studies summarized in Table 1 present Αβ as a potent proinflammatory, proapoptotic, and proatherogenic molecule affecting the function of endothelial cells, platelets, vascular smooth muscle cells, and macrophages (Figure 3).
Interventions Affecting Aβ Metabolism
Lifestyle modifications
A healthy lifestyle, including adherence to Mediterranean diet, omega-3 fatty acids, and caloric restriction may reduce Aβ brain deposits and exert antiamyloidogenic properties (Online Table 4). We recently demonstrated that increased daily activity assessed by accelerometer recordings and lower physical fitness, as assessed by VO2 max, in 3,266 participants without CVD from the Dallas Heart Study were independently associated with plasma levels of Aβ1-40 (40). However, changes of Aβ peptide blood levels over time in response to physical activity have not been assessed. Yet, accumulating evidence suggests that an unhealthy lifestyle such as a high-fat diet and cigarette smoking (64) may enhance the amyloidogenic pathway (Online Table 4). These findings suggest that cardiovascular effects of lifestyle modifications may be partly mediated by altering Aβ metabolism, but further research should explore these effects in humans, particularly with regards to Aβ1-40 as a direct effector molecule in cardiovascular disease.
Cardiovascular medical treatment
Statins
Experimental evidence indicates that statins reduce brain and intracellular Aβ levels in vitro and in vivo, by down-regulating its upstream pathway, reducing cellular uptake of Aβ peptides, and enhancing its clearance through the blood brain barrier (Table 2). However, results of 2 randomized clinical studies evaluating blood Αβ1-40 peptides after statin treatment were inconsistent, possibly due to statins’ effect on equilibrium between brain and circulating Αβ (Table 2).
Table 2.
Off-Target Effects of Statins on Aβ Metabolism and Accumulation
| Intervention/Condition | Cell Type/Population | Effects on Aβ Metabolism | Ref. # |
|---|---|---|---|
| Lovastatin (escalating doses 10–60 mg OD) | Double-blind, randomized, placebo-controlled clinical study of 94 patients with hypercholesterolemia, 12 weeks | Serum levels of total Aβ are reduced in a dose-dependent manner | (92) |
| Simvastatin (20 mg OD) | Prospective interventional clinical trial of 19 patients with AD, 12 weeks | CSF levels of alpha and beta-secretase-cleaved APP decreased, no change in plasma levels of Aβ1-42 | (93) |
| Pravastatin (10 mg OD) | Prospective observational clinical study of 46 patients with hyperlipidemia, 6 months | No change in plasma levels of Aβ1-40 and Αβ1-42 | (94) |
| Simvastatin (20–80 mg OD) or Atorvastatin (20–80 mg OD) | Prospective interventional randomized clinical trial of 39 patients with hypercholesterolemia, 9 months | No change in plasma levels of Aβ1-40, Aβ1-42, or total Aβ | (95) |
| Simvastatin (escalating 40–80 mg OD) | Prospective open-label trial of 12 patients with AD or mild cognitive impairment and hypercholesterolemia, 12 weeks | No change in plasma levels of Aβ1-40 | (96) |
| Simvastatin Lovastatin |
Neuronal cell culture, Guinea pigs |
Decreased production of Aβ1-40 and Αβ1-42 in neurons in vitro Decreased CSF levels of Aβ1-40 (−47%) and Aβ1-42 (−62%) |
(97) |
| Lovastatin Simvastatin |
HEK cells | Inhibited dimerization of β-secretase Decreased intracellular production of total Aβ |
(98) |
| Fluvastatin | C57BL/6 mice neurons HBME cells |
Increased APP-CTF clearance to the lysosome in neurons Increased LRP-1 and Aβ uptake in HBME |
(99) |
| Simvastatin | PBCE cells 3x Tg AD mice |
Increased LRP1 and apoJ expression Reduced Aβ uptake by PBCEC Decreased production of APP-CTFs in brain capillary endothelial cells of mice neurons |
(100) |
Aβ = amyloid beta; AD = Alzheimer’s disease; apoJ = apolipoprotein J; APP = amyloid precursor protein; APP-CTF = amyloid precursor protein C-terminal fragment; CSF = cerebrospinal fluid; HBME = human brain micro-endothelial cells; HEK cells = human embryonic kidney cells; LRP = low density lipoprotein receptor-related protein; OD = oral dose; PBCE = porcine brain capillary endothelial cells; 3x Tg AD mice = transgenic Alzheimer’s disease mice.
Antihypertensive and heart failure drug treatment
Most classes of antihypertensive drugs used in clinical practice influence APP/Aβ metabolism (Table 3). Inhibition of the angiotensin-converting enzyme increases Aβ1-40 or Aβ1-42 availability due to attenuation of its breakdown (65) or through blockade of Aβ1-42 conversion to Aβ1-40 (65), respectively. Consequently, plasma levels of Aβ1-42 were found to increase after angiotensin-converting enzyme inhibition, but results of Aβ1-40 levels were not consistent, showing either increase or no change (Table 3). The favorable effects of angiotensin receptor antagonists on Aβ metabolism shown in the central nervous system (Table 3) have not been investigated on the cardiovascular system in humans, similar to the effect of β-blockers, calcium-channel blockers, and diuretic agents (Table 3).
Table 3.
Off-Target Effects of Antihypertensives and Heart Failure Treatment on Aβ Metabolism and Accumulation
| Intervention/Condition | Cell Type/Population | Effects on Aβ Metabolism | Ref. # |
|---|---|---|---|
| ACE Inhibitors | |||
| Captopril | CHO cells, HEK293 cells |
ACE degrades Aβ1-40 and -42 ACE inhibition increases total Aβ levels |
(65) |
| Captopril | Tg2576 mice, Post-mortem human brain tissue |
ACE converts Aβ1-42 to Aβ1-40 ACE inhibition increases Aβ1-42 deposition in human and mice neurons |
(101) |
| Trandolapril | Tg2576 mice | Decreased brain Aβ1-40 and Aβ1-42 Increased plasma Aβ1-40 and Aβ1-42 (x2.5) |
(102) |
| Lisinopril (2.5–80 mg daily) Enalapril (10 mg daily) Benazepril (10 mg daily) |
Observational clinical study of 22 patients with mild cognitive impairment | Increased Aβ1-42 levels and Aβ1-42/-40 ratio in plasma | (103) |
| ARBs | |||
| Losartan | SHRSP rats | Decreased content of Aβ1-40 (−30%) and Aβ1-42 (−25%) by enhancing insulin-degrading enzyme, neprilysin, and transthyretin expression in brain | (104) |
| Olmesartan | APP23 transgenic mice | Olmesartan prevents Aβ1-40 induced elevation of ROS Aβ burden not reduced in brain microvessels |
(105) |
| Candesartan | Primary neuron cultures from Tg2576 mouse embryos | Prevents Αβ1-40 and -42 aggregation and Aβ1-42 oligomerization in neurons | (106) |
| Losartan | Tg2576 mice | Reduced plasma and brain Aβ1-42 (−20%), while no changes in Aβ1-40 levels | (102) |
| Candesartan, irbesartan, olmesartan, valsartan, losartan, telmisartan eprosartan | Healthy elderly Cross-sectional study (n = 871) Prospective study (n = 124) |
Increased clearance of Aβ1-42 from the brain into CSF | (107) |
| ARNIs | |||
| Sacubitril/valsartan (400 mg OD) | Double-blind, randomized, placebo-controlled clinical study of 43 healthy subjects | Treatment increased CSF Aβ1-38 peptide and plasma Aβ1-40 levels (+50%) | (108) |
| B-Blockers | |||
| ICI 118,551 (beta-blocker used in experimental conditions) | C57 mice | β2 adrenergic receptor blockade attenuates acute stress-induced Aβ1-40 (−20%) and Aβ1-42 (−5%) in neurons | (109) |
| Propranolol | SAMP8 mice | Propranolol attenuates increases in Aβ1-42 and BACE1 and decreases in IDE expression by shifting APP cleavage to nonamyloidogenic pathway in neurons | (110) |
| Propranolol Carvedilol |
Tg2576 mice | Propranolol reduces plasma and brain Aβ1-40 (−40%) and Αβ1-42 (−50%) Carvedilol reduces brain Aβ1-40 and -42 levels |
(102) |
| Carvedilol | N2a cells | Protective against endogenous Aβ-induced neurotoxicity in neuronal N2a cells | (111) |
| CCBs | |||
| Nilvadipine, nitrendipine, amlodipine | TgPS1/APPsw mice or B6/SJL F1 mice | Nilvadipine and nitrendipine but not amlodipine (acute treatment) reduce brain content of Aβ probably by stimulating clearance through BBB | (112) |
| Nilvadipine (chronic treatment) reduces amyloid plaque burden in mouse brain | (112) | ||
| Nilvadipine, amlodipine, nifedipine, nitrendipine | TgPS1/APPsw mice | Nilvadipine and nitrendipine increase Aβ1-40 and Aβ1-42 plasma levels, while amlodipine and nifedipine had no effect on Aβ1-40 or Aβ1-42 plasma levels | (112) |
| Amlodipine, diltiazem, felodipine, isradipine, nifedipine, nicardipine, nimodipine, nisoldipine | H4 neuroglioma cells | Nifedipine reduces production of Aβ1-42 (−40%), by increasing α-secretase and diminishing γ-secretase activity | (113) |
| Nicardipine | Tg2576 mice | Nicardipine reduces plasma Aβ1-40 (−30%) and Αβ1-42 (−50%) | (102) |
| Nitrendipine | Primary neuron cultures generated from Tg2576 mouse embryos | Nitrendipine prevents Αβ1-40 and -42 aggregation and Aβ1-42 oligomerization in vitro | (106) |
| Diuretic Agents | |||
| Furosemide | Tg2576 mice | Aβ1-40 and -42 brain content decreased Plasma Aβ1-40 and -42 increased (×2) |
(102) |
| Furosemide | Neurons of Tg2576 mice | Furosemide prevents Αβ oligomerization in vitro and reduces amyloid burden (−30%) by dissociating pre-aggregated Aβ1-42 oligomers | (106) |
| Hemodialysis | |||
| Hemodialysis | Cross-sectional study of 30 CKD patients under hemodialysis | Hemodialysis removes blood Aβ1-40 and -42 while plasma Aβ remains decreased longitudinally | (114) |
| Hemodialysis | Prospective study of 26 CKD patients under hemodialysis | Plasma levels Aβ1-40 (−35%) and Αβ1-42 (−22%) reduced after 1 hemodialysis session | (115) |
| Hemodialysis | Prospective clinical study of 30 CKD hemodialysis patients | Long-term hemodialysis leads to reduced or unchanged plasma Aβ1-40 while plasma Aβ1-42 remains unchanged or increases | (116) |
| Hemodialysis | Cross-sectional study of 47 patients with CKD | Plasma levels of Aβ1-40 and -42 are reduced | (117) |
| Peritoneal dialysis | Cross-sectional study of 30 patients with CKD | Peritoneal dialysis decreases plasma levels Aβ1-40 and -42 | (118) |
Aβ = amyloid beta; ACE = angiotensin-converting enzyme; ARBs = angiotensin receptor blockers; ARNIs = angiotensin receptor/neprilysin inhibitors; BBB = blood brain barrier; CCBs = calcium-channel blockers; CHO cells = Chinese hamster ovary cells; CKD = chronic kidney disease; CSF = cerebrospinal fluid; HEK cells = human embryonic kidney cells; IDE = insulin degrading enzyme; ROS = reactive oxygen species; SAMP8 = senescence-accelerated mouse model; SHRSP rats = stroke-prone spontaneously hypertensive rats.
A new heart failure drug class, the angiotensin receptor-neprilysin inhibitors, involves the inhibition of neprilysin, which is an Αβ degrading enzyme and thus may increase Aβ1-40 plasma levels (Table 3). In light of new evidence showing that Aβ1-40 blood levels are associated with increased mortality in patients with heart failure not receiving angiotensin receptor-neprilysin inhibitors (54) and that Aβ1-40 is widely expressed in the myocardium of patients with heart failure (48), it remains unknown whether some beneficiary effects of angiotensin receptor-neprilysin inhibitors may be partly offset due to increased systemic Aβ1-40 availability. This may be particularly important in regard to long-term outcomes, as deposition diseases need time to evolve. Peritoneal dialysis and hemodialysis reduce plasma levels of Aβ1-40 and -42 (Table 3), supporting the significance of Αβ renal clearance indicating a definite interventional target on Aβ1-40 availability.
Antithrombotic agents
Although some evidence indicates that at low concentrations, anticoagulant agents may increase Aβ metabolism, most experimental studies indicate that mainly due to their glycosaminoglycan structure, heparin and enoxaparin inhibit Aβ neurotoxic effects by affecting APP function and BACE1 activity (Online Table 5). However, whether these protective effects are extended systemically to the cardiovascular system merits further investigation. In contrast, 1 experimental study showed that treatment of C57BL/6 mice with anticoagulants greatly increased plasma levels of Aβ (>20-fold) (66) through down-regulation of the factor XII–factor VII pathway, which is involved in Aβ degradation (66). Clopidogrel or aspirin may interfere with APP/Aβ generation from platelets, but further studies are needed to confirm this relationship (Online Table 5).
Finally, although most phase III trials assessing antiamyloid-specific, targeted therapies were negative regarding efficacy in AD (67), their impact on CVD is unknown and merits further investigation.
Conclusions and Future Directions
Several issues merit clarification. Although patients with CAD are more likely to develop AD-like neuropathological lesions than those without CAD (68), whether atherogenesis occurs in parallel or independently from brain parenchyma amyloid load in humans is unknown. In B6Tg2576 mice, brain Aβ load is positively correlated with the area of aortic atherosclerotic lesions, while APP23/ApoE−/− mice developed aortic atherosclerotic lesions well before any parenchymal brain depositions (62). The association between Αβ1-40 and normal or premature cardiovascular aging needs to be further elucidated. Understanding the mechanisms responsible for the vascular preference of Aβ1-40 over -42 can elucidate the precise biological role of this peptide in the complex pathophysiology of vascular inflammation.
A pathophysiological role of Aβ1-40 across the continuum of cardiovascular disease is suggested through its independent association with a broad spectrum of vascular and cardiac involvement from early functional vascular alterations and subclinical atherosclerosis to overt symptomatic CAD, ACS, and heart failure. This is robustly supported by experimental evidence that APP and Aβ1-40 are critically involved in vascular inflammation, vascular and cardiac aging, and atherothrombosis. The association of Aβ1-40 with mortality has been consistently shown in a total population of about 5,000 patients in 6 independent cohorts derived from 8 countries. Thus, Aβ1-40 fulfills several criteria for consideration as a new biomarker for risk stratification in cardiovascular disease, including proof of concept, clinical utility, prospective validation, incremental and reclassification value for risk prediction, and ease of use. The implementation of a universally accepted method of sampling, preparation, storage, and measurement of circulating Aβ1-40 in plasma and the definition of normal and reference values as well as the conduction of studies with strict protocols of measurement in well-defined populations will allow the clinical application of this peptide as a new risk biomarker in patients with established cardiovascular disease. Interestingly, the association of Aβ1-40 with subclinical functional vascular alterations in healthy individuals and its association with all-cause mortality in the general population indicate that it should be further tested as a possible biomarker of cardiovascular risk in primary prevention as well. Most importantly, multiple lines of evidence clearly indicate that manipulating APP/Aβ turnover and aggregation or blocking its inflammatory reactions is feasible, potentially improving our understanding and means to simultaneously protect the brain, heart, and vessels during physiological or premature aging.
Acknowledgment
The authors express their gratitude to Dr. Kerida Shook for proofreading the manuscript.
Footnotes
This work was supported by the European Research Council (MODVASC grant) and the DFG SFB834 (grant number 75732319) (to Dr. Stellos). Dr. Stellos has received fees for being on the regional advisory board for Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For an expanded Methods section and supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Gottesman R.F., Albert M.S., Alonso A. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deschaintre Y., Richard F., Leys D., Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–680. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- 3.Roher A.E., Tyas S.L., Maarouf C.L. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement. 2011;7:436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breteler M.M., Claus J.J., Grobbee D.E., Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 6.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 7.Koyama A., Okereke O.I., Yang T., Blacker D., Selkoe D.J., Grodstein F. Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassar R., Bennett B.D., Babu-Khan S. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 9.Tanzi R.E., Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Kang J., Lemaire H.G., Unterbeck A. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D.K., Choi S.H., Washicosky K.J. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf1059. 340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Nostrand W.E., Schmaier A.H., Farrow J.S., Cunningham D.D. Platelet protease nexin-2/amyloid beta-protein precursor. Possible pathologic and physiologic functions. Ann N Y Acad Sci. 1991;640:140–144. doi: 10.1111/j.1749-6632.1991.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 13.Obregon D., Hou H., Deng J. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Kant R., Goldstein L.S. Cellular functions of the amyloid precursor protein from development to dementia. Dev Cell. 2015;32:502–515. doi: 10.1016/j.devcel.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Mawuenyega K.G., Sigurdson W., Ovod V. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casoli T., Di Stefano G., Giorgetti B. Release of beta-amyloid from high-density platelets: implications for Alzheimer's disease pathology. Ann N Y Acad Sci. 2007;1096:170–178. doi: 10.1196/annals.1397.082. [DOI] [PubMed] [Google Scholar]
- 17.Burgermeister P., Calhoun M.E., Winkler D.T., Jucker M. Mechanisms of cerebrovascular amyloid deposition: lessons from mouse models. Annals of the New York Academy of Sciences. 2000;903:307–316. doi: 10.1111/j.1749-6632.2000.tb06381.x. [DOI] [PubMed] [Google Scholar]
- 18.Biffi A., Greenberg S.M. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisniewski H., Wegiel J., Vorbrodt A., Mazur-Kolecka B., Frackowiak J. Part I. Alzheimer's disease: vascular concepts, cellular issues, and genetics (plenary lectures)-role of perivascular cells and myocytes in vascular amyloidosis. Ann N Y Acad Sci. 2000;903:6–18. doi: 10.1111/j.1749-6632.2000.tb06344.x. [DOI] [PubMed] [Google Scholar]
- 20.Roher A.E., Esh C.L., Kokjohn T.A. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokjohn T.A., Van Vickle G.D., Maarouf C.L. Chemical characterization of pro-inflammatory amyloid-beta peptides in human atherosclerotic lesions and platelets. Biochim Biophys Acta. 2011;1812:1508–1514. doi: 10.1016/j.bbadis.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGowan E., Pickford F., Kim J. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer J.D., Simmons K., Parsadanian M. Human apolipoprotein E4 alters the amyloid-β 40: 42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert J., Button E.B., Yuen B. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. Eife. 2017;6 doi: 10.7554/eLife.29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Meyer G.R., De Cleen D.M., Cooper S. Platelet phagocytosis and processing of beta-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ Res. 2002;90:1197–1204. doi: 10.1161/01.res.0000020017.84398.61. [DOI] [PubMed] [Google Scholar]
- 26.Kitazume S., Yoshihisa A., Yamaki T. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets: a novel biomarker for acute coronary syndrome. J Biol Chem. 2012;287:40817–40825. doi: 10.1074/jbc.M112.398578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melchor J.P., Van Nostrand W.E. Fibrillar amyloid beta-protein mediates the pathologic accumulation of its secreted precursor in human cerebrovascular smooth muscle cells. J Biol Chem. 2000;275:9782–9791. doi: 10.1074/jbc.275.13.9782. [DOI] [PubMed] [Google Scholar]
- 28.Liu C.-C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verghese P.B., Castellano J.M., Garai K. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laina A., Stellos K., Stamatelopoulos K. Vascular ageing: underlying mechanisms and clinical implications. Exp Gerontol. 2018;109:16–30. doi: 10.1016/j.exger.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Deane R., Du Yan S., Submamaryan R.K. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 32.Park L., Zhou P., Pitstick R. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niwa K., Porter V.A., Kazama K., Cornfield D., Carlson G.A., Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Zhao C., Zhao A., Li M., Ren J., Qu X. New insights in amyloid beta interactions with human telomerase. J Am Chem Soc. 2015;137:1213–1219. doi: 10.1021/ja511030s. [DOI] [PubMed] [Google Scholar]
- 35.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 36.Hughes T.M., Kuller L.H., Barinas-Mitchell E.J. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes T.M., Kuller L.H., Barinas-Mitchell E.J. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatelopoulos K., Sibbing D., Rallidis L.S. Amyloid-beta (1-40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol. 2015;65:904–916. doi: 10.1016/j.jacc.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Bucerius J., Barthel H., Tiepolt S. Feasibility of in vivo (18)F-florbetaben PET/MR imaging of human carotid amyloid-beta. Eur J Nucl Med Mol Imaging. 2017;44:1119–1128. doi: 10.1007/s00259-017-3651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatelopoulos K., Pol C.J., Ayers C. Amyloid-beta (1-40) peptide and subclinical cardiovascular disease. J Am Coll Cardiol. 2018;72:1060–1061. doi: 10.1016/j.jacc.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janelidze S., Stomrud E., Palmqvist S. Plasma beta-amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roeben B., Maetzler W., Vanmechelen E. Association of plasma Abeta40 peptides, but not Abeta42, with coronary artery disease and diabetes mellitus. J Alzheimers Dis. 2016;52:161–169. doi: 10.3233/JAD-150575. [DOI] [PubMed] [Google Scholar]
- 43.Chong Y.H., Sung J.H., Shin S.A., Chung J.H., Suh Y.H. Effects of the beta-amyloid and carboxyl-terminal fragment of Alzheimer's amyloid precursor protein on the production of the tumor necrosis factor-alpha and matrix metalloproteinase-9 by human monocytic THP-1. J Biol Chem. 2001;276:23511–23517. doi: 10.1074/jbc.M009466200. [DOI] [PubMed] [Google Scholar]
- 44.Stamatelopoulos K., Mueller-Hennessen M., Georgiopoulos G. Amyloid-beta (1-40) and mortality in patients with non-ST-segment elevation acute coronary syndrome: a cohort study. Ann Intern Med. 2018;168:855–865. doi: 10.7326/M17-1540. [DOI] [PubMed] [Google Scholar]
- 45.Gabelle A., Schraen S., Gutierrez L.A. Plasma beta-amyloid 40 levels are positively associated with mortality risks in the elderly. Alzheimers Dement. 2015;11:672–680. doi: 10.1016/j.jalz.2014.04.515. [DOI] [PubMed] [Google Scholar]
- 46.Roffi M., Patrono C., Collet J.P. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 47.Amsterdam E.A., Wenger N.K., Brindis R.G. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Greco S., Zaccagnini G., Fuschi P. Increased BACE1-AS long noncoding RNA and beta-amyloid levels in heart failure. Cardiovasc Res. 2017;113:453–463. doi: 10.1093/cvr/cvx013. [DOI] [PubMed] [Google Scholar]
- 49.Troncone L., Luciani M., Coggins M. Abeta amyloid pathology affects the hearts of patients with Alzheimer's disease: mind the heart. J Am Coll Cardiol. 2016;68:2395–2407. doi: 10.1016/j.jacc.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnini S., Solito R., Cetti E. Abeta peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis. FASEB J. 2010;24:2385–2395. doi: 10.1096/fj.09-146456. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi S., Sato N., Yamamoto A. Alzheimer disease-associated peptide, amyloid beta40, inhibits vascular regeneration with induction of endothelial autophagy. Arterioscler Thromb Vasc Biol. 2009;29:1909–1915. doi: 10.1161/ATVBAHA.109.188516. [DOI] [PubMed] [Google Scholar]
- 52.Saido T.C., Yokota M., Maruyama K. Spatial resolution of the primary beta-amyloidogenic process induced in postischemic hippocampus. J Biol Chem. 1994;269:15253–15257. [PubMed] [Google Scholar]
- 53.Thomas T., Thomas G., McLendon C., Sutton T., Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 54.Bayes-Genis A., Barallat J., de Antonio M. Bloodstream Amyloid-beta (1-40) Peptide, Cognition, and Outcomes in Heart Failure. Rev Esp Cardiol (Engl Ed) 2017;70:924–932. doi: 10.1016/j.rec.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Loffredo F.S., Nikolova A.P., Pancoast J.R., Lee R.T. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsiao K., Chapman P., Nilsen S. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 57.Davis J., Xu F., Deane R. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 58.Giokarini T., Bonafini L., Shearman M.S., Hill R.G., Longmore J. Beta-Amyloid (A beta 1-40)-evoked changes in vascular reactivity are mediated via an endothelium-specific mechanism: studies using rabbit isolated aorta. Ann N Y Acad Sci. 1997;826:475–478. doi: 10.1111/j.1749-6632.1997.tb48507.x. [DOI] [PubMed] [Google Scholar]
- 59.Niwa K., Younkin L., Ebeling C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L., Cao D., Garber D.W., Kim H., Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol. 2003;163:2155–2164. doi: 10.1016/s0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarre A., Gowert N.S., Donner L. Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer's disease. Cell Signal. 2014;26:2040–2050. doi: 10.1016/j.cellsig.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 62.Tibolla G., Norata G.D., Meda C. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis. 2010;210:78–87. doi: 10.1016/j.atherosclerosis.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 63.Van De Parre T.J., Guns P.J., Fransen P. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis. 2011;216:54–58. doi: 10.1016/j.atherosclerosis.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Gonzalez I., Estrada L.D., Sanchez-Mejias E., Soto C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer's disease. Nat Commun. 2013;4:1495. doi: 10.1038/ncomms2494. [DOI] [PubMed] [Google Scholar]
- 65.Hemming M.L., Selkoe D.J. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem. 2005;280:37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L., Bhattacharya A., Li Y., Zhang Y. Anticoagulants inhibit proteolytic clearance of plasma amyloid beta. Oncotarget. 2018;9:5614–5626. doi: 10.18632/oncotarget.23718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makin S. The amyloid hypothesis on trial. Nature. 2018;559:S4–S7. doi: 10.1038/d41586-018-05719-4. [DOI] [PubMed] [Google Scholar]
- 68.de la Torre J.C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 69.Austin S.A., Sens M.A., Combs C.K. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci. 2009;29:14451–14462. doi: 10.1523/JNEUROSCI.3107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vukic V., Callaghan D., Walker D. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fonseca A.C., Ferreiro E., Oliveira C.R., Cardoso S.M., Pereira C.F. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim Biophys Acta. 2013;1832:2191–2203. doi: 10.1016/j.bbadis.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Price J.M., Chi X., Hellermann G., Sutton E.T. Physiological levels of beta-amyloid induce cerebral vessel dysfunction and reduce endothelial nitric oxide production. Neurol Res. 2001;23:506–512. doi: 10.1179/016164101101198758. [DOI] [PubMed] [Google Scholar]
- 73.Miller T.W., Isenberg J.S., Shih H.B., Wang Y., Roberts D.D. Amyloid-beta inhibits No-cGMP signaling in a CD36- and CD47-dependent manner. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sondag C.M., Combs C.K. Amyloid precursor protein mediates proinflammatory activation of monocytic lineage cells. J Biol Chem. 2004;279:14456–14463. doi: 10.1074/jbc.M313747200. [DOI] [PubMed] [Google Scholar]
- 75.Sondag C.M., Combs C.K. Adhesion of monocytes to type I collagen stimulates an APP-dependent proinflammatory signaling response and release of Abeta1-40. J Neuroinflammation. 2010;7:22. doi: 10.1186/1742-2094-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiala M., Zhang L., Gan X. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood-brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- 77.Giri R.K., Selvaraj S.K., Kalra V.K. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- 78.Giri R.K., Rajagopal V., Shahi S., Zlokovic B.V., Kalra V.K. Mechanism of amyloid peptide induced CCR5 expression in monocytes and its inhibition by siRNA for Egr-1. Am J Physiol Cell Physiol. 2005;289:C264–C276. doi: 10.1152/ajpcell.00461.2004. [DOI] [PubMed] [Google Scholar]
- 79.Bamberger M.E., Harris M.E., McDonald D.R., Husemann J., Landreth G.E. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giri R., Shen Y., Stins M. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 81.Schulz B., Liebisch G., Grandl M., Werner T., Barlage S., Schmitz G. Beta-amyloid (Abeta40, Abeta42) binding to modified LDL accelerates macrophage foam cell formation. Biochim Biophys Acta. 2007;1771:1335–1344. doi: 10.1016/j.bbalip.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 82.El Khoury J.B., Moore K.J., Means T.K. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moore K.J., El Khoury J., Medeiros L.A. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 84.Henry A., Li Q.X., Galatis D. Inhibition of platelet activation by the Alzheimer's disease amyloid precursor protein. Br J Haematol. 1998;103:402–415. doi: 10.1046/j.1365-2141.1998.01005.x. [DOI] [PubMed] [Google Scholar]
- 85.Herczenik E., Bouma B., Korporaal S.J. Activation of human platelets by misfolded proteins. Arterioscler Thromb Vasc Biol. 2007;27:1657–1665. doi: 10.1161/ATVBAHA.107.143479. [DOI] [PubMed] [Google Scholar]
- 86.Shen M.Y., Hsiao G., Fong T.H., Chou D.S., Sheu J.R. Expression of amyloid beta peptide in human platelets: pivotal role of the phospholipase Cgamma2-protein kinase C pathway in platelet activation. Pharmacol Res. 2008;57:151–158. doi: 10.1016/j.phrs.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Sonkar V.K., Kulkarni P.P., Dash D. Amyloid beta peptide stimulates platelet activation through RhoA-dependent modulation of actomyosin organization. FASEB J. 2014;28:1819–1829. doi: 10.1096/fj.13-243691. [DOI] [PubMed] [Google Scholar]
- 88.Canobbio I., Guidetti G.F., Oliviero B. Amyloid beta-peptide-dependent activation of human platelets: essential role for Ca2+ and ADP in aggregation and thrombus formation. Biochem J. 2014;462:513–523. doi: 10.1042/BJ20140307. [DOI] [PubMed] [Google Scholar]
- 89.Gowert N.S., Donner L., Chatterjee M. Blood platelets in the progression of Alzheimer's disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canobbio I., Catricala S., Di Pasqua L.G. Immobilized amyloid Abeta peptides support platelet adhesion and activation. FEBS Lett. 2013;587:2606–2611. doi: 10.1016/j.febslet.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 91.Zamolodchikov D., Renne T., Strickland S. The Alzheimer's disease peptide beta-amyloid promotes thrombin generation through activation of coagulation factor XII. J Thromb Haemost. 2016;14:995–1007. doi: 10.1111/jth.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Friedhoff L.T., Cullen E.I., Geoghagen N.S., Buxbaum J.D. Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol. 2001;4:127–130. doi: 10.1017/S1461145701002310. [DOI] [PubMed] [Google Scholar]
- 93.Sjogren M., Gustafsson K., Syversen S. Treatment with simvastatin in patients with Alzheimer's disease lowers both alpha- and beta-cleaved amyloid precursor protein. Dement Geriatr Cogn Disord. 2003;16:25–30. doi: 10.1159/000069989. [DOI] [PubMed] [Google Scholar]
- 94.Ishii K., Tokuda T., Matsushima T. Pravastatin at 10 mg/day does not decrease plasma levels of either amyloid-beta (Abeta) 40 or Abeta 42 in humans. Neurosci Lett. 2003;350:161–164. doi: 10.1016/s0304-3940(03)00895-4. [DOI] [PubMed] [Google Scholar]
- 95.Hoglund K., Wiklund O., Vanderstichele H., Eikenberg O., Vanmechelen E., Blennow K. Plasma levels of beta-amyloid(1-40), beta-amyloid(1-42), and total beta-amyloid remain unaffected in adult patients with hypercholesterolemia after treatment with statins. Arch Neurol. 2004;61:333–337. doi: 10.1001/archneur.61.3.333. [DOI] [PubMed] [Google Scholar]
- 96.Serrano-Pozo A., Vega G.L., Lutjohann D. Effects of simvastatin on cholesterol metabolism and Alzheimer disease biomarkers. Alzheimer Dis Assoc Disord. 2010;24:220–226. doi: 10.1097/WAD.0b013e3181d61fea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fassbender K., Simons M., Bergmann C. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parsons R.B., Price G.C., Farrant J.K., Subramaniam D., Adeagbo-Sheikh J., Austen B.M. Statins inhibit the dimerization of beta-secretase via both isoprenoid- and cholesterol-mediated mechanisms. Biochem J. 2006;399:205–214. doi: 10.1042/BJ20060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinohara M., Sato N., Kurinami H. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem. 2010;285:22091–22102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zandl-Lang M., Fanaee-Danesh E., Sun Y. Regulatory effects of simvastatin and apoJ on APP processing and amyloid-beta clearance in blood-brain barrier endothelial cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:40–60. doi: 10.1016/j.bbalip.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Zou K., Yamaguchi H., Akatsu H. Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27:8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J., Zhao Z., Lin E. Unintended effects of cardiovascular drugs on the pathogenesis of Alzheimer's disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Regenold W.T., Blumenthal J.B., Loreck D.J. Elevated plasma Abeta42 in cognitively impaired individuals taking ACE inhibitor antihypertensives. Am J Alzheimers Dis Other Demen. 2017;32:347–352. doi: 10.1177/1533317517707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drews H.J., Yenkoyan K., Lourhmati A. Intranasal losartan decreases perivascular beta amyloid, inflammation, and the decline of neurogenesis in hypertensive rats. Neurotherapeutics. 2019;16:725–740. doi: 10.1007/s13311-019-00723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takeda S., Sato N., Takeuchi D. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 106.Zhao W., Wang J., Ho L., Ono K., Teplow D.B., Pasinetti G.M. Identification of antihypertensive drugs which inhibit amyloid-beta protein oligomerization. J Alzheimers Dis. 2009;16:49–57. doi: 10.3233/JAD-2009-0925. [DOI] [PubMed] [Google Scholar]
- 107.Nation D.A., Ho J., Yew B. for the Alzheimer's Disease Neuroimaging Initiative. Older adults taking AT1-receptor blockers exhibit reduced cerebral amyloid retention. J Alzheimers Dis. 2016;50:779–789. doi: 10.3233/JAD-150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Langenickel T.H., Tsubouchi C., Ayalasomayajula S. The effect of LCZ696 (sacubitril/valsartan) on amyloid-beta concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol. 2016;81:878–890. doi: 10.1111/bcp.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu N.N., Wang X.X., Yu J.T. Blocking beta2-adrenergic receptor attenuates acute stress-induced amyloid beta peptides production. Brain Res. 2010;1317:305–310. doi: 10.1016/j.brainres.2009.12.087. [DOI] [PubMed] [Google Scholar]
- 110.Dobarro M., Orejana L., Aguirre N., Ramirez M.J. Propranolol restores cognitive deficits and improves amyloid and Tau pathologies in a senescence-accelerated mouse model. Neuropharmacology. 2013;64:137–144. doi: 10.1016/j.neuropharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 111.Liu J., Wang M. Carvedilol protection against endogenous Abeta-induced neurotoxicity in N2a cells. Cell Stress Chaperones. 2018;23:695–702. doi: 10.1007/s12192-018-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Paris D., Bachmeier C., Patel N. Selective antihypertensive dihydropyridines lower Abeta accumulation by targeting both the production and the clearance of Abeta across the blood-brain barrier. Mol Med. 2011;17:149–162. doi: 10.2119/molmed.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lovell M.A., Abner E., Kryscio R., Xu L., Fister S.X., Lynn B.C. Calcium channel blockers, progression to dementia, and effects on amyloid beta peptide production. Oxid Med Cell Longev. 2015;2015:787805. doi: 10.1155/2015/787805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitaguchi N., Tatebe H., Sakai K. Influx of tau and amyloid-beta proteins into the blood during hemodialysis as a therapeutic extracorporeal blood amyloid-beta removal system for Alzheimer's disease. J Alzheimers Dis. 2019;69:687–707. doi: 10.3233/JAD-190087. [DOI] [PubMed] [Google Scholar]
- 115.Tholen S., Schmaderer C., Chmielewski S. Reduction of Amyloid-beta plasma levels by hemodialysis: an anti-amyloid treatment strategy? J Alzheimers Dis. 2016;50:791–796. doi: 10.3233/JAD-150662. [DOI] [PubMed] [Google Scholar]
- 116.Kitaguchi N., Hasegawa M., Ito S. A prospective study on blood Abeta levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer's disease. J Neural Transm. 2015;122:1593–1607. doi: 10.1007/s00702-015-1431-3. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y.H., Xiang Y., Wang Y.R. Association between serum amyloid-beta and renal functions: implications for roles of kidney in amyloid-beta clearance. Mol Neurobiol. 2015;52:115–119. doi: 10.1007/s12035-014-8854-y. [DOI] [PubMed] [Google Scholar]
- 118.Jin W.S., Shen L.L., Bu X.L. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017;134:207–220. doi: 10.1007/s00401-017-1721-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.